Abstract
Inhibition is of fundamental importance to regulate activity in cortical circuits. Inhibition is mediated through a diversity of different interneurones and γ-aminobutyric acid A receptor (GABAAR) subtypes. Here we employed paired-pulse transcranial magnetic stimulation (TMS) to measure short interval intracortical inhibition (SICI), a GABAAR-mediated inhibition in human motor cortex, to address the question of which GABAAR subtype is responsible for this form of inhibition. It has been shown that classical benzodiazepines (diazepam and lorazepam) have a non-selective affinity profile at different α-subunit-bearing subtypes of the GABAAR while zolpidem has a 10-fold greater affinity to the α1-subunit-bearing GABAAR compared with those bearing the α2- or α3-subunit. We found that, in seven healthy subjects, a single oral dose of 20 mg of diazepam or 2.5 mg of lorazepam significantly increased SICI, whereas 10 mg of zolpidem did not change SICI. This dissociation occurred despite equal sedation by all three drugs, an α1-subunit GABAAR-mediated effect. The findings strongly suggest that SICI is not mediated by the α1-subunit-bearing subtype of the GABAAR but by those bearing either the α2- or α3-subunit. This study represents an attempt by means of TMS to identify GABAAR subtype-specific action at the systems level of human cortex, a highly relevant issue because the different α-subunit-bearing subtypes of the GABAAR are differently involved in benzodiazepine-mediated effects such as sedation, amnesia or anxiolysis, in developmental cortical plasticity, and in neurological disorders such as epilepsy.
Transcranial magnetic stimulation (TMS) provides an opportunity to study the effects of CNS active drugs on the intact human brain. By using paired-pulse TMS protocols, or by coupling of peripheral nerve stimulation with TMS of the contralateral motor cortex, it is possible to recruit specific neuronal circuits of the human brain and to evaluate in vivo the effects of drugs on several neurotransmitter systems that influence these circuits (Ziemann, 2004). One important paired-pulse TMS measure is the short interval intracortical inhibition (SICI) (Kujirai et al. 1993; Ziemann et al. 1996b; Di Lazzaro et al. 1998; Ilic et al. 2002). There exists strong evidence that SICI originates at the level of motor cortex rather than at a subcortical or spinal level because it can be elicited by low-intensity conditioning pulses that are subthreshold for activation of corticospinal neurones (Di Lazzaro et al. 1998). In addition, there is strong evidence that SICI is mediated through a motor cortical inhibitory interneuronal circuit that employs γ-aminobutyric acid A receptors (GABAAR) because benzodiazepines, positive allosteric modulators of the GABAAR, enhance SICI (Ziemann et al. 1996a; Di Lazzaro et al. 2000, 2005a,b; Ilic et al. 2002). GABAARs are pentamers that constitute an extraordinary structural heterogeneity. Benzodiazepine-sensitive GABAARs in the CNS bear one α1-, α2-, α3- or α5-subunit in combination with β- and γ-subunits. These different GABAARs subtypes are responsible for different benzodiazepine effects (Möhler et al. 2002, 2004). The α1-subunit-bearing subtype of the GABAAR mediates the sedative, amnestic and, to a large extent, the anticonvulsant action of benzodiazepine site agonists while the α2-subunit-bearing subtype of the GABAAR mediates anxiolytic action (Möhler et al. 2002, 2004). The different GABAAR subtypes are targeted by different subtypes of inhibitory interneurones (Nusser et al. 1996; Fritschy et al. 1998; Nyiri et al. 2001; Klausberger et al. 2002). Therefore, identification of the GABAAR subtype that mediates SICI is of great interest because it would allow the linking of a TMS measure to a specific cortical interneuron circuit and its associated function. In order to accomplish this goal, we tested here the effects of two classical benzodiazepines, diazepam and lorazepam, and of a novel agonist at the benzodiazepine site, zolpidem. While diazepam has high affinity to all α-subunit-bearing subtypes of the GABAAR, zolpidem has a 10-fold greater affinity to the α1-subunit-bearing GABAAR than α2- or α3-subunit-bearing GABAARs and very low affinity to the α5-subunit-bearing GABAAR (Möhler et al. 2002, 2004). The affinity profile of lorazepam is unknown but it is considered a classical benzodiazepine with a broad affinity to all α-subunit-bearing subtypes of the GABAAR. The different GABAAR affinity profiles of diazepam and zolpidem constitute the rationale for this study: if given in doses that match with respect to sedative effects (mediated by the α1-subunit-bearing subtype of the GABAAR) then similar enhancing effects on SICI would strongly suggest that SICI is mediated through an interneurone circuit that uses the α1-subunit-bearing subtype of the GABAAR. In contrast, if an enhancement of SICI is obtained with diazepam but not with zolpidem then this would strongly point to mediation of SICI through a different GABAAR subtype, most likely the one that bears the α2- or α3-subunit, while the ones bearing the α5-subunit are unlikely to be involved due to their low prevalence in the CNS (Möhler et al. 2002).
Methods
Subjects
Seven right-handed healthy volunteers (mean age, 28.6 ± 5.7 years; 5 male) participated in the experiments. All gave their written informed consent. The study was performed according to the Declaration of Helsinki and approved by the ethics committee of the Medical Faculty of the Catholic University of Rome.
Transcranial magnetic stimulation (TMS)
TMS was performed with a high power Magstim 200 magnetic stimulator with a monophasic current waveform (Magstim Co., Whitland, Dyfed, UK). A figure-of-eight coil with external loop diameters of 9 cm was held over the right motor cortex at the optimum scalp position to elicit motor-evoked potentials (MEPs) in the contralateral left first dorsal interosseous (FDI) muscle. The induced current in the brain flowed in a posterior-to-anterior direction. Muscle responses were recorded with two 9 mm diameter Ag–AgCl surface electrodes with the active electrode over the motor point of the muscle and the reference on the metacarpophalangeal joint of the index finger. EMG responses were amplified and filtered (bandwidth 3 Hz–3 kHz) by D360 amplifiers (Digitimer, Welwyn Garden City, Herts, UK). Data were collected on a computer with a sampling rate of 10 kHz per channel and stored for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK). Resting motor threshold (RMT) was defined as the minimum stimulus intensity that produced a liminal MEP (> 50 μV in at least 50% of 10 trials) at rest. Stimulus intensity will be given as a percentage of maximum stimulator output (%MSO). Active motor threshold (AMT) was defined as the minimum stimulus intensity that produced a small MEP (> 200 μV in 50% of 10 trials) during isometric contraction of the tested muscle at about 20% of maximum voluntary contraction. A constant level of voluntary contraction was maintained with reference to an oscilloscope display of the EMG signal in front of the subject. Auditory feedback of the EMG activity was also provided. In order to minimize the recording time for each protocol, both single and paired-pulse TMS of the motor cortex were performed with the stimulator(s) connected to the BiStim Module (Magstim Co.) throughout all measurements.
SICI was studied using an established protocol (Kujirai et al. 1993). Two magnetic stimuli were given to the motor cortex through the same stimulating coil, using the Bistim module, and the effect of the first stimulus (conditioning stimulus, CS) on the second stimulus (test stimulus, TS) was investigated. The CS intensity was set to 5% of MSO below AMT. The TS intensity was adjusted to elicit a MEP in the relaxed FDI with a peak-to-peak amplitude of on average 1 mV when given alone. Interstimulus intervals (ISIs) of 2 and 3 ms were investigated. Five stimuli were delivered at each ISI. For SICI recordings, maintenance of full muscle relaxation is very important (Ridding et al. 1995). This was ensured by providing the subjects with audio-visual feedback of the raw EMG at high gain (μV). SICI was calculated by normalizing the mean amplitude of the conditioned MEPs to the mean amplitude of the unconditioned test MEPs. After drug intake, TS intensity was adjusted whenever necessary to ensure that the test MEPs were matched in amplitude to the test MEP before drug intake (see TS intensity and MEP amplitude in Table 1). This is important because SICI varies with the amplitude of the unconditioned test MEP (Daskalakis et al. 2002).
Table 1.
Motor thresholds, CS and TS intensities, and MEP amplitude before (t0) and after (t1–t3) drug intake
| Measure | t0 | t1 | t2 | t3 |
|---|---|---|---|---|
| Zopidem | ||||
| RMT (% MSO) | 41.1 ± 7.6 | 42.1 ± 8.1 | 41.4 ± 7.7 | 41.0 ± 7.6 |
| AMT (% MSO) | 30.9 ± 5.8 | 29.9 ± 6.9 | 30.1 ± 6.1 | 30.1 ± 5.9 |
| CS (% MSO) | 26.9 ± 5.3 | 25.6 ± 6.3 | 26.3 ± 5.3 | 26.1 ± 4.9 |
| TS (% MSO) | 51.4 ± 10.3 | 52.9 ± 11.2 | 54.3 ± 11.4 | 53.9 ± 11.4 |
| MEP (mV) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.4 | 1.6 ± 0.8 |
| Lorazepam | ||||
| RMT (% MSO) | 41.9 ± 8.5 | 41.0 ± 7.7 | 41.4 ± 8.8 | 41.6 ± 6.9 |
| AMT (% MSO) | 30.3 ± 6.2 | 29.3 ± 6.0 | 28.4 ± 6.1 | 29.1 ± 5.9 |
| CS (% MSO) | 26.6 ± 5.3 | 26.0 ± 5.3 | 25.9 ± 5.1 | 26.3 ± 4.4 |
| TS (% MSO) | 54.9 ± 13.3 | 56.1 ± 14.2 | 55.3 ± 14.9 | 55.3 ± 14.9 |
| MEP (mV) | 1.1 ± 0.4 | 1.1 ± 0.6 | 1.1 ± 0.6 | 1.3 ± 0.5 |
| Diazepam | ||||
| RMT (% MSO) | 43.7 ± 7.3 | 43.4 ± 7.3 | 44.7 ± 6.7 | 44.6 ± 7.3 |
| AMT (% MSO) | 32.1 ± 6.1 | 32.4 ± 6.6 | 32.4 ± 6.5 | 31.6 ± 6.0 |
| CS (% MSO) | 27.7 ± 5.6 | 27.7 ± 5.8 | 28.2 ± 5.8 | 27.6 ± 5.7 |
| TS (% MSO) | 55.0 ± 10.3 | 56.9 ± 10.9 | 60.7 ± 15.6 | 57.1 ± 13.1 |
| MEP (mV) | 1.3 ± 0.6 | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.3 ± 0.5 |
Values are means ±s.d.
Experimental design
All measurements were done at baseline (t0, before drug intake) and at three time points after drug intake (t1–t3). t1 was 1.5 h for diazepam, and 2.0 h for lorazepam and zolpidem, according to differences in drug pharmacokinetics (plasma peak concentration: diazepam, 1–1.5 h (Shader et al. 1984); lorazepam, 1.5–2.5 h (Kyriakopoulos et al. 1978); zolpidem, 0.75–2.6 h (Salva & Costa, 1995)). Measurements at t2 and t3 were always performed 6 h and 24 h after drug intake, respectively. Drugs were taken orally as a single oral dose (diazepam, 20 mg; lorazepam 2.5 mg, zolpidem, 10 mg). Doses were selected according to previous reports that showed a significant enhancement of SICI under 20 mg of diazepam (Ilic et al. 2002; Di Lazzaro et al. 2005b) or 2.5 mg of lorazepam (Ziemann et al. 1996a; Di Lazzaro et al. 2000, 2005a,b). The dose of 10 mg of zolpidem was chosen because this is the commonly used daily dose, and we expected it to cause a similar level of sedation compared with diazepam and lorazepam. All subjects were tested for all three drugs in a randomised crossover design. The intersession interval for a given subject was at least 1 month to exclude interaction between sessions.
The sedative effects of diazepam, lorazepam and zolpidem were evaluated at the presumed peak-plasma concentration time (1.5 h after diazepam intake, 2 h after lorazepam or zolpidem intake) using a visual analog scale with a self rating of the subjective state of sedation. The subjects marked a point on a 100 mm line that represented the full range of the subject's level of sedation (with 0 meaning ‘very alert’ and 100 meaning ‘very sedated’).
Statistical analysis
The effects of drug were tested separately for the TMS measures (RMT, AMT, MEP amplitude and SICI) and TMS parameters (CS and TS intensity) using repeated measures analysis of variance (ANOVA) with drug (three levels, diazepam, lorazepam, zolpidem) and time (four levels, t0–t3) as within-subject factors. For SICI, the additional within-subject factor of ISI (two levels, 2 and 3 ms) was introduced. Non-sphericity was corrected by the Huynh-Feldt method. In the case of significant F values, post hoc paired Student's t tests were applied to further analyse the main effects. For the comparison of the sedative drug effects as expressed on the visual analog scale we used the Friedman test. Possible correlations between sedative drug effects and change in SICI were explored by linear regression analysis. Significance was assumed whenever P < 0.05.
Results
There was no significant effect of drug, time or their interaction on RMT, AMT, CS intensity or test MEP amplitude (Table 1). There was a significant effect of time on TS intensity (F3,6 = 3.67, P = 0.03) that was explained by a small increase in TS intensity after drug intake to maintain MEPs of on average 1 mV in amplitude. However, there was no significant effect of drug or the interaction of drug with time on TS intensity, indicating that the increase in TS intensity over time was similar for all three drugs (Table 1). There was no difference between drugs at baseline (t0) with respect to RMT, AMT, CS intensity, TS intensity, test MEP amplitude or SICI (Table 1, Fig. 1).
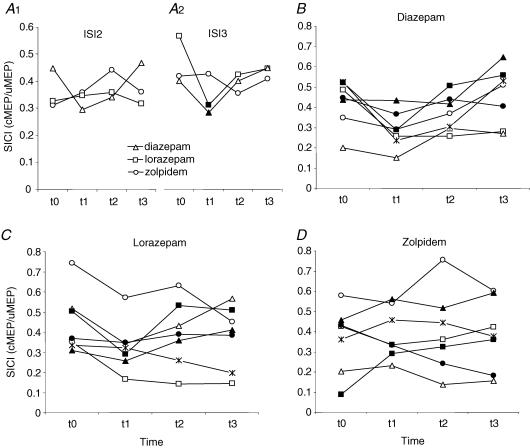
Figure 1. Short latency intracortical inhibition (SICI).
A, mean SICI (n = 7) expressed as the ratio of conditioned over unconditioned MEP (cMEP/uMEP) before (t0) and at three time points (t1–t3) after intake of a single oral dose of 20 mg of diazepam (triangles), 2.5 mg of lorazepam (squares) or 10 mg of zolpidem (circles), tested at interstimulus intervals (ISI) of 2 ms (A1) and 3 ms (A2). Filled symbols indicate significant change from t0 (P < 0.05). B–D, same data as in A but with all seven tested subjects shown individually. Each symbol denotes a single subject tested in randomised order for diazepam (B), lorazepam (C) and zolpidem (D). Note that diazepam and lorazepam but not zolpidem led to a consistent increase in SICI at t1 when compared with t0.
The three-way ANOVA with SICI as the dependent measure revealed no effect of ISI or its interactions with drug (F2,12 = 3.57, P = 0.06), time (F3,18 = 1.20, P = 0.34), or drug and time (F6,36 = 1.89, P = 0.11) (Fig. 1A1 and A2). The trend towards a significant interaction between ISI and drug was explained by an increase of SICI by diazepam and lorazepam only at the ISI of 3 ms (Fig. 1A1 and A2), consistent with previous observations (Ziemann et al. 1996a; Di Lazzaro et al. 2000, 2005a). For further analysis, individual averages of the SICI values at ISIs of 2 and 3 ms were calculated to obtain a single value of SICI. This revealed a significant interaction between drug and time (F6,36 = 2.57, P = 0.036) while there were no main effects of drug (F2,6 = 0.001, P = 0.99) or time (F2.56,6 = 2.97, P = 0.07) (Fig. 1). The interaction between drug and time was explained by a significant increase of SICI at t1 after diazepam (P = 0.02) and lorazepam (P = 0.01) but not zolpidem (P = 0.53) (Fig. 1).
The mean visual analog scale sedation score at t1 was 57.6 ± 20.7 after diazepam, 40.3 ± 12.0 after lorazepam and 55.7 ± 27.9 after zolpidem. The differences between scores were not significant (F2,6 = 1.87, P = 0.20). In addition, the individual visual analog scale sedation scores for either of the three drugs did not correlate with the individual change in SICI (difference of SICI between t1 and t0) (P > 0.4).
Discussion
The present results provide evidence that SICI, a TMS measure of excitability of inhibitory circuitry in human motor cortex, is differently affected by benzodiazepine receptor ligands (diazepam, lorazepam, zolpidem) that have different affinity at various subtypes of the GABAAR: diazepam and lorazepam but not zolpidem enhanced SICI. Diazepam and lorazepam are considered as classical benzodiazepines with non-selective affinity to all benzodiazepine-sensitive subtypes of the GABAAR (bearing the α1-, α2-, α3- or α5-subunit) while zolpidem has 10-fold higher affinity to the α1-subunit compared with the α2- and α3-subunit-bearing subtypes of the GABAAR and no affinity to the α5-subunit-bearing subtype (Langer et al. 1992). In the following discussion, we propose an explanation of the observed dissociated effects of the three study drugs on SICI on the basis of their differential affinity on the various subtypes of the GABAAR.
The important negative and novel finding of this study is that zolpidem had no effect on SICI, in contrast to the enhancement of SICI by diazepam and lorazepam shown here and in previous papers (Ziemann et al. 1996a; Di Lazzaro et al. 2000, 2005a,b; Ilic et al. 2002). It is possible that a significant enhancement of SICI by zolpidem was not seen due to a floor effect, i.e. that SICI was already maximal and could not be driven further by the study drug. However, this is rather unlikely because diazepam and lorazepam were tested here and in previous studies (Di Lazzaro et al. 2005 a,b) under the same SICI protocol, and baseline SICI did not differ between drugs (Fig. 1). Alternatively, it might be argued that the dose of zolpidem was insufficient to produce an increase of SICI. While this cannot be fully excluded because dose–response curves were not obtained, zolpidem caused similar sedation compared with diazepam and lorazepam. The sedative effects of benzodiazepines are mediated via the α1-subunit-bearing GABAAR (Rudolph et al. 1999; Crestani et al. 2000). Hence, it can be concluded that zolpidem was available at the α1-subunit-bearing subtype of the GABAAR in the CNS in a biologically relevant concentration because it produced a level of sedation that was equivalent to the one produced by diazepam and lorazepam. Therefore, insufficient dosing does not seem likely to explain the failure of zolpidem to affect SICI.
Rather, the dissociation of effects of zolpidem versus diazepam and lorazepam on SICI suggests that SICI is not mediated via the α1-subunit-bearing subtype of the GABAAR. Based on the different GABAAR affinity profiles of zolpidem versus classical benzodiazepines, a straightforward explanation for the observed dissociation of the effects of zolpidem versus diazepam on SICI is that the α2-subunit-bearing subtype rather than the α1-subunit-bearing subtype of the GABAAR mediates SICI.
Certain specialized subtypes of inhibitory interneurones preferentially target this subtype of the GABAAR expressed on postsynaptic pyramidal cells, while others do not. In particular, this receptor is targeted by parvalbumin-negative basket cells on the soma, and by axo-axonic (chandelier) cells on the axon initial segment in the hippocampus (Nusser et al. 1996; Fritschy et al. 1998; Nyiri et al. 2001; Klausberger et al. 2002) and neocortex (Fritschy et al. 1998). Chandelier cells produce strong inhibition of the pyramidal target cells and control their output more efficaciously than any other cell type (Howard et al. 2005). The physiological properties of SICI, in particular its very low threshold (Ziemann et al. 1996b; Di Lazzaro et al. 1998; Ilic et al. 2002) is consistent with this strategically important form of inhibition.
Recently, it was reported that SICI increases after zolpidem but does not change after diazepam administration (Mohammadi et al. 2006). These results are opposite to ours. While the lack of effect of diazepam in their study might be explained by the lower dosage (5 mg in their study, 20 mg in the present study), it is more difficult to understand the reason for the opposite findings after zolpidem administration. There are several possible explanations for this discrepancy. Those authors performed SICI testing at a different time after drug administration (1 h versus 2 h in the present study), they investigated only one interstimulus interval (3 ms versus 2 and 3 ms in the present study), and they related the intensity of the conditioning stimulus to resting motor threshold rather than to active motor threshold. More importantly, it should also be noted that, in their study, zolpidem resulted in a significant decrease (by more than 30%) of the amplitude of the unconditioned test MEP that was not corrected for, whereas we matched test MEP amplitudes before and after zolpidem. Since variation in test MEP amplitude strongly influences the magnitude of SICI in a U-shaped fashion (Daskalakis et al. 2002; Ilic et al. 2002), it is well possible that the variation in test MEP amplitude in their study was a confounding factor that contributed significantly to the difference in findings with the present study.
Finally, while the affinity of lorazepam at the different subtypes of benzodiazepine-sensitive GABAARs is not known, lorazepam is considered as a classical benzodiazepine similar to diazepam. Our data are consistent with this view since the enhancing effect of lorazepam on SICI would suggest that lorazepam has relevant affinity to the α2-subunit-bearing subtype of the GABAAR.
The current findings open up the promising avenue to study GABAAR subtype-specific inhibition in human cortex at the systems level. The present experiments go beyond those of one recent study that showed a dissociated effect of diazepam versus lorazepam on short latency afferent inhibition, a different form of cortical inhibition (Di Lazzaro et al. 2005b). The mechanisms of that effect could only be speculated upon because, in contrast to zolpidem, the affinity profile of lorazepam on the different GABAAR subtypes is not known. The current data are important because the different GABAAR subtypes are differently involved in fundamental biological processes such as sedation and amnesia (α1-subunit-bearing subtype of the GABAAR) or anxiolysis (α2-subunit-bearing subtype of the GABAAR) (Möhler et al. 2002, 2004), developmental cortical plasticity (Fagiolini et al. 2004), and epilepsy (Cossette et al. 2002).
References
- Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131:1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali PA. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005a;564:661–668. doi: 10.1113/jphysiol.2004.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short latency afferent inhibition. J Physiol. 2005b;569:315–323. doi: 10.1113/jphysiol.2005.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABAA receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos AA, Greenblatt DJ, Shader RI. Clinical pharmacokinetics of lorazepam: a review. J Clin Psychiatry. 1978;39:16–23. [PubMed] [Google Scholar]
- Langer SZ, Faure-Halley C, Seeburg P, Graham D, Arbilla S. The selectivity of zolpidem and alpidem for the alpha1-subunit of the GABAA receptor. Eur Neuropsychopharmacol. 1992;2:232–234. [Google Scholar]
- Mohammadi B, Krampfl K, Petri S, Bogdanova D, Kossev A, Bufler J, Dengler R. Selective and nonselective benzodiazepine agonists have different effects on motor cortex excitability. Muscle Nerve. 2006;33:778–784. doi: 10.1002/mus.20531. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Crestani F, Hensch T, Rudolph U. Specific GABAA circuits in brain development and therapy. Biochem Pharmacol. 2004;68:1685–1690. doi: 10.1016/j.bcp.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of α2-subunit-containing GABAA receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Salva P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29:142–153. doi: 10.2165/00003088-199529030-00002. [DOI] [PubMed] [Google Scholar]
- Shader RI, Pary RJ, Harmatz JS, Allison S, Locniskar A, Greenblatt DJ. Plasma concentrations and clinical effects after single oral doses of prazepam, clorazepate, and diazepam. J Clin Psychiatry. 1984;45:411–413. [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996b;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]