Abstract
Previous work shows that sleep deprivation impairs hippocampal-dependent learning and long-term potentiation (LTP). Brain-derived neurotrophic factor (BDNF), cAMP response-element-binding (CREB) and calcium–calmodulin-dependent protein kinase II (CAMKII) are critical modulators of hippocampal-dependent learning and LTP. In the present study we compared the effects of short- (8 h) and intermediate-term (48 h) sleep deprivation (sd) on the expression of BDNF and its downstream targets, Synapsin I, CREB and CAMKII in the neocortex and the hippocampus. Rats were sleep deprived using an intermittent treadmill system which equated total movement in the SD and control treadmill animals (CT), but permitted sustained periods of rest in CT animals. Animals were divided into SD (treadmill schedule: 3 s on/12 s off) and two treadmill control groups, CT1 (15 min on/60 min off) and CT2 (30 min on/120 min off – permitting more sustained sleep). Real-time Taqman RT-PCR was used to measure changes in mRNA; BDNF protein levels were determined using ELISA. In the hippocampus, 8 h treatments reduced BDNF, Synapsin I, CREB and CAMKII gene expression in both SD and control groups. Following 48 h of experimental procedures, the expression of all these four molecular markers of plasticity was reduced in SD and CT1 groups compared to the CT2 and cage control groups. In the hippocampus, BDNF protein levels after 8 h and 48 h treatments paralleled the changes in mRNA. In neocortex, neither 8 h nor 48 h SD or control treatments had significant effects on BDNF, Synapsin I and CAMKII mRNA levels. Stepwise regression analysis suggested that loss of REM sleep underlies the effects of SD on hippocampal BDNF, Synapsin I and CREB mRNA levels, whereas loss of NREM sleep underlies the effects on CAMKII mRNA.
Several hypotheses regarding the functions of sleep have been proposed. One of these views suggests that sleep periods are favourable for brain plasticity (Benington & Frank, 2003). This capacity of the brain to display plasticity enables it to achieve new functions by changing the core elements of its internal niche and/or connectivity in response to environmental constraints (Vaynman & Gomez-Pinilla, 2005). It has been suggested that the role of sleep in favouring brain plasticity may be multidimensional and encompass processes ranging from reactivation of neuronal ensembles during post-training sleep to molecular changes (Maquet, 2001).
Recent studies have provided evidence that the expression of certain genes involved in synaptic plasticity is affected by sleep/wake state. For example, the phosphorylated form of the cAMP response element binding protein (CREB) transcription factor is present at higher levels after periods of waking than periods of sleep (Cirelli & Tononi, 1998). Short-term total sleep deprivation (8 h) increases brain-derived neurotrophic factor (BDNF) mRNA levels in the cortex (Cirelli & Tononi, 2000; Taishi et al. 2001), and selective REM sleep deprivation (6 h) suppresses BDNF protein levels in the cerebellum and brainstem without producing changes in the hippocampus (Sei et al. 2000). Thus, although sleep deprivation (SD) may impair memory, and could be predicted to reduce the expression of plasticity-related genes (Maquet, 2001), recent attempts to examine the problem have produced inconsistent results. However, previous work examining the effects of SD on plasticity-related gene expression has focused on short-term treatments. Increased BDNF expression in the neocortex, compared to sleep, was seen during spontaneous waking as well as after 8 h of SD (Cirelli et al. 2004), and may reflect the effect of waking state rather than SD More sustained SD may be needed to induce functional deficits including hippocampal-dependent task deficits (McDermott et al. 2003).
In the present study we compare the effects of 8 h and 48 h of SD. We examine the expression of genes involved in plasticity both in neocortex and hippocampus. These include BDNF, two genes that are targets of BDNF, Synapsin I and CREB, and calcium–calmodulin-dependent protein kinase II (CAMKII). We also measured effects of SD on BDNF protein levels.
BDNF is known to play a key role in the survival, growth and maintenance of neurons during development (Barde, 1994), and to modulate synaptic plasticity in the adult brain (Lo, 1995). BDNF affects synaptic plasticity through (1) the regulation of axonal and dendritic branching and remodelling (McAllister et al. 1996); (2) synaptogenesis in arborizing axon terminals (Alsina et al. 2001); (3) increasing the efficacy of synaptic transmission (Boulanger & Poo, 1999); and (4) the functional maturation of excitatory and inhibitory synapses (Vicario-Abejon et al. 1998). BDNF also plays a prominent role in learning and memory processes. Spatial learning (Kesslak et al. 1998) or contextual learning (Hall et al. 2000) induces the expression of BDNF mRNA in the hippocampus, a region involved with the acquisition of new memories. BDNF influences the synthesis and the phosphorylation (Jovanovic et al. 1996, 2000) of Synapsin I. Synapsin I is a member of a family of nerve terminal-specific phosphoproteins implicated in neurotransmitter release, axonal elongation, and formation and maintenance of synaptic contacts. Therefore, Synapsin I is a useful marker to evaluate the role of BDNF on synaptic function. CREB is a transcription factor that mediates many of the actions of the cAMP cascade on gene expression (Meyer et al. 1993). The phosphorylation of CREB is affected by several protein kinases including protein kinase C and CAMKII among other kinases. CAMKII plays a key role in neurotransmission, gene expression and plasticity (Soderling, 2000).
One factor limiting sleep deprivation studies has been the lack of a well-controlled procedure suitable for intermediate-term deprivation. Studies involving use of novelty or gentle handling are typically uncontrolled and labour-intensive. The small-pedestal and rotating-drum methods are stressful (Meerlo et al. 2002; Machado et al. 2004). In the present study we have used an intermittent treadmill method for sleep deprivation. With this method, total treadmill movement is equated in sleep-deprived and control animals, and stress hormonal levels are at basal levels after 48 h of deprivation (Guzman-Marin et al. 2003).
Methods
Animal preparation
All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. All animal protocols were reviewed and approved by the Internal Animal Care and Use Committee of the VA Greater Los Angeles Healthcare System. Male Sprague-Dawley rats weighing 300–350 g were used for this experiment. Animals were housed individually in Plexiglas cages (27 × 29 × 30 cm) and kept under 12: 12 light/dark cycle with access to water and food ad libitum. Under deep anaesthesia (ketamine 80 mg kg−1, i.p. + xylazine 10 mg kg−1, i.p.) and aseptic conditions, rats were surgically prepared for sleep–wake cycle monitoring. Five stainless steel screw electrodes were implanted in the skull (frontal and parietal bones) for electroencephalogram (EEG) recording, and four stainless steel wires were inserted into nuchal muscles for electromyogram (EMG) recording. All electrodes were connected with a plug and fixed to the skull with dental acrylic.
Experimental procedure and recording
Animals were divided into four groups of eight rats each: treadmill sleep deprived (SD), control treadmill 1 (CT1), control treadmill 2 (CT2) and cage control (CC). After one week of recovery from surgery and one week of acclimatizion to experimental conditions, the procedures were started. Sleep deprivation and control procedures were accomplished using an intermittent treadmill method. CT1, CT2 and SD animals were studied in a Plexiglas enclosure (30 × 30 × 40 cm) with open top and bottom, which was positioned over a treadmill (90 × 60 × 15 cm). The treadmill speed was set at 10 cm s−1, and timed to move with either a 3 s on/12 s off (SD) or 15 min on/60 min off (CT1), or 30 min on/120 min off (CT2) periodicity during the entire recording session (48 h). This schedule permitted sustained periods of rest in CT groups, but equated the total treadmill movement in SD and CT groups in each 24 h. Based on our previous sleep data (Guzman-Marin et al. 2003, 2005) we noted that the CT1 group is partially sleep deprived when compared to the cage control. The changes found in the CT1 group prompted us to include an additional control group that was permitted more sustained periods for sleep. The direction of treadmill movement was switched every 15 min. For the CC group, rats were housed individually in Plexiglas cages. Animals had continuous access to food and water and were maintained on a 12 h: 12 h light/dark cycle.
For sleep–wake cycle recordings, rats were connected to amplifiers through an overhead counterbalanced cable and slip ring. EEG and EMG signals were filtered at 1.0 and 30 Hz and 30–300 Hz, respectively, and digitized with the Gamma sleep recording and analysis software system (Grass Telefactor, West Warwick RI, USA). Sleep–wake states were scored in 10 s epochs on the basis of the predominant state within the epoch. Wake was identified by low-voltage high-frequency EEG activity and sustained elevated neck muscle tone. A high-amplitude low-frequency EEG with decreased muscle activity was defined as non-rapid eye movement (NREM) sleep, whereas rapid eye movement (REM) sleep was identified by moderate-amplitude EEG with dominant theta frequency (4–8 Hz), combined with low muscle tone. The percentages of each state were calculated for each 24 h period, and the total 48 h period.
In addition to determining the total duration of each sleep–wake state over the course of the 48 h of the experiment, we measured the number and duration of sleep bouts in order to determine whether sleep bouts were different in the CT1 and CT2 animals. It has been shown that the duration of a sleep bout can affect objective sleepiness and decreased psychomotor performance even when total sleep was normal (Bonnet & Arand, 2003). The duration of sleep bouts in each rat was calculated for each 24 h period and the total 48 h period.
In the first experiment, animals were killed by decapitation at ZT8, 8 h after the beginning of the experiments (ZT0). In the second experiment, animals were killed at ZT0 after 2 days of experimental manipulation. The cortex and hippocampal regions were bilaterally dissected by inserting a fine blunt needle into the ventricular cavity just dorsal to the hippocampus, and removing overlying cortex and callosum. The surface of the hippocampus and dentate gyrus was used to guide removal of cortex along the septotemporal axis. The exposed hippocampus and dentate gyrus was pulled free of the hemisphere in a ventral-to-dorsal direction. The dorsoanterior aspect of each hippocampus was trimmed free of septum and dorsal fornix, immediately placed into an Ependorf plastic tube and stored at −80°C. The dissection includes a small part of the subiculum adjacent to CA1, and occasionally a small strand of the fimbria. As with the hippocampus the cerebral cortices were cut and stored in Ependorf plastic tubes and stored at −80°C. To minimize variability, the first author performed all dissections.
Real time TaqMan RT-PCR
Total RNA was isolated using the RNA STAT-60 kit (TEL-TEST, Friendswood, TX, USA) according to the manufacturer's protocol. Total RNA (100 ng) was converted into cDNA using TaqMan EZ RT-PCR Core reagents (Perkin-Elmer, Branchburg, NJ, USA). The mRNAs for BDNF, Synapsin I, CREB and CAMKII were measured by real-time TaqMan quantitative reverse transcription polymerase chain reaction (RT-PCR) using an ABI PRISM 7700 Sequence Detection System (Perkin-Elmer, Applied Biosystems). The technique is based on the ability to directly detect the RT-PCR product with no downstream processing. This is accomplished with the monitoring of the increase in fluorescence of a dye-labelled DNA probe specific for each factor under study, plus a probe specific for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene used as an endogenous control for the assay. The sequences of probes, forward and reverse primers, designed by Integrated DNA Technologies (Coralville, IA) were:
BDNF: 5′-AGTCATTTGCGCACAACTTTAAAA GTCTGCATT-3′), forward 5′-GGACATATCCATGACCAGAAAGAAA-3′, reverse 5′-GCAACAAACCACAACATTATCGAG-3′; Synapsin I: 5′-CATGGCACGTAATGGAGACTACCGCA-3′, forward 5′-CCGCCAGCTGCCTTC-3′, reverse 5′-TGCAGCCCAATGACCAAA-3′; CREB: 5′-CATGGCACGTAATGGAGACTACCGCA-3′, forward 5′-CCGCCAGCATGCCTTC-3′, reverse 5′-TGCAGCCCAATGACCAAA-3′;
CAMKII: 5′-CTCCACTGTGGCCTCCTGCATGC-3′, forward 5′-AGCACCCCTGGATCTCGC-3′, reverse 5′-TTCTTCAGGCAGTCCACGGT-3′.
The RT reaction conditions were 2 min at 50°C as the initial step to activate uracil glycosylase (UNG), followed by 30 min at 60°C as the reverse transcription and completed by an UNG deactivation at 95°C for 5 min. The 40 cycles of the two-step PCR-reaction conditions were 20 s at 94°C and 1 min at 62°C.
Protein measurements
Hippocampal extracts were prepared in lysis buffer (137 mm NaCl, 20 mm Tris–HCl pH 8.0, 1% NP-40, 10% glycerol, 1 mm phenylmethylsulphonyl fluoride, 10 μg ml−1 aprotinin, 1 μg ml−1 leupeptin, 0.5 mm sodium vanadate). Homogenates were centrifuged to remove insoluble material (12 000 r.p.m. for 20 min at 4°C), and total protein concentration was determined according to the MicroBCA procedure (Pierce, Rockford, IL, USA). BDNF protein was quantified using an enzyme-linked immunosorbent assay (ELISA; BDNF Emax ImmunoAssay system Kit; Promega Inc., Madison, WI, USA) according to the manufacturer's protocol.
Statistical analysis
Results are expressed as means ± standard errors of means (s.e.m.) Comparisons between groups were made using one-way analysis of variance (ANOVA) followed by Bonferroni's test (multiple comparisons). The correlation between BDNF, Synapsin I, CREB and CAMKII mRNA levels and sleep parameters was studied using the Pearson product moment correlation and stepwise regression analysis (Statistica 6). Differences were considered significant at P < 0.05.
Results
Effects of 8 h and 48 h of intermittent treadmill treatment on sleep parameters
The method of SD used in this study had marked effects on the stages of the sleep–wake cycle. Both 8 h and 48 h treadmill treatments had significant effects on the percentages of total sleep (F(3,28) = 116.0 and F(3,28) = 186.2, respectively; P < 0.001), NREM sleep (F(3,28) = 103.0 and F(3,28) = 168.1, respectively; P < 0.001), and REM sleep (F(3,28) = 151.9 and F(3,28) = 94.01, respectively; P < 0.001).
In the 8 h treatment study (Table 1) SD animals, compared to CC, exhibited 86% reduction in total sleep, 82% reduction in NREM sleep, and a complete suppression of REM sleep (P < 0.001, Bonferroni's post hoc test). Sleep architecture in control treadmill animals was also affected by treadmill treatment. In the CT2 group, compared to CC, total sleep, NREM sleep and REM sleep were reduced by 41%, 32% and 79% (P < 0.001, Bonferroni's post hoc test), whereas in the CT1 group only the percentage of REM sleep was significantly reduced (89%, P < 0.001, Bonferroni's post hoc test). The CT2 group had significantly lower percentages of total sleep and NREM sleep with respect to the CT1 group (P < 0.001, Bonferroni's post hoc test).
Table 1.
Sleep-wake cycle parameters in the 8 h experiment
| Wake | NREM sleep | REM sleep | Total sleep | |
|---|---|---|---|---|
| sd | 91.41 ± 0.43*†‡ | 8.59 ± 0.44*†‡ | 0*†‡ | 8.59 + 0.44*†‡ |
| CT1 | 51.24 ± 0.84 | 47.5 ± 1.04† | 1.25 ± 0.2* | 48.76 ± 0.84† |
| CT2 | 60.62 ± 3.1* | 33.25 ± 2.78*‡ | 2.48 ± 0.76* | 35.73 ± 3.28*‡ |
| CC | 39.55 ± 3.73 | 48.75 ± 3.51 | 11.7 ± 0.6 | 60.45 ± 3.73 |
The table shows the percentage of time spent in the different stages of the sleep–wake cycle. CC, cage control group; SD, sleep deprivation group; CT1, control treadmill 1 group (15 min on/60 min off); CT2, control treadmill 2 group (30 min on/120 min off). Data are means ±s.e.m. for groups of 8 rats each.
P < 0.001 versus CC
P < 0.001 versus CT2
P < 0.001 versus CT1. One-way ANOVA, followed by Bonferroni's post hoc test.
In the 48 h treatment study, SD animals, compared to CC, exhibited 87%, 85%, and almost 100% reduction in total sleep, NREM sleep, and REM sleep, respectively (P < 0.001, Bonferroni's post hoc test). In the CT1 group, total sleep, NREM and REM sleep were reduced by 29%, 20% and 73%, respectively (P < 0.001, Bonferroni's post hoc test), compared to CC, whereas in the CT2 group, total sleep and REM sleep were reduced by 13% and 57% (P < 0.05), respectively, and the percentage of NREM sleep changed insignificantly compared to CC. The sleep–wake cycle parameters in the 48 h study can be seen in Table 2.
Table 2.
Sleep–wake cycle parameters in the 48 h experiment
| Wake | NREM | REM sleep | Total sleep | |
|---|---|---|---|---|
| sd | 93.9 ± 0.75*†‡ | 6.61 ± 0.75*†‡ | 0.004 ± 0.001*†‡ | 6.62 ± 0.75*†‡ |
| CT1 | 62.97 ± 2.11*† | 34.58 ± 1.76*† | 2.47 ± 0.59* | 37.04 ± 2.11*† |
| CT2 | 54.38 ± 1.52‡ | 41.76 ± 1.34‡ | 3.88 ± 0.35 | 45.64 ± 1.51‡ |
| CC | 47.79 ± 1.18 | 43.18 ± 1.21 | 9.08 ± 0.40 | 52.26 ± 1.19 |
The table shows the percentage of time spent in the different stages of the sleep–wake cycle. Results are presented as average for the entire 48 h recording session. CC, cage control group; SD, sleep deprivation group; CT1, control treadmill 1 group (15 min on/60 min off); CT2, control treadmill 2 group (30 min on/120 min off). Data are means ±s.e.m. for groups of 8 rats each.
P < 0.001 versus CC
P < 0.001 versus CT2
P < 0.001 versus CT1. One-way ANOVA, followed by Bonferroni's post hoc test.
We also quantified the number and length of wake, NREM and REM sleep bouts in animals subjected to 48 h experimental and control procedures (Table 3). The SD group exhibited an increased number and duration of wake bouts and decreased number and duration of NREM and REM sleep bouts compared to all the control groups (P < 0.001, Bonferroni's post hoc test). Control groups did not show significant differences in wake bout duration. CT1 animals, compared to CC and CT2, had an increased number of wakefulness bouts (P < 0.001, Bonferroni's post hoc test) as well as an increased number of NREM sleep bouts of shorter duration (P < 0.001, Bonferroni's post hoc test). The number of REM sleep bouts in the CT1 group was significantly lower compared to the CC group (P < 0.001, Bonferroni's post hoc test), and their duration was shorter compared to both CC (P < 0.001) and CT2 groups (P < 0.01, Bonferroni's post hoc test). CT2 animals, compared to CC, did not exhibit significant differences either in number or duration of wakefulness and NREM sleep bouts, but showed reduced number (P < 0.001) and duration (P < 0.05) of REM sleep bouts. In summary, during 48 h SD, the CT2 group, compared to the CT1 group, had smaller reductions in total sleep, NREM sleep amounts and bout duration, and REM sleep amount and bout duration.
Table 3.
Parameters of the sleep–wake states in the 48 h experiment
| Wake bouts | NREM sleep bouts | REM sleep bouts | ||||
|---|---|---|---|---|---|---|
| Number | Length (min) | Number | Length (min) | Number | Length (min) | |
| sd | 215.88 ± 13.76*†‡ | 6.45 ± 0.5*†‡ | 217.13 ± 13.76*†‡ | 0.44 ± 0.04*†‡ | 0.58 ± 0.18*†‡ | 0.07 ± 0.02*†‡ |
| CT1 | 513.5 ± 16.64*† | 1.78 ± 0.08 | 517.13 ± 16.58*† | 0.97 ± 0.06*† | 42.25 ± 8.84* | 0.86 ± 0.12*† |
| CT2 | 384.75 ± 12.68‡ | 2.06 ± 0.1 | 388.75 ± 12.15‡ | 1.55 ± 0.05‡ | 46.0 ± 3.24* | 1.21 ± 0.05*‡ |
| CC | 422.88 ± 8.42 | 1.63 ± 0.03 | 420.38 ± 8.88 | 1.49 ± 0.07 | 86.89 ± 5.09 | 1.52 ± 0.05 |
Results are presented as average for the entire 48 h recording session. Average duration of wakefulness, NREM and REM sleep episodes in each of the four groups.
P < 0.001 versus CC
P < 0.001 versus CT2
P < 0.001 versus CT1. One-way ANOVA, followed by Bonferroni's post hoc test.
Effects of 8 h and 48 h of intermittent treadmill treatments on BDNF, Synapsin I, CREB and CAMKII expression
We first investigated whether the expression of BDNF, Synapsin I, CREB and CAMKII in the hippocampus was affected by 8 h of intermittent treadmill treatment. Expression of the mRNA levels of BDNF, Synapsin I and CREB were reduced in the hippocampus in SD, CT1 and CT2 groups when compared with the CC (Fig. 1A and Fig. 2A–C)). BDNF mRNA levels were reduced by 20–32% (Fig. 1A); Synapsin I mRNA levels were reduced by 38–48% (Fig. 2A) and CREB mRNA levels were reduced by 22–33% (Fig. 2B) when compared to CC. All reductions were statistically significant (P < 0.05). There were no significant differences among SD, CT1 and CT2 groups in the expression of these transcripts. With respect to the expression of CAMKII mRNA, the SD and CT2 groups exhibited an approximated 25% reduction when compared to CC and CT1 (P < 0.05; Fig. 2C).
Figure 1. Effects of 8 h and 48 h of SD on the expression of BDNF (A and C) and its protein (B and D) determined by Taqman RT-PCR and ELISA in the hippocampus.
Each value is expressed as a percentage of the cage control value (100%), and represents the mean ±s.e.m. of the group of eight animals. CC, cage control group; SD, sleep deprivation; CT1, control treadmill 1 (15 min on/60 min off); CT2, control treadmill 2 (30 min on/120 off). *P < 0.05, **P < 0.01, One-way ANOVA followed by Bonferroni's post hoc test.
Figure 2. Effects of 8 h and 48 h of SD on mRNA expression of Synapsin I (A and D), CREB (B and E) and CAMKII (C and F) in the hippocampus.
Data are presented as percent of the cage control (CC) group. SD, sleep deprivation; CT1, control treadmill 1 (15 min on/60 min off); CT2, control treadmill 2 (30 min on/120 min off). Note that the CT2 group has comparable values to those of the CC. n = 8, *P < 0.05, **P < 0.01, one-way ANOVA followed by Bonferroni's post hoc test.
BDNF, Synapsin I and CAMKII mRNA levels were quantified in the neocortex from the same brains used for hippocampal gene expression measurement in the 8 h treatment groups. None of the mRNA levels were statistically different in any of the groups when compared to the CC (Fig. 3A–C).
Figure 3. Effects of 8 h and 48 h of SD on the expression of BDNF (A and D), Synapsin I (B and E) and CAMKII (C) mRNA as determined by Taqman RT-PCR in the neocortex from the same group of animals used for measurements of these transcripts in the hippocampus.
Each value is expressed as a percentage of the cage control value (100%), and represents the mean ±s.e.m. for a group of eight animals.
Following 48 h of SD, we found that the mRNA levels of BDNF, Synapsin I, CREB and CAMKII in the hippocampus were reduced by 25%, 30%, 35% and 27% (P < 0.05), respectively, compared to CC (Figs 1C and 2D-F). Expression of each of these transcripts was also significantly reduced in the SD group compared to the CT2 group (P < 0.05). Two days of experimental procedures also reduced the BDNF, Synapsin I, CREB and CAMKII levels in the CT1 group by 35%, 40%, 45% and 22%, respectively, when compared to the CC. However in the CT2 group mRNA levels of all these four molecules were not significantly different from the CC (Fig. 2D–F).
In neocortex, neither the 48 h SD group nor the control treatments had significant effects on BDNF or Synapsin I mRNA levels (Fig. 3D and E)
Effects of 8 and 48 h of intermittent treadmill treatment on BDNF protein levels in the hippocampus
Figure 1B and D shows protein levels derived from ELISA for the hippocampus in the same brains used for the gene expression analysis in the 8 h and 48 h experiments, respectively. We performed an ELISA to determine whether the changes in BDNF mRNA levels affected protein levels. In both 8 h and 48 h conditions, protein levels exhibited changes similar to those exhibited by gene expression. In the 8 h experimental condition, BDNF protein levels were reduced by 26%, 16% and 14% in the SD, CT1 and CT2 groups when compared with the CC (P < 0.05), whereas no differences were seen between these groups. In the 48 h condition BDNF protein levels were reduced in the SD and CT1 groups by 39% and 37% with respect to the CC (P < 0.05). BDNF protein was also lower in the SD compared to the CT2 group. There was no difference between the CT2 and the CC group (Fig. 1D).
Sleep–wake parameters and gene expression
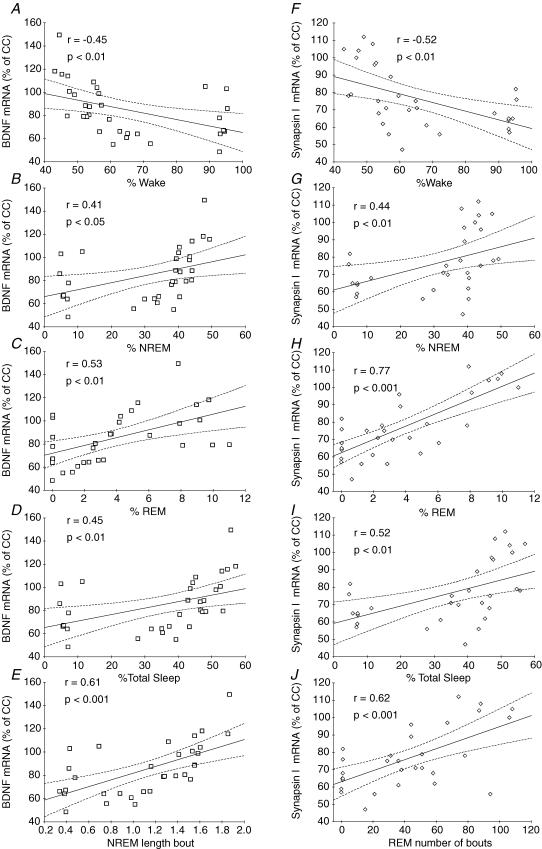
Across all animals in the 48 h treatment groups, BDNF mRNA levels (Fig. 4A–E) in the hippocampus were correlated positively with the NREM sleep bout length (r = 0.61; P < 0.001), the percentages of NREM (r = 41; P < 0.05), REM (r = 0.53; P < 0.01) and total sleep (r = 0.45; P < 0.01), and negatively correlated with the percentage of wakefulness (r = −0.45; P < 0.01). Synapsin I (Fig. 4F–J), CREB (Fig. 5A–D) and CAMKII (Fig. 5E–I) mRNA levels also exhibited significant correlations with the same set of parameters of the sleep–wake cycle.
Figure 4. Correlations between sleep–wake cycle parameters and BDNF and Synapsin 1 mRNA levels.
A–E, across all the experiments in the 48 h group, BDNF mRNA levels in the hippocampus correlate negatively with the percentage of time spent in waking (r =−0.45; P < 0.01; A) and positively with the percentages of NREM (r = 41; P < 0.05; B), REM (r = 0.53; P < 0.01, C), total sleep (r = 0.45; P < 0.01, D) and the NREM sleep bout duration (r = 0.61; P < 0.001, E). F–J, correlations between sleep–wake cycle parameters and Synapsin I mRNA levels. Synapsin I mRNA levels in the hippocampus correlated negatively with the percentage of time spent in waking (r =−0.52; P < 0.01, F), and positively with the percentages of NREM (r = 0.44; P < 0.01, G), REM (r = 0.77; P < 0.001, H), total sleep (r = 0.52; P < 0.01, I) and the number of REM sleep bouts (r = 0.62; P < 0.001, J).
Figure 5. Correlations between sleep–wake cycle parameters and CREB and CAMKII mRNA levels.
A–D, CREB mRNA levels correlated positively with the NREM length bout (r = 0.41; P < 0.05, A), the number of REM sleep bouts (r = 0.4; P < 0.05, B), the REM sleep bout duration (r = 0.4; P < 0.05, C) and the percentage of REM sleep (r = 0.55; P < 0.001, D). E–I, correlations between sleep–wake cycle parameters and CAMKII mRNA levels. CAMKII mRNA levels showed a negative correlation with the percentage of waking (r =−0.42; P < 0.05, E) and a positive correlation with the percentage of time spent in NREM sleep (r = 0.4; P < 0.05, F), REM sleep (r = 0.45; P < 0.01, G), total sleep (r = 0.42; P < 0.05, H) and the NREM bout length (r = 0.41; P < 0.05, I).
To determine the relative contributions of NREM and REM sleep, we performed a stepwise regression analysis. This analysis revealed that if the contribution of NREM percentage is removed, BDNF mRNA levels continue to be related to the NREM sleep bout length (r = 0.72; P < 0.05), the percentage of REM sleep (r = 0.4; P < 0.05), total sleep (r = 0.4; P < 0.05), and the percentage of wakefulness (r =−0.4; P < 0.05). However, when controlling for the percentage of REM sleep we found that the correlations between BDNF levels and the NREM sleep bout length (r = 0.19), the percentages of NREM and total sleep (r = 0) as well as wakefulness (r = 0) were no longer significant (P > 0.05).
Similarly, significant correlations between sleep parameters and Synapsin I and CREB mRNA levels continue to be significant if the contribution of NREM sleep is removed. Controlling for the percentage of REM we found that the correlations between Synapsin I and the percentage of wake (r =− 0.3), NREM (r = 0.3), total sleep (r = 0.3) and the number of REM bouts (r = 0.35) were no longer significant (P > 0.05). In the case of CREB mRNA, when controlling for the percentage of REM, the correlation between CREB mRNA and NREM sleep bout length (r = 0) and the number of bouts (r = 0.13) and length of REM episodes (r = 0) were no longer significant (P > 0.05).
In contrast, when the regression analysis was carried out to analyse the contributions of NREM or REM sleep to the expression of CAMKII mRNA, no changes in correlations (Fig. 5E–I) were found when controlling for the percentage of REM sleep. However if the contribution of NREM percentage is removed, the correlations between CAMKII mRNA and the percentage of wake (r =−0.2), REM (r = 0.2), total sleep (r = 0.3) and the lengths of NREM sleep bouts (r = 0.11) were no longer significant (P > 0.05).
Discussion
The main findings were that, in the hippocampus BDNF, Synapsin I, CREB and CAMKII mRNA levels were reduced in both SD and treadmill control groups after 8 h treadmill treatment. However, following 48 h SD, BDNF, Synapsin I, CAMKII and CREB mRNA levels were reduced in the SD group compared to both CC and CT2 group. Each of these transcripts was unchanged in the CT2 group when compared to CC. The effects of SD on BDNF protein levels at 8 h and 48 h followed the same profile as observed for the mRNA. The results of stepwise regression analysis showed that REM sleep amounts had independent correlations with BDNF, Synapsin I and CREB gene expression. The level of NREM sleep had an independent correlation with CAMKII gene expression in the hippocampus. Neither 8 h nor 48 h SD or control treatments had significant effects on BDNF, Synapsin I and CAMKII mRNA levels in the neocortex.
Our analysis showed that changes in sleep–waking cycle parameters in SD and treadmill control animals account for the effects of treadmill treatments on plasticity-related genes' expression. In spite of quiescent periods that allowed sustained periods of rest, treadmill control treatments resulted in partial sleep loss. As could be expected, this loss in control groups was greater during the 8 h than the 48 h treatments. During the 48 h treatment, sleep parameters were related to the duration of quiescent time on the treadmill in control groups (60 min in CT1, 120 min in CT2). The characteristics of sleep in the CT2 group were closer to characteristics found in CC group. CT2 animals did not differ from CC in number and duration of wake and NREM sleep bouts, and had very mild reduction of the total sleep time, but had a significant reduction of REM sleep. The CT1 animals compared to CT2 exhibited a reduction in total sleep, greater NREM sleep fragmentation and a reduced duration of REM sleep bouts.
The sensitivity of BDNF, Synapsin I and CREB expression to REM sleep is a possible explanation for the differential changes in gene expression in control groups in the 48 h experiment. The SD, CT1 and CT2 groups all had reduced REM sleep compared to the CC group. The CT2 group, which did not exhibit differences in gene expression compared to CC, had significantly longer REM bouts. The CT2 group also had more sustained NREM sleep bouts compared to the CT1 group. We conclude that sustained REM periods are critical for expression of certain plasticity-related genes, and suggest that NREM sleep may be critical for others, i.e. CAMKII in our study.
There is a large body of evidence indicating that selective REM sleep deprivation has deleterious effects on spatiallearning and memory. In the rat, REM sleep deprivation impairs reference, but not the working memory in a radial arm maze task (Smith et al. 1998). In addition, REM sleep deprivation impairs spatial learning as measured in the Morris water maze (Youngblood et al. 1997, 1999; Smith & Rose, 1996; Smith & Rose, 1997), and the eight-box task (Bjorness et al. 2005), but not in non-spatial memory using the visible version of the maze (Smith & Rose, 1997). Moreover, long-term potentiation (LTP) was inhibited following 72 h of REM deprivation (McDermott et al. 2003).
The hippocampus is an area crucial for learning and memory, which expresses an abundance of BDNF and its receptor (Rocamora et al. 1996) and is responsive to manipulations in BDNF levels. BDNF gene inhibition or deletion (Figurov et al. 1996; Kang et al. 1997) produces a deficit in hippocampal LTP, an electrophysiological correlate of learning and memory (Nguyen & Kandel, 1996). Restitution of hippocampal BDNF by exogenous application (Patterson et al. 1996) or overexpression (Korte et al. 1995) of BDNF corrected these deficits in hippocampal plasticity. CREB has a key role in memory storage and synaptic plasticity (Silva et al. 1998). Moreover, it has been shown that BDNF and pCREB levels and other synaptic-related molecules are increased in the hippocampus following learning (Ulloor & Datta, 2005). The reduction in BDNF, Synapsin I, CREB and CAMKII gene expression induced by SD in our studies could explain the disturbances in spatial memory function induced by SD. The reduction in the levels of BDNF in the hippocampus is consistent with a recent finding showing that SD reduces the protein levels of the phosphorylated form of the extracellular signal-regulated kinase 2 (phospho-ERK2), which is one of the targets of the BDNF receptor activation (Guan et al. 2004).
Three features of hippocampal function during REM may underlie its role in plasticity. During REM sleep, hippocampal neurons exhibit temporally structured discharge patterns mimicking those present during prior spatial learning tasks (Wilson & McNaughton, 1994). This replay may help stabilize synaptic connections by critical synaptic proteins. A second feature is the prominent theta burst activity of neurons in REM. The theta-burst stimulation pattern is most effective in inducing hippocampal LTP, and is also significantly effective in releasing native BDNF (Kramar et al. 2004). Running consistently increases BDNF expression in the hippocampus (Gomez-Pinilla et al. 2002), and also elicits a typical theta rhythm pattern (Buzsaki, 2002). Therefore, it is possible that reduced theta activity resulting from SD, particularly REM suppression could lead to a reduction in BDNF expression. A third possibility stems from studies showing that brainstem phasic wave (P-wave) occurrence during REM is critical for retention of shuttle box avoidance learning across 6 h sleep periods (Mavanji & Datta, 2003). In the hippocampus, pCREB, BDNF, and Arc proteins are increased following shuttle box learning; the increases are correlated with P-wave density (Saha & Datta, 2005; Ulloor & Datta, 2005). In addition, the induction of LTP during waking led to expression of zif-268 in the hippocampus following subsequent REM; this transcription factor regulates the expression of many genes (Ribeiro et al. 1999). Further studies need to address these alternative hypotheses.
Stress may affect hippocampal plasticity-related gene expression (Smith et al. 1995). However, we showed previously that corticosterone levels were not increased after 48 h SD imposed by the intermittent treadmill method (Guzman-Marin et al. 2003), probably because we carefully adapt our animals to the procedure. Thus, it is unlikely that the stress accounts for changes in gene expression in the 48 h treatment groups, although we cannot rule out an effect of stress in the 8 h treatment study.
Previous studies of total sleep or REM deprivation on hippocampal BDNF levels have been inconsistent. For example, by using ‘gentle handling’ to achieve total sleep deprivation for 8 h Taishi et al. (2001) showed no change in BDNF mRNA levels following deprivation, and an increase following recovery sleep after sleep deprivation. However, Fujihara et al. (2003) showed that both mRNA and protein levels were increased in this region by 1–2 h SD induced by stroking the back of the rat. Interestingly, following 3 h of recovery from SD, BDNF mRNA levels continue to be elevated. In young rats, after total sleep deprivation by gentle handling for 1.5–3 h, BDNF levels were increased when these manipulations were conducted at postnatal day 24, but not at 16 or 20 days (Hairston et al. 2004). Finally in a study of 6 h selective REM deprivation by stroking the back of the rat, a decrease in BDNF protein in the brainstem and cerebellum was reported, whereas no changes were seen in the hippocampus (Sei et al. 2000). Following an 8 h light phase shift, hippocampal BDNF protein was increased (Sei et al. 2003) at a time when REM sleep was also increased (Sei et al. 1994). Thus, in previous studies, the effects of SD were variable, but BDNF expression was consistently increased during recovery sleep. Our results also show that sleep may promote BDNF expression.
In neocortex, 8 h of total sleep deprivation through gentle handling increased BDNF mRNA following deprivation, but no changes were found following sleep recovery after deprivation (Taishi et al. 2001). Another study using gene array technology showed that BDNF expression was increased both by sleep deprivation through exposure to novel stimuli and following spontaneous waking (Cirelli et al. 2004). In young rats, total sleep deprivation by gentle handling increased BDNF levels at postnatal day 20 and 24, but not at day 16 (Hairston et al. 2004).
The different findings reported in these studies may reflect differing methodology. Studies using stroking the back of the rat, gentle handling or the introduction of new objects do not incorporate controls, based on the assumption that these interventions do not induce stress or have other confounding effects. However there is evidence that gentle handling is not stress free (Gip et al. 2004). The introduction of new objects has been shown to play a role in the induction and maintenance of synaptic plasticity in the hippocampus (Davis et al. 2004). Indeed, there is evidence that BDNF expression is increased in the cortex and hippocampus when animals are exposed to an enriched environment in which novel objects are placed into their home cages (Young et al. 1999; Ickes et al. 2000). Thus, it is possible that the increases in BDNF expression observed in some previous studies might be associated with factors other than suppression of sleep.
The results presented here show that SD has a differential effect on the BDNF and Synapsin I levels in the cortex and in the hippocampus. These differences may reflect a different susceptibility in these brain regions to the effects of SD. For example, super-oxide dimutase activity was reduced after prolonged SD in the hippocampus, but not in the cortex (Ramanathan et al. 2002). The present data support the view that the hippocampus is more vulnerable to the effects of SD.
In summary our findings demonstrate that sleep deprivation results in a reduction in the expression of BDNF and its protein levels, as well as, Synapsin I and CAMKII in the hippocampus, whereas no changes were observed in the expression of these molecules in the neocortex. Plasticity-related gene expression was sensitive to subtle features of sleep architecture. Loss of sustained REM periods seemed to underlie some effects of SD on the expression of some plasticity-related genes, but loss of NREM sleep underlies others.
Acknowledgments
We gratefully acknowledge Lisa Boehmer for providing software for sleep bout analysis. The authors wish to thank Bryan Angara for his excellent technical assistance. This research was made possible by a grant from the American Sleep Medicine Foundation, a foundation of the American Academy of Sleep Medicine to RGM. It was supported by the US Department of Veterans Affairs Medical Research service and US National Institutes of Health grants MH 47480, HL 60296.
References
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol. 2003;69:71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- Bjorness TE, Riley BT, Tysor MK, Poe GR. REM restriction persistently alters strategy used to solve a spatial task. Learn Mem. 2005;12:352–359. doi: 10.1101/lm.84805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- Boulanger L, Poo MM. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;8:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differences in gene expression between sleep and waking as revealed by mRNA differential display. Brain Res Mol Brain Res. 1998;56:293–305. doi: 10.1016/s0169-328x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CD, Jones FL, Derrick BE. Novel environments enhance the induction and maintenance of long-term potentiation in the dentate gyrus. J Neurosci. 2004;24:6497–6506. doi: 10.1523/JNEUROSCI.4970-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Fujihara H, Sei H, Morita Y, Ueta Y, Morita K. Short-term sleep disturbance enhances brain-derived neurotrophic factor gene expression in rat hippocampus by acting as internal stressor. J Mol Neurosci. 2003;21:223–232. doi: 10.1385/jmn:21:3:223. [DOI] [PubMed] [Google Scholar]
- Gip P, Hagiwara G, Sapolsky RM, Cao VH, Heller HC, Ruby NF. Glucocorticoids influence brain glycogen levels during sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1057–R1062. doi: 10.1152/ajpregu.00528.2003. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111–2116. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Stewart DR, Gong H, Szymusiak R, Mc G, Inty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563–571. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston IS, Peyron C, Denning DP, Ruby NF, Flores J, Sapolsky RM, Heller HC, O'Hara BF. Sleep deprivation effects on growth factor expression in neonatal rats: a potential role for BDNF in the mediation of delta power. J Neurophysiol. 2004;91:1586–1595. doi: 10.1152/jn.00894.2003. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfanati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czemik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I–actin interactions. Proc Natl Acad Sci. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czemik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles of TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo DC. Neurotrophic factors and synaptic plasticity. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Datta S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur J Neurosci. 2003;17:359–370. doi: 10.1046/j.1460-9568.2003.02460.x. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Koehl M, Van Der Borgh T, Turek FW. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J Neuroendocrinol. 2002;14:397–402. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- Meyer T, Waeber G, Lin J, Beckmann W, Habener JF. The promoter of the gene encoding 3-,5-cyclic adenosine monophosphate (cAMP) response element binding protein contains cAMP response elements: evidence for positive auto regulation of gene transcription. Endocrinology. 1993;132:770–780. doi: 10.1210/endo.132.2.8381074. [DOI] [PubMed] [Google Scholar]
- Nguyen P, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13:1387–1390. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Goyal V, Mello C, Pavlides C. Brain gene expression during REM sleep depends on prior waking experience. Learn Mem. 1999;6:500–508. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocamora N, Pascual M, Acsady L, de Lecea L, Freund TF, Soriano E. Expression of NGF and NT3 mRNAs in hippocampal interneurons innervated by the GABAergic septohippocampal pathway. J Neurosci. 1996;16:3991–4004. doi: 10.1523/JNEUROSCI.16-12-03991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Datta S. Two-way active avoidance training-specific increases in phosphorylated cAMP response element-binding protein in the dorsal hippocampus, amygdala, and hypothalamus. Eur J Neurosci. 2005;21:3403–3414. doi: 10.1111/j.1460-9568.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- Sei H, Fujihara H, Ueta Y, Morita K, Kitahama K, Morita Y. Single eight-hour shift of light-dark cycle increases brain-derived neurotrophic factor protein levels in the rat hippocampus. Life Sci. 2003;73:53–59. doi: 10.1016/s0024-3205(03)00251-0. [DOI] [PubMed] [Google Scholar]
- Sei H, Kiuch T, Chang H, Seno H, Sano A, Morita Y. Response of the sleep-wake rhythm to an 8-hour advance of the light-dark cycle in the rat. Chronobiol Int. 1994;11:293–300. doi: 10.3109/07420529409057245. [DOI] [PubMed] [Google Scholar]
- Sei H, Saitoh D, Yamamoto K, Morita K, Morita Y. Differential effect of short-term REM sleep deprivation on NGF and BDNF protein levels in the rat brain. Brain Res. 2000;877:387–390. doi: 10.1016/s0006-8993(00)02708-6. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the morris water maze. Physiol Behav. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;111:1197–1204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- Smith C, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working memory in the radial arm maze task. Neurobiol Learning Memory. 1998;69:211–217. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- Soderling T. CaM-kinases: modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Taishi P, Sanchez C, Wang Y, Fang J, Harding JW, Krueger JM. Conditions that affect sleep alter the expression of molecules associated with synaptic plasticity. Am J Physiol Regul Integr Comp Physiol. 2001;281:R839–R845. doi: 10.1152/ajpregu.2001.281.3.R839. [DOI] [PubMed] [Google Scholar]
- Ulloor J, Datta S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: a mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. J Neurochem. 2005;95:418–428. doi: 10.1111/j.1471-4159.2005.03378.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Collin C, McKay RDG, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultural hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Youngblood BD, Smagin GN, Elkins PD, Ryan DH, Harris RB. The effects of paradoxical sleep deprivation and valine on spatial learning and brain 5-HT metabolism. Physiol Behav. 1999;67:643–649. doi: 10.1016/s0031-9384(99)00120-1. [DOI] [PubMed] [Google Scholar]
- Youngblood BD, Zhou J, Smagin GN, Ryan DH, Harris RB. Sleep deprivation by the flowerpot technique and spatial reference memory. Physiol Behav. 1997;61:249–256. doi: 10.1016/s0031-9384(96)00363-0. [DOI] [PubMed] [Google Scholar]