Abstract
AII amacrine cells play a crucial role in retinal signal transmission under scotopic conditions. We have used rat retinal slices to investigate the functional properties of inhibitory glycine receptors on AII cells by recording spontaneous IPSCs (spIPSCs) in whole cells and glycine-evoked responses in outside-out patches. Glycinergic spIPSCs displayed fast kinetics with an average 10–90% rise time of ∼500 μs, and a decay phase best fitted by a double-exponential function with τfast ∼ 4.8 ms (97.5% amplitude contribution) and τslow ∼ 33 ms. Decay kinetics were voltage dependent. Ultrafast application of brief (∼2–5 ms) pulses of glycine (3 mm) to patches, evoked responses with fast deactivation kinetics best fitted with a double-exponential function with τfast ∼ 4.6 ms (85% amplitude contribution) and τslow ∼ 17 ms. Double-pulse experiments indicated recovery from desensitization after a 100-ms pulse of glycine with a double-exponential time course (τfast ∼ 71 ms and τslow ∼ 1713 ms). Non-stationary noise analysis of spIPSCs and patch responses, and directly observed channel gating yielded similar single-channel conductances (∼41 to ∼47 pS). In addition, single-channel gating occurred at ∼83 pS. These results suggest that the fast glycinergic spIPSCs in AII cells are probably mediated by α1β heteromeric receptors with a contribution from α1 homomeric receptors. We hypothesize that glycinergic synaptic input may target the arboreal dendrites of AII cells, and could serve to shunt excitatory input from rod bipolar cells and transiently uncouple the transcellular current through electrical synapses between AII cells and between AII cells and ON-cone bipolar cells.
In the central nervous system, diversity among postsynaptic neurotransmitter receptors generates diversity in synaptic transmission properties and appears to be an important mechanism for neural signal processing, e.g. by filtering similar presynaptic signals through different postsynaptic receptors (DeVries, 2000). Glycine is an important inhibitory neurotransmitter in spinal cord, brainstem and retina, and exerts its action by activating receptors with an integral chloride-selective channel (reviewed by Lynch, 2004). Five different subunits (α1–α4, β) have been cloned, and functional glycine receptors are pentameric receptors, either α homomers or αβ heteromers. Depending on the subunit composition, glycine receptors display marked variability, including single-channel conductance and kinetic properties (reviewed by Legendre, 2001). In the spinal cord and brainstem, there is strong evidence for differential expression of glycine receptor subunits during development, but much less so in the mature brain. During development, glycine receptor expression changes from α2 homomeric or α2β heteromeric channels with slow kinetics to α1β heteromeric channels with fast kinetics (Becker et al. 1988; Takahashi et al. 1992). This switch is accompanied by a functional change from slowly to rapidly decaying glycinergic synaptic responses (Takahashi et al. 1992; Singer et al. 1998).
In the mammalian retina, glycine is employed as an inhibitory neurotransmitter in ∼50% of all amacrine cells (Pourcho, 1996; Menger et al. 1998). Amacrine cells are local circuit interneurons that receive synaptic input from bipolar cells and other amacrine cells, and send output to bipolar cells, ganglion cells and other amacrine cells. In contrast to the spinal cord and brainstem, there is strong evidence for differential expression of α1-, α2-, α3- and β-subunits among neurons in the mature retina (Grünert & Wässle, 1993; Haverkamp et al. 2003, 2004), and there is some ultrastructural evidence for synaptic localization (Sassoè-Pognetto et al. 1994). Glycinergic synaptic currents have been detected in amacrine cells (Frech et al. 2001), bipolar cells (Cui et al. 2003; Ivanova et al. 2006) and ganglion cells (Protti et al. 1997; Tian et al. 1998), with little evidence for differences in kinetic properties of glycinergic synaptic currents within a class of cells. Indeed, it has been suggested that amacrine cells only express glycine receptors with slow kinetic properties (Frech et al. 2001).
In this study, we have investigated the functional characteristics of glycine receptors in the narrow-field AII amacrine cell. This cell plays a crucial role in retinal signal transmission in the rod pathway. It receives excitatory input from rod bipolar cells and sends its output to ON-cone bipolar cells via electrical synapses, and to OFF-cone bipolar cells via glycinergic, inhibitory synapses (Bloomfield & Dacheux, 2001). Surprisingly, glycinergic, spontaneous inhibitory postsynaptic currents (spIPSCs) in AII amacrine cells displayed very fast decay kinetics, best fitted by a double-exponential function (τfast ∼ 4.8 ms and τslow ∼ 33 ms). Correspondingly, ultrafast application of brief (∼2–5 ms) pulses of glycine (3 mm) to patches evoked responses with similar, fast deactivation kinetics (τfast≈ 4.6 ms and τslow≈ 17 ms). Non-stationary noise analysis of spIPSCs and patch responses, and directly observed channel gating yielded similar single-channel conductances (∼41 to ∼47 pS). In addition, single-channel gating occurred at ∼83 pS.
Methods
General aspects of the methods have previously been described in detail (Hartveit, 1996; Veruki et al. 2003). Albino rats (4–7 weeks postnatal) were deeply anaesthetized with halothane in oxygen and killed by cervical dislocation (procedure approved under the surveillance of the Norwegian Animal Research Authority). Retinal slices were visualized with a ×40 water immersion objective and infrared differential interference contrast videomicroscopy. When filled with intracellular solution, recording pipettes typically had resistances of 4–6 MΩ for recordings in the whole-cell configuration and 5–8 MΩ for recordings in the outside-out patch configuration. All patches were isolated from the soma of the recorded cells. For some outside-out recordings, pipettes were coated with dental wax and fire-polished immediately before use. All recordings were carried out at room temperature (20–23°C), except where explicitly indicated at an elevated temperature of 34°C (Veruki et al. 2003).
Solutions and drugs
The extracellular perfusing solution was continuously bubbled with 95% O2–5% CO2 and had the following composition (mm): 125 NaCl, 25 NaHCO3, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 10 glucose, pH 7.4. The recording pipettes (whole-cell and patch) were filled with (mm): 130 KCl, 10 Hepes, 1 CaCl2, 8 NaCl, 5 ethylene glycol-O,O′-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA), 4 magnesium adenosine 5′-triphosphate (MgATP), 2 N-(2,6- dimethylphenylcarbamoylmethyl)triethylammonium bromide (QX-314; Tocris Cookson, Bristol, UK). pH was adjusted to 7.3 with KOH. In experiments where the membrane potential was varied in order to examine current-voltage (I–V) relationships and reversal potentials (Erev) of currents, the pipette solution contained (mm): 125 CsCl, 10 Hepes, 1 CaCl2, 8 NaCl, 5 EGTA, 4 MgATP, 15 tetraethylammonium chloride. Alternatively, an intracellular solution containing reduced chloride was used (mm): 125 CsCH3SO3, 8 NaCl, 10 Hepes, 1 CaCl2, 5 EGTA, 4 MgATP, 15 tetraethylammonium chloride. pH was adjusted to 7.3 with CsOH. Lucifer yellow was added at a concentration of 1 mg ml−1 to the intracellular solutions for visualization of cells at the end of the recordings (whole-cell and patch). Theoretical liquid junction potentials were calculated with the computer program JPCalcW (Molecular Devices, Sunnyvale, CA, USA), and membrane holding potentials were automatically corrected for liquid junction potentials online.
Drugs were added directly to the extracellular solution used to perfuse the slices. The concentrations of the drugs were as follows (μm; supplied by Tocris Bioscience, Avonmouth, UK, unless otherwise noted): 10 bicuculline methchloride, 10 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 0.3 strychnine (Research Biochemicals, Natick, MA, USA) and 0.3 tetrodotoxin (TTX). For recordings of inhibitory postsynaptic currents (PSCs), the extracellular solution contained 10 μm CNQX to block non-NMDA receptors and 0.3 μm TTX to block voltage-gated Na+ channels (Boos et al. 1993). Solutions were either made up freshly for each experiment or prepared from concentrated aliquots stored at −20°C. CNQX was first dissolved at 100 mm in dimethylsulfoxide (Sigma, St Louis, MO, USA), and then diluted to the final concentration by sonication. Pressure application of γ-aminobutyric acid (GABA) from a multibarrel pipette complex was performed as described in Hartveit (1996).
Fast drug application
Ultrafast drug application was performed according to the description of Jonas (1995), and as detailed in Veruki et al. (2003). Drugs were applied from a theta-tube application pipette (septum thickness ∼117 μm; final tip diameter ∼300 μm; Hilgenberg, Malsfeld, Germany). The pipette tip with the outside-out patch was positioned near the interface between the control solution and agonist-containing solution continuously flowing out of each barrel, about 100 μm away from the tip of the application pipette. Concentration jumps of agonist to the patch were performed by rapidly moving the application pipette and thus the interface between the two solutions. Drugs were dissolved in Hepes-buffered solution containing (mm): 145 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 5 hemisodium-Hepes, 10 glucose, pH adjusted to 7.4 with HCl. For any concentration of glycine (May and Baker Ltd, Dagenham, UK), it replaced an equimolar concentration of NaCl. Agonist pulses were applied every 3–10 s. The solution exchange time was measured as previously described (Veruki et al. 2003). Under optimal conditions, the 20–80% rise time of the solution exchange ranged from 175 to 500 μs.
Electrophysiological recording and data acquisition
Voltage-clamp recordings were made with either an EPC9-dual or EPC10-triple amplifier (HEKA Elektronik, Lambrecht, Germany) controlled by either Pulse or PatchMaster software (HEKA Elektronik). Cells and patches were held at a potential of −60 mV. The signals were typically low-pass filtered with a corner frequency (−3 dB) of 5 kHz, and sampled at 50 kHz. Capacitative currents caused by the recording pipette capacitance (Cfast) and the cell membrane capacitance (Cslow) were measured with the automatic capacitance neutralization network feature of the amplifier that also estimated the series resistance (Rseries). The average capacitance in the whole-cell recordings was 13.6 ± 0.6 (s.e.m.) pF (n = 55). Throughout every continuous recording of spontaneous synaptic currents, Rseries was regularly monitored (every 60 s) by acquiring the responses to a series of 20 mV hyperpolarizing voltage pulses (16 ms duration). During such stimulation, the Cslow neutralization circuitry was transiently disabled. The capacitative transients were analysed offline by averaging consecutive responses (typically 15–20) and fitting the decay with triple-exponential functions in order to estimate the peak capacitative current and calculate Rseries. The average Rseries was 17.0 ± 0.7 MΩ (n = 55). For cells included in kinetic analysis, the average Rseries was 15.5 ± 0.8 MΩ (n = 21). Cells with Rseries above 20 MΩ, or cells where Rseries varied by more than 20% during the recording, were excluded from kinetic analysis. Electronic compensation of Rseries has proven difficult with AII amacrine cells, presumably because of the extensive electrical coupling (Veruki & Hartveit, 2002a), and was not attempted for any of the recordings. Cells with a holding current larger than −70 pA at a holding potential of −60 mV were excluded from analysis. The average holding current was −34 ± 2.7 pA (n = 55).
For establishment of I–V relationships, sampling was repeated at a series of membrane command potentials. The potential was stepped to a new, constant value before the start of sampling (1–5 s for whole-cell recordings, 250 ms for patch recordings) in order to allow the membrane current to relax to a plateau level.
General data analysis
Data were analysed with PulseFit/PulseTools, FitMaster (HEKA Elektronik), Igor Pro (WaveMetrics, Lake Oswego, OR, USA), AxoGraph (Molecular Devices) and TAC (Bruxton Corp., Seattle, WA, USA). Spontaneous PSCs were detected with a threshold of 5–7 pA, depending on the noise level (MiniAnalysis; Synaptosoft, Decatur, GA, USA) and verified by eye. For amplitude and interevent interval analyses, complex PSCs, i.e. PSCs consisting of temporally overlapping events, were analysed as described by Veruki et al. (2003). Because of the low frequency of events in general, such complex PSCs were rare. For kinetic and non-stationary noise analyses, we included only well-separated (interevent intervals ≥ 40 ms), monophasic PSCs which appeared to rise in a monotonic fashion without visible deviation of the rising phase (cf. Traynelis et al. 1993), and which decayed exponentially. For kinetic analysis of averaged PSCs, the number of individual events for each cell ranged between 58 and 331. Before averaging, individual spPSCs were aligned along the point of steepest rise.
The decay time course of individual and averaged PSCs, as well as responses evoked by the ultrafast application of agonist, was estimated by curve fitting with exponential functions. For single-exponential functions we used the function:
| (1) |
where I(t) is the current as a function of time, A is the amplitude at time 0, τ is the time constant, and Iss is the steady-state current amplitude (typically zero). For double-exponential functions we used the function:
| (2) |
where I(t) is the current as a function of time, A1 and A2 are the amplitudes of the first and second exponential components, τ1 and τ2 are the time constants of the first (fast) and second (slow) exponential components and Iss is the steady-state current amplitude (typically zero). Fitting was started 300–750 μs after the peak amplitude (typically 500 μs for PSCs). For double-exponential functions the amplitude contribution was calculated as 100%× (Ax/(A1+A2)). The weighted decay time constant was calculated as (a1τ1+a2τ2), where a1 and a2 are the relative amplitudes of the two exponential components, and τ1 and τ2 are the corresponding time constants. For waveforms fitted with double-exponential functions (spontaneous PSCs and evoked responses in patches), we defined time 0 as the start of the response (determined by eye as the point in time at which the current rose from the baseline noise) (Otis et al. 1996). Temperature coefficients (Q10 values) for decay time constants, 10–90% rise times and peak amplitudes were calculated as in Veruki et al. (2003).
For measurements of Erev values, data points of I–V relationships were fitted with straight lines (for ECl ∼ 0 mV) or by third-order polynomial functions (for ECl ∼−43 mV). Erev was calculated as the intersection of the fitted line with the abscissa. For analysis of voltage-dependent kinetics, plots of the decay time constant versus membrane potential were fitted with the function:
| (3) |
where τ is the decay time constant, τ0 is the time constant at 0 mV, Em is the membrane potential and H is the change in membrane potential (in mV) for an e-fold increase in τ (Magleby & Stevens, 1972).
Concentration–response data were normalized to the response at a fixed concentration and fitted with a Hill-type equation of the following form:
| (4) |
where I is the response at a given concentration of agonist ([A]), Imax is the maximum response, EC50 is the agonist concentration giving rise to half-maximal response and nH is the Hill coefficient. For experiments with the glycine receptor antagonist strychnine, the glycine-evoked response at each concentration of strychnine was normalized to the response in the absence of strychnine, and data points were plotted as glycine-evoked response versus strychnine concentration. Concentration–inhibition curves were estimated by fitting with an equivalent equation as above, yielding values for IC50 (strychnine concentration giving rise to half-maximal inhibition) and nH.
In cases where step transitions corresponding to single-channel gating could be directly observed in the later portions of glycine responses, all-point amplitude histograms were constructed from selected epochs, and fitted with sums of Gaussian distributions to obtain the mean current of the open level (TAC; maximum likelihood algorithm). The single-channel current was taken as the difference between the open-channel peak and the baseline. The single-channel chord conductance was calculated as:
| (5) |
from the known holding potential (Em; −60 mV) and assuming Erev = 0 mV.
Data are presented as means ±s.e.m. (n = number of cells, events or patches), and percentages are presented as percentage of control. Statistical analyses with comparisons between groups were performed using Student's paired t test (two-tailed, unless otherwise stated) or the Kolmogorov–Smirnov test where appropriate. Correlations (either between time and a parameter for stability analysis or between two parameters) were performed using Spearman's rank order correlation test. Differences were considered significant at the P < 0.05 level. For illustration purposes, most raw data records were low-pass filtered (digital non-lagging Gaussian filter; −3 dB at 1–4 kHz; 1 kHz for traces with discrete single-channel transitions). Unless otherwise noted, the current traces in the figures represent individual traces.
Non-stationary noise analysis
To obtain the conductance of glycine receptor-channels in outside-out patches, we applied non-stationary noise analysis (Sigworth, 1980) to responses evoked by ultrafast applications of glycine (3 mm; 2 or 5 ms pulses; 23–150 repetitions evoked every 3 s). The sampling frequency was 50 kHz and the records were low-pass filtered at 5 or 10 kHz. Only epochs without rundown were used for non-stationary noise analysis. The ensemble mean response was binned into 30–50 segments along the ordinate, such that each bin, on the average, corresponded to an equal number of channel closings during the decay phase (Traynelis et al. 1993). The ensemble variance was plotted against the mean current (omitting the rising phase of the response) and fitted with the function:
| (6) |
where i is the apparent single-channel current, I is the mean current, N is the number of available channels in the patch and σ2b is the variance of the background noise. The open probability (Popen) at any given time is determined by the equation Popen = I/iN. The single-channel conductance was calculated by eqn (5).
To obtain the conductance of synaptic glycine receptor-channels, we applied peak-scaled non-stationary noise analysis (Traynelis et al. 1993) to ensembles of spPSCs (low-pass filtered at 5 kHz and sampled at 50 kHz). For selection of spPSCs for noise analysis, we followed the procedure described by Momiyama et al. (2003). Briefly, individual spPSCs were first low-pass filtered at 2 kHz (digital Gaussian filter in AxoGraph) and analysed by measuring peak amplitude, 10–90% rise time and decay time constant. For the latter measurement, we used a single-exponential function, despite the finding (see Results) that ensemble averages for most cells were best fitted with double-exponential functions. Measured parameters were then numbered according to the event number and tested by Spearman's rank order correlation test for time stability (using a variable size, sliding window algorithm implemented in Igor Pro, code adapted from that in the Neuromatic package; http://www.physiol.ucl.ac.uk/research/silver_a/). From the complete recording of an individual cell, the algorithm returned a maximum number of consecutive spPSCs whose parameters did not display any significant correlations (P > 0.05). After testing for correlations between rise time and amplitude, between amplitude and decay time constant and between rise time and decay time constant (Spearman's rank order correlation test; see Results), spPSCs were used for noise analysis without the imposed digital (2 kHz) Gaussian filter. The number of events for each cell that passed these criteria and were used for noise analysis generally represented 95–100% of the total number of events recorded from a given cell.
To correct for quantal variability (Traynelis et al. 1993), the synaptic currents were analysed with peak-scaled non-stationary noise analysis. Before calculating the ensemble mean PSC, the individual spPSCs were aligned along the point of steepest rise between onset and peak. The peak of the mean current response waveform was scaled to the response value at the corresponding point in time of each individual event before subtraction to generate the difference waveforms. The ensemble mean PSC was binned (see above) and variance versus mean curves were plotted for the decay phase of the PSC. The curves typically displayed a parabolic shape, thus a large portion (>50%) of each curve was fitted with eqn (6) to obtain estimates for the single-channel current (i). The single-channel chord conductance was calculated from eqn (5).
Results
Identification of AII amacrine cells in retinal slices
This study includes results from 101 AII amacrine cells (55 whole-cell recordings and 46 outside-out patch recordings). All cells were visually targeted according to the following criteria: (1) location of the cell body at and across the border between the inner nuclear layer and the inner plexiform layer; (2) medium size of the cell body; and (3) a relatively thick primary dendrite that tapers as it descends into the inner plexiform layer. All cells were filled with Lucifer yellow and after both whole-cell and outside-out patch recordings, fluorescence microscopy allowed unequivocal identification of each cell's morphology (Fig. 1A). In most cells we observed characteristic unclamped action currents evoked by 5 mV depolarizing test pulses from a holding potential of −60 mV (Mørkve et al. 2002). When the intracellular pipette solution contained QX-314, a blocker of voltage-gated Na+-channels, spontaneous and evoked action currents disappeared 30–60 s after break-in.
Figure 1. Properties of spontaneous inhibitory postsynaptic currents (spIPSCs) in AII amacrine cells in the rat retinal slice preparation.
A, an AII amacrine cell in an in vitro slice preparation from rat retina. The cell is shown as a composite fluorescence photomicrograph (after filling with Lucifer yellow) overlaid on a retinal slice visualized with infrared differential interference contrast videomicroscopy. Scale bar, 10 μm. Data from same AII amacrine cell in B–E. B, two examples of rapidly decaying spIPSCs. C, average waveform of spIPSCs (166 events). D, interevent interval histogram of spIPSCs; double-exponential fit indicated by white line; bin width 120 ms. E, amplitude distribution of spIPSCs; notice skew towards larger amplitudes; bin width 2.5 pA. One event with a peak amplitude of 129 pA is not shown. Here and later, the noise distribution is shown as an unfilled histogram (peak scaled to the peak of the IPSC amplitude distribution). F, bath application of 300 nm strychnine rapidly blocks spIPSCs in an AII amacrine cell (because of the slow time scale, spIPSCs appear as downward spikes). G, block by bath application of 300 nm strychnine of spIPSCs in an AII amacrine cell is complete, and reverses slowly during washout of strychnine. Activity of spIPSCs measured as cumulative peak amplitude of all spIPSCs occurring during 60-s-long periods. H, concentration–response curve for block by strychnine (30–1000 nm) of spIPSCs in AII amacrine cells. Activity of spIPSCs measured as in G and normalized to the activity in the control condition (without strychnine; leftmost data point) for each concentration of strychnine. Data points plotted as mean ±s.e.m. The number of cells tested at each concentration of strychnine is indicated in the graph. Data points have been fitted with eqn (4). I, adding TTX (300 nm) to the bath does not change peak amplitude (left panel), 10–90% rise time (centre panel) or decay time constant (τdecay; single-exponential fit; right panel) of spIPSCs in an AII amacrine cell. Each graph shows the cumulative probability (relative frequency distribution) of each parameter for the population of events recorded in the control condition (continuous line; n = 146 events) and in the presence of TTX (broken line; n = 264 events).
Spontaneous PSCs in AII amacrine cells
To isolate the action of glycine receptors, whole-cell recordings were carried out in the presence of CNQX and TTX in order to block non-NMDA receptors and voltage-gated Na+ channels, respectively. In this condition, ∼50% of all AII amacrine cells recorded (93/177) displayed spPSCs (Fig. 1B and C). Cells without spPSCs, or cells that did not display more than 15 spPSCs over a time period of 3–5 min, were excluded from analysis. For the cells included in the material, the spPSCs occurred at a low, irregular frequency (0.45 ± 0.05 Hz, range 0.06–1.8 Hz, n = 55). Interevent interval and amplitude histograms were constructed from all spPSCs for 11 AII amacrine cells, each with more than 100 spPSCs (range 103–331 events). Histograms of interevent intervals could be well fitted by double-exponential functions with average time constants τ1 = 0.4 ± 0.2 s and τ2 = 1.6 ± 0.4 s (n = 11), indicating a tendency towards clustering of spPSCs (Fig. 1D). Amplitude histograms were skewed towards larger values. For the example shown in Fig. 1E, the mode was ∼10 pA and the mean was 25.1 ± 1.5 pA (range 4.7–129 pA). The coefficient of variation was 0.76 for this cell. For the 11 AII amacrine cells, the mode occurred at 12.3 ± 1.7 pA (range 7.5–25 pA), and the mean was 22.5 ± 2.0 pA (range 14–39 pA). The coefficient of variation varied between 0.51 and 0.80 (average 0.61 ± 0.03).
The specific glycine receptor antagonist strychnine has been shown to block responses evoked by exogenously applied glycine in AII amacrine cells (Boos et al. 1993). In the presence of 300 nm strychnine, a concentration that is selective for glycine receptors in the spinal cord (Jonas et al. 1998), spPSCs in AII amacrine cells were completely blocked (Fig. 1F and G; n = 5). The block was slowly reversible and the spPSCs recovered completely when cells could be held long enough to wash out the strychnine (Fig. 1G; n = 2). In order to estimate an IC50 for the effect of strychnine on the synaptic glycine receptors, we recorded spPSCs in the control condition and for a range of strychnine concentrations (30 nm−1 μm; Fig. 1H). The degree of block was calculated as the difference between the time-averaged cumulative amplitude of spPSCs in control and in strychnine, relative to control. The IC50 was estimated to be 27 nm (Fig. 1H). Based on this evidence, we consider the spPSCs recorded in the conditions described here to be glycinergic IPSCs. Recordings in the presence of 10 μm bicuculline (to block GABAA receptors), revealed a small, but reproducible reduction of the peak amplitude of the spIPSCs (27 ± 7%; n = 6; not shown). We consider this to be an effect of bicuculline on glycine receptors (cf. Protti et al. 1997; Jonas et al. 1998). All recordings from AII amacrine cells were therefore done without bicuculline. In separate experiments, however, we verified expression of functional GABAA receptors by AII amacrine cells (Boos et al. 1993; Contini & Raviola, 2003). Similar to the results of Boos et al. (1993), we found that exogenous application of GABA (200 μm) by pressure from a multibarrel complex evoked strong responses in all cells tested (peak amplitude 322 ± 64 pA; n = 6; data not shown). This suggests that in the present experiments, GABAA receptors on AII amacrine cells were not synaptically activated, but that the reason for this is probably presynaptic as opposed to postsynaptic.
The glycinergic spIPSCs recorded in AII amacrine cells are most likely caused by synaptic release from glycinergic amacrine cells. In order to examine a potential role of presynaptic Na+-dependent spiking with respect to transmitter release, we recorded spIPSCs in AII amacrine cells both in the absence and presence of TTX. For the cell illustrated in Fig. 1I, there was no significant difference with respect to peak amplitude, 10–90% rise time, or time constant of decay (single-exponential function) between the two conditions (Kolmogorov–Smirnov test: P = 0.65 for peak amplitude, P = 0.06 for 10–90% rise time, P = 0.62 for time constant of decay). Similar results were observed for three other cells. Our results suggest that, at least under the present recording conditions, any contribution of presynaptic Na+-dependent spiking to transmitter release does not alter the amplitudes or kinetic properties of the synaptic responses.
Time course of spontaneous glycinergic IPSCs in AII amacrine cells
To examine the kinetic properties of the glycinergic spIPSCs, we selected well-separated events with monophasic waveforms. For 11 AII amacrine cells, each with more than 100 spIPSCs, we measured kinetic parameters for individual events and for ensemble averages. For the representative example illustrated in Fig. 2A–H, the peak amplitude of individual spIPSCs varied from 3 to 69 pA (Fig. 2A; average 18.3 ± 0.8 pA). The 10–90% rise time of the individual spIPSCs varied from 102 to 1713 μs (Fig. 2B; average 475 ± 16 μs). The decay phase of individual spIPSCs was reasonably well fitted by single-exponential functions (Fig. 2D), with τdecay varying from 0.7 to 12.5 ms (Fig. 2C; average 3.5 ± 0.1 ms). For the average spIPSC, the decay phase was better fitted with a double-exponential function (Fig. 2E; τfast = 3.6 ms, τslow = 11.4 ms; amplitude contributions 97% and 3%, respectively) than with a single-exponential function (not shown; τdecay = 3.8 ms).
Figure 2. Kinetics of spIPSCs in AII amacrine cells.
A, distribution of peak amplitude for spIPSCs; bin width 2.5 pA. Data from the same AII amacrine cell in A–H. B, distribution of 10–90% rise time for spIPSCs; bin width 0.025 ms. C, distribution of τdecay (single-exponential) for spIPSCs; bin width 0.25 ms. D, four overlaid spIPSCs (dotted lines), aligned by the point of steepest rise. A single-exponential fit (continuous line) has been overlaid on each spIPSC. E, average waveform of spIPSCs (dotted line; 204 events); double-exponential fit indicated by continuous line. F, relation between spIPSC 10–90% rise time and τdecay. G, relation between spIPSC peak amplitude and 10–90% rise time. H, relation between spIPSC peak amplitude and τdecay. I, spontaneous IPSCs recorded at ambient temperature (23°C; upper panel) and after heating to 34°C (lower panel). J, average waveforms of spIPSCs recorded at 23°C and 34°C (same cell as I), aligned at onset. Waveforms plotted at same scale (upper panel) and after normalization of peak amplitudes (lower panel). Notice reduced decay time, as well as increased peak amplitude, after increasing the temperature.
For 10 of the cells, there was no correlation between 10-90% rise time and τdecay (Fig. 2F; Spearman's R varied between −0.12 and 0.20; P = 0.27 ± 0.09; n = 10), suggesting that the spIPSCs were not differentially influenced by electrotonic filtering. These 10 cells were used for non-stationary noise analysis (see below). For six of the ten cells, there was no correlation between peak amplitude and 10–90% rise time (Fig. 2G), but for the other four cells there was a significant positive correlation (Spearman's R varied between 0.15 and 0.21; P = 0.01 ± 0.01; n = 4). For sevenof the ten cells, there was a significant positive correlation between peak amplitude and τdecay (Fig. 2H; single-exponential fit; Spearman's R varied between 0.21 and 0.35; P = 0.01 ± 0.01; n = 7). These correlations are the opposite of what one would expect if they were due to differential electrotonic filtering, but suggest that there could be variation between or within release sites with respect to the mean current waveform, possibly related to inter- and intrasite variability with respect to the temporal transmitter concentration profile (cf. Barberis et al. 2004).
Kinetic properties of ensemble averages were measured for a total of 21 AII amacrine cells (58–331 individual events). The population average of the 10–90% rise time was 501 ± 17 μs (range 368–673 μs). For comparison with patch responses (see below), the 20–80% rise time was 319 ± 10 μs (range 239–405 μs). In most cases (18/21 cells), the decay phase of the average IPSC was best fitted by a double-exponential function. The average τfast was 4.8 ± 0.2 ms (97.5 ± 0.5% amplitude contribution), and the average τslow was 32.9 ± 5.2 ms (2.5 ± 0.5% amplitude contribution; n = 18). The amplitude-weighted τdecay was 5.4 ± 0.3 ms. When the decay phase was fitted with a single-exponential function, the average τdecay was 5.0 ± 0.2 ms (n = 21).
We also examined the kinetic and amplitude characteristics of spIPSCs at a temperature closer to that in vivo. Figure 2I illustrates spIPSCs from an AII amacrine cell recorded at room temperature (23°C, upper panel) and 34°C (lower panel). The averaged waveforms for each condition (Fig. 2J) indicate that at physiological temperatures the 10–90% rise time decreased (from 505 to 402 μs), the decay time constant decreased (from 4.3 to 2.9 ms; single-exponential fit), and the amplitude increased (from 23 to 44 pA). Similar results were seen in three other cells. The average 10–90% rise time decreased from 562 ± 25 to 418 ± 37 μs, the average τdecay decreased from 5.6 ± 0.4 to 2.9 ± 0.1 ms, and the average amplitude increased from 19.3 ± 2.0 to 36.0 ± 4.7 pA (n = 4; P < 0.05 for all conditions; paired t test). The corresponding Q10 values were 1.3 ± 0.1 for the 10–90% rise time, 1.8 ± 0.1 for the decay time constant, and 1.8 ± 0.2 for the amplitude. These values are similar to the Q10 for τdecay of glycinergic IPSPs in goldfish Mauthner cells (Titmus et al. 1996).
Erev and voltage dependence of spontaneous glycinergic IPSCs
To examine the voltage dependence of the glycinergic spIPSCs, we varied the membrane holding potential in steps of 10 mV between −80 and +40 mV. The cell illustrated in Fig. 3 shows that with symmetrical intra- and extracellular chloride, the spIPSCs reversed from inward to outward currents close to 0 mV (Fig. 3A and B). To construct an I–V relationship, we averaged individual spIPSCs occurring during periods ranging from 60 to 240 s (n = 4–43 events for the example in Figs 3A and B; n = 2–107 for all cells analysed) at each holding potential, and plotted the peak amplitude of each averaged IPSC (Fig. 3B; •) against the membrane potential (Fig. 3C; •). The I–V curve was approximately linear and we estimated Erev for this cell to be 2.6 mV (average 4.8 ± 1.0 mV; n = 7). An ensemble I–V relationship constructed by pooling results for seven cells after normalizing the response values for each cell to the cell's response at −60 mV, had an Erev of 3.6 mV. These values for Erev are very close to the calculated equilibrium potential for chloride (ECl = 2.7 mV). When the intracellular concentration of chloride was reduced (ECl =−43 mV), Erev changed to −48.1 ± 2.6 mV (n = 5). Erev was very similar when calculated from an ensemble I–V relationship (−48.9 mV). These results indicate that Erev for the glycinergic spIPSCs followed changes in ECl, suggesting that the relevant channels display a relatively high selectivity for chloride.
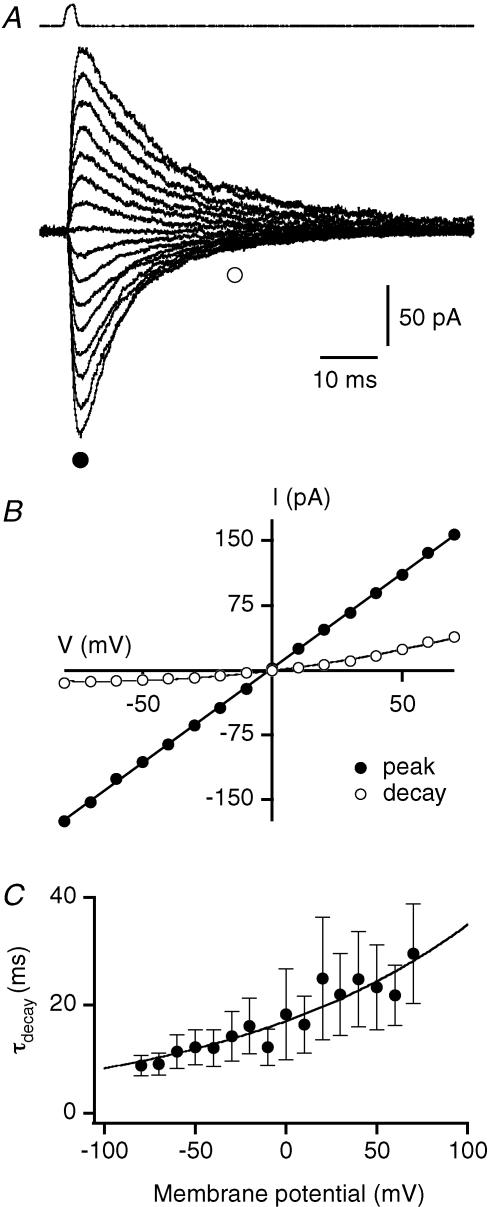
Figure 3. Voltage dependence of spIPSCs in AII amacrine cells.
A, traces with spIPSCs in an AII amacrine cell at three different holding potentials; notice reversal of current and slower decay at positive holding potentials (upper trace). B, averaged spIPSCs at a series of holding potentials (from −80 to +40 mV; 20 mV steps). Notice slower decay at positive compared to negative holding potentials. • and ○ (peak and decay phase, respectively) indicate time points used for I–V relationships in C. C, I–V relationships of averaged spIPSCs, measured for peak (•) and decay phase (○) of responses in B, fitted with a straight line and with a third-order polynomial function, respectively. D, average waveforms for spIPSCs (different cell than A–C) recorded at −80 (dotted line) and +40 mV (continuous line), plotted as absolute current after normalization of peak amplitudes. Notice longer decay time at +40 compared to −80 mV. E, average τdecay (mean ±s.e.m.; n = 6 cells) measured for averaged spIPSCs at −80 and +40 mV.
We also noticed a voltage dependence of the decay time course of the spIPSCs, with more prolonged decay at positive membrane potentials (Fig. 3B and D). Correspondingly, there was a tendency towards outward rectification when the I–V relationship (Fig. 3C; ○) was constructed from response values at the end of the decay of the spIPSCs (Fig. 3B; ○), but the difficulty of holding the cells at membrane potentials more depolarized than +40 mV for long enough periods of time, precluded a more complete analysis. The voltage-dependent time course of decay was analysed by fitting the mean spIPSCs at −80 mV and +40 mV with single-exponential functions (Fig. 3D). The τdecay at +40 mV (8.1 ± 0.6 ms) was significantly longer than the τdecay at −80 mV (4.5 ± 0.2; P = 4.8 × 10−4; n = 5; paired t test; Fig. 3E).
Non-stationary noise analysis of glycinergic spIPSCs
For 10 AII amacrine cells, each with more than 100 events, there was no correlation between 10%-90% rise time and τdecay (see above). For each of these cells, the time stability of the ensemble of spIPSCs had been ensured by systematically searching the recording for a consecutive series of spIPSCs with no time-dependent changes in either peak amplitude, 10–90% rise time or τdecay (Fig. 4A–C). For these 10 cells, Spearman's R varied between −0.15 and 0.14 for peak amplitude, between −0.07 and 0.18 for 10–90% rise time and between −0.03 and 0.18 for τdecay (P = 0.38 ± 0.10 for peak amplitude; P = 0.35 ± 0.07 for 10–90% rise time; P = 0.34 ± 0.11 for τdecay).
Figure 4. Non-stationary noise analysis of glycinergic spIPSCs in an AII amacrine cell.
A–C, plots of peak amplitude (A), 10–90% rise time (B) and τdecay (C) for 197 consecutive spIPSCs; no time-dependent correlation (linear fit in each graph). D, three individual spIPSCs from the population in A–C, superimposed mean spIPSC (smooth curves) after peak-scaling waveform to each individual spIPSC. E, three difference currents calculated from corresponding individual spIPSCs and peak-scaled mean spIPSC in D. F, the mean spIPSC. Broken horizontal lines indicate amplitude intervals used for binning mean current and variance (see Methods). G, ensemble current variance (without binning) for the spIPSCs, calculated from the difference traces between individual spIPSCs and the peak-scaled mean spIPSC (as in E). H, plot of ensemble current variance calculated with peak-scaling (G) versus mean current (F; after binning). Time range used for the variance versus mean plot corresponds to data points from the peak of the mean spIPSC to the end of the decay phase. The data points were fitted with eqn (6). I, plot of ensemble current variance calculated without peak-scaling versus mean current (F; after binning). Time range used for the variance versus mean plot as in H. Data points (corresponding to the inital slope to the left) have been fitted with a straight line.
Because of quantal variability, with the number of available receptor channels varying from one spIPSC to the next, we employed peak-scaled non-stationary noise analysis (Traynelis et al. 1993) and scaled the peak of the ensemble mean waveform (Fig. 4F) to each individual spIPSC (Fig. 4D) before calculating the difference currents (Fig. 4E) and the ensemble variance (Fig. 4G). For the cell illustrated in Fig. 4, we obtained a parabolic variance versus mean curve (Fig. 4H). Fitting with eqn (6) (∼75% of the curve), gave a unitary current (i) of 2.8 pA, corresponding to a unitary chord conductance (γ) of 47.5 pS. For the 10 cells, peak-scaled non-stationary noise analysis gave a unitary chord conductance of 40.5 ± 2.6 pS (range 28.7–55.5 pS).
When non-stationary noise analysis was performed without peak-scaling, the variance versus mean curve deviated strongly upwards (Fig. 4I), but the initial slope of the variance versus mean curve should still correspond to the unitary current amplitude (i) (Hartveit & Veruki, 2006). For the cell illustrated in Fig. 4, this resulted in i = 3.1 pA (average 2.7 ± 1.7 pA), corresponding to γ = 51.1 pS (average 44.1 ± 2.5 pS, range 30.9–56.0 pS; n = 10 cells).
There was no correlation between our estimate of the single-channel conductance and the cut-off frequency of the corresponding RC filter (the combination of series resistance and cell membrane capacitance) for a given whole-cell recording (P = 0.39; Spearman's rank order correlation test).
Deactivation and desensitization kinetics of glycine receptors in outside-out patches
In order to directly investigate the kinetics of glycine receptor channels in AII amacrine cells, we measured responses evoked by ultrafast application of glycine to somatic outside-out patches. This technique permits agonist concentrations to be changed with a rise time of a few hundred microseconds, and has provided insights into glycine receptor kinetic properties that could not have been achieved with slower application techniques and whole-cell recordings (reviewed by Legendre, 2001). Figure 5A illustrates the response evoked by a short (∼2 ms) pulse of glycine (3 mm) to a patch from an AII amacrine cell. The response rose rapidly to a peak with a 20–80% rise time of 369 μs. For eight patches, the 20–80% rise time of responses evoked by brief (∼2 ms) pulses was 698 ± 65 μs (range 369–870 μs). At the end of the pulse, the response decayed rapidly. This deactivation, which reflects the closure of channels after removal of agonist, was well fitted by a double-exponential function. For the patch shown in Fig. 5A, τfast was 4.3 ms and τslow was 30 ms. The amplitude contributions were 96% and 4%, respectively. The average τfast was 4.6 ± 0.5 ms (85 ± 5% amplitude contribution) and the average τslow was 17.4 ± 2.3 ms (15 ± 5% amplitude contribution; n = 8). For the patch illustrated in Fig. 5A, the amplitude weighted τdecay was 5.4 ms (average = 6.2 ± 0.8 ms). When the time course of deactivation was fitted with a single-exponential function, we obtained a τdecay of 4.7 ms (average 6.5 ± 0.9 ms).
Figure 5. Deactivation and desensitization kinetics of glycine receptors in AII amacrine cells.
A, response (lower trace; dotted line; average of 22 trials) of outside-out patch from an AII amacrine cell to brief (∼2 ms), ultrafast application of glycine (3 mm), overlaid with double-exponential fit to decay phase (continuous line). Here, and in subsequent figures, the upper trace illustrates either the exchange time course (measured as a change in liquid junction current with an open tip pipette after breaking the patch; see Methods) or, alternatively, the amplifier stimulus output when the exchange time was not measured for each type of protocol. B, response (lower trace; dotted line, average of 20 trials) of outside-out patch (same as in A) to long (1 s), ultrafast application of glycine (3 mm), overlaid with double-exponential fit to the decay phase (white continuous line). C, the difference between fast deactivation and slow desensitization kinetics is particularly well illustrated by the overlaid responses (lower traces) of an AII outside-out patch to a series of applications (3 mm glycine) of variable duration (2, 20, 50 and 100 ms). Each trace is the average of 20–25 trials. D, overlaid responses (lower traces) of an AII outside-out patch evoked by two brief (∼2 ms) pulses of glycine (3 mm), separated by recovery intervals from 10 to 165 ms. Each trace is the average of nine trials. Notice lack of fast desensitization after first pulse. E, overlaid responses (lower traces) of an AII outside-out patch evoked by two pulses of glycine (3 mm), interval between first (conditioning; 100 ms) pulse and second (test; 20 ms) pulse separated by recovery intervals from 10 to 280 ms. Each trace is the average of six trials. Notice fast and slow desensitization during first pulse, and fast and slow recovery from desensitization. F, no depression of the response to the second pulse of glycine (as in D) after a brief conditioning pulse. Response plotted (mean ±s.e.m.) as a function of the interval between the first and second pulse (n = 6 patches; 3–9 responses at each interval for each patch). Broken line indicates mean peak current activated by the first pulse (100%). G, depression of the response to the second pulse of glycine (as in E) after a longer conditioning pulse. Response plotted (mean ±s.e.m.) as a function of the recovery interval between the first and the second pulse (n = 6 patches). Data points have been fitted with a double-exponential function.
We also applied longer pulses of glycine (3 mm) in order to study desensitization. With a pulse duration of 1000 ms, the rise times and peak amplitudes were very similar to the corresponding values obtained for short pulses. However, the time course of desensitization, reflecting the closure of channels in the maintained presence of glycine, was considerably slower than the time course of deactivation. For the AII amacrine cell responses illustrated in Fig. 5B (same patch as in Fig. 5A), the decay could be well fitted with a double-exponential function, resulting in time constants of 53 ms (τfast) and 1750 ms (τslow). The amplitude contributions were 21% and 79%, respectively. For a total of six patches, the average τfast was 40.0 ± 14.5 ms (30 ± 3% amplitude contribution), and the average τslow was 883 ± 311 ms (70 ± 3% amplitude contribution). The average amplitude-weighted τdecay was 621 ± 218 ms. The equilibrium response to 3 mm glycine was measured as the current at the end of the 1000 ms application, and was on average 49 ± 2% of the peak response (range 46–57%). To further study deactivation and desensitization of the decay phase of the glycine receptors, we applied a succession of pulses of variable duration to outside-out patches from AII amacrine cells. In the example illustrated in Fig. 5C, it can be seen that the decay time course changed instantly from the slow desensitization to the faster deactivation upon removal of glycine.
The time course of desensitization suggests that brief pulses of glycine, mimicking single vesicle release at synaptic sites, are unlikely to cause significant receptor desensitization. We examined this by employing a double-pulse protocol with application of two brief (∼2 ms) pulses of glycine (3 mm) separated by a series of increasing recovery intervals (from 3 to 645 ms; interval measured from the end of the first to the beginning of the second pulse). Figure 5D shows a representative example from one patch, with a series of superimposed traces with interpulse intervals ranging from 10 to 165 ms. For these pulses, there was no evidence that the second (test) pulse evoked a smaller peak response than the first (conditioning) pulse. When we pooled data for six patches and plotted the response to the test pulse relative to the response to the first pulse (taking into account the baseline current measured at onset of the response evoked by the test pulse) as a function of the recovery interval between the two pulses, the result confirmed the lack of desensitization (Fig. 5F).
A similar double-pulse protocol, with a longer (100 ms) conditioning pulse that caused significant desensitization, was employed to study the recovery from desensitization. For the patch illustrated in Fig. 5E, the first pulse caused approximately 30% desensitization and suppressed the response to the test pulse (20 ms duration). With increasing recovery intervals (interpulse intervals ranging from 10 ms to 7.3 s), the response to the test pulse recovered to the control level. When we plotted the response to the test pulse relative to the response to the first pulse (taking into account the baseline current measured at onset of the response evoked by the test pulse) as a function of the recovery interval between the two pulses (Fig. 5G), the time course of the response recovery was well fitted by a double-exponential function (τfast = 71 ms and τslow = 1713 ms; pooled data from six patches). The amplitude contributions were 73% (τfast) and 27% (τslow).
Concentration–response properties of glycine receptors in outside-out patches
Experiments with ultrafast application of glycine (3 μm to 10 mm) to somatic outside-out patches were used to study the concentration–response relationship of the glycine receptors of AII amacrine cells. Figure 6A illustrates the responses evoked by pulses of glycine (10 μm to 3 mm) to an outside-out patch. At low concentrations, from 10 to ∼100 μm, glycine evoked a response that rose slowly to a steady-state plateau. At higher concentrations, from ∼300 μm to 10 mm, the evoked responses displayed considerably faster rise times, and after the initial peak they started to desensitize. The peak amplitude of the response at each concentration was used to construct a concentration–response curve and the averaged concentration–response relationship for 11 patches is shown in Fig. 6B. For each concentration, the pulse duration was adjusted such that the response reached steady-state or started to desensitize. The EC50 was 357 μm, and the Hill coefficient was 1.4. The EC50 is similar to the value measured for glycine responses in patches from spinal cord neurons (Jonas et al. 1998), but higher than values measured in other systems (Harty & Manis, 1998; Legendre, 1998).
Figure 6. Concentration–response relationships of glycine-evoked currents in AII amacrine cells.
A, currents activated in an outside-out patch by application of different concentrations of glycine. Pulse duration adjusted for each concentration to reach either a steady-state response or the beginning of desensitization. B, concentration–response relationship of peak current evoked by glycine. The glycine-activated current at each concentration is plotted as mean ±s.e.m. Data points normalized to the current at 3 mm. Here and in C, the number of patches for each data point is indicated in the graph, and data points have been fitted with eqn (4). C, concentration–response curve for block by strychnine (10–1000 nm) of currents evoked by brief (∼2 ms) pulses of glycine (3 mm). The glycine-activated current at each concentration of strychnine is plotted as mean ±s.e.m. Data points normalized to the response in the absence of strychnine (leftmost data point).
With outside-out patches, we were also able to study the block by strychnine of responses evoked by brief (∼2 ms) pulses of glycine (3 mm). When patches were equilibrated with varying concentrations of strychnine (10–1000 nm), the peak response evoked by glycine was reduced in a concentration-dependent manner (Fig. 6C). The IC50 was 12 nm, similar to the IC50 estimated for strychnine block of glycinergic spIPSCs in whole-cell recordings (27 nm; see above). This supports our conclusion that the spIPSCs blocked by 300 nm strychnine were glycinergic spIPSCs.
Voltage dependence of deactivation kinetics of glycine receptor-mediated currents in outside-out patches
The recordings of spIPSCs in AII amacrine cells at different membrane holding potentials suggested that the synaptic glycine receptors are voltage sensitive. We investigated the voltage dependence of the current activated by brief (∼2 ms) pulses of glycine (3 mm) at holding potentials from −80 mV to +70 mV in 10 mV increments. For each patch we averaged between three and nine responses at each membrane potential. A representative example of a series of responses is shown in Fig. 7A. The I–V relationship for the peak response (Fig. 7A; •) was approximately linear and reversed at −1.0 mV which is close to ECl at −2.5 mV (Fig. 7B; •; average −1.9 ± 2.5 mV; n = 6 patches). From the individual responses (Fig. 7A), it can be seen that the time course of decay was voltage dependent, with a slower decay at more positive membrane potentials. Corresponding to this, the I–V relationship for the decay phase (Fig. 7A; ○) showed outward rectification (Fig. 7B; ○). We further examined the voltage dependence of the decay phase by fitting each averaged response with a single-exponential function and plotting the decay time constant against the membrane potential (Fig. 7C). The data could be well fit with eqn (3), giving a value for H of 139 mV (the change in membrane potential for an e-fold increase in τdecay).
Figure 7. Voltage dependence of glycine receptors in AII amacrine cells.
A, overlaid responses (lower traces) of outside-out patch to brief (∼2 ms), ultrafast application of glycine (3 mm) at a series of holding potentials (from −80 to +70 mV; 10 mV steps; each trace is the average of nine trials). Notice slower decay at positive compared to negative holding potentials. • and ○ (peak and decay phase, respectively) indicate time points used for I–V relationship in B. B, I–V relationships of responses in A, measured for peak (•) and decay phase (○) and fitted with a straight line and with a third-order polynomial function, respectively. C, deactivation time constant (single-exponential function) as a function of holding potential (plotted as mean ± s.e.m.; n = 5–6 patches for each data point). Data points have been fitted with eqn (3).
Non-stationary noise analysis of transient currents evoked by glycine in outside-out patches
When spIPSCs were analysed by non-stationary noise analysis, peak-scaling precluded any measurement of Popen. In order to estimate the single-channel current and maximum Popen of glycine receptors, we used non-stationary noise analysis of responses evoked by ultrafast application of brief pulses of 3 mm glycine to outside-out patches from AII amacrine cells. Figure 8A shows three individual records evoked by glycine, together with the superimposed ensemble mean response (Fig. 8C). The corresponding differences between each individual record and the ensemble mean are shown in Fig. 8B, and were used to calculate the ensemble variance (Fig. 8D; n = 46 responses). The variance versus mean plot of the data points corresponding to the decaying phase is shown in Fig. 8E, and has a clear parabolic shape, indicating that the maximum Popen reached a value larger than 0.5. When the data points were fitted with
Figure 8. Non-stationary noise analysis of glycine-evoked responses in an outside-out patch from an AII amacrine cell.
A, three individual records obtained by brief (∼5 ms) pulses of glycine (3 mm), superimposed ensemble mean waveform (smooth curves). B, three difference currents calculated from corresponding individual records (A) and mean waveform. C, the mean current of all 46 glycine-evoked responses. Broken horizontal lines indicate amplitude intervals used for binning mean current and variance (see Methods). D, ensemble current variance (without binning) for the glycine-evoked responses, calculated from the difference between individual records and the ensemble mean waveform (as in B). E, plot of ensemble current variance (D) versus mean current (C; after binning). Time range used for the variance versus mean plot corresponds to data points from the peak of the mean waveform to the end of the decay phase. The data points were fitted with eqn (6).
eqn (6), the apparent glycine-activated single-channel current was 2.86 pA, corresponding to an apparent single-channel chord conductance of 47.7 pS. The number of active channels was estimated as 23.6, corresponding to a maximum Popen at the peak response of 0.83 (Fig. 8E). For 10 patches tested with glycine (3 mm), the mean single-channel chord conductance was 41.7 ± 2.1 pS (range 30.8–51.0 pS), and the mean number of available channels was 46.7 ± 8.0 (range 15.1–94.3). The average maximum Popen was 0.82 ± 0.03 (range 0.71–0.98).
Direct observations of single-channel gating in patch responses and spIPSCs
The estimates from non-stationary noise analysis potentially represent weighted averages of different conductance levels, irrespective of whether they correspond to different channels or different (sub)conductance levels of the same channels. Cull-Candy et al. (1988) demonstrated that the weights are determined by the relative number of channels, the single-channel conductance(s) for each channel type, and their open probability. It is therefore important to investigate the relation between the apparent single-channel conductance values estimated by non-stationary noise analysis (spIPSCs or outside-out patches) and directly observed single-channel conductance levels. For several outside-out patches with low noise levels, discrete transitions between open and closed states of varying levels were apparent during later phases of individual current responses. Figure 9A shows the response of an outside-out patch to a brief pulse of glycine (3 mm), with several discrete transitions between open and closed states. We estimated the single-channel conductance by constructing an all-point amplitude histogram (Fig. 9A; inset) from a selected segment of the current trace (Fig. 9A; legend). For the analysed segment, the single-channel current was ∼2.8 pA, corresponding to a single-channel chord conductance of ∼47 pS. For the same patch, non-stationary noise analysis yielded an apparent conductance of ∼45 pS. Observations of similar single-channel openings (42–50 pS) were made for several patches. In addition, several patches from AII amacrine cells displayed single-channel transitions with a larger amplitude of ∼5 pA (Fig. 9B), corresponding to a single-channel chord conductance of ∼83 pS. Amplitude transitions less than 2.8 pA were also observed, but they were not analysed further. Transitions with an amplitude of ∼5 pA were also observed during the decay phase of spIPSCs in several AII amacrine cells (Fig. 9C).
Figure 9. Single-channel conductance of glycine receptor channels in patches and spIPSCs in AII amacrine cells.
A, current response evoked by a brief (∼2 ms) pulse of glycine (3 mm) to an outside-out patch from an AII amacrine cell. Here, and in panel B, the peak of response has been truncated for clarity. Notice directly resolvable single-channel gating in response to application of glycine. Here and below, broken lines indicate either baseline current (0 pA; leak current has been subtracted) or inward current during channel opening (as indicated). Inset shows all-point amplitude histogram for data points between the vertical arrow and the end of the trace (not shown). Histogram has been fitted with the sum of two Gaussian distributions (broken line) and the interval between the two peaks was taken as the single-channel current amplitude (∼2.8 pA). B, three individual current responses evoked by a brief (2 or 5 ms) pulse of glycine (3 mm) to outside-out patches from AII amacrine cells. Notice directly resolvable single-channel gating in response to application of glycine. C, three individual spIPSCs from AII amacrine cells. Notice directly resolvable single-channel gating during the decay phase of the spIPSCs.
Heteromeric and homomeric glycine receptors
Glycine receptor single-channel openings at ∼5 pA (corresponding to a conductance of ∼83 pS) suggest the presence of α1 homomeric receptors (Bormann et al. 1993; see Discussion). Because these openings were observed in both patch responses and spIPSCs of AII amacrine cells (Fig. 9B and C), it is possible that such receptors are present in both extrasynaptic and synaptic receptors of these cells. In order to investigate this, we examined the effects of picrotoxin on patch responses and spIPSCs in AII amacrine cells. It has previously been demonstrated that relatively low concentrations of picrotoxin can block α1 homomeric glycine receptors (IC50 10 μm), but that α1β heteromeric receptors require considerably higher concentrations to be blocked (IC50 > 1000 μm; Pribilla et al. 1992). As illustrated by the example in Fig. 10A, 10 μm picrotoxin reversibly suppressed the response evoked by application of brief (∼5 ms) pulses of glycine (3 mm) to AII amacrine cell patches (peak amplitude reduced by 25.4 ± 6.5%; range 3.3–48.4%; n = 7; P = 0.003; one-tailed paired t test). When the concentration of picrotoxin was increased to 100 μm, the block increased to 60.4 ± 6.6% (range 39.3–79.7%; n = 6; P = 0.03; one-tailed paired t test; data not shown). These results suggest that α1 homomeric glycine receptors contribute to the population of extrasynaptic receptors on AII amacrine cells.
Figure 10. Partial block of glycine receptors in AII amacrine cells to low concentrations of picrotoxin.
A, average responses evoked by application of brief (∼5 ms) pulses of glycine to an outside-out patch from an AII amacrine cell: control (thin continuous line; n = 25 responses), after equilibrating the patch with 10 μm picrotoxin (thick continuous line; n = 23 responses) and after returning the patch to control solution (recovery; dotted line; n = 25 responses). B, averaged spIPSCs from an AII amacrine cell: control (thin continuous line; n = 136 events), after adding 10 μm picrotoxin to the bath (thick continuous line; n = 117 events) and after washout of picrotoxin (recovery; dotted line; n = 264 events).
When 10 μm picrotoxin was applied to AII amacrine cells during whole-cell recording, there was a reversible reduction of the peak amplitude of spIPSCs in all cells recorded (Fig. 10B; average reduction 23.2 ± 2.3%; range 7.2–33.8%; n = 9; P = 0.001; one-tailed paired t test). When the concentration of picrotoxin was increased to 100 μm, the average reduction of peak amplitude increased to 47.3 ± 6.0% (range 31.2–67.8%; n = 5; P = 0.02; one-tailed paired t test). Taken together, the presence of large single-channel openings (∼5 pA) in both outside-out patch responses to glycine and glycinergic spIPCS, and the sensitivity to low concentrations of picrotoxin (10 μm), suggest that α1 homomeric glycine receptors contribute to both synaptic and extrasynaptic receptors of AII amacrine cells.
Discussion
In this study we have investigated the functional properties of glycinergic spIPSCs in AII amacrine cells, and compared them with the functional properties of extrasynaptic glycine receptors in somatic outside-out patches as studied with ultrafast application. The spIPSCs seemed to belong to a unimodal population of events with fast rise and decay times, and were blocked by low concentrations of the specific glycine receptor antagonist strychnine (IC50 27 nm). The Erev was very close to ECl and followed changes in ECl. These results strongly suggest that the recorded spIPSCs are mediated by glycine receptor ion channels.
Kinetic properties of glycinergic spIPSCs in AII amacrine cells
The glycinergic spIPSCs in AII amacrine cells occurred at a relatively low frequency, such that few events overlapped in time. Spontaneous IPSCs recorded at room temperature displayed fast kinetics with a 10–90% rise time of ∼500 μs and a decay phase that was well fitted by a double-exponential function with τfast ∼ 4.8 ms and τslow ∼ 33 ms, and amplitude contributions of 97.5% and 2.5%, respectively. At an elevated temperature of 34°C, the 10–90% rise time decreased to ∼400 μs, and the decay time constant decreased to 2.9 ms (single-exponential fit). These values are among the fastest reported for glycinergic spIPSCs (Takahashi et al. 1992; Legendre, 1998; Smith et al. 2000), albeit not as fast as for glycinergic spIPSCs in the medial nucleus of the trapezoid body (Awatramani et al. 2004). The decay kinetics of glycinergic spIPSCs in AII amacrine cells are much faster than those reported by Frech et al. (2001) for unspecified amacrine cells in the mouse retina. Presumably, the cells studied by Frech et al. (2001) did not include AII amacrine cells. Thus, it is likely that there is considerable heterogeneity among amacrine cells with respect to the kinetic properties of their glycine receptors.
We observed that the time course of decay was voltage dependent, with slower decay at positive than at negative membrane potentials. For glycine-evoked currents in outside-out patches, we were able to measure the voltage sensitivity, which was relatively low, with ∼139 mV change in membrane potential required for an e-fold change in τdecay (single-exponential fit). Similar voltage-dependent decay has previously been reported for glycine receptor channels in zebrafish hindbrain (Legendre, 1999). It is unlikely that the weak voltage dependence is of functional importance for synaptic transmission in this pathway, but we notice that the effect of voltage on the decay kinetics of glycinergic responses is qualitatively similar to that observed for AMPA-type glutamate receptors in the same cells (Veruki et al. 2003). The mechanism for the voltage-dependent decay remains to be determined.
Molecular identity of glycine receptors expressed by AII amacrine cells
The glycinergic spIPSCs in AII amacrine cells displayed fast kinetics, and it is likely that the kinetic properties of the receptors themselves play an important role in determining the kinetics of the spIPSCs. We examined the receptor kinetics by ultrafast application of glycine to outside-out patches excised from the somata of AII amacrine cells. Like the spIPSCs, the glycine receptor channels in patches displayed fast kinetics with a deactivation time course that was best fitted by a double-exponential function. The time constants were 4.6 ms (τfast) and 17 ms (τslow), and the amplitude contributions were 85% and 15%, respectively. The deactivation was voltage dependent with slower decay at positive membrane potentials than at negative membrane potentials (see above). The desensitization time course was markedly slower. With a pulse duration of 1000 ms, we could resolve two components with average time constants of 40 ms (τfast) and 883 ms (τslow). The equilibrium response at the end of the 1000 ms application was ∼50% of the peak response. After a conditioning pulse of 100 ms, the receptors recovered from desensitization with a biexponential time course (τfast = 71 ms, τslow = 1713 ms). When the glycine receptors were activated with brief (∼2 ms) pulses of agonist, we found no evidence for fast desensitization, consistent with the findings of Legendre (1998) and Singer & Berger (1999). These results indicated that the decay time course of spIPSCs in AII amacrine cells is relatively similar to the time course of deactivation in outside-out patches. The difference in time constants and amplitude contributions might be attributed to differences in the exact molecular composition or, possibly, to differences in the state of modulatory phosphorylation between synaptic and extrasynaptic receptors. However, the similarity between the kinetic properties of synaptic and extrasynaptic receptors suggest that the properties of the latter in general are fairly representative of the former. Because of the similarity between the spIPSC decay kinetics and the deactivation kinetics of extrasynaptic receptors, combined with the much slower desensitization kinetics of extrasynaptic receptors, it is likely that desensitization does not play a role in determining the time course of glycinergic spIPSCs. This conclusion is also supported by our finding of voltage-dependent kinetics of spIPSCs (cf. Legendre, 1999), and is similar to that reached for other glycinergic synapses in the CNS (Legendre, 1998; Singer & Berger, 1999). It is possible, however, that with longer-lasting, repetitive release of glycine at individual synapses, the postsynaptic receptors might be driven into longer-lived desensitized states.
The individual estimates of glycine receptor single-channel conductance by non-stationary noise analysis ranged from 29 to 56 pS for spIPSCs, and from 31 to 51 pS for patches. We directly identified single-channel transitions between closed and open states in both types of responses. For patch responses, we observed openings corresponding to a single-channel conductance of ∼47 pS and ∼83 pS, as well as lower amplitudes that we did not attempt to measure. For spIPSCs, the signal to noise ratio was lower, but we could directly identify openings with a single-channel conductance of ∼83 pS. The fast decay kinetics of spIPSCs and patch responses suggest that α1-subunits, and possibly α3-subunits, but not α2-subunits are involved (Takahashi & Momiyama, 1991). A single-channel conductance of ∼47 pS suggests the presence of heteromeric αβ receptors (44–54 pS; Bormann et al. 1993), in this case α1β and possibly α3β receptors. However, the higher single-channel conductance of ∼83 pS is consistent with homomeric α1 (85 pS), but not with homomeric α3 receptors (100–110 pS; Bormann et al. 1993). The most parsimonious interpretation is that the glycine receptors of AII amacrine cells are made up of α1β heteromeric and α1 homomeric receptors. The reported lower subconductance levels for heteromeric αβ receptors (20–30 pS; Bormann et al. 1993) could possibly explain some of the lower apparent conductances obtained with non-stationary noise analysis. Previous evidence has suggested the presence of homomeric receptors in extrasynaptic, but not synaptic glycine receptors (Takahashi et al. 1992). In our case, however, the single-channel openings of ∼83 pS, as well as the sensitivity to a relatively low concentration of picrotoxin (10 μm), suggest a contribution of α1 homomeric receptors to both synaptic and extrasynaptic receptor populations in AII amacrine cells. This is similar to the conclusions reached for synaptic glycine receptors in zebrafish hindbrain (Legendre, 1997).
Although the ability of immunocytochemical investigations at the light microscopic level to attribute punctate labelling of specific receptor subunits to the correct cell type(s) has recently been questioned (Ivanova et al. 2006), it is interesting to note that Sassoè-Pognetto et al. (1994) reported a colocalization of the α1-subunit with a cellular marker for AII amacrine cells (parvalbumin) in both the inner and outer part of the inner plexiform layer of rat retina. While immuno-electron microscopy verified localization of the α1-subunit to axon terminals of OFF-cone bipolar cells in the outer part of the inner plexiform layer, postsynaptic to the lobular appendages of AII amacrine cells, Sassoè-Pognetto et al. (1994) suggested that the α1-subunit was also localized to the arboreal dendrites of AII amacrine cells in the inner plexiform layer. This is consistent with the physiological evidence presented in our study. Immunocytochemical studies in primate retina, however, suggested that arboreal dendrites of AII amacrine cells express the α2-subunit (Jusuf et al. 2005).
Non-stationary noise analysis of both spIPSCs and patch responses yielded an average apparent single-channel conductance of ∼42 pS. With an average spIPSC peak amplitude of ∼23 pA, this corresponds to ∼9 glycine receptor channels being open at the peak of a single spIPSC. With a saturating concentration of agonist, the glycine receptors in patches reached a maximum Popen of 0.82. If the synaptic receptors reach a similar maximum Popen, these estimates suggest that 10–11 receptors are available to bind transmitter after release of a single vesicle.
Functional importance of glycinergic inhibition in AII amacrine cells
Glycinergic amacrine cells comprise 10–20 different types, most of which are narrow-field amacrine cells with dendritic trees spanning varying heights of the inner plexiform layer (MacNeil et al. 1999; Menger et al. 1998). While glycinergic inhibition is involved in transforming the visual responses of retinal ganglion cells (e.g. Belgum et al. 1984) and in the signal transfer between AII amacrine cells and OFF-cone bipolar cells (Pourcho & Goebel, 1985; Sassoè-Pognetto et al. 1994; Ivanova et al. 2006), there is little evidence for a specific role of glycinergic input to AII amacrine cells. Under scotopic conditions, AII amacrine cells display a robust ON-centre/OFF-surround receptive field organization (Nelson, 1982; Dacheux & Raviola, 1986; Bloomfield & Xin, 2000), but there is evidence that the surround-mediated response is generated by GABAergic feedback inhibition to rod bipolar cell axon terminals (Völgyi et al. 2002), and that glycinergic inhibitory input to the AII amacrine cell is not involved (Bloomfield & Xin, 2000).
Regardless of the specific involvement of glycinergic input to AII amacrine cells in visual signal processing, the functional characteristics of the glycine receptors documented in our study will constrain their physiological role. Specifically, the kinetic properties of spIPSCs in AII amacrine cells will impact the integrative properties of these cells and determine how inhibitory inputs shape their response properties. The fast decay kinetics of the spIPSCs will require a relatively high input frequency in order for summation of inhibitory inputs to occur. A related prediction is that AII amacrine cells will not be very effective as integrators of glycinergic input. Accordingly, the timing of the inhibitory input, relative to that of the excitatory input, becomes critically important for the functional impact of inhibition. Precisely timed inhibition might therefore be important for the function of these cells. AII amacrine cells express AMPA-type glutamate receptors with very fast kinetics (Veruki et al. 2003), and the fast kinetics of their glycine receptors might be an adaptation to this property.
Electron microscopic investigations indicate that AII amacrine cells are postsynaptic to other amacrine cells at two morphologically distinct types of inputs (Strettoi et al. 1992). One input targets the cell body region and originates from dopaminergic amacrine cells which use GABA as a cotransmitter (Wässle & Chun, 1988). The numerically largest input targets the arboreal dendrites with synapses located close to gap junctions (that interconnect AII amacrines or connect AII amacrines with ON-cone bipolar cells) and glutamatergic, excitatory ribbon synapses established by the axonal endings of rod bipolar cells (Strettoi et al. 1992). The amacrine input to the arboreal dendrites possibly originates from a single class of amacrine cells (Strettoi et al. 1992), and we speculate that it generates the glycinergic spIPSCs identified in the present study. Activation of these glycinergic synapses will reduce the membrane resistance of the arboreal dendrites close to the chemical and electrical synapses. In addition to reducing the depolarization caused by excitatory input from rod bipolar cells, the resulting shunt could lead to a transient uncoupling of the transcellular current, similar to what has been suggested for electrical synapses in the inferior olive (Llinás, 1974; Lang et al. 1996). This could be a mechanism for short-term, dynamic regulation of the effective electrical coupling between AII amacrine cells (Veruki & Hartveit, 2002a) and between AII amacrine cells and ON-cone bipolar cells (Veruki & Hartveit, 2002b), and an alternative to the presumably slower, direct modulatory control of the electrical junction conductance (Hampson et al. 1992; Mills & Massey, 1995). Of the glycinergic amacrine cells described in the rat retina (Menger et al. 1998), several have dendritic trees that stratify in the inner part of the inner plexiform layer (ON-sublamina) where they could target the arboreal dendrites of AII amacrine cells. Because some of the cells have dendritic trees restricted to the ON-sublamina, while others have dendritic trees spanning both the ON- and the OFF-sublamina, it is not possible to predict the visual response polarity (ON versus ON/OFF) of the glycinergic amacrine cell(s) presynaptic to AII amacrine cells. An important goal for future work should be to functionally identify the glycinergic amacrine cells presynaptic to AII amacrine cells and determine how presynaptic activity impacts the integrative properties of their postsynaptic target.
Acknowledgments
Financial support from the Norwegian Research Council (NFR 161217/V40), the Meltzer fund (University of Bergen) and the Faculty of Medicine at the University of Bergen (fellowships for M.L.V. and S.B.G.) is gratefully acknowledged. S.B.G. is supported by the medical student research program (‘Forskerlinje’) of the Faculty of Medicine at the University of Bergen. We thank Dr Svein H. Mørkve for valuable advice on ultrafast drug application.
References
- Awatramani GB, Turecek R, Trussell LO. Inhibitory control at a synaptic relay. J Neurosci. 2004;24:2647–2643. doi: 10.1523/JNEUROSCI.5144-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A, Petrini EM, Cherubini E. Presynaptic source of quantal size variability at GABAergic synapses in rat hippocampal neurons in culture. Eur J Neurosci. 2004;20:1803–1810. doi: 10.1111/j.1460-9568.2004.03624.x. [DOI] [PubMed] [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgum JH, Dvorak DR, McReynolds JS. Strychnine blocks transient but not sustained inhibition in mudpuppy retinal ganglion cells. J Physiol. 1984;354:273–286. doi: 10.1113/jphysiol.1984.sp015375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Ret Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. J Physiol. 2000;523:771–783. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos R, Schneider H, Wässle H. Voltage- and transmitter-gated currents in AII-amacrine cells in a slice preparation of the rat retina. J Neurosci. 1993;13:2874–2888. doi: 10.1523/JNEUROSCI.13-07-02874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J, Rundström N, Betz H, Langosch D. Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 1993;12:3729–3737. doi: 10.1002/j.1460-2075.1993.tb06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini M, Raviola E. GABAergic synapses made by a retinal dopaminergic neuron. Proc Natl Acad Sci U S A. 2003;100:1358–1363. doi: 10.1073/pnas.0337681100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Ma Y-P, Lipton SA, Pan Z-H. Glycine receptors and glycinergic synaptic input at the axon terminals of mammalian retinal rod bipolar cells. J Physiol. 2003;553:895–909. doi: 10.1113/jphysiol.2003.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Howe JR, Ogden DC. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Frech MJ, Pérez-León J, Wässle H, Backus KH. Characterization of the spontaneous synaptic activity of amacrine cells in the mouse retina. J Neurophysiol. 2001;86:1632–1643. doi: 10.1152/jn.2001.86.4.1632. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. Immunocytochemical localization of glycine receptors in the mammalian retina. J Comp Neurol. 1993;335:523–537. doi: 10.1002/cne.903350405. [DOI] [PubMed] [Google Scholar]
- Hampson ECGM, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E. Membrane currents evoked by ionotropic glutamate receptor agonists in rod bipolar cells in the rat retinal slice preparation. J Neurophysiol. 1996;76:401–422. doi: 10.1152/jn.1996.76.1.401. [DOI] [PubMed] [Google Scholar]
- Hartveit E, Veruki ML. Studying properties of neurotransmitter receptors by non-stationary noise analysis of spontaneous synaptic currents. J Physiol. 2006;574:751–785. doi: 10.1113/jphysiol.2006.111856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty TP, Manis PB. Kinetic analysis of glycine receptor currents in ventral cochlear nucleus. J Neurophysiol. 1998;79:1891–1901. doi: 10.1152/jn.1998.79.4.1891. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Müller U, Harvey K, Harvey RJ, Betz H, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the α3 subunit. J Comp Neurol. 2003;465:524–539. doi: 10.1002/cne.10852. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Müller U, Zeilhofer HU, Harvey RJ, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the α2 subunit. J Comp Neurol. 2004;477:399–411. doi: 10.1002/cne.20267. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Müller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Jonas P. Fast application of agonists to isolated membrane patches. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2. New York, London: Plenum Press; 1995. pp. 231–243. [Google Scholar]
- Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Jusuf PR, Haverkamp S, Grünert U. Localization of glycine receptor alpha subunits on bipolar and amacrine cells in primate retina. J Comp Neurol. 2005;488:113–128. doi: 10.1002/cne.20555. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Llinás R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–275. doi: 10.1152/jn.1996.76.1.255. [DOI] [PubMed] [Google Scholar]
- Legendre P. Pharmacological evidence for two types of postsynaptic glycinergic receptors on the Mauthner cell of 52-h-old zebrafish larvae. J Neurophysiol. 1997;77:2400–2415. doi: 10.1152/jn.1997.77.5.2400. [DOI] [PubMed] [Google Scholar]
- Legendre P. A reluctant gating mode of glycine receptor channels determines the time course of inhibitory miniature synaptic events in zebrafish hindbrain neurons. J Neurosci. 1998;18:2856–2870. doi: 10.1523/JNEUROSCI.18-08-02856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P. Voltage dependence of the glycine receptor-channel kinetics in the zebrafish hindbrain. J Neurophysiol. 1999;82:2120–2129. doi: 10.1152/jn.1999.82.5.2120. [DOI] [PubMed] [Google Scholar]
- Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. Eighteenth Bowditch lecture. Motor aspects of cerebellar control. Physiologist. 1974;17:19–46. [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. [PubMed] [Google Scholar]
- Magleby KL, Stevens CF. The effect of voltage on the time course of end-plate currents. J Physiol. 1972;223:151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wässle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998;401:34–46. doi: 10.1002/(sici)1096-9861(19981109)401:1<34::aid-cne3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Momiyama A, Silver RA, Häusser M, Notomi T, Wu Y, Shigemoto R, Cull-Candy SG. The density of AMPA receptors activated by a transmitter quantum at the climbing fibre-Purkinje cell synapse in immature rats. J Physiol. 2003;549:75–92. doi: 10.1113/jphysiol.2002.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørkve SH, Veruki ML, Hartveit E. Functional characteristics of non-NMDA-type ionotropic glutamate receptor channels in AII amacrine cells in rat retina. J Physiol. 2002;542:147–165. doi: 10.1113/jphysiol.2002.020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol. 1982;47:928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
- Otis TS, Wu Y-C, Trussell LO. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. J Neurosci. 1996;16:1634–1644. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho RG. Neurotransmitters in the retina. Curr Eye Res. 1996;15:797–803. doi: 10.3109/02713689609003465. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol. 1985;233:473–480. doi: 10.1002/cne.902330406. [DOI] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the β subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Gerschenfeld HM, Llano I. GABAergic and glycinergic IPSCs in ganglion cells of rat retinal slices. J Neurosci. 1997;17:6075–6085. doi: 10.1523/JNEUROSCI.17-16-06075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoè-Pognetto M, Grünert U, Wässle H. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the α1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth FJ. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. J Neurophysiol. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. J Physiol. 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurons. Neuron. 1991;7:965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Tian N, Hwang TN, Copenhagen DR. Analysis of excitatory and inhibitory spontaneous synaptic activity in mouse retinal ganglion cells. J Neurophysiol. 1998;80:1327–1340. doi: 10.1152/jn.1998.80.3.1327. [DOI] [PubMed] [Google Scholar]
- Titmus MJ, Korn H, Faber DS. Diffusion, not uptake, limits glycine concentration in the synaptic cleft. J Neurophysiol. 1996;75:1738–1752. doi: 10.1152/jn.1996.75.4.1738. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Silver RA, Cull-Candy SG. Estimated conductance of glutamate receptor channels activated during EPSCs at the cerebellar mossy fiber-granule cell synapse. Neuron. 1993;11:279–289. doi: 10.1016/0896-6273(93)90184-s. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. AII (rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron. 2002a;33:935–946. doi: 10.1016/s0896-6273(02)00609-8. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci. 2002b;22:10558–10566. doi: 10.1523/JNEUROSCI.22-24-10558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Functional properties of spontaneous EPSCs and non-NMDA receptors in rod amacrine (AII) cells in rat retina. J Physiol. 2003;549:759–774. doi: 10.1113/jphysiol.2003.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Xin D, Bloomfield SA. Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. J Physiol. 2002;539:603–614. doi: 10.1113/jphysiol.2001.013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Chun M-H. Dopaminergic and indoleamine-accumulating amacrine cells express GABA-like immunoreactivity in the cat retina. J Neurosci. 1988;8:3383–3394. doi: 10.1523/JNEUROSCI.08-09-03383.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]