Abstract
Changing arterial oxygen content (CaO2) has a highly sensitive influence on the rate of peripheral locomotor muscle fatigue development. We examined the effects of CaO2 on exercise performance and its interaction with peripheral quadriceps fatigue. Eight trained males performed four 5 km cycling time trials (power output voluntarily adjustable) at four levels of CaO2 (17.6–24.4 ml O2 dl−1), induced by variations in inspired O2 fraction (0.15–1.0). Peripheral quadriceps fatigue was assessed via changes in force output pre- versus post-exercise in response to supra-maximal magnetic femoral nerve stimulation (Δ Qtw; 1–100 Hz). Central neural drive during the time trials was estimated via quadriceps electromyogram. Increased CaO2 from hypoxia to hyperoxia resulted in parallel increases in central neural output (43%) and power output (30%) during cycling and improved time trial performance (12%); however, the magnitude of Δ Qtw (−33 to −35%) induced by the exercise was not different among the four time trials (P > 0.2). These effects of CaO2 on time trial performance and Δ Qtw were reproducible (coefficient of variation = 1–6%) over repeated trials at each FIO2 on separate days. In the same subjects, changing CaO2 also affected performance time to exhaustion at a fixed work rate, but similarly there was no effect of Δ CaO2 on peripheral fatigue. Based on these results, we hypothesize that the effect of CaO2 on locomotor muscle power output and exercise performance time is determined to a significant extent by the regulation of central motor output to the working muscle in order that peripheral muscle fatigue does not exceed a critical threshold.
Increases and decreases in arterial O2 content (CaO2) and therefore in systemic O2 transport achieved via changes in FIO2 and/or Hb concentration are well documented to influence maximum work rate (Knight et al. 1993; Richardson et al. 1999; Calbet et al. 2003) as well as endurance exercise capacity (Adams & Welch, 1980; Koskolou & McKenzie, 1994; Peltonen et al. 1995). However, exactly how changes in CaO2 might affect whole body exercise performance is complex, because influences of CaO2 on O2 transport occur throughout the organism. Thus, with alterations in CaO2, changes in performance may occur because of ‘central’ fatigue mechanisms dictating an increase or decrease in volitional motor output to the locomotor muscles. Peripheral locomotor muscle fatigue may also be a limiting factor to exercise performance with changes in CaO2. Alternatively, both central and peripheral fatigue mechanisms may be important.
We have recently demonstrated that prevention of even a small (< 10%) reduction in arterial O2 saturation (SaO2) and CaO2, as normally observed during heavy sustained exercise to exhaustion in a normoxic environment, significantly alleviated exercise-induced peripheral quadriceps fatigue in humans (Romer et al. 2006a). These data were obtained by comparing the reductions in quadriceps muscle force output in response to supra-maximal femoral nerve stimulation, immediately following exercise of equal power output and duration at SaO2 92% (FIO2 0.21)versusSaO2 98%(FIO2 0.27). These direct measurements of peripheral muscle fatigue following exercise are consistent with earlier findings which showed greater recruitment of quadriceps EMG during heavy sustained exercise in hypoxia versus normoxia, again comparing exercise of equal work rates and durations (Mateika et al. 1996; Taylor et al. 1997). We recently confirmed and extended these findings over a wide range of CaO2 from hypoxia to hyperoxia by combining supra-maximal nerve stimulation pre- and post-exercise with locomotor muscle EMG during exercise (Amann et al. 2006). Combined, these data support a significant and sensitive effect of Δ CaO2 on the rate of development of peripheral muscle fatigue during sustained heavy intensity endurance exercise. In turn, these findings are consistent with the reported effects of hypoxaemia on the rate of muscle metabolite accumulation during progressive exercise (Katz & Sahlin, 1987), even with small muscle groups (Bylund-Fellenius et al. 1981; Chasiotis et al. 1983; Hogan et al. 1999).
The current study attempts to explore how this documented effect of Δ CaO2 on peripheral fatigue and rate of metabolite accumulation relates to central fatigue and endurance exercise performance. To this end, we applied a wide range of CaO2 (via changes in FIO2) during 5 km cycling time trials, a ‘closed-loop’ design (Gandevia, 2001) in which the exercise distance is predetermined. Thus, the time trial is a performance test whereby the subjects are continuously able to ‘choose’ their force output as they attempt to complete the distance in as short a time as possible. Given the findings cited above, we predicted that with variations in CaO2, peripheral muscle fatigue would influence time trial performance; however, we were unsure about whether or not peripheral and central fatigue were linked. Based on the observed effects of Δ CaO2 on performance time and mean power output during the time trial and their correspondence with the level of peripheral muscle fatigue achieved, we formulated a hypothesis that exercise performance time is determined to a significant extent by the regulation of central motor output to the working muscle in order that peripheral muscle fatigue does not exceed a critical threshold. Then, in the same subjects we tested this hypothesis by applying it to a constant work rate performance test to the limit of exhaustion under varying levels of CaO2. This ‘open-loop’ design (Gandevia, 2001) has no fixed end-point leaving the termination of exercise to the subject's volition.
Methods
Subjects
Eight healthy, trained male cyclists volunteered for this project (mean ±s.e.m.; age 22.5 ± 1.7 years, body weight 69.8 ± 2.8 kg, height 173.9 ± 3.0 cm, peak oxygen consumption 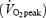 63.3 ± 1.3 ml kg−1 min−1). All subjects had normal resting pulmonary function. Written informed consent was obtained from each participant. All procedures conformed to the standards set by the Declaration of Helsinki and the protocol was approved by the institution's human subjects committee.
63.3 ± 1.3 ml kg−1 min−1). All subjects had normal resting pulmonary function. Written informed consent was obtained from each participant. All procedures conformed to the standards set by the Declaration of Helsinki and the protocol was approved by the institution's human subjects committee.
Protocol
At two preliminary visits to the laboratory subjects were thoroughly familiarized with the procedures used to assess quadriceps muscle function and (a) performed a preliminary 5 km time trial; and (b) performed a maximal incremental exercise test (FIO2 0.21; 20 W + 25 W min−1; Amann et al. 2004) on a computer-controlled electrically braked cycle ergometer (Velotron, Elite Model, Racer Mate, Seattle, WA, USA) for the determination of peak workload (Wpeak) and 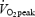 . On later occasions, subjects completed four 5 km cycling time trials in randomized order at the same time of the day and separated by at least 48 h. During these trials, subjects were exposed to either room air (normoxia, FIO2 0.21) or a humidified gas mixture (hypoxia, FIO2 0.15; iso-oxia, FIO2 0.24–0.30; hyperoxia, FIO2 1.0). During the ‘iso-oxia’ trial, supplemental O2 was given to prevent any decrease in arterial O2 saturation below resting values, as estimated using pulse oximetry. The subjects were blinded to the respective FIO2. Subjects were free to shift gears during the time trials. Constant feedback regarding performance time and distance covered was made available to the subjects on a monitor. Electromyographic activity of the vastus lateralis was recorded during all time trials.
. On later occasions, subjects completed four 5 km cycling time trials in randomized order at the same time of the day and separated by at least 48 h. During these trials, subjects were exposed to either room air (normoxia, FIO2 0.21) or a humidified gas mixture (hypoxia, FIO2 0.15; iso-oxia, FIO2 0.24–0.30; hyperoxia, FIO2 1.0). During the ‘iso-oxia’ trial, supplemental O2 was given to prevent any decrease in arterial O2 saturation below resting values, as estimated using pulse oximetry. The subjects were blinded to the respective FIO2. Subjects were free to shift gears during the time trials. Constant feedback regarding performance time and distance covered was made available to the subjects on a monitor. Electromyographic activity of the vastus lateralis was recorded during all time trials.
On different days separated by at least 48 h, subjects performed three constant-load trials to the limit of exhaustion (Tlim) while either breathing room-air (normoxia, FIO2 0.21) or a humidified gas mixture (hypoxia, FIO2 0.15; hyperoxia, FIO2 1.0) in randomized order. Mean power output and pedal cadence from their previous normoxic time trial were used to set the fixed workload (313.8 ± 12.9 W, equals 82.5 ± 1.7% normoxic Wpeak; 105.7 ± 2.5 rev min−1); subjects were again blinded to FIO2. Throughout all exercise tests, subjects remained seated to minimize changes in muscle recruitment. To avoid initial peak power outputs in the time trials and Tlim trials, subjects were instructed to slowly pick up their pace, the recording period started after the mean power output and pedal cadence, adopted from the familiarization time trial, was reached (< 6 s). Neuromuscular function was assessed before, 2.5 min, and 35 min (only after time trials) after exercise (see below). All trials were preceded by a 10 min individualized warm-up at 1.5 W (kg body weight)−1.
Physiological response to exercise
Ventilation and pulmonary gas exchange were measured breath-by-breath at rest and throughout exercise using an open-circuit system (Harms et al. 1998) The averaging interval for the respiratory variables was 20 s. Oxygen consumption during the hyperoxic trials is not reported since conventional equations for computing 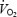 from expired gas data cannot be determined by Haldane's transformation equation when using FIO2 of 1.0 (Stanek et al. 1979; Welch & Pedersen, 1981). Heart rate (HR) was measured from the R–R interval of an electrocardiogram using a three lead arrangement. Ratings of perceived exertion (RPE, dyspnoea and limb discomfort) were obtained at the end of each exercise trial using Borg's modified CR10 scale (Borg, 1998). HbO2 saturation was estimated using a pulse oximeter (SpO2) with optodes placed on the forehead (Nellcor OxiMax, Pleasanton, CA, USA). During all time trials, earlobe capillary blood samples were obtained every 750 m and immediately at end-exercise using an electrochemical analyser (YSI 1500 Sport, OH, USA). During the time trial in normoxic conditions arterial blood was obtained throughout exercise using standard procedures (Harms et al. 1998). Arterial samples were maintained on ice and analysed within 20 min for PO2 (PaO2), PCO2 (PaCO2), and pH using a blood-gas analyser calibrated with tonometered blood (Radiometer ABL300, Copenhagen, Denmark). Arterial HbO2 saturation (SaO2) and [Hb] were measured with a co-oximeter (Radiometer OSM3). Body temperature changes during exercise were measured with a thermocouple placed pernasally in the lower one-third of the oesophagus (Mon-a-therm, Mallinckrodt, St Louis, MO, USA). PaO2, PaCO2 and pH were corrected for in vivo temperature changes using standard procedures (Severinghaus, 1966). CaO2 (ml O2 (dl blood)−1) was calculated as follows: (1.39 ×[Hb]×SaO2/100) + 0.003 ×PaO2. We calculated the proportion of the decrease in SaO2 due to changes in temperature, pH, or PaO2 by comparing measured SaO2 values with the SaO2 values calculated with temperature, pH and PaO2 held constant at pre-exercise baseline values (Severinghaus, 1966). CaO2 during the remaining time trials as well as during the constant workload trials (see below) was calculated based on the subject's [Hb] obtained during the time trial in normoxia (FIO2 0.21). Arterial PO2 in these remaining trials was estimated by subtracting the alveolar-to-arterial O2 difference, as measured during the normoxic time trial, from end-tidal PO2 (PETO2) and HbO2 saturation was estimated from SpO2. Although some random error will certainly occur with these estimates, we reasoned that even a 20 mmHg error would only have a minimal effect on dissolved O2 and therefore on our calculation of CaO2.
from expired gas data cannot be determined by Haldane's transformation equation when using FIO2 of 1.0 (Stanek et al. 1979; Welch & Pedersen, 1981). Heart rate (HR) was measured from the R–R interval of an electrocardiogram using a three lead arrangement. Ratings of perceived exertion (RPE, dyspnoea and limb discomfort) were obtained at the end of each exercise trial using Borg's modified CR10 scale (Borg, 1998). HbO2 saturation was estimated using a pulse oximeter (SpO2) with optodes placed on the forehead (Nellcor OxiMax, Pleasanton, CA, USA). During all time trials, earlobe capillary blood samples were obtained every 750 m and immediately at end-exercise using an electrochemical analyser (YSI 1500 Sport, OH, USA). During the time trial in normoxic conditions arterial blood was obtained throughout exercise using standard procedures (Harms et al. 1998). Arterial samples were maintained on ice and analysed within 20 min for PO2 (PaO2), PCO2 (PaCO2), and pH using a blood-gas analyser calibrated with tonometered blood (Radiometer ABL300, Copenhagen, Denmark). Arterial HbO2 saturation (SaO2) and [Hb] were measured with a co-oximeter (Radiometer OSM3). Body temperature changes during exercise were measured with a thermocouple placed pernasally in the lower one-third of the oesophagus (Mon-a-therm, Mallinckrodt, St Louis, MO, USA). PaO2, PaCO2 and pH were corrected for in vivo temperature changes using standard procedures (Severinghaus, 1966). CaO2 (ml O2 (dl blood)−1) was calculated as follows: (1.39 ×[Hb]×SaO2/100) + 0.003 ×PaO2. We calculated the proportion of the decrease in SaO2 due to changes in temperature, pH, or PaO2 by comparing measured SaO2 values with the SaO2 values calculated with temperature, pH and PaO2 held constant at pre-exercise baseline values (Severinghaus, 1966). CaO2 during the remaining time trials as well as during the constant workload trials (see below) was calculated based on the subject's [Hb] obtained during the time trial in normoxia (FIO2 0.21). Arterial PO2 in these remaining trials was estimated by subtracting the alveolar-to-arterial O2 difference, as measured during the normoxic time trial, from end-tidal PO2 (PETO2) and HbO2 saturation was estimated from SpO2. Although some random error will certainly occur with these estimates, we reasoned that even a 20 mmHg error would only have a minimal effect on dissolved O2 and therefore on our calculation of CaO2.
Contractile function, myoelectrical activity and membrane excitability
Electromyography
Quadriceps electromyogram (EMG) was recorded from the right vastus lateralis (VL), vastus medialis (VM), and rectus femoris (RF) using monitoring electrodes with full-surface solid adhesive hydrogel (Kendall H59P, Mansfield, MA, USA), with on-site amplification. Electrodes were placed in a bipolar electrode configuration on the belly of the muscle with an inter-electrode distance between 20 and 100 mm. The position of the EMG electrodes was marked with indelible ink to ensure that they were placed in the same location at subsequent visits. In order to minimize movement artifacts, electrode cables were fastened to the subject's quadriceps using medical adhesive tape and wrapped with an elastic bandage. The surface EMG electrodes were used to asses (a) the magnetically evoked compound muscle action potentials (M-waves) for VL, VM and RF to evaluate changes in M-wave properties, and (b) the VL EMG continuously throughout all time trials to estimate changes in central neural command. Membrane excitability was assessed before and immediately after exercise for VL, VM and RF using M-wave properties evoked by supramaximal magnetic stimuli. The characteristics measured included peak amplitude, duration and conduction time (Caquelard et al. 2000; Sandiford et al. 2005). The duration was defined as the time from baseline to baseline from the beginning to the end of the biphasic M-wave, where the beginning is defined as a positive deflection two standard deviations above baseline harmonic mean and the end as a return to baseline. The conduction time was defined as the time between the stimulus artifact and peak.
Raw EMG signals from VL corresponding to each muscle contraction during the constant-workload trials and the pre- and post-exercise MVC manoeuvres were recorded for later analysis. The EMG signal was amplified and filtered by a Butterworth band pass filter (BMA −830, CWE, Inc., Ardmore, PA, USA) with a low pass cutoff frequency of 10 Hz and a high pass cutoff frequency of 1 kHz. The slope of the filters was −6 dB octave−1. The filtered EMG signal was sampled at 2 kHz by a 16 bit A/D converter (PCI-MIO-16XE-50, National Instruments, Austin, TX, USA) with custom software (Labview 6.0, National Instruments). A computer algorithm identified the onset of activity where the rectified EMG signal deviated by more than two standard deviations above the baseline for at least 100 ms. Each EMG burst was visibly inspected to verify the timing identified by the computer. For data analysis, the integral of each burst (integrated EMG (iEMG)) was calculated using the formula:
where m is the raw EMG signal.
Magnetic stimulation
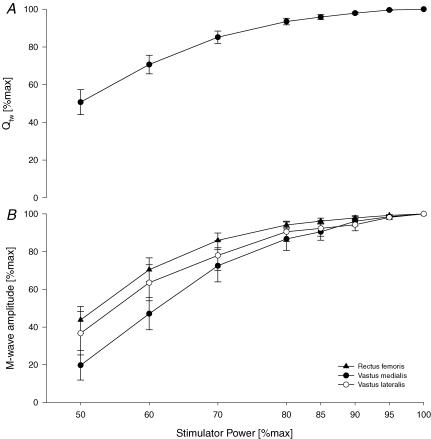
For a detailed description we refer the reader to a previous study from our laboratory (Romer et al. 2006a). Briefly, subjects lay supine on a table with the right thigh resting in a preformed holder and the knee joint angle set at 1.57 rads (90 deg) of flexion. Two magnetic stimulators (Magstim 200, The Magstim Company Ltd, Wales, UK) were used to stimulate the femoral nerve (Polkey et al. 1996; Kufel et al. 2002), the evoked quadriceps twitch force (Qtw) was obtained from a load cell (Interface, Model SM 1000, Scottsdale, AZ, USA). We used single (1 Hz) and paired stimuli (interstimulus intervals: 100 ms (10 Hz), 50 ms (20 Hz), 10 ms (100 Hz)) to discriminate between low and high frequency fatigue (Yan et al. 1993; Polkey et al. 1997). To determine whether nerve stimulation was supramaximal, three single twitches were obtained every 30 s at 50, 60, 70, 80, 85, 90, 95 and 100% of maximal stimulator power output at the beginning of every experiment. A near plateau in baseline Qtw and M-wave amplitudes with increasing stimulus intensities was observed in every subject, indicating maximal depolarization of the femoral nerve (Fig. 1). Following a 20 min rest period, six maximal voluntary contractions (MVCs) of the right quadriceps, separated by 30 s, were performed for 5 s each. To obtain potentiated twitch force (Qtw,pot), Qtw in response to a single twitch was measured 5 s after each MVC. Next, paired stimuli (100, 50 and 10 Hz) were repeated four times for each frequency, followed by eight single stimuli (1 Hz). The stimulations were each separated by 30 s. The entire assessment procedure took 15 min to complete and was performed before exercise (∼30 min) and again 2.5 min and 35 min (only after time trials) after exercise. Qtw in response to each stimulus was analysed. To compare force development characteristics, contraction time (CT), maximal rate of force development (MRFD), half-relaxation time (RT0.5) and maximal relaxation rate (MRR) were analysed for all single twitches (Lepers et al. 2002; Sandiford et al. 2005).
Figure 1. Quadriceps twitch force (Qtw;A) and M-wave amplitudes (B) as a direct response to magnetic stimulation of the femoral nerve applying single twitches (1 Hz) at increasing stimulator power settings (FIO20.21).
The incremental protocol was applied after a 10 min rest period and completed 15 min before the pre-exercise assessment of neuromuscular function. The electromyographic activity was recorded from three pairs of surface electrodes (rectus femoris, vastus medialis, vastus lateralis) and M-wave amplitudes were analysed using a customized software program. n = 8.
We assumed that the motor nerve input to the quadriceps, via the femoral nerve, was the same and supramaximal before and after exercise for each of the comparisons. Based on the 2–3% increment in M-wave amplitude and twitch force beyond 85% of maximum stimulus intensity (Fig. 1) it is likely that we were within 3% of a truly supramaximal stimulus intensity, which may have slightly underestimated the true magnitude of exercise-induced quadriceps fatigue (Bigland-Ritchie & Vollestad, 1988). An additional underestimation of peripheral fatigue might have been caused by the fixed 2.5 min delay between end-exercise and post-exercise neuromuscular measurements, which represented the time needed to instrument the subjects. However, we assumed that any effect of force recovery would have been similar across the variations in CaO2.
Reliability measurements
At separate visits to the laboratory, subjects were removed from the testing apparatus after baseline measurements of muscle function had been obtained and rested in a chair for 30 min without contracting the quadriceps, after which they were attached to the testing apparatus and measurements of quadriceps muscle function were repeated. There was no systematic bias in the baseline measurements either within or between days. Mean within-day within-subject coefficients of variation for resting Qtw across all frequencies were 1.4 ± 0.2 (range: 0.2–3.2) and 4.5 ± 1.7 (range: 0.0–8.8) for MVC. Mean between-day within-subject coefficients of variation for Qtw across all frequencies were 4.2 ± 0.9 (range: 2.1–6.3) and 6.0 ± 0.6 (range: 3.9–7.1) for MVC.
Technical considerations
The limitations of surface EMG measurements, the potential underestimation of fatigue through the use of magnetic femoral nerve stimulation, as well as temperature and muscle potentiation effects on peripheral quadriceps fatigue assessment can be found in the online Supplemental material and in published reports (Enoka & Stuart, 1992; Amann et al. 2006; Romer et al. 2006a).
Statistical analysis
One-way within-subject ANOVA was used to determine the effects of the different levels of CaO2. If ANOVA yielded a significant result, follow-up pairwise comparisons using the Holm's sequential Bonferroni procedure were conducted. Results are presented as mean ±s.e.m. The α level was set at 0.05 a priori.
Results
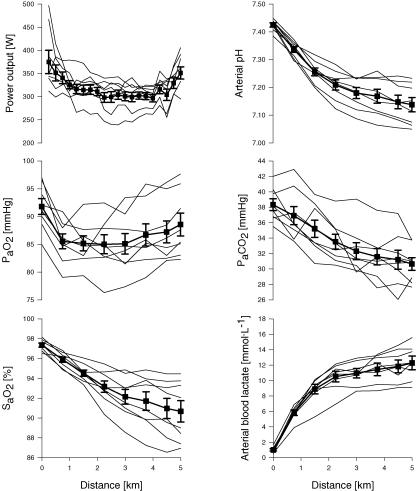
Arterial blood gases during the time trial in normoxia (Fig. 2)
Figure 2. Effects of 5 km time trial in normoxia (FIO20.21) on power output and arterial blood measurements.
Thick lines represent group mean data; thin lines represent individual subject data (n= 8). Mean time for the time trial was 483.4 ± 7.5 s (range 437.5–478.4 s). [Hb] was 14.4 ± 0.5 g l−1 and CaO2 was 19.8 ± 0.8 ml O2 dl−1 at rest, and 16.4 ± 0.7 g l−1 and 20.9 ± 1.0 ml O2 dl−1 at 5 km.
Power output fell progressively over the initial 1.5 km in the time trial (P < 0.05), plateaued for the next 3 km and rose in the final 0.5 km ‘sprint’ (P < 0.01). Mean SaO2 fell progressively from 98 to 91% throughout the time trial, with substantial variability among subjects (−4 to −11%). Reductions in PaO2 below rest were small (3–9 mmHg). Thus, the exercise-induced HbO2 desaturation was due primarily to the rightward shift in the HbO2 dissociation curve, 57% of which was accounted for by a progressive metabolic acidosis (arterial pH range: 7.05–7.23, arterial blood lactate range: 9.1–15.6 mmol l−1 at end-exercise) and the remainder by a 2.1 ± 0.1°C rise in temperature. Mean [Hb] and CaO2 increased from rest (14.4 ± 0.5 g l−1; 19.8 ± 0.8 ml dl−1) to end-exercise (16.4 ± 0.7 g l−1; 20.9 ± 1.0 ml dl−1) (P < 0.01).
All subjects showed a progressive but variable hyperventilation throughout the time trial as PaCO2 was reduced at end-exercise by 5–11 mmHg below rest. The alveolar-to-arterial O2 difference widened almost threefold from rest to 1.5 km (P < 0.001) and narrowed slightly towards the end of the time trial (P < 0.01).
Effects of FIO2 on time trial CaO2, performance time, power output and iEMG (Table 1, Fig. 3)
Table 1.
Physiological response to 2.5 and 5.0 km of cycling at maximal self-selected effort (time trial, TT)
| TT–hypoxia | TT–normoxia | TT–iso-oxia | TT–hyperoxia | |||||
|---|---|---|---|---|---|---|---|---|
| 2.5 km | 5 km | 2.5 km | 5 km | 2.5 km | 5 km | 2.5 km | 5 km | |
| FIO2 | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.21 ± 0.00 | 0.21 ± 0.00 | 0.24 ± 0.00 | 0.28 ± 0.01 | 1.0 ± 0.00 | 1.0 ± 0.00 |
| CaO2 (ml O2 dl−1) | 18.0 ± 0.81,2,4 | 17.6 ± 0.81,2,4 | 21.7 ± 0.72,3,4 | 20.9 ± 0.92,3,4 | 22.7 ± 0.81,3,4 | 22.6 ± 0.81,3,4 | 24.6 ± 0.81,2,3 | 24.4 ± 0.91,2,3 |
| Exercise time (s) | 233.1 ± 3.71,2,4 | 483.4 ± 7.51,2,4 | 224.7 ± 2.73,4 | 458.4 ± 6.82,3,4 | 222.4 ± 3.43,4 | 451.4 ± 6.81,3,4 | 217.5 ± 2.91,2,3 | 438.9 ± 6.91,2,3 |
| Power output (W) | 257.3 ± 10.51,2,4 | 269.3 ± 7.61,2,4 | 299.1 ± 11.62,3,4 | 351.1 ± 13.22,3,4 | 318.5 ± 13.41,3,4 | 399.5 ± 20.01,3,4 | 342.4 ± 11.51,2,3 | 414.5 ± 17.41,2,3 |
| SpO2 (%) | 80.0 ± 1.51,2,4 | 77.9 ± 1.51,2,4 | 95.2 ± 0.72,3,4 | 91.2 ± 0.32,3,4 | 98.2 ± 0.41,3,4 | 97.7 ± 0.41,3,4 | 100 ± 0.51,2,3 | 100 ± 0.51,2,3 |
| SaO2 (%) | — | — | 93.2 ± 0.6 | 90.7 ± 1.1 | — | — | — | — |
| HR (beats min−1) | 175.4 ± 4.0 | 182.3 ± 3.71,2,4 | 179.8 ± 3.0 | 188.4 ± 3.23 | 178.8 ± 3.9 | 187.7 ± 4.03 | 177.5 ± 3.5 | 190.0 ± 3.93 |
| RPE (dyspnoea) | — | 9.1 ± 0.2 | — | 9.1 ± 0.3 | — | 8.6 ± 0.4 | — | 8.4 ± 0.5 |
| RPE (limb) | — | 9.3 ± 0.2 | — | 9.3 ± 0.2 | — | 8.6 ± 0.3 | — | 9.0 ± 0.3 |
| fR (breaths min−1) | 56.5 ± 7.0 | 67.9 ± 10.9 | 52.1 ± 5.0 | 70.5 ± 10.5 | 54.5 ± 6.5 | 70.7 ± 9.9 | 56.8 ± 11.3 | 71.5 ± 9.5 |
| VT (l) | 2.8 ± 0.2 | 2.6 ± 0.24 | 2.8 ± 0.1 | 2.6 ± 0.24 | 2.9 ± 0.2 | 2.6 ± 0.34 | 3.1 ± 0.3 | 2.9 ± 0.31,2,3 |
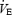 (l min−1) (l min−1) |
154.6 ± 4.7 | 167.3 ± 5.74 | 146.9 ± 5.5 | 171.1 ± 1.54 | 155.1 ± 6.2 | 178.5 ± 5.44 | 164.3 ± 7.1 | 206.5 ± 4.81,2,3 |
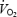 (l min−1) (l min−1) |
3.6 ± 0.11,2 | 3.6 ± 0.11,2 | 4.1 ± 0.22,3 | 4.4 ± 0.22,3 | 4.6 ± 0.31,3 | 4.8 ± 0.31,3 | — | — |
| 43.4 ± 1.21,2 | 46.7 ± 2.01,2 | 36.1 ± 1.43 | 39.7 ± 1.63 | 34.5 ± 2.3 | 37.1 ± 1.73 | — | — | |
| PETO2 (mmHg) | 79.2 ± 0.81,2,4 | 81.2 ± 0.71,2,4 | 111.5 ± 1.42,3,4 | 115.7 ± 1.32,3,4 | 146.9 ± 7.31,3,4 | 163.3 ± 4.31,3,4 | 622.3 ± 4.61,2,3 | 629.5 ± 5.11,2,3 |
| PETCO2 (mmHg) | 31.0 ± 0.71,2,4 | 26.9 ± 0.82,4 | 34.4 ± 1.43,4 | 28.9 ± 1.34 | 35.7 ± 1.53 | 30.9 ± 1.53 | 37.7 ± 1.71,3 | 32.1 ± 1.61,3 |
| PaO2 (mmHg) * | — | — | 85.0 ± 1.7 | 88.6 ± 2.1 | — | — | — | — |
| PaCO2 (mmHg) * | — | — | 33.5 ± 1.0 | 30.6 ± 0.8 | — | — | — | — |
| Capillary [La−]B (mmol l−1) | 9.7 ± 0.7 | 11.4 ± 0.62,4 | 8.7 ± 0.4 | 10.6 ± 0.5 | 8.8 ± 0.4 | 9.8 ± 0.53 | 8.0 ± 0.5 | 9.0 ± 0.63 |
| Arterial [La−]B (mmol l−1)* | — | — | 10.5 ± 0.7 | 12.3 ± 0.8 | — | — | — | — |
| [Hb] (g l−1)* | — | — | 16.3 ± 0.6 | 16.4 ± 0.7 | — | — | — | — |
| Arterial pH* | — | — | 7.209 ± 0.019 | 7.138 ± 0.026 | — | — | — | — |
| Oesophageal temp. (°C) | 38.5 ± 0.1† | 39.1 ± 0.1† | 38.4 ± 0.2 | 39.1 ± 0.1 | 38.5 ± 0.2† | 39.3 ± 0.2† | 39.0 ± 0.2† | 39.7 ± 0.1† |
n = 8. VT, tidal volume.
P < 0.05 versus normoxia
P < 0.05 versus iso-oxia
P < 0.05 versus hypoxia
P < 0.05 versus hyperoxia.
At rest, in normoxia and iso-oxia: CaO2 19.8 ± 0.8 ml O2 dl−1, SaO2 97.4 ± 0.2%, PaO2 92.8 ± 1.4 mmHg, PaCO2 38.3 ± 0.8 mmHg, arterial pH 7.423 ± 0.008, arterial blood lactate ([La−]B) 1.0 ± 0.2 mmol l−1, [Hb] 14.4 ± 0.5 g l−1; and in hypoxia and hyperoxia: CaO2 18.3 ± 0.6 ml O2 dl−1, SaO2 90.4 ± 0.4% and 21.7 ± 0.7 ml O2, 100 ± 0.0%, respectively.
n= 2.
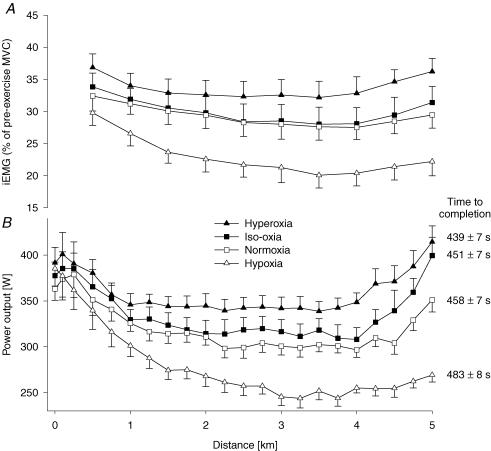
Figure 3. Effect of ΔCaO2 on motor output and muscle power output during the 5 km time trial.
A, effects of various CaO2 values on group mean integrated EMG (iEMG) of vastus lateralis normalized to the iEMG obtained during pre-exercise maximal voluntary contractions (MVCs). Each point represents the mean iEMG of the preceding 0.5 km section. Mean iEMG during the time trial was significantly increased from hypoxia to hyperoxia (P < 0.05). B, group mean variations in power output during the 5 km time trial in four different conditions. Group mean power output was 356.5 ± 12.5 W, 331.0 ± 12.9 W, 313.8 ± 12.9 W and 275.0 ± 9.7 W (P < 0.05) for hyperoxia (FIO2, SpO2, CaO2) 1.0, 100%, 24 ml dl−1; iso-oxia 0.28, 98%, 23 ml dl−1; normoxia 0.21, 91%, 21 ml dl−1; and hypoxia 0.15, 77%, 18 ml dl−1, respectively. n= 8.
Changing FIO2 provided a range of PETO2 and SaO2 and therefore of CaO2 during exercise. Group mean performance time in the time trials decreased significantly with each increment in CaO2 from hypoxia to hyperoxia. The effects of Δ CaO2 on performance time were quite consistent among participants. All eight subjects increased performance time in hypoxia (4–8%) and reduced their time in hyperoxia (−3 to −6%), while 7 of 8 reduced performance time from normoxia to iso-oxia (−1 to −5%).
Mean power output during the time trial was also highly sensitive to CaO2 (Fig. 3). The profile for power output of an initial fall, plateau and a terminal rise were somewhat similar for hyperoxia, normoxia and iso-oxia, but in hypoxia the power output fell markedly and progressively over the initial 3 km of exercise and remained low throughout the final 2 km of the trial.
Figure 3 illustrates changes in iEMG during the four time trials; each point represents a 500 m segment of the time trial. Mean iEMG of the vastus lateralis was normalized to the iEMG obtained from pre-exercise MVC manoeuvres on each day. Over the course of the time trial, iEMG fell progressively (P < 0.05) from 0.5 km to 3.5 km in hyperoxia (13 ± 4%), iso-oxia (15 ± 2%) and hypoxia (33 ± 3%), whereas in normoxia, iEMG decreased until 4 km (16 ± 3%) (P < 0.05). Average iEMG over the entire time trial was increased (P < 0.05) with each increment in CaO2, i.e. from hypoxia to normoxia (28.4 ± 2.8%), normoxia to iso-oxia (2.5 ± 0.6%), and iso-oxia to hyperoxia (12.5 ± 1.3%). This increase in average iEMG from hypoxia to hyperoxia occurred in all 8 subjects.
Effects of time trials and Δ CaO2 on peripheral quadriceps fatigue (Table 2 and Fig. 4)
Table 2.
Effects of 5 km time trial performances (TT, self-selected effort) on fatigue variables
| TT–hypoxia (CaO2 17.6 ml dl−1; 483 s; 275 W) | TT–normoxia (CaO2 20.9 ml dl−1; 458 s; 314 W) | TT–iso-oxia (CaO2 22.6 ml dl−1; 451 s; 331 W) | TT–hyperoxia (CaO2 24.4 ml dl−1; 438 s; 357 W) | P | |
|---|---|---|---|---|---|
| Twitch variables | |||||
| 1 Hz potentiated (N) | −34.3 ± 4.7 | −35.1 ± 4.2 | −33.4 ± 2.1 | −35.4 ± 1.3 | 0.19 |
| 1 Hz (N) | −27.1 ± 2.6 | −28.6 ± 3.8 | −28.5 ± 2.2 | −29.8 ± 2.5 | 0.78 |
| 10 Hz (N) | −29.6 ± 4.6 | −32.5 ± 4.2 | −31.1 ± 2.4 | −32.8 ± 3.0 | 0.64 |
| 50 Hz (N) | −26.5 ± 4.6 | −26.2 ± 4.0 | −25.3 ± 1.9 | −27.9 ± 1.5 | 0.52 |
| 100 Hz (N) | −27.3 ± 4.6 | −25.4 ± 3.5 | −24.7 ± 1.7 | −27.8 ± 2.3 | 0.43 |
| Mean of 4 frequenciesa (N) | −27.6 ± 4.1 | −27.9 ± 3.6 | −27.0 ± 1.5 | −29.4 ± 1.8 | 0.39 |
| Within-twitch variables | |||||
| MRFDb (N s−1) | −25.8 ± 3.9 | −25.6 ± 3.2 | −25.9 ± 2.1 | −28.3 ± 2.3 | 0.70 |
| MRRb (N s−1) | −29.1 ± 5.1 | −26.6 ± 3.7 | −29.1 ± 3.5 | −30.3 ± 2.6 | 0.67 |
| CTb (s) | −3.3 ± 0.7 | −4.5 ± 1.2 | −4.2 ± 0.8 | −4.0 ± 0.8 | 0.52 |
| RT0.5b (s) | 8.3 ± 1.9 | 8.1 ± 2.7 | 8.0 ± 1.4 | 7.9 ± 1.2 | 0.98 |
| MVC (N) | −12.5 ± 4.3 | −13.9 ± 1.6 | −12.5 ± 1.8 | −10.8 ± 2.0 | 0.64 |
Values are expressed as percentage change from pre-exercise baseline measurement to 2.5 min post-exercise. All variables changed significantly, P < 0.05 (n= 8).
1–100 Hz
based on 1 Hz non-potentiated (single twitch); MRFD, maximal rate of force development; MRR, maximal rate of relaxation; CT, contraction time; RT0.5, half-relaxation time; MVC, maximal voluntary contraction. Pre-exercise, resting mean values for twitch forces (1–100 Hz) are represented in Fig. 4. Pre-exercise, resting mean values for MRFD, MRR, CT, RT0.5 and MVC were 1063 ± 16 N s−1, −784 ± 11 N s−1, 0.27 ± 0.02 s, 0.12 ± 0.01 s, and 537 ± 5 N, respectively.
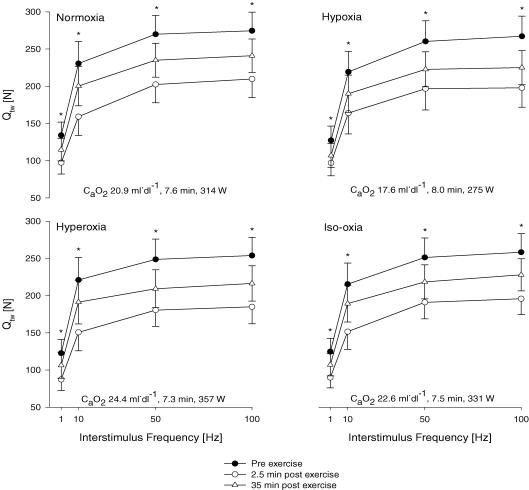
Figure 4. Effects of Δ CaO2during the 5 km time trial on absolute values for group mean force–frequency responses of the quadriceps muscle pre-, 2.5 min and 35 min post-time-trial (various workloads and performance times) in four conditions elicited by supramaximal magnetic femoral nerve stimulation.
Normoxia: (FIO2, SpO2) 0.21, 91%; hypoxia: 0.15, 77%; hyperoxia: 1.00, 100%; iso-oxia: 0.28, 98%. Quadriceps twitch forces (Qtw) are represented as a direct response to single (1 Hz) and three paired twitches with various interstimulus durations (100 ms ∼10 Hz, 20 ms ∼50 Hz, 10 ms ∼100 Hz). *Significant differences between Qtw pre-, 2.5 min post- and 35 min post-exercise (P < 0.01). n= 8.
M-waves
As a measure of membrane excitability we examined pre- versus post-exercise M-wave characteristics in conjunction with the muscle mechanical properties for VL, VM and RF in all conditions and for each exercise trial. None of these changes were significantly different among the various CaO2 values. The absence of changes in M-wave properties (P > 0.2) from pre- to post-exercise indicates that the exercise-induced reductions in Qtw are mainly due to changes within the quadriceps and that peripheral failure of electrical transmission might be excluded.
Contractile function
Quadriceps twitch force (Qtw). Immediately after each time trial, Qtw across the various stimulation frequencies decreased between −25.3 and −35.4% below pre-exercise baseline (P < 0.01) and, despite some recovery, remained reduced from baseline (P < 0.01) 35 min after various time trials (Table 2, Fig. 4). Low frequency fatigue was indicated by the significantly greater loss in Qtw (Δ Qtw) associated with low frequency stimulation (1 and 10 Hz) as compared to high frequency stimulation (50 and 100 Hz). Reductions in Qtw across all frequencies assessed 2.5 min (and 35 min) following the time trials were not significantly different between conditions of varying CaO2 (Table 2).
Within-twitch measurements
All within-twitch measurements (MRFD, MRR, CT, RT0.5) were significantly reduced from baseline immediately post-exercise (Table 2). No differences in these variables were observed up to 35 min post-exercise between the four time trials (P= 0.19–0.90).
MVC force
Peak force during the 5-s MVC manoeuvres was significantly decreased from baseline after all trials (P < 0.01) (Table 2). Thirty-five minutes following each time trial, MVC force was still significantly reduced from baseline (P < 0.01). Reductions in MVC force did not differ among the time trials at varying CaO2 (Table 2).
Effects of CaO2 during time trials on 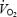 , ventilation and lactate (Table 1, Fig. 5)
, ventilation and lactate (Table 1, Fig. 5)
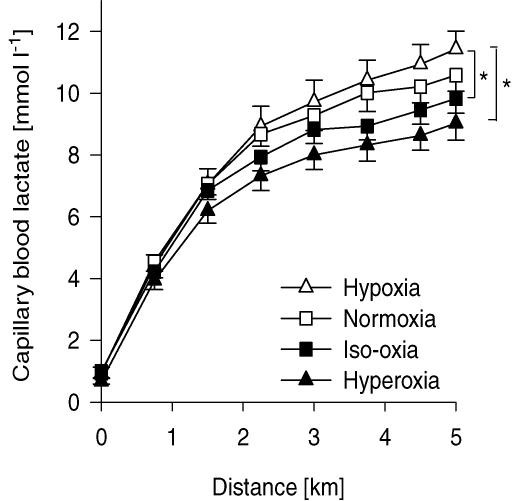
Figure 5. Group mean capillary blood lactate obtained during the 5 km time trials in various conditions.
The asterisks indicate significance at 5 km (*P < 0.05). Hypoxia: (FIO2, SpO2, CaO2) 0.15, 77%, 18 ml dl−1; normoxia: 0.21, 91%, 21 ml dl−1; iso-oxia: 0.28, 98%, 23 ml dl−1; hyperoxia: 1.0, 100%, 24 ml dl−1. n = 8.
During normoxia, 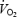 reached 90.0 ± 2.5% of
reached 90.0 ± 2.5% of 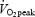 at 1 km and gradually increased to 98.7 ± 1.1% of
at 1 km and gradually increased to 98.7 ± 1.1% of 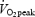 at the end of the trial. Starting at 500 m,
at the end of the trial. Starting at 500 m, 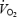 was 14.7 ± 1.5% lower during hypoxia relative to normoxia, whereas
was 14.7 ± 1.5% lower during hypoxia relative to normoxia, whereas 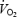 during iso-oxia, starting at 500 m, was on average 10.4 ± 2.5% higher relative to normoxia. The significantly lower PETCO2 at the end of the hypoxic time trial indicates a relative hyperventilation as compared to iso-oxia, normoxia and hyperoxia. Absolute minute ventilation
during iso-oxia, starting at 500 m, was on average 10.4 ± 2.5% higher relative to normoxia. The significantly lower PETCO2 at the end of the hypoxic time trial indicates a relative hyperventilation as compared to iso-oxia, normoxia and hyperoxia. Absolute minute ventilation 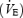 was significantly higher in hyperoxia as compared to the other conditions starting at 3 km (P < 0.05) coinciding with the substantially higher work rate in hyperoxia. Capillary blood lactate concentration tended to be highest in hypoxia and lowest in hyperoxia beginning at the 3 km distance of the time trial and these differences achieved statistical significance at the 4.5 km distance (Fig. 5).
was significantly higher in hyperoxia as compared to the other conditions starting at 3 km (P < 0.05) coinciding with the substantially higher work rate in hyperoxia. Capillary blood lactate concentration tended to be highest in hypoxia and lowest in hyperoxia beginning at the 3 km distance of the time trial and these differences achieved statistical significance at the 4.5 km distance (Fig. 5).
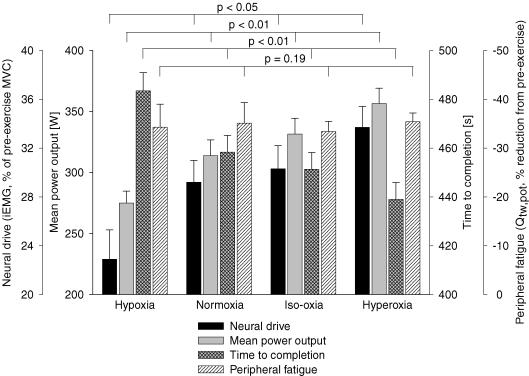
Summary of CaO2 effects on time trial performance and peripheral fatigue
As summarized in Figs 3 and 6, increasing CaO2 over the range of 17.6–24.4 ml dl−1 caused significant increases in neural drive (P < 0.05) and mean power output (P < 0.01) from hypoxia to hyperoxia and resulted in significantly shorter performance times (P < 0.01) with increasing CaO2. Despite these significant effects of arterial oxygenation, exercise-induced decreases in quadriceps twitch force were nearly identical (−33 to −35% pre- to post-exercise for Δ Qtw,pot) for all CaO2 levels.
Figure 6. Summary ofCaO2effects on time trial group mean neural drive (iEMG, vastus lateralis), power output, time to completion and end-exercise peripheral quadriceps fatigue.
Peripheral locomotor muscle fatigue is represented as percentage reduction in potentiated single twitch force (Qtw,pot) from pre-exercise baseline. Hypoxia: (FIO2, SpO2, CaO2) 0.15, 77%, 18 ml dl−1; normoxia: 0.21, 91%, 21 ml dl−1; iso-oxia: 0.28, 98%, 23 ml dl−1; hyperoxia: 1.0, 100%, 24 ml dl−1. n = 8.
Effects of Δ CaO2 during constant work rate trials (Tlim) on exercise time and peripheral fatigue (Tables 3 and 4)
Table 3.
Physiological response to the final minute of constant-load exercise (314 ± 13 W) to the limit of tolerance (Tlim)
| Tlim–hypoxia | Tlim–normoxia | Tlim–hyperoxia | |
|---|---|---|---|
| FIO2 | 0.15 ± 0.0 | 0.21 ± 0.0 | 1.0 ± 0.0 |
| CaO2 (ml O2 dl−1) | 18.2 ± 0.61,3 | 21.3 ± 0.52,3 | 24.5 ± 0.61,2 |
| Exercise time (s) | 269.7 ± 21.91,3 | 488.5 ± 37.32,3 | 1162.3 ± 237.91,2 |
| SpO2 (%) | 81.6 ± 1.91,3 | 93.3 ± 0.82,3 | ∼1001,2 |
| HR (beats min−1) | 182.0 ± 3.8 | 187.7 ± 3.9 | 184.6 ± 3.2 |
| RPE (dyspnoea) | 9.2 ± 0.2 | 8.3 ± 0.2 | 7.8 ± 0.9 |
| RPE (limb) | 9.5 ± 0.2 | 9.4 ± 0.2 | 9.0 ± 0.4 |
| fR (breaths min−1) | 66.7 ± 10.4 | 68.9 ± 8.7 | 57.0 ± 4.0 |
| VT (l) | 2.8 ± 0.2 | 2.6 ± 0.2 | 2.5 ± 0.3 |
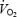 (l min−1) (l min−1) |
176.5 ± 4.73 | 174.3 ± 3.23 | 146.7 ± 11.21,2 |
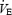 (l min−1) (l min−1) |
3.8 ± 0.11 | 4.4 ± 0.22 | — |
| PETO2 (mmHg) | 81.9 ± 1.51,3 | 115.8 ± 0.92,3 | 622.4 ± 4.7 1,2 |
| PETCO2 (mmHg) | 29.8 ± 1.73 | 30.6 ± 0.6 | 33.1 ± 1.62 |
n= 8.
P < 0.05 versus normoxia
P < 0.05 versus hypoxia
P < 0.05 versus hyperoxia. (See legend to Table 1 for mean resting values.)
Table 4.
Effects of constant-load exercise to the limit of exhaustion (Tlim) on fatigue variables
| Tlim–hypoxia (CaO2 18.2 ml dl−1; 270 s; 314 W) | Tlim–normoxia (CaO2 21.3 ml dl−1; 489 s; 314 W) | Tlim–hyperoxia (CaO2 24.5 ml dl−1; 1162 s; 314 W) | P | |
|---|---|---|---|---|
| Twitch variables | ||||
| 1 Hz potentiated (N) | −31.7 ± 2.2 | −34.2 ± 3.6 | −31.7 ± 2.9 | 0.44 |
| 1 Hz (N) | −27.0 ± 1.2 | −28.1 ± 4.4 | −27.6 ± 3.4 | 0.98 |
| 10 Hz (N) | −30.3 ± 2.2 | −31.8 ± 5.3 | −31.3 ± 4.6 | 0.97 |
| 50 Hz (N) | −25.1 ± 2.1 | −24.6 ± 2.4 | −24.2 ± 2.7 | 0.94 |
| 100 Hz (N) | −23.2 ± 2.2 | −23.5 ± 2.1 | −23.5 ± 1.8 | 0.98 |
| Mean of 4 frequenciesa (N) | −26.1 ± 2.3 | −26.5 ± 3.3 | −26.2 ± 2.8 | 0.98 |
| Within-twitch variables | ||||
| MRFDb (N s−1) | −25.0 ± 1.5 | −25.9 ± 4.3 | −23.8 ± 4.3 | 0.92 |
| MRRb (N s−1) | −26.3 ± 2.4 | −28.1 ± 4.3 | −23.4 ± 4.9 | 0.58 |
| CTb (s) | −3.1 ± 0.4 | −3.4 ± 1.0 | −3.0 ± 0.9 | 0.76 |
| RT0.5b (s) | 6.5 ± 0.9 | 7.1 ± 1.2 | 6.9 ± 1.4 | 0.92 |
| MVC (N) | −11.0 ± 2.4 | −9.1 ± 3.0 | −9.4 ± 3.2 | 0.85 |
Values are expressed as percentage change from pre-exercise baseline measurement to 2.5 min post-exercise. All variables changed significantly, P < 0.05 (n= 8).
1–100 Hz
based on 1 Hz non-potentiated (single twitch); MRFD, maximal rate of force development; MRR, maximal rate of relaxation; CT, contraction time; RT0.5, half-relaxation time; MVC, maximal voluntary contraction. Percentage SpO2 values at end-exercise in hypoxia, normoxia and hyperoxia were 81.6 ± 1.9%, 93.3 ± 0.8% and 100 ± 0.1%, respectively.
Similar values for PETO2, SpO2 and CaO2 to those obtained during the time trials were also obtained (again, by varying FIO2) during constant-load heavy intensity exercise (314 ± 13 W) to exhaustion in the same eight subjects (Table 3).
The time to exhaustion was reduced by 44.4 ± 3.0% from normoxia to hypoxia and increased by 131.4 ± 35.5% from normoxia to hyperoxia. The effects of hypoxia versus normoxia on performance time (Tlim) were quite consistent across all eight subjects (−39 to −52%). However, the effects of hyperoxia versus normoxia were more variable, as six of the subjects showed a fairly consistent 1.6- to 2-fold longer performance time whereas two of the subjects increased Tlim 2.8- and 3.8-fold.
On average, as shown by PETCO2 measurements, subjects hyperventilated more in hypoxia and less in hyperoxia. In 7 of the 8 subjects, 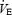 was reduced in hyperoxia compared to normoxia (P < 0.05) and hypoxia (P < 0.05). Oxygen consumption reached 86.0 ± 2.0% of
was reduced in hyperoxia compared to normoxia (P < 0.05) and hypoxia (P < 0.05). Oxygen consumption reached 86.0 ± 2.0% of 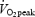 after 2 min in normoxia and progressively increased to 97.3 ± 1.8% at the end of exercise.
after 2 min in normoxia and progressively increased to 97.3 ± 1.8% at the end of exercise.
The results of femoral nerve stimulation pre- versus 2.5 min post-constant-load exercise to exhaustion are shown in Table 4. M-wave properties were again not altered post- versus pre-exercise in any of the CaO2 conditions. Immediately after each Tlim trial, Qtw across the different stimulation frequencies decreased between −23.2 (at 100 Hz) and −34.2% (at 1 Hz, potentiated) below pre-exercise baseline (P < 0.01) (Table 4). Exercise to the limit of exhaustion also reduced MRFD, MRR and CT (P < 0.05) and increased RT0.5 (P < 0.05) from pre-exercise baseline (Table 4). These exercise-induced changes in Qtw amplitude and within-twitch characteristics were not affected by CaO2.
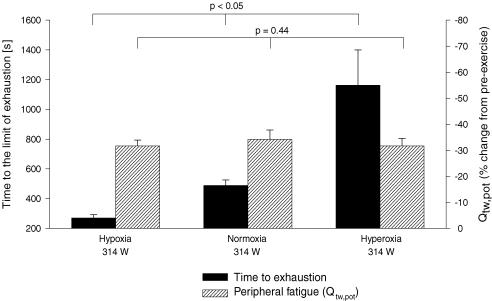
The key data for constant work load tests are summarized in Fig. 7. Despite the marked changes in time to exhaustion from hypoxia to normoxia to hyperoxia, values of peripheral quadriceps fatigue – as indicated here by changes in the potentiated twitch pre- to post-exercise – were nearly identical at all levels of CaO2.
Figure 7. Performance characteristics of various time to the limit of exhaustion (Tlim) trials.
Group mean Tlim, mean constant power output (313.8 ± 12.9 W) and end-exercise peripheral quadriceps fatigue. Peripheral locomotor muscle fatigue is represented as percentage reduction in potentiated single twitch force (Qtw,pot) from pre-exercise baseline. Hypoxia: (FIO2, SpO2, CaO2) 0.15, 82%, 18 ml dl−1; normoxia: 0.21, 93%, 21 ml dl−1; hyperoxia: 1.0, 100%, 25 ml dl−1. n = 8.
Reproducibility of CaO2 effects on exercise-induced quadriceps fatigue, power output, and time trial performance (Table 5)
Table 5.
Reproducibility of time trial performances and peripheral muscle fatigue assessment
| TT–hypoxia | TT–normoxia | TT–iso-oxia | TT–hyperoxia | |||||
|---|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 1* | Trial 2* | Trial 1 | Trial 2 | Trial 1 | Trial 2 | |
| Performance time (s) | 477.8 ± 2.1 | 481.2 ± 2.6 | 458.4 ± 6.8 | 460.0 ± 5.9 | 437.9 ± 1.9 | 438.9 ± 1.3 | 426.3 ± 2.9 | 427.1 ± 2.8 |
| CV (%) | 1.0 ± 0.2 (0.4–1.7) | 0.6 ± 0.1 (0.1–0.8) | 0.6 ± 0.1 (0.4–0.7) | 0.2 ± 0.1 (0.0–0.4) | ||||
| Mean power output (W) | 282 ± 7 | 278 ± 6 | 314 ± 13 | 312 ± 10 | 338 ± 6 | 335 ± 5 | 373 ± 7 | 374 ± 8 |
| CV (%) | 1.6 ± 0.4 (0.9–2.9) | 1.3 ± 0.3 (0.7–3.4) | 2.3 ± 0.7 (1.0–4.3) | 0.8 ± 0.2 (0.0–1.2) | ||||
| Δ Qtw,pot (% reduction) | −34.1 ± 4.1 | −34.6 ± 4.1 | −35.1 ± 4.2 | −33.1 ± 4.6 | −33.9 ± 3.1 | −34.4 ± 3.0 | −35.5 ± 2.7 | −35.1 ± 2.6 |
| CV (%) | 5.9 ± 1.4 (0.3–10.5) | 4.9 ± 1.5 (0.2–10.3) | 6.2 ± 1.7 (0.9–11.7) | 4.4 ± 1.1 (0.1–10.1) | ||||
All 8 subjects (*) repeated the normoxic time trial twice (separate days, with and without arterial catheterization). Four subjects repeated each 5 km time trial twice (TT; same inspirate, 2 separate days). The table represents group mean performance variables (time and mean power output) and the corresponding change for potentiated single twitch force (Δ Qtw,pot), expressed as percentage change from pre-exercise baseline value. Group mean values for power output, performance time and peripheral fatigue between the two trials at equal FIO2 did not differ significantly (P > 0.2). CV, coefficient of variation. Hypoxia: (FIO2, SpO2, CaO2) 0.15, 77%, 18 ml dl−1; normoxia: 0.21, 91%, 21 ml dl−1; iso-oxia: 0.28, 98%, 23 ml dl−1; hyperoxia: 1.0, 100%, 24 ml dl−1.
All eight subjects repeated the normoxic time trial twice on different days (with and without arterial catheterization) and four of the eight subjects repeated the hypoxic, iso-oxic and hyperoxic time trial on two different days. At any given FIO2 and CaO2 no systematic changes occurred between trials in group mean values for performance time, mean power output during exercise, or percentage Δ Qtw (pre- to post-exercise) (P > 0.3). Between-day coefficients of variation at any given FIO2 averaged 4–6% for Δ Qtw across the range of stimulation frequencies, 0.2–1.0% for performance time and 0.8–2.3% for mean power output during exercise.
Discussion
Hypothesis: linking peripheral fatigue, central motor output and exercise performance
We asked if the effect of Δ CaO2 on exercise performance was related to exercise-induced peripheral locomotor muscle fatigue. Using a time trial performance test in which the subject had the option to determine their second-by-second muscle force output and/or velocity of shortening we found that Δ CaO2 had marked parallel effects on central motor output and muscle power output, with inverse effects on performance time; however, the magnitude of peripheral muscle fatigue developed at end-exercise was identical. Similar effects of Δ CaO2 were obtained as a result of fixed high-intensity exercise to exhaustion. We interpret these data to mean that a critical ‘threshold’ magnitude of peripheral muscle fatigue is an important dependent variable during whole body exercise which is protected by up or down regulation of central motor drive to the locomotor muscles and therefore of locomotor muscle force/power output. In turn, peripheral muscle fatigue and/or the rate of development of peripheral muscle fatigue, acting primarily via its influence over central motor output to the muscle, is a significant determinant of exercise performance.
Our rationale for this hypothesis is as follows. We know, based on comparisons of percentage Δ Qtw and rate of rise of quadriceps EMG at high intensity work rates of equal force output and duration, that reduced CaO2 enhances and increased CaO2 constrains the rate of development of peripheral quadriceps fatigue. These effects on peripheral fatigue are highly sensitive to even very small changes in CaO2 (Taylor et al. 1997; Amann et al. 2006; Romer et al. 2006a). Therefore, during the time trial test at, for example, reduced FIO2 and CaO2, if power output had been maintained equal to that in normoxia, the rate of peripheral fatigue development would have been greatly accelerated. But the fact that the rate of central neural drive and muscle force/power output were down-regulated in the presence of reduced CaO2 ensured that the rate of development of peripheral fatigue was slowed and prevented from exceeding a certain critical threshold. Gandevia has referred to this theoretical fatigue threshold as a ‘sensory tolerance limit’ (Gandevia, 2001). This hypothesis requires that the changed rate of fatigue development with Δ CaO2 be manifested and ‘sensed’ in the working muscle and that feedback pathways are available to influence motor output (see below).
Effects of O2 availability on peripheral fatigue
There are several reported findings in humans consistent with the idea that Δ CaO2 affects exercise-induced changes in the metabolic determinants of muscle fatigue and force production which coincide with our observed effects on peripheral fatigue. Thus, during incremental cycling exercise to voluntary exhaustion in normoxia versus hypoxia, changes in sarcoplasmic reticulum Ca2+ cycling (Duhamel et al. 2004) and Na+–K+-ATPase (Sandiford et al. 2005) determined from muscle biopsies obtained at end-exercise were identical, despite marked differences in performance time (∼13%) and peak work rate (∼19%). Previous studies also found no significant effects of severe (Romer et al. 2005) or mild (Sandiford et al. 2005) hypoxaemia versus normoxia on end-exercise neuromuscular fatigue, despite marked differences in exercise time to exhaustion. Furthermore, magnetic resonance imaging measurements during incremental plantar flexion exercise to voluntary exhaustion showed hypoxia-induced acceleration and hyperoxia-induced slowing of intracellular Pi accumulation and phosphocreatine depletion in calf muscle (Hogan et al. 1999). Identical levels of these muscle metabolites were achieved at end-exercise, despite markedly different peak work rates and exercise times (Hogan et al. 1999). These authors also observed that the rate of rise of muscle [H+] with increasing exercise was influenced by Δ CaO2; although not significant, at end-exercise [H+] was lowest in hyperoxia (highest peak work rate) and highest in hypoxia (lowest peak work rate). We found a similar effect of Δ CaO2 during exercise on the rate of plasma lactate accumulation during the time trials (see Fig. 5).
There are multiple potential causes of peripheral muscle fatigue associated with the changing FIO2 that we have not attempted to distinguish in this study (for further discussion of these effects see Amann et al. 2006; Romer et al. 2006a). For example, we do not know whether a changing CaO2 (and O2 transport) or arterial PO2 is the critical mechanism influencing muscle mitochondrial oxygenation and metabolite production, as FIO2 is altered during heavy intensity exercise (Roach et al. 1999; Gonzalez-Alonso et al. 2001). Furthermore, PaCO2 differs slightly but significantly at the varying levels of CaO2 and this is likely to exert some influence on muscle pH and therefore fatigue development (Fitts, 1994). Finally, the work of and/or fatigue incurred by the respiratory muscles during exercise has been shown to have significant effects on muscle sympathetic nerve activity, vascular resistance and blood flow to working skeletal muscles (Harms et al. 1997; St Croix et al. 2000). In turn, mechanically unloading the respiratory muscles by 50–60% during high intensity exercise in normoxia prevented a significant portion of exercise-induced quadriceps fatigue (Romer et al. 2006b). Accordingly, this influence might have contributed in a minor way to peripheral fatigue development during the constant workload (Tlim) trials in which hypoxia caused an increase in absolute 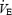 . However, in the time trials, absolute
. However, in the time trials, absolute 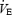 was quite similar across hypoxic to normoxic conditions and even higher in the hyperoxic trials. The higher
was quite similar across hypoxic to normoxic conditions and even higher in the hyperoxic trials. The higher 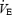 in hyperoxia, despite much higher PaO2, was probably due to the substantially higher work rates achieved (see Table 1).
in hyperoxia, despite much higher PaO2, was probably due to the substantially higher work rates achieved (see Table 1).
Sensory muscle afferent fibres and exercise performance
How might these CaO2-dependent effects on the rate of development of peripheral muscle fatigue and its determinants be ‘sensed’ and in turn impact central motor output? Group III and IV muscle afferents innervate free nerve endings distributed widely throughout muscle. Metabolic byproducts of muscular contractions associated with fatigue, including H+ and Pi, have been shown to increase the spontaneous discharge of both group III and IV mechanoreceptors and nociceptors (Mense, 1977; Kniffki et al. 1978; Rotto & Kaufman, 1988; Gandevia, 1998). As mentioned above, the rate of accumulation of these fatigue-related (and sensory fibre-stimulating) metabolites have been shown to be accelerated in hypoxia and attenuated in hyperoxia (Hogan et al. 1999) and thus the metaboreflex, regulated via sensory muscle afferents, might be similarly influenced via Δ CaO2. It is generally accepted that the central actions of group III and IV muscle afferents potentially inhibit central motor output and voluntary muscular performance via a reduced activation of the motoneuron pool (i.e. central fatigue) (Bigland-Ritchie et al. 1986; Garland, 1991; Gandevia, 2001). A cortical manifestation of the feedback influences may be found in the CaO2-dependent rate of rise of conscious perceptions of effort during constant work rate exercise (Romer et al. 2005; Amann et al. 2006), which would be expected to affect moment to moment voluntary decisions concerning the magnitude of central motor output.
Our previous findings comparing Δ CaO2 effects at an equal fixed work rate and duration provide support for the above-described feedback influence (Amann et al. 2006). When subjects were required to maintain a constant high-intensity muscle force output, central neural drive over time was increased, presumably to recruit more motor units. In these experiments, the lower the CaO2, the greater was the rate of development of peripheral fatigue (as indicated by greater Δ Qtw pre- versus post-exercise) and the greater the time-dependent rise in iEMG.
Quantifying exercise-induced peripheral muscle fatigue
Since a major finding in support of our hypothesis was that the magnitude of exercise-induced peripheral quadriceps muscle fatigue was equal in hypoxia through hyperoxia despite marked differences in exercise time and force output, it is important to verify that our magnetic nerve stimulation technique and experimental design were capable of detecting significant changes in exercise-induced peripheral muscle fatigue. Accordingly, we recently reported that variations in exercise duration at constant heavy intensity work rate and CaO2 (Romer et al. 2006a), as well as changes in respiratory muscle work at equal exercise duration and work rate, (Romer et al. 2006b) were capable of eliciting significant differences of 5–17% in exercise-induced reductions in quadriceps twitch force (Romer et al. 2006a,b). Most relevant, when we compared the effects of Δ CaO2 at equal cycling work rates and durations we found significant differences in percentage Δ Qtw (pre- versus post-exercise) with each increment in CaO2 (range =Δ Qtw 2.8 ± 0.9% at CaO2 23 versus 24 ml dl−1, to Δ Qtw 13.2 ± 1.5% at CaO2 18 versus 24 ml dl−1) (Amann et al. 2006). These data show both that CaO2 has a highly sensitive influence on the rate of development of exercise-induced peripheral muscle fatigue (Δ Qtw) and also that our magnetic stimulation methods are capable of detecting relatively small significant differences in exercise-induced peripheral muscle fatigue. We also note that all measures derived from magnetic stimulation, both within-twitch parameters and Δ Qtw at all stimulation frequencies, were internally consistent in showing equivalent changes in exercise-induced peripheral fatigue pre- to post-time trial at each FIO2 (see Table 2).
Finally, two types of between-day reproducibility data are strongly supportive of the validity of our peripheral fatigue findings. First, the control (pre-exercise) force: frequency curves were highly reproducible within subjects, between days (Amann et al. 2006; Romer et al. 2006a). Secondly, the magnitude of the exercise-induced peripheral muscle fatigue was nearly identical between repeat time trials conducted at each FIO2 and the random variation in Δ Qtw between trials was less than 7% (see Table 5). Thus, in total these data show that our methods are capable of detecting even small, significant attenuations in peripheral quadriceps fatigue as induced by variations in CaO2 during exercise.
Additional (central) causes of Δ CaO2 effects on central motor output and exercise performance
It is important to emphasize that a ‘threshold’ for peripheral muscle fatigue as proposed above is certainly not the only potential source of inhibitory influences on central motor output and muscle force output during these complex conditions of high intensity exercise in the face of a changing CaO2. A significant influence of brain hypoxia on performance has also been indirectly implicated in several studies. Thus, in conditions of relatively severe acute hypoxia with end-exercise PaO2 25–34 mmHg and SaO2 51–66%: (a) Kjaer et al. (1999) showed that blockade of afferent feedback via lumbar epidural anaesthesia did not influence exercise time to exhaustion; and (b) Calbet et al. (2003) reported that switching from severe hypoxia to normoxia at the point of exhaustion immediately allowed exercise to continue at the required work rate. The marked severity of the cerebral hypoxia imposed in these studies was evidenced by the waning consciousness reported in these exercising subjects (Kjaer et al. 1999). These data strongly suggest that hypoxic-sensitive sources of inhibition of central motor output exist outside any influences related to peripheral muscle fatigue and its associated afferent feedback.
Metabolic turnover of neurotransmitters such as 5-hydroxytryptamine in cerebral tissue is especially sensitive to oxygen lack and even to intense exercise per se, and may influence central motor output during exercise (Davis & Bailey, 1997). Perhaps the relative influence of these CNS versus peripheral influences on central motor output is critically dependent upon the severity of the perturbation in systemic and cerebral O2 transport across the range of severe hypoxia to hyperoxia. In addition to changes in CaO2, reductions in cerebral blood flow secondary to the hypocapnia attending high intensity endurance exercise (see fig. 2) would also be expected to compromise cerebral O2 transport. Accordingly, while even relatively small increases and decreases in CaO2 do exert highly sensitive effects on the rate of development of exercise-induced peripheral fatigue (Amann et al. 2006; Romer et al. 2006a), we would predict that the relative influence of the proposed feedback effect from fatiguing muscle on central motor output and exercise performance will diminish as CaO2 is reduced substantially below normoxic levels and CNS hypoxia increases in influence. Alternatively, the feedback from peripheral muscle fatigue may increase in relative importance as CaO2 is increased above more moderate levels of hypoxia through hyperoxia. Consistent with this idea are the findings by Arbogast et al. (2000) showing a depressive effect of severe hypoxia and a stimulating action of hyperoxia on group IV muscle afferent responses to a fixed level of muscular fatigue. However, this speculation requires further experimentation, utilizing a broad range of arterial oxygenation.
Conclusion
We interpret our findings to indicate that the rate of peripheral locomotor muscle fatigue development – as affected by CaO2– is a significant determinant of the magnitude of central motor output during exercise in order to prevent ‘excessive’ development of peripheral muscle fatigue beyond a critical threshold or sensory tolerance limit. Thus, acting via inhibitory neural feedback to higher motor areas of the central nervous system, the rate of peripheral fatigue development influences central motor drive and exercise performance, i.e. power output and time to task completion in ‘closed-loop’ designs and time to the limit of exhaustion in ‘open-loop’ designs. We hypothesize that the CaO2 and O2-transport-sensitive rate of peripheral muscle fatigue development operates as a (dose-dependent) trigger of ‘central fatigue’, i.e. reductions in central motor drive, which in turn influences exercise performance. Finally, we need to emphasize that peripheral fatigue development, as studied here, presents as just one of the many potential mechanisms – CNS hypoxia being another – available to influence central motor output and performance as O2 availability is altered during high intensity exercise. Quantifying the relative contributions of these central fatigue-causing mechanisms remains a formidable task.
Acknowledgments
This research was supported by a National Heart, Lung, and Blood Institute (NHLBI) RO1 grant (HL-15469). A. T. Lovering and M. K. Stickland were supported by a NHLBI Training Grant (T32 HL-07654) and NSERC, respectively. We thank Mr Anthony J. Jacques and Mr C. Joel Hess for valuable assistance with analysing the EMG data.
Supplemental material
The online version of this paper can be accessed at:DOI: 10.1113/jphysiol.2006.113936 http://jp.physoc.org/cgi/content/full/jphysiol.2006.113936/DC1 and contains supplemental material entitled ‘Technical considerations’.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Adams RP, Welch HG. Oxygen uptake, acid-base status, and performance with varied inspired oxygen fractions. J Appl Physiol. 1980;49:863–868. doi: 10.1152/jappl.1980.49.5.863. [DOI] [PubMed] [Google Scholar]
- Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ, Dempsey JA. The effects of arterial oxygen content upon peripheral locomotor muscle fatigue. J Appl Physiol. 2006;101:119–127. doi: 10.1152/japplphysiol.01596.2005. [DOI] [PubMed] [Google Scholar]
- Amann M, Subudhi A, Foster C. Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc. 2004;36:613–622. doi: 10.1249/01.mss.0000122076.21804.10. [DOI] [PubMed] [Google Scholar]
- Arbogast S, Vassilakopoulos T, Darques JL, Duvauchelle JB, Jammes Y. Influence of oxygen supply on activation of group IV muscle afferents after low-frequency muscle stimulation. Muscle Nerve. 2000;23:1187–1193. doi: 10.1002/1097-4598(200008)23:8<1187::aid-mus5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Vollestad N. Hypoxia and fatigue: how are they related? In: Sutton JR, Houston CS, Coates G, editors. Hypoxia: the Tolerable Limits. Indianapolis, IL, USA: Benchmark; 1988. pp. 315–325. [Google Scholar]
- Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL, USA: Human Kinetics; 1998. [Google Scholar]
- Bylund-Fellenius AC, Walker PM, Elander A, Holm S, Holm J, Schersten T. Energy metabolism in relation to oxygen partial pressure in human skeletal muscle during exercise. Biochem J. 1981;200:247–255. doi: 10.1042/bj2000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R291–R303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Caquelard F, Burnet H, Tagliarini F, Cauchy E, Richalet JP, Jammes Y. Effects of prolonged hypobaric hypoxia on human skeletal muscle function and electromyographic events. Clin Sci (Lond) 2000;98:329–337. [PubMed] [Google Scholar]
- Chasiotis D, Hultman E, Sahlin K. Acidotic depression of cyclic AMP accumulation and phosphorylase b to a transformation in skeletal muscle of man. J Physiol. 1983;335:197–204. doi: 10.1113/jphysiol.1983.sp014528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29:45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Duhamel TA, Green HJ, Sandiford SD, Perco JG, Ouyang J. Effects of progressive exercise and hypoxia on human muscle sarcoplasmic reticulum function. J Appl Physiol. 2004;97:188–196. doi: 10.1152/japplphysiol.00958.2003. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Neural control in human muscle fatigue: changes in muscle afferents, motoneurones and motor cortical drive [corrected] Acta Physiol Scand. 1998;162:275–283. doi: 10.1046/j.1365-201X.1998.0299f.x. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Garland SJ. Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol. 1991;435:547–558. doi: 10.1113/jphysiol.1991.sp018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol. 1998;507:619–628. doi: 10.1111/j.1469-7793.1998.619bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999;86:1367–1373. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- Katz A, Sahlin K. Effect of decreased oxygen availability on NADH and lactate contents in human skeletal muscle during exercise. Acta Physiol Scand. 1987;131:119–127. doi: 10.1111/j.1748-1716.1987.tb08213.x. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Hanel B, Worm L, Perko G, Lewis SF, Sahlin K, Galbo H, Secher NH. Cardiovascular and neuroendocrine responses to exercise in hypoxia during impaired neural feedback from muscle. Am J Physiol. 1999;277:R76–R85. doi: 10.1152/ajpregu.1999.277.1.R76. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978;31:511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Koskolou MD, McKenzie DC. Arterial hypoxemia and performance during intense exercise. Eur J Appl Physiol Occup Physiol. 1994;68:80–86. doi: 10.1007/BF00599246. [DOI] [PubMed] [Google Scholar]
- Kufel TJ, Pineda LA, Mador MJ. Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve. 2002;25:438–444. doi: 10.1002/mus.10047. [DOI] [PubMed] [Google Scholar]
- Lepers R, Maffiuletti NA, Rochette L, Brugniaux J, Millet GY. Neuromuscular fatigue during a long-duration cycling exercise. J Appl Physiol. 2002;92:1487–1493. doi: 10.1152/japplphysiol.00880.2001. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Essif E, Fregosi RF. Effect of hypoxia on abdominal motor unit activities in spontaneously breathing cats. J Appl Physiol. 1996;81:2428–2435. doi: 10.1152/jappl.1996.81.6.2428. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977;267:75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen JE, Rantamaki J, Niittymaki SP, Sweins K, Viitasalo JT, Rusko HK. Effects of oxygen fraction in inspired air on rowing performance. Med Sci Sports Exerc. 1995;27:573–579. [PubMed] [Google Scholar]
- Polkey M, Kyroussis D, Hamnegard C, Hughes P, Rafferty G, Moxham J, Green M. Paired phrenic nerve stimuli for the detection of diaphragm fatigue in humans. Eur Respir J. 1997;10:1859–1864. doi: 10.1183/09031936.97.10081859. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve. 1996;19:549–555. doi: 10.1002/(SICI)1097-4598(199605)19:5<549::AID-MUS1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JAL, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol. 1999;276:H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA. Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2006a;290:R365–R375. doi: 10.1152/ajpregu.00332.2005. [DOI] [PubMed] [Google Scholar]
- Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of severe hypoxia on endurance capacity and quadriceps muscle fatigue in healthy humans. Med Sci Sports Exerc. 2005;37:S296. [Google Scholar]
- Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006b;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiford SD, Green HJ, Duhamel TA, Schertzer JD, Perco JD, Ouyang J. Muscle Na-K-pump and fatigue responses to progressive exercise in normoxia and hypoxia. Am J Physiol Regul Integr Comp Physiol. 2005;289:R441–R449. doi: 10.1152/ajpregu.00652.2004. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW. Blood gas calculator. J Appl Physiol. 1966;21:1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- Stanek KA, Nagle FJ, Bisgard GE, Byrnes WC. Effect of hyperoxia on oxygen consumption in exercising ponies. J Appl Physiol. 1979;46:1115–1118. doi: 10.1152/jappl.1979.46.6.1115. [DOI] [PubMed] [Google Scholar]
- Taylor AD, Bronks R, Smith P, Humphries B. Myoelectric evidence of peripheral muscle fatigue during exercise in severe hypoxia: some references to m. vastus lateralis myosin heavy chain composition. Eur J Appl Physiol Occup Physiol. 1997;75:151–159. doi: 10.1007/s004210050140. [DOI] [PubMed] [Google Scholar]
- Welch HG, Pedersen PK. Measurement of metabolic rate in hyperoxia. J Appl Physiol. 1981;51:725–731. doi: 10.1152/jappl.1981.51.3.725. [DOI] [PubMed] [Google Scholar]
- Yan S, Gauthier A, Similowski T, Faltus R, Macklem P, Bellemare F. Force-frequency relationships of in vivo human and in vitro rat diaphragm using paired stimuli. Eur Respir J. 1993;6:211–218. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be accessed at:DOI: 10.1113/jphysiol.2006.113936 http://jp.physoc.org/cgi/content/full/jphysiol.2006.113936/DC1 and contains supplemental material entitled ‘Technical considerations’.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com