Abstract
In skeletal muscle, Ca2+ is implicated in contraction, and in regulation of gene expression. An alteration of [Ca2+]i homeostasis is responsible, at least partially, for the muscle degeneration that occurs after eccentric contractions in Duchenne muscular dystrophy, a disease characterized by the loss of the cytoskeletal protein dystrophin. Using patch clamp in the cell-attached configuration, we characterized the store-operated channels (SOCs) and the stretch-activated channels (SACs) present in isolated mouse skeletal muscle. SOCs were voltage independent, had a unitary conductance between 7 and 8 pS (110 mm Ca2+ in the pipette), and their open probability increased when the sarcoplasmic reticulum was depleted by thapsigargin. These SOCs were identical to those previously described in the pathophysiology of Duchenne muscular dystrophy. Under the same experimental conditions, we detected a channel activity that was increased by applying a negative pressure to the patch electrode. The SACs responsible for this current had the same unitary conductance and current–voltage relationship as those observed for SOCs. SOCs and SACs had a similar sensitivity to pharmacological agents such as Gd3+, SKF-96365, 2-aminoethoxydiphenyl borate and GsMTx4 toxin. Moreover, stimulation with IGF-1 increased the occurrence of the activity of both channel types. Together, these observations suggest that SOCs and SACs might belong to the same population or share common constituents. From a functional point of view, treatment of soleus muscle with SKF-96365 or GsMTx4 toxin increased its sensitivity to a fatigue protocol, suggesting that the influx of Ca2+ that occurs through these channels during contraction is also involved in force maintaining during repeated stimulations.
In most cells, depletion of Ca2+ from intracellular Ca2+ stores causes Ca2+ entry across the plasma membrane. This process has been generally termed capacitative Ca2+ entry or store-operated Ca2+ entry (Putney, 1986; Parekh & Penner, 1997), and is considered to play an important role in Ca2+ homeostasis. Candidate channels for this store-operated entry of Ca2+ are proteins of the transient receptor potential (TRP) superfamily, which were initially recognized in Drosophila (Montell & Rubin, 1989) and then also in mammalian cells (Birnbaumer et al. 1996; Okada et al. 1998; Putney, 1999). TRP proteins are cation channels presenting a similarity of structure (six transmembrane domains) and some sequence homology. In mammals, the TRP superfamily contains six subfamilies. Four of them share substantial sequence identity in the transmembrane domains: classical (TRPC), vanilloid (TRPV), melastatin (TRPM), and ANKTM1 (TRPA). The last two subgroups, i.e. muclopins (TRPML) and polycystins (TRPP) are only distantly related to other subfamilies. Studying the precise function of these channels has been rendered difficult because of the lack of specific inhibitors. Different drugs have been used such as trivalent lanthanides (La3+ and Gd3+), and SKF-96365 (1-[β-[3-(4-methoxyphenylpropoxy]-4-methoxyphenetyl]-1H-imidazole) (Putney, 2001), but they lack specificity (Bales et al. 1999). Even 2-aminoethoxy-diphenyl borate (2-APB), initially used as an inositol-trisphosphate-receptor blocker (Ma et al. 2000), has the ability to block thapsigargin-induced Ca2+ entry, which varies from cell to cell (Kukkonen et al. 2001; Ma et al. 2001), and obviously has multiple targets (Peppiatt et al. 2003).
So far, the TRP channels identified as possibly involved in store-operated influxes of Ca2+ belong to the TRPC and TRPV subfamilies. Indeed, using a methodology of heterologous expression, TRPC1, 2, 3, 4 and 5, and TRPV6, have been shown to be possibly activated by store depletion (Groschner et al. 1998; Philipp et al. 1998; Vannier et al. 1999; Warnat et al. 1999; Liu et al. 2000; Yue et al. 2001). However, other authors showed that heterologous expression of these proteins also gave channels whose activation was independent of store depletion (Zitt et al. 1997; Hofmann et al. 1999; Schaefer et al. 2000; Wu et al. 2000; Bodding et al. 2003). This might be due to the fact that the mechanism of channel activation depends on their expression level (Vazquez et al. 2003). Strategies using repression with antisense oligonucleotides were then used to demonstrate the possible involvement of TRPC1, 2 and 3, and TRPC4, in store-operated entry of Ca2+ (Philipp et al. 2000; Wu et al. 2000; Gailly & Colson-Van Schoor, 2001). Finally, the involvement of TRPC4 in store-operated Ca2+ current was demonstrated by showing that mice deficient in TRPC4 lack this current in endothelial cells and exhibit reduced vasorelaxation (Freichel et al. 2001).
In a previous work, we have shown that store-dependent channels are present in skeletal muscles and that their activity is abnormally increased in Duchenne muscular dystrophy (Vandebrouck et al. 2002), a myopathy due to the lack of a cytoskeletal protein called dystrophin.
TRP channels also respond to stimuli other than store depletion. Indeed, TRPC proteins have been shown to respond to agonists independently of store depletion, and TRPV proteins are sensitive to heat and cold, pH changes, osmolarity or volume changes, or are constitutively active and responsible for Ca2+ reabsorption in kidney and duodenum (TRPV5 and 6) (reviewed in Benham et al. 2002; Minke & Cook, 2002). In the present study, we characterize the pharmacological profile and the different modes of activation of voltage-independent Ca2+ channels present in adult skeletal muscle fibres, and we assess their possible involvement in the normal mechanical response of muscle to electrical stimulation.
Methods
Muscle mechanics
Adult wild-type (C57) mice were deeply anaesthetized with a solution (10 ml kg−1, intraperitoneally) containing ketamine (10 mg ml−1) and xylazine (1 mg ml−1) in order to preserve muscle perfusion during dissection of both soleus muscles. Depth of anaesthesia was assessed by the abolition of eyelid and pedal reflexes. After dissection, the animals were killed by rapid neck dislocation. This protocol has been approved by the Animal Ethics Committee of the Catholic University of Louvain, Brussels. Soleus muscles were bathed in a 1 ml horizontal chamber continuously superfused with oxygenated Krebs solution (O2 95%/CO2 5%) containing (mm): NaCl 118, NaHCO3 25, KCl 5, KH2PO4 1, CaCl2 2.5, MgSO4 1, glucose 5, maintained at a temperature of 20 ± 0.1°C. One end of the muscle was tied to an isometric force transducer, and the other end to an electromagnetic motor and length transducer. Stimulation was delivered through platinum electrodes running parallel to the muscles. Muscle length was carefully adjusted for maximal isometric force using 0.35 s maximally fused tetani. Force was recorded on a high-speed pen recorder (Sanborn model 320).
Isolation of adult skeletal muscle fibres
The flexor digitorum brevis (FDB) muscles were removed and incubated for 38 min at 37°C in an oxygenated ‘Krebs–Hepes’ solution (see composition below) containing 0.2% collagenase type IV (Sigma, St Louis, MO, USA). Muscles were then washed twice in Krebs buffer, suspended in DMEM/HAM F12 (Sigma) supplemented with 2% fetal bovine serum (Sigma), and mechanically dissociated by repeated passages through fire-polished Pasteur pipettes of progressively decreasing diameter. Dissociated fibres were plated onto tissue culture dishes coated with extracellular matrix basement membrane (Harbor Bio-Products, Norwood, MA, USA) and allowed to adhere to the bottom of the dish for 2 h. For Ca2+ measurements, cells were plated on circular glass coverslips. Culture dishes were kept in an incubator, with 5% CO2 at 30°C.
Measurements of cytosolic [Ca2+]
Muscle fibres were loaded for 1 h at room temperature with the membrane-permeant Ca2+-indicator Fura-PE3/AM (1 μm) and Pluronic F-127 (0.004%). Fura-PE3/AM was preferred to Fura-2/AM as it is stable during long-lasting experiments, with little or no compartmentation (Vorndran et al. 1995). Fibres were illuminated through an inverted Nikon microscope (×40-magnification objective) alternately at 340 and 380 nm, and the fluorescent light emitted at 510 nm was measured using a Deltascan spectrofluorimeter (Photon Technology International). The ratio R340/380 of the fluorescence intensity emitted at the two excitation wavelengths was calculated, and cytosolic concentration of Ca2+ ([Ca2+]i) was determined with a calibration previously described (Vandebrouck et al. 2002). The Krebs–Hepes solution contained (mm): NaCl 135.5, MgCl2 1.2, KCl 5.9, glucose 11.5, Hepes 11.5, CaCl2 1.8 (pH 7.3). When necessary, CaCl2 was omitted and replaced by 50 μm sodium EGTA, and osmolarity was adjusted with sucrose. The potassium aspartate solution contained (mm): potassium aspartate 150, MgCl2 5, EGTA 10 and Hepes 10 (pH 7.3).
Electrophysiological methods
Single-channel activity was recorded from cell-attached patches using the technique described by Hamill et al. (1981). Patch electrodes were pulled on a DMZ-Universal (Zeitz-Instruments) puller in three stages from borosilicate glass capillaries (1.5 mm in diameter; Harvard Apparatus) to a tip diameter of 1–2 μm. Patch electrodes had a resistance of 2–5 MΩ. Cells were viewed under phase contrast with a Diaphot Nikon inverted microscope. The activity was recorded at a constant holding potential of −60 mV and at room temperature, using a HEKA EPC-9 amplifier. This holding potential value takes into account the basal membrane potential (measured independently at −50 mV) (Cahalan & Neher, 1992). Current records were filtered with a Bessel filter at 3 kHz and digitized at 10 kHz. Data were analysed using Pulse-Fit, Pulse-Tools and Origin 6.1 software. Most of the patches contained more than one channel. Therefore, the global open probability (n.Po) was calculated (ratio of total open time to total time for a given patch).
The intrapipette solution contained (mm): CaCl2 110, Hepes 10 and DIDS (4,4′-diisothyocyanatostilbene-2,2′-disulphonic acid) 0.1. The bathing solution contained (mm): NaCl 124, MgCl2 1.2, KCl 5.9, glucose 11.5, HepesNa 11.5, EGTA 10. The osmolarity (measured with a microosmometer type 13/13DR Roebling) of these solutions was adjusted to 320–330 mosmol l−1 by adding water or sucrose, and adjusted to pH 7.3 with NaOH. DIDS was used to block possible chloride conductances.
Thapsigargin (TG) was dissolved in DMSO and diluted 1:2000 into the bath to a final concentration of 1 μm. Channels activity was recorded during 90 s (30 sweeps of 3 s) in the absence of TG (control condition) and was prolonged for 3–5 min after application of the drug on the same patch. The solvent alone had no effect on channel activity. Mechanical stimulation was performed by applying on the patch pipette a suction the pressure of which was measured with a mercury-filled U-shape manometer. Channel mechanosensitivity was therefore only studied under stationary conditions. As the response to stretch was fast, channels activity was only recorded during 30–60 s for each chosen pressure (one or two series of 10 sweeps of 3 s).
Reagents
The GsMTx4 toxin, isolated from Grammostola spatulata spider (Suchyna et al. 2000), was obtained from Peptides International (Louisville, KY, USA); SKF-96365 and 2-APB were from Alexis Corporation (Lausen, Switzerland); and Fura-PE3/AM was from Calbiochem (Darmstadt, Germany). All other reagents were of analytical grade and purchased from Sigma. Channel inhibitors were added to the pipette solution in order to have access to the external face of the channel in cell-attached configuration.
Statistics
Data are presented as means ± s.e.m.). The χ2 test, Fisher exact test, ANOVA and Student's t test were used to determine statistical significance.
Results
Characterization of TG-induced Ca2+ currents in skeletal muscle fibres
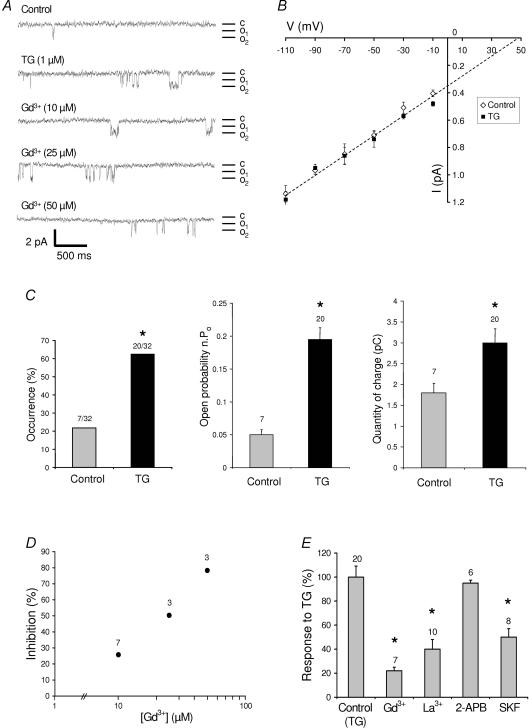
In a previous paper we showed the presence, in muscle fibres, of voltage-independent Ca2+ channels that are activated by store depletion. Under the conditions used here (cell-attached configuration, muscle fibres in physiological medium), we detected a basal Ca2+ channel activity with a unitary conductance of 7.4 ± 0.47 pS (n = 7, in 110 mm Ca2+) and a reversal potential at +43 mV (Fig. 1A and B). TG was used to deplete the stores by inhibiting the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA). After 90 s of patch recording under control conditions, 1 μm TG was added to the bath and recording was prolonged by 3 min. As previously shown (Vandebrouck et al. 2002), TG did not induce significant modifications of the properties of these channels (unitary conductance of 7.1 ± 0.68 pS, n = 20, reversal potential of +48 mV). However, their occurrence (number of patches in which a Ca2+ current is recorded/number of patches sampled) was significantly increased by a factor of 2.8 in the presence of TG (the channel activity appearing in patches devoid of activity in the absence of TG, Fig. 1C). The open channel probability (Po) was also significantly increased after TG application (0.05 ± 0.02 (n = 7) versus 0.19 ± 0.08 (n = 20)) and the quantity of charge passing through the channels (integration of the current extrapolated over a period of 120 s of observation) approximately doubled (Fig. 1C). The current passing through these channels was efficiently inhibited by 50 μm Gd3+, 50 μm La3+ or 30 μm SKF-96365, but not by 100 μm 2-APB (Fig 1A, D and E). A dose–response relationship is presented for Gd3+, showing an IC50 value of around 30 μm (Fig. 1D). All these properties were consistent with previously described properties of voltage-independent Ca2+ channels found in skeletal muscle (Vandebrouck et al. 2002), and presented some similarities with the channels studied previously by Franco-Obregon and Lansman and reported to be mechanosensitive (Franco-Obregon & Lansman, 1994). This prompted us to investigate whether the store-operated channels detected here were also mechanosensitive.
Figure 1. Characterization of thapsigargin-induced Ca2+ currents in skeletal muscle fibres.
A, examples of current traces (patch-clamp; cell-attached configuration at −60 mV holding potential) recorded (in different patches) under control conditions, after stimulation with 1 μm thapsigargin (TG), or in the presence of TG and various concentrations of Gd3+. Mean unitary currents between 0.65 and 0.73 pA. c, closed state; o, open states. B, current–voltage relationship in control (⋄) and TG-treated cells (▪). Voltages indicated were determined from the applied holding potential and the estimated resting potential (−50 mV). C, the proportion of patches with a channel activity (occurrence), the open state probability (n.Po), and the quantity of charge obtained by integration of the current observed extrapolated over a period of 120 s under control condition and after application of 1 μm TG (same patch studied). *Significant difference (P < 0.05) between control and TG-treated cells; the number of patches under each condition is indicated above the histograms. χ2 test (for comparison of occurrences) and paired t test (for comparisons of open probabilities and quantities of charge). D, dose–response relationship of the inhibition of TG-induced currents (the number of patches under each condition is indicated; s.e.m. is smaller than symbols). E, response (in percentage) to 1 μm TG in the absence or in the presence of 50 μm Gd3+, 50 μm La3+, 100 μm 2-APB and 30 μm SKF-96365. *Significantly different (P < 0.05) from control conditions (TG without inhibitors; one-way ANOVA followed by a Tukey test).
Characterization of stretch-induced Ca2+ currents in skeletal muscle fibres
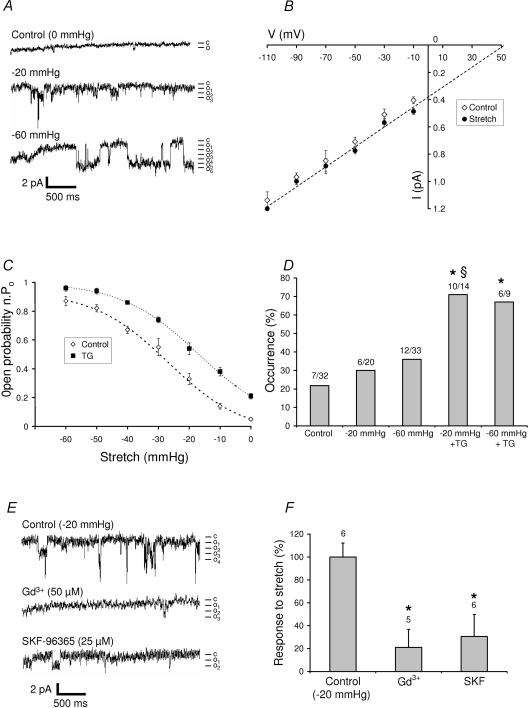
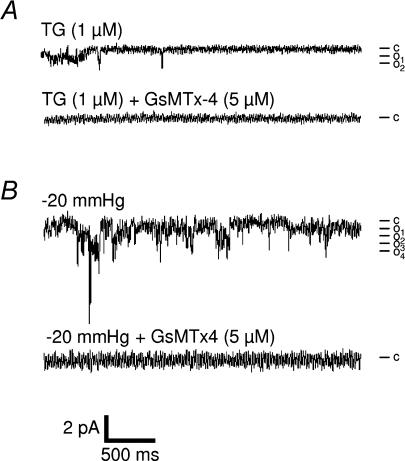
Figure 2 shows that applying a negative pressure to the patch electrode increased the Po of voltage-independent Ca2+ channels but had no significant effect on their conductance (7.1 ± 0.39 pS, n = 6) or on their reversal potential (+52 mV), which were similar to those described above (Fig. 2A and B). The relationship between the amount of pressure applied to the patch electrode and channel Po was well fitted by a Boltzmann equation (Fig. 2C). In contrast, the occurrence of channel activity was not significantly modified by mechanical stretch (Fig. 2D). We also compared the response to mechanical stretch in the presence and the absence of TG. Figure 2C shows that channel activity recorded from muscle fibres under the two experimental conditions increased with the pressure of suction applied to the patch electrode. The occurrence (Fig. 2D) and the total quantity of charge (not shown) also significantly increased. To further determine whether store-dependent channels and mechanosensitive channels belong to the same population, we studied the sensitivity of these channels to pharmacological agents. We found that inhibitors of store-operated channels, such as Gd3+ (50 μm, i.e. just above the EC50 value for SOC inhibition, see above) and SKF-96365 (30 μm) which inhibited, respectively, 78 and 50% of the current induced by TG (Fig. 1E) also inhibited 78 and 69%, respectively, of the current triggered by pressure application (Fig. 2F). We also tested GsMTx4, a peptide toxin from the tarantula Grammostola spatulata, reported to specifically block mechanosensitive channels (Suchyna et al. 2000). In the presence of 5 μm GsMTx4, no basal activity was detected. As expected, 5 μm GsMTx4 inhibited the response to stretch (suction from −20 to −60 mmHg), but interestingly, also completely abolished the response to TG (no activity detected in eight experiments, representing a total recording of more than 43 min in the presence of TG; Fig. 3). We concluded that adult muscle fibres have SOCs and SACs that share similar pharmacological profiles. In particular, both channels are inhibited by the GsMTx4 toxin which has been shown to inhibit TRPC1 channels.
Figure 2. Characterization of stretch-induced Ca2+ currents in skeletal muscle fibres.
A, examples of current traces (patch clamp; cell-attached configuration at −60 mV holding potential) recorded before (control) and after the indicated pressures applied to the patch electrode (same patch recorded). Mean unitary currents of 0.84 pA. c, closed state; o, open states. B, current–voltage relationships in control (⋄) and stretch-activated (▪) cells. Voltages indicated were determined from the applied holding potential and the estimated resting potential (−50 mV). C, effects of the pressure level of suction applied to the patch electrode on n.Po) in the absence (⋄, n = 3) or in the presence (▪, n = 5) of 1 μm TG. Boltzmann fit (dashed line). D, occurrence of channel activity recorded under control conditions, during stretches (−20 and −60 mmHg suction), and during the same stretches but after 5 min application of 1 μm TG. *Significantly different (P < 0.05) from control (χ2 test, 4 degrees of freedom; results then compared two-by-two, χ2 test or Fisher exact tests). §Significantly different (P < 0.05) from −20 mmHg pressure (Fisher exact test). The proportion of patches in which an activity is detected is indicated above each column of the histogram. E, pharmacological characterization of stretch-activated single-channel currents. Examples of current traces (patch-clamp; cell-attached configuration at −60 mV holding potential) recorded under control condition (during a −20 mmHg suction) or in the presence of Gd3+ (50 μm) and SKF-96365 (25 μm). Mean unitary currents of 0.87 pA. F, response (in percentage) to −20 mmHg stretch in the absence and presence of 50 μm Gd3+ or 30 μm SKF-96365. *Significantly different (P < 0.05) from control conditions (stretch without inhibitors; one-way ANOVA followed by a Tukey test).
Figure 3. Effects of GsMTx4 on TG- and stretch-induced Ca2+ currents in skeletal muscle fibres.
Representative records of current traces (patch clamp; cell-attached configuration at −60 mV holding potential) recorded in the absence and in the presence of 5 μm GsMTx4, after stimulation by 1 μm TG (A) and during a −20 mmHg suction (B). Mean unitary current of 0.61 pA. c, closed state; o, open states.
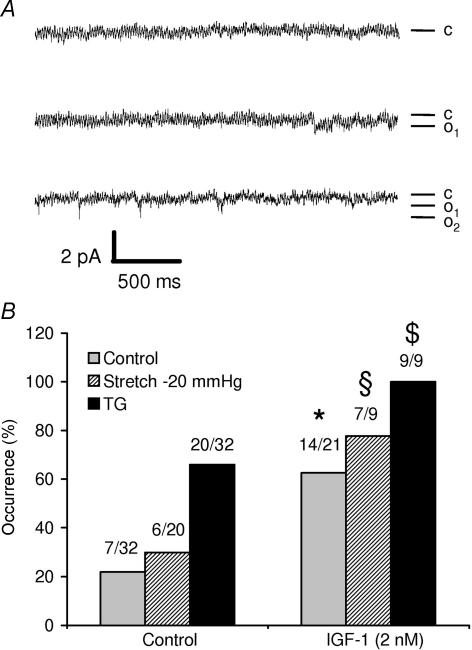
Among the stretch-activated channels described in skeletal muscle, the growth-factor-regulated channel (GRC, now named TRPV2) has been shown to translocate to plasma membrane upon IGF-1 stimulation (Kanzaki et al. 1999; Iwata et al. 2003). Interestingly, we observed that the occurrence of channel activity (measured under basal conditions or after stimulation by −20 mmHg stretch or by 1 μm TG) increased when the cells were pretreated for 5 min with 2 nm IGF-1, suggesting that the channels detected here might also be constituted, at least partially, of TRPV2 (Fig. 4).
Figure 4. Effects of IGF-1 on TG- and stretch-induced Ca2+ currents in skeletal muscle fibres.
A, representative records of current traces (patch clamp; cell-attached configuration at −60 mV holding potential) recorded 2 min (upper trace), 2.5 min (middle trace) and 3 min (lower trace) after stimulation with 2 nm IGF-1. Mean unitary currents of 0.6 pA. c, closed state; o, open states. B, effects of 5 min stimulation with 2 nm IGF-1 on the channel activity measured at rest, after a stretch of −20 mmHg or after stimulation with 1 μm TG. *Significantly different from control (P < 0.05, χ2 test); §significantly different from the −20 mmHg stimulation in the absence of IGF-1 (P < 0.05, χ2 test); $significantly different from the stimulation with TG in the absence of IGF-1 (P < 0.05, Fisher exact test). The proportion of patches in which an activity is detected is indicated above each bar.
Physiological activation of store-operated channels
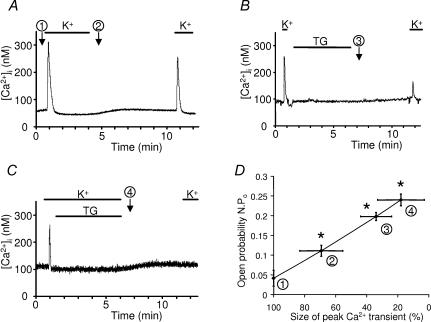
Experimentally, sarcoplasmic reticulum can be fully depleted by a combined and prolonged action of 20 mm caffeine and 1 μm TG in the absence of external Ca2+ (10 mm EGTA). Re-introduction of Ca2+ in the external medium induces a large entry of Ca2+ which is inhibited by Gd3+ (data not shown). But do the channels studied here function as store-operated channels in vivo? The following experiments were designed to examine the level of store-depletion necessary to activate voltage-independent Ca2+ channels. Ca2+ measurements and patch-clamp studies were performed in parallel on cells maintained in Krebs medium that were first transferred to potassium aspartate medium. Depolarization in this solution induced a [Ca2+]i transient that was taken as an index of the amount of releasable Ca2+ (Fig. 5A, procedure no.1). Fibres were then kept for 5 min in this solution in the absence or in the presence of 1 μm thapsigargin (Fig. 5A and C, procedures no.2 and no. 4); alternatively, fibres were repolarized rapidly after the first peak of Ca2+ and TG was applied for 5 min in a Krebs solution (Fig. 5B, procedure no.3). Whatever the treatment, the fibres were then rinsed in Krebs medium (to prepare for the next depolarization) containing 50 μm EGTA to avoid any refilling of the stores during this period of time. The return to potassium aspartate solution produced a second peak of [Ca2+]i the amplitude of which could be compared with the initial transient of [Ca2+]i in order to estimate the decrease of releasable Ca2+ staying in the stores. Compared with the control situation, these three different procedures significantly reduced the content of releasable Ca2+ to 69, 34 and 18% of their initial content (Fig. 5D). The level of activity of Ca2+ channels was studied under similar conditions and a relationship between the content of releasable Ca2+versus the Po of store-operated channels is presented in Fig. 5D. It turns out that a threshold of 30% of depletion seems sufficient to activate store-operated channels.
Figure 5. Level of store depletion and activation of voltage-independent Ca2+ channels.
A, B and C, depolarization in potassium aspartate solution induced a [Ca2+]i transient that was taken as an index of the amount of releasable Ca2+ (procedure no. 1). Fibres were then kept for 5 min in potassium aspartate solution in the absence or in the presence of 1 μm TG (A and C, procedures no. 2 and no. 4); alternatively, fibres were repolarized rapidly after the first peak of Ca2+, and TG was applied for 5 min in a Krebs solution (B, procedure no. 3). After repolarization in Krebs medium (5 min), the amount of releasable Ca2+ was re-estimated (see details in the text). D, relationship between the Po measured by patch-clamp (at the time and under the conditions indicated by the encircled numbers) and the amount of releasable Ca2+ from the stores in C57 mice muscle fibres. *Significantly different from control (P < 0.05): sizes of peak Ca2+ transients compared by a t test (paired experiments); Po values were compared by a one-way ANOVA followed by a Tukey test. Procedures are indicated by encircled numbers. K+, potassium aspartate solution.
Functional role of SOCs/SACS in muscle fatigue
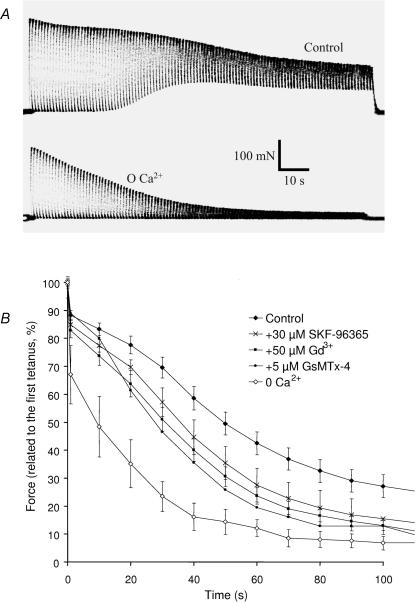
Muscles stimulated maximally and repeatedly present a progressive decrease in tension production, in shortening velocity and in relaxation speed. These observations, commonly grouped under the concept of muscle fatigue, were classically attributed to the accumulation of intracellular lactic acid (Hill & Kupalov, 1929) resulting in acidosis, but it is now clear that this effect contributes little to muscle fatigue (Allen, 2004) and that intracellular ionic changes also play major roles in this process (see Discussion; Stephenson et al. 1998). The following experiments were designed to assess the role of the influx of Ca2+ in muscle fatigue. Soleus muscles were chosen for their dependence on oxidative metabolism, thus limiting the role of anaerobic processes in ionic changes and their contribution to muscle fatigue. These muscles were subjected to 50 Hz stimulation trains of 500 ms duration at 1 s intervals (50% duty cycle). After 2 min, the muscles were allowed to recover and were stimulated at 10 s intervals for 10 min. Under control conditions, maximal force was maintained during the first 30 s and then progressively declined. At the same time, relaxation became incomplete during the 0.5 s separating two successive stimulation periods (Fig. 6A). Recovery occurred during the first 5 min after the protocol of fatigue. This recovery was complete and a second fatigue protocol could be performed without any modification in comparison to the first protocol. In the absence of extracellular Ca2+, the maximal force was not maintained, declined significantly faster and the relaxation stayed complete during the whole protocol (Fig. 6A). Recovery was significantly slower and less complete (data not shown). In order to investigate the possible involvement of the store-dependent and mechanosensitive channels, the same protocol was followed in the presence of extracellular Ca2+ but in the absence and then in the presence of SKF-96365, Gd3+ or GsMTx4 toxin (paired experiments). The presence of Gd3+ (50 μm) significantly accentuated the decrease of force during the fatigue protocol (Fig. 6B; P < 0.05, t test on paired experiments). The presence of SKF-96365 also accentuated the decrease of force albeit a little later (effect significant after 30 s). Finally, the GsMTx4 toxin (5 μm; tested only two times because of its cost) had a similar effect and partially mimicked the absence of extracellular Ca2+ (Fig. 6B).
Figure 6. Involvement of Ca2+ entry in muscle fatigue.
A, representative examples of force records. Muscles stimulated in the presence (upper panel) or in the absence (lower panel) of Ca2+. Tetani of 500 ms every second for 2 min (50 Hz stimulation frequency). B, quantification of the loss of force during the protocol of fatigue (force measured every 10th tetanus). Results expressed relative to the maximal force produced during the first tetanus. Five paired experiments performed for each condition, except for GsMTx4 toxin (n = 2). Statistical significance indicated in the text.
Discussion
In the present paper, we show that adult muscle fibres have SACs and SOCs that share several biophysical and pharmacological properties. Indeed, they present the same unitary conductance, support currents having the same reversal potential and have a similar sensitivity to Gd3+, SKF-96365, 2-APB and GsMTx4 toxin. Besides, the occurrence of both channels is increased after IGF-1 stimulation. Together, these observations might suggest that they belong to the same population or share common constituents.
Possible molecular identity of SOCs and SACs
SACs in muscle fibres might be constituted of the TRPC1 isoform, which has been shown to form such stretch-activated cation channels in vertebrate cells (Maroto et al. 2005). Accordingly, this channel is completely inhibited by the spider venom toxin GsMTx4, which, to date, has only been shown to block SACs encoded by TRPC1 (Suchyna et al. 2000; Gottlieb et al. 2006). We have previously suggested the involvement of this protein as a constituent of SOCs in normal and dystrophic skeletal muscle fibres (Vandebrouck et al. 2002). The similarity of the properties of SACs and SOCs, in particular their similar sensitivity to GsMTx4 toxin, suggests that both channels might be constituted, at least partially, of TRPC1 protein. Similarly, SACs and SOCs might also involve TRPV2 protein (previously named GRC or VRL1), a channel which translocates to the membrane upon IGF-1 stimulation and which is elevated in the membrane of dystrophic patients (Kanzaki et al. 1999; Iwata et al. 2003; Muraki et al. 2003; Yeung et al. 2005). Finally, they might implicate the TRPV4 isoform which is activated by cell swelling through a phospholipase A2 (PLA2)-dependent mechanism (Vriens et al. 2004). Interestingly, we have previously shown that the channels described here have an abnormally high open probability in dystrophic muscle fibres (Gailly, 2002; Vandebrouck et al. 2002), a situation in which the activity of PLA2 has been reported to be increased by a factor of 40 (Lindahl et al. 1995). Precise identification of the isoform(s) involved is under study.
Functional role of SACs and SOCS in skeletal muscle fibres
It is not obvious that store depletion occurs in vivo because adult muscle fibres do not exchange much Ca2+ with the extracellular medium. Indeed, skeletal muscle fibres have huge amounts of sarcoplasmic reticulum which is extremely rich in Ca2+-pumps (SERCA) and which contains a high buffering capacity (calsequestrin). Ca2+ extrusion through the plasma membrane seems also very slow (reviewed in Martonosi & Pikula, 2003). So almost all of the Ca2+ released from the sarcoplasmic reticulum is rapidly restored to the sarcoplasmic reticulum after stimulation; accordingly, twitch contractions can thus be produced repeatedly in the absence of extracellular Ca2+ (Armstrong et al. 1972). However, evaluations of Ca2+ influx using the Ca45 uptake technique indicate that each twitch contraction induces a small increase of Ca2+ entry (Bianchi & Shanes, 1959), the mechanism of which is unknown. Here, we show that a decrease of the order of 30% of the Ca2+ stores is sufficient to induce an entry of Ca2+. Interestingly, it has been evaluated that a single action potential triggers the release of 0.2–0.3 mm from the sarcoplasmic reticulum to the cytoplasm (Baylor et al. 1983), which corresponds to more than a quarter of the Ca2+ contents present in the sarcoplasmic reticulum (Endo, 1977). Thus it seems reasonable to think that store-operated channels are indeed activated during in vivo contraction. This is corroborated by the fatigue experiments. In the absence of external Ca2+ or when SOCs are inhibited, a faster decline of force is observed, suggesting that a sustained activity, such as the one observed in tonic muscles as soleus, requires a constant repletion of the stores by a subsequent entry of Ca2+. A partial failure of the sarcoplasmic reticulum to release Ca2+ during tetanus has been proposed as a possible cause of fatigue (Allen & Westerblad, 2001; Allen, 2004). This seems to be due to the accumulation of Ca2+ phosphate in the sarcoplasmic reticulum (Kabbara & Allen, 1999; Dutka et al. 2005). It is interesting to note that, at rest, the sarcoplasmic reticulum of slow twitch fibres (as in soleus muscle) is saturated with Ca2+ while the sarcoplasmic reticulum of fast twitch muscle fibres is only about one-third full (Fryer & Stephenson, 1996), suggesting that the amount of Ca2+ in the stores might be more critical for the physiological function of slow twitch fibres. Our results emphasize the importance of the entry of Ca2+ during sustained trains of contractions of slow twitch muscle. Such importance of Ca2+ handling in muscle fatigue has also been suggested in fast and slow muscles deficient in mitsugumin, a protein expressed at the triad junction, the lack of which leads to a disorganization of the T-tubules and sarcoplasmic relationship and to a susceptibility to fatigue and to a dysfunction of store-operated entry of Ca2+ (observed in embryonic and neonatal muscles but not in adult fast fibres) (Nishi et al. 1999; Nagaraj et al. 2000; Kurebayashi & Ogawa, 2001; Kurebayashi et al. 2003; Ma & Pan, 2003).
Whether the channels studied here are also activated by stretch during contraction is difficult to evaluate. Indeed, the force applied to membrane patches in the experiments presented here represents only about 5% of the force developed by muscle during contraction. However, there are no data on the possible transmission of force (produced during contraction) to the cell membrane.
Acknowledgments
This work was supported by the Association française contre les myopathies (AFM), the Association belge contre les maladies neuro-musculaires (ABMM), and by a grant ARC 05/10-328 from the General Direction of Scientific Research of the French Community of Belgium. T.D. was supported by the Francqui Foundation.
References
- Allen DG. Skeletal muscle function: role of ionic changes in fatigue, damage and disease. Clin Exp Pharmacol Physiol. 2004;31:485–493. doi: 10.1111/j.1440-1681.2004.04032.x. [DOI] [PubMed] [Google Scholar]
- Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol. 2001;536:657–665. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis-(β-aminoethylether)-N,N′-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Bales PJ, Zerbes M, Powis DA, Marley PD. Effect of Gd3+ on bradykinin-induced catecholamine secretion from bovine adrenal chromaffin cells. Br J Pharmacol. 1999;128:1435–1444. doi: 10.1038/sj.bjp.0702933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Chandler WK, Marshall MW. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Davis JB, Randall AD. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002;42:873–888. doi: 10.1016/s0028-3908(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Bianchi CP, Shanes AM. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959;42:803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci U S A. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodding M, Fecher-Trost C, Flockerzi V. Store-operated Ca2+ current and TRPV6 channels in lymph node prostate cancer cells. J Biol Chem. 2003;278:50872–50879. doi: 10.1074/jbc.M308800200. [DOI] [PubMed] [Google Scholar]
- Cahalan M, Neher E. Patch clamp techniques: an overview. Methods Enzymol. 1992;207:3–14. doi: 10.1016/0076-6879(92)07003-7. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Cole L, Lamb GD. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C1502–C1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Jr, Lansman JB. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J Physiol. 1994;481:299–309. doi: 10.1113/jphysiol.1994.sp020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/– mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P. New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim Biophys Acta. 2002;1600:38–44. doi: 10.1016/s1570-9639(02)00442-9. [DOI] [PubMed] [Google Scholar]
- Gailly P, Colson-Van Schoor M. Involvement of trp-2 protein in store-operated influx of calcium in fibroblasts. Cell Calcium. 2001;30:157–165. doi: 10.1054/ceca.2001.0221. [DOI] [PubMed] [Google Scholar]
- Gottlieb P, Suchyna TM, Bowman CL, Sachs F. The mechanosensitive TRPC1 ion channel activity is modulated by cytoskeleton and inhibited by the peptide GsMTx4. Biophys J. 2006;90(1):1545–plat. [Google Scholar]
- Groschner K, Hingel S, Lintschinger B, Balzer M, Romanin C, Zhu X, Schreibmayer W. Trp proteins form store-operated cation channels in human vascular endothelial cells. FEBS Lett. 1998;437:101–106. doi: 10.1016/s0014-5793(98)01212-5. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill A, Kupalov P. Anaerobic and aerobic activity in isolated muscle. Proc Royal Soc London Series B. 1929;105:313–322. [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara AA, Allen DG. The role of calcium stores in fatigue of isolated single muscle fibres from the cane toad. J Physiol. 1999;519:169–176. doi: 10.1111/j.1469-7793.1999.0169o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Lund PE, Akerman KE. 2-Aminoethoxydiphenyl borate reveals heterogeneity in receptor-activated Ca2+ discharge and store-operated Ca2+ influx. Cell Calcium. 2001;30:117–129. doi: 10.1054/ceca.2001.0219. [DOI] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N, Takeshima H, Nishi M, Murayama T, Suzuki E, Ogawa Y. Changes in Ca2+ handling in adult MG29-deficient skeletal muscle. Biochem Biophys Res Commun. 2003;310:1266–1272. doi: 10.1016/j.bbrc.2003.09.146. [DOI] [PubMed] [Google Scholar]
- Lindahl M, Backman E, Henriksson KG, Gorospe JR, Hoffman EP. Phospholipase A2 activity in dystrophinopathies. Neuromuscul Disord. 1995;5:193–199. doi: 10.1016/0960-8966(94)00045-b. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O'Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- Ma J, Pan Z. Junctional membrane structure and store operated calcium entry in muscle cells. Front Biosci. 2003;8:d242–255. doi: 10.2741/977. [DOI] [PubMed] [Google Scholar]
- Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- Ma HT, Venkatachalam K, Li HS, Montell C, Kurosaki T, Patterson RL, Gill DL. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem. 2001;276:18888–18896. doi: 10.1074/jbc.M100944200. [DOI] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Martonosi AN, Pikula S. The network of calcium regulation in muscle. Acta Biochim Pol. 2003;50:1–30. [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- Nagaraj RY, Nosek CM, Brotto MA, Nishi M, Takeshima H, Nosek TM, Ma J. Increased susceptibility to fatigue of slow- and fast-twitch muscles from mice lacking the MG29 gene. Physiol Genomics. 2000;4:43–49. doi: 10.1152/physiolgenomics.2000.4.1.43. [DOI] [PubMed] [Google Scholar]
- Nishi M, Komazaki S, Kurebayashi N, Ogawa Y, Noda T, Iino M, Takeshima H. Abnormal features in skeletal muscle from mice lacking mitsugumin29. J Cell Biol. 1999;147:1473–1480. doi: 10.1083/jcb.147.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998;273:10279–10287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL. 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium. 2003;34:97–108. doi: 10.1016/s0143-4160(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalie A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp S, Trost C, Warnat J, Rautmann J, Himmerkus N, Schroth G, Kretz O, Nastainczyk W, Cavalie A, Hoth M, Flockerzi V. TRP4 (CCE1) protein is part of native calcium release-activated Ca2+-like channels in adrenal cells. J Biol Chem. 2000;275:23965–23972. doi: 10.1074/jbc.M003408200. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 1986;48:75–88. doi: 10.1146/annurev.ph.48.030186.000451. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci U S A. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. The pharmacology of capacitative calcium entry. Mol Interv. 2001;1:84–94. [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Lamb GD, Stephenson GM. Events of the excitation–contraction–relaxation (E-C-R) cycle in fast- and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiol Scand. 1998;162:229–245. doi: 10.1046/j.1365-201X.1998.0304f.x. [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proc Natl Acad Sci U S A. 1999;96:2060–2064. doi: 10.1073/pnas.96.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez G, Wedel BJ, Trebak M, St John Bird G, Putney JW., Jr Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J Biol Chem. 2003;278:21649–21654. doi: 10.1074/jbc.M302162200. [DOI] [PubMed] [Google Scholar]
- Vorndran C, Minta A, Poenie M. New fluorescent calcium indicators designed for cytosolic retention or measuring calcium near membranes. Biophys J. 1995;69:2112–2124. doi: 10.1016/S0006-3495(95)80082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnat J, Philipp S, Zimmer S, Flockerzi V, Cavalie A. Phenotype of a recombinant store-operated channel: highly selective permeation of Ca2+ J Physiol. 1999;518:631–638. doi: 10.1111/j.1469-7793.1999.0631p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Babnigg G, Villereal ML. Functional significance of human trp1 and trp3 in store-operated Ca2+ entry in HEK-293 cells. Am J Physiol Cell Physiol. 2000;278:C526–C536. doi: 10.1152/ajpcell.2000.278.3.C526. [DOI] [PubMed] [Google Scholar]
- Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium-release- activated calcium channel. Nature. 2001;410:705–709. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- Zitt C, Obukhov AG, Strubing C, Zobel A, Kalkbrenner F, Luckhoff A, Schultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]