Abstract
Brief, intense exercise training may induce metabolic and performance adaptations comparable to traditional endurance training. However, no study has directly compared these diverse training strategies in a standardized manner. We therefore examined changes in exercise capacity and molecular and cellular adaptations in skeletal muscle after low volume sprint-interval training (SIT) and high volume endurance training (ET). Sixteen active men (21 ± 1 years, 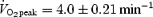 ) were assigned to a SIT or ET group (n = 8 each) and performed six training sessions over 14 days. Each session consisted of either four to six repeats of 30 s ‘all out’ cycling at ∼250%
) were assigned to a SIT or ET group (n = 8 each) and performed six training sessions over 14 days. Each session consisted of either four to six repeats of 30 s ‘all out’ cycling at ∼250% 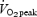 with 4 min recovery (SIT) or 90–120 min continuous cycling at ∼65%
with 4 min recovery (SIT) or 90–120 min continuous cycling at ∼65% 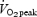 (ET). Training time commitment over 2 weeks was ∼2.5 h for SIT and ∼10.5 h for ET, and total training volume was ∼90% lower for SIT versus ET (∼630 versus∼6500 kJ). Training decreased the time required to complete 50 and 750 kJ cycling time trials, with no difference between groups (main effects, P ≤ 0.05). Biopsy samples obtained before and after training revealed similar increases in muscle oxidative capacity, as reflected by the maximal activity of cytochrome c oxidase (COX) and COX subunits II and IV protein content (main effects, P ≤ 0.05), but COX II and IV mRNAs were unchanged. Training-induced increases in muscle buffering capacity and glycogen content were also similar between groups (main effects, P ≤ 0.05). Given the large difference in training volume, these data demonstrate that SIT is a time-efficient strategy to induce rapid adaptations in skeletal muscle and exercise performance that are comparable to ET in young active men.
(ET). Training time commitment over 2 weeks was ∼2.5 h for SIT and ∼10.5 h for ET, and total training volume was ∼90% lower for SIT versus ET (∼630 versus∼6500 kJ). Training decreased the time required to complete 50 and 750 kJ cycling time trials, with no difference between groups (main effects, P ≤ 0.05). Biopsy samples obtained before and after training revealed similar increases in muscle oxidative capacity, as reflected by the maximal activity of cytochrome c oxidase (COX) and COX subunits II and IV protein content (main effects, P ≤ 0.05), but COX II and IV mRNAs were unchanged. Training-induced increases in muscle buffering capacity and glycogen content were also similar between groups (main effects, P ≤ 0.05). Given the large difference in training volume, these data demonstrate that SIT is a time-efficient strategy to induce rapid adaptations in skeletal muscle and exercise performance that are comparable to ET in young active men.
Regular endurance training induces numerous physiological adaptations that facilitate improved exercise capacity, i.e. the ability to sustain a given submaximal workload for a longer period of time or achieve a higher average power output over a fixed distance or time (Coyle, 1995; Hawley, 2002). One of the most prominent adaptations to training is a change in skeletal muscle substrate metabolism (Holloszy & Coyle, 1984). For example, even a short period of endurance training (5–7 days) increases glycogen availability but reduces the rate of glycogen catabolism during matched-work exercise (Green et al. 1992; Chesley et al. 1996), resulting in improved endurance capacity (Green et al. 1995). Training-induced shifts in substrate utilization are classically attributed to the improved respiratory control sensitivity that results from an increase in mitochondrial density, as reflected by changes in the maximal activity or protein content of enzymes in the tricarboxylic acid cycle and electron transport chain (Saltin & Gollnick, 1983; Holloszy & Coyle, 1984). However, other factors must also contribute to the training response, as shown by studies that show metabolic (Green et al. 1992; Clark et al. 2004) or performance adaptations (Coyle et al. 1988; Weston et al. 1997) despite no change in muscle oxidative capacity.
In contrast to traditional endurance training (ET), high-intensity sprint interval training (SIT) is generally thought to have less of an effect on muscle oxidative capacity, substrate utilization and endurance performance (Gleeson, 2000; Kubukeli et al. 2002). However, SIT increases the maximal activities of mitochondrial enzymes (Henriksson & Reitman, 1976; Saltin et al. 1976; Burgomaster et al. 2005), reduces glycogen utilization and lactate accumulation during matched-work exercise (Harmer et al. 2000; Clark et al. 2004; Burgomaster et al. 2006) and improves performance during tasks that primarily rely on aerobic metabolism (Burgomaster et al. 2005; Burgomaster et al. 2006; Eddy et al. 1977). SIT may also be more effective than ET for improving other important determinants of endurance performance, such as muscle buffering capacity (Weston et al. 1997; Edge et al. 2006). Low volume SIT may therefore represent a time-efficient strategy to induce muscle and performance adaptations similar to high volume ET (Coyle, 2005). However, no study has directly compared these diverse training approaches in a standardized manner.
The unique purpose of the present study was to compare changes in exercise capacity and molecular and cellular adaptations in skeletal muscle after low volume SIT and high volume ET. The SIT protocol was based on recent work from our laboratory (Burgomaster et al. 2005, 2006) and consisted of six sessions of brief, repeated ‘all out’ 30 s cycling efforts, interspersed with a short recovery, over 14 days. The ET protocol was modelled after work by others (Green et al. 1992; Spina et al. 1996) and consisted of six sessions of 90–120 min of moderate intensity cycling exercise, with 1–2 days of recovery interspersed between training sessions. As a result, subjects in both groups performed the same number of training sessions on the same days with the same number of recovery days; however, total training volume was ∼90% lower in the SIT group. Needle biopsy samples were obtained before and after training to examine changes in muscle factors related to exercise tolerance, including muscle oxidative capacity and buffering capacity (Hawley, 2002). Performance tests included 50 kJ and 750 kJ cycling time trials, which required ∼2 min and ∼1 h to complete, respectively, and thus differed considerably in the relative energy contribution from oxidative and non-oxidative metabolism. We hypothesized that both SIT and ET would increase muscle oxidative capacity and 750 kJ time trial performance, given the major contribution from aerobic metabolism during this task. In contrast, we hypothesized that SIT but not ET would increase muscle buffering capacity and 50 kJ time trial performance given the large contribution from non-oxidative metabolism during this task.
Methods
Subjects
Sixteen healthy men were recruited to take part in the experiment (Table 1). All subjects were physically active students at McMaster University who took part in some form of recreational exercise two to three times per week (jogging, cycling, etc.). None of the subjects were engaged in regular training for a particular sporting event. Eight subjects were randomly assigned to a SIT group and the other eight were assigned to an ET group. Following routine medical screening, subjects were advised of the purpose of the study and associated risks, and all provided written informed consent. The experimental protocol was approved by the McMaster University and Hamilton Health Sciences Research Ethics Board and conformed to the Declaration of Helsinki.
Table 1.
Subject characteristics
| Variable | SIT group | ET group |
|---|---|---|
| Age (years) | 22 ± 1 | 21 ± 1 |
| Weight (kg) | 78 ± 2 | 81 ± 4 |
| Height (cm) | 183 ± 2 | 184 ± 2 |
| Body mass index | 23.3 ± 0.5 | 24.0 ± 1.0 |
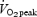 (l min−1) (l min−1) |
4.1 ± 0.2 | 4.0 ± 0.3 |
Values are means ± s.e.m., n = 8 per group. SIT, sprint interval training: ET, endurance training: 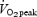 , peak oxygen uptake. There were no differences between groups in any descriptive characteristic.
, peak oxygen uptake. There were no differences between groups in any descriptive characteristic.
Pre-experimental procedures
Prior to baseline measurements, subjects made several familiarization visits to the laboratory in order to become orientated with the testing procedures and training devices. During one of these visits, subjects performed an incremental test to exhaustion on an electronically braked cycle ergometer (Lode Excalibur Sport V2.0, Groningen, the Netherlands) to determine 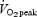 using an online gas collection system (Moxus modular oxygen uptake system, AEI technologies, Pittsburgh, PA, USA). Following a 5 min warm-up at 50 W, the test began with the workload increasing by 1 W every 2 s until volitional exhaustion. The value used for
using an online gas collection system (Moxus modular oxygen uptake system, AEI technologies, Pittsburgh, PA, USA). Following a 5 min warm-up at 50 W, the test began with the workload increasing by 1 W every 2 s until volitional exhaustion. The value used for 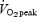 corresponded to the highest value achieved over a 30 s collection period. All subjects also performed a 50 kJ time trial and 750 kJ time trial in order to become familiarized with the exercise tests employed during the main experimental trials. Subjects in the SIT group also performed a familiarization Wingate test, and subjects in the ET group performed a submaximal exercise test to determine the workload that elicited ∼65%
corresponded to the highest value achieved over a 30 s collection period. All subjects also performed a 50 kJ time trial and 750 kJ time trial in order to become familiarized with the exercise tests employed during the main experimental trials. Subjects in the SIT group also performed a familiarization Wingate test, and subjects in the ET group performed a submaximal exercise test to determine the workload that elicited ∼65%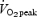 . All exercise tests performed during the familiarization period were performed on separated days, separated by at least 24 h, and at least 3 days prior to baseline testing.
. All exercise tests performed during the familiarization period were performed on separated days, separated by at least 24 h, and at least 3 days prior to baseline testing.
Details of exercise performance tests
Time trials
Subjects were instructed to complete 50 and 750 kJ self-paced laboratory time trials on an electronically braked cycle ergometer (Lode) as quickly as possible with no temporal, verbal or physiological feedback. The only feedback provided during the time trials was work completed, which was presented as ‘distance covered’ on a computer monitor (i.e. 50 kJ was equated to 2 km, and 750 kJ was equated to 30 km, such that visual feedback at any point during the time trial was presented in units of distance rather than work completed). Exercise duration and average power were recorded upon completion of each test.
Wingate test
Subjects in the SIT group completed a 30 s maximal effort on an electronically braked cycle ergometer (Lode) at a resistance equivalent to 7.5% of their body mass. The ergometer was interfaced with a computer loaded with software (Wingate Software Version 1.11, Lode BV) that applied the appropriate load for each subject. Subjects were instructed to begin pedalling as fast as possible ∼2 s before the computer applied the load and received extensive verbal encouragement throughout the test. Peak power, mean power and fatigue index were calculated and recorded by an online data acquisition system.
Experimental protocol
The experimental protocol consisted of (i) baseline testing (i.e. following familiarization); (ii) a 2 week training intervention, and (iii) post-training procedures.
Baseline testing
Prior to training, all subjects underwent a resting needle muscle biopsy procedure. The lateral portion of one thigh was anaesthetized (1% xylocaine) and a small incision made through the skin and underlying fascia in order to obtain a tissue sample from the vastus lateralis muscle. The muscle sample was immediately frozen in liquid nitrogen after removal from the leg. Subjects also performed two baseline performance tests and the timing of the tests was standardized for all subjects. Subjects performed a 50 kJ cycling time trial 1 h after the biopsy procedure, followed 48 h later by a 750 kJ cycling time trial.
Training
The training protocol commenced ∼48 h after the 750 kJ time trial and consisted of six sessions spread over 14 days, with 1–2 days recovery between training sessions (Table 2). Both groups performed training on Mondays, Wednesdays and Fridays for 2 weeks. For the SIT group, training consisted of repeated 30 s maximal cycling efforts, interspersed with 4 min of recovery (rest or light cycling at 30 W). Training progression was implemented by increasing the number of repeats from four repetitions during sessions 1 and 2, to five repetitions during sessions 3 and 4, and finally to six repetitions during sessions 5 and 6. For the ET group, training consisted of 90–120 min of continuous cycling at an intensity corresponding to 65% of 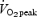 . Training progression in the ET group was implemented by increasing the duration of exercise from 90 min during sessions 1 and 2, to 105 min during sessions 3 and 4, and finally to 120 min during sessions 5 and 6. All training sessions for both groups were directly supervised by one of the study investigators.
. Training progression in the ET group was implemented by increasing the duration of exercise from 90 min during sessions 1 and 2, to 105 min during sessions 3 and 4, and finally to 120 min during sessions 5 and 6. All training sessions for both groups were directly supervised by one of the study investigators.
Table 2.
Training protocols
| Parameter | SIT group | ET group |
|---|---|---|
| Work intensity | ‘All out’ supramaximal (∼700 w) | 65% 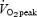 (∼175 w) (∼175 w) |
| Exercise protocol (per session) | 30 s × 4–6 repeats, 4 min recovery | 90–120 min of continuous exercise |
| Total exercise/training time | 2–3 min (intervals only) | 90–120 min |
| commitment per session | 18–27 min (incl. recovery) | |
| Total exercise/training time | 15 min (intervals only) | 630 min |
| commitment over 2 weeks | 135 min (incl. recovery) | |
| Total exercise volume1 | ∼630 kJ (intervals only) | ∼6500 kJ |
| over 2 weeks | ∼950 kJ (incl. recovery)2 |
SIT, sprint interval training; ET, endurance training; 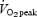 , peak oxygen uptake.
, peak oxygen uptake.
Based on average workloads sustained during training
assuming subjects cycled at the highest workload permitted during recovery (30 W) for the maximum duration (4 min) after every interval performed during training (total of 30 intervals over 2 weeks).
Post-training procedures
The nature and timing of the post-training tests was identical in all respects to the pre-training procedures. A resting needle muscle biopsy sample was obtained ∼72 h after the final training session, using the same leg as for the pre-training sample but separated by a minimum of 5 cm from the first incision. A 50 kJ time trial was performed 1 h after the biopsy procedure, followed 48 h later by the 750 kJ time trial.
Physical activity and nutritional controls
Subjects were instructed to continue their normal dietary and physical activity practices throughout the experiment. Subjects were also instructed to refrain from any exercise aside from activities of daily living for 2 days prior to the biopsy procedures and exercise performance tests. In order to minimize diet-induced variability in muscle metabolism, subjects were also instructed to consume the same types and quantities of food for 2 days prior to the resting muscle biopsy and the time trials. Subjects were required to complete food diaries prior to the baseline biopsy and performance tests. The food diaries were collected, photocopied and returned to the subjects before the post-training tests so that subjects could replicate their individual pattern of food intake.
Muscle analyses
Frozen wet muscle samples were initially sectioned into several pieces under liquid nitrogen and stored at –80°C. One piece of frozen muscle was freeze dried, powdered and dissected free of all non-muscle elements and stored at –80°C.
Enzyme activity
One piece of frozen wet muscle was homogenized using a glass tissue grinder in a buffer containing 50 mm K2HPO4, 1 mm EDTA and 1 mm DTT (pH 7.4). The maximal activity of cytochrome c oxidase (COX) was determined on a spectrophotometer (Cary BIO 300, Varian Cary Instruments, Toronto, ON, Canada) using previously described methods (Carter et al. 2001). Reactions were carried out over a 3 min period and all assays were performed in duplicate. Protein content of the muscle homogenate was determined by the method of Lowry et al. (1951) and enzyme activity was calculated in mol (kg protein)−1 h−1.
Western blotting
The amounts of COX subunit II (mitochondrial encoded) and COX subunit IV (nuclear encoded) were quantified using Western blotting as previously described (Raha et al. 2002). One piece of frozen wet muscle was homogenized and proteins were separated using 12.5% Tris–glycine polyacrylamide gel electrophoresis for 1.5 h at 110 V constant voltage (Bio-Rad Protean III, Bio-Rad Laboratories Ltd, Mississauga, ON, Canada). The electrophoretogram was transferred to nitrocellulose using 100 V for 1 h in 1 × transfer buffer (25 mm Tris base, 192 mm glycine, 24% v/v methanol and 0.1% SDS). The nitrocellulose was blocked with 5% skim milk in Tris-buffered saline (TBST: 137 mm NaCl, 2.7 mm KCl, 25 mm Tris-Cl, pH 8.0, 0.1% Tween-20) for 2 h at room temperature. The membrane was incubated with monoclonal antibodies against COX II and COX IV (Mitosciences, Eugene, OR, Canada) using 1: 2500 and 1: 1000 dilutions, respectively, and incubated in TBST supplemented with 3% skim milk. The two antibodies were used simultaneously to probe the Western blot in a volume of 10 ml at 4°C for 16 h. The blots were developed using horseradish peroxidase conjugated secondary antibody targeted to mouse and/or rabbit in conjunction with an enhanced chemiluninescence kit (ECL Plus, Amersham Biosciences, Laval, PQ, Canada) and analysed using a software package (Image J, NIH, Bethesda, MD, USA).
RNA extraction, reverse transcription and RT-PCR
Total RNA was extracted from wet muscle using a commercially available TRIzol Reagent (Invitrogen Canada, Burlington, ON, Canada) following the manufacturer's instructions. The concentration and purity of the RNA was determined spectrophotometrically by measuring the absorbance at 260 (OD260) and 280 (OD280) with an OD260/OD280 ratio of 1.5–1.6. The integrity of RNA samples were assessed by RNA agarose–formaldehyde gel electrophoresis and evaluating the ratio of 28S to 18S rRNA bands.
One microgram of RNA, DNase treated (DNA-free, Ambion Inc., Austin, TX, USA), was converted to cDNA using a commercially available kit (1st Strand cDNA Synthesis Kit for RT-PCR (AMV), Roche Applied Science, Laval, PQ, Canada). Briefly, the reaction volume of 20 μl for RT contained: 1 × reaction buffer, 5 mm MgCl2, 1 mm dNTP mixture, 3.2 μg of random primer p(dN)6, 50 U RNase inhibitor, 0.8 μl avian myeloblastosis virus (AMV) reverse transcriptase, and 1 μg DNA-free RNA. Reverse transcription was performed in a thermal cycler (Applied Biosystems, Foster City, CA, USA): 25°C for 10 min, 42°C for 60 min, 99°C for 5 min, and 4°C for 5 min. All RNA samples were converted to cDNA together to prevent technical variation. Negative controls (no RNA or no reverse transcriptase enzyme) were run simultaneously with samples to control for RNA and genomic DNA contamination.
The primers for real-time PCR were designed using Primer3 software (Whitehead Institute for Biomedical Research) and their thermodynamic specificity was determined using BLAST sequence alignments (NCBI) and Oligo analyser software (Integrated DNA Technologies, Coralville, IA, USA). Real-time PCR was performed using an iCycler real-time PCR system (Bio-Rad Laboratories, CA) using SYBR Green 1 chemistry (iQ SYBR Green Supermix) according to the manufacturer's instructions. Briefly, the reaction volume of 25 μl for PCR contained: 1 × iQ SYBR Green Supermix, forward and reverse primers (Table 3) and 10 ng cDNA template. The application was performed for 1 cycle (50°C for 2 min, 95°C for 10 min) followed by 40 cycles (95°C for 15 s, 60°C for 60 s) and the beta2-microglobulin (β2M) signal was used as a housekeeping gene to normalize threshold cycle (CT) values. All samples were run in duplicate simultaneously with RNA- and RT-negative controls. In addition, the melting point dissociation curve generated by the instrument was also used to confirm the specificity of the amplified product.
Table 3.
Primer sequences
| Gene | Forward primer | Reverse primer |
|---|---|---|
| β2M | ggctatccagcgtactccaa | gatgaaacccagacacatagca |
| COX II | cgactacggcggactaatct | tcgattgtcaacgtcaagga |
| COX IV | cgagcaatttccacctctgt | ggtcacgccgatccatataa |
β2M, beta2-microglobulin; COX, cyctochrome c oxidase.
Muscle buffering capacity
The in vitro method of Marlin & Harris (1991) was employed, except that NaF as opposed to iodoacetic acid was used to inhibit glycolysis because it is acid–base neutral and would not affect the initial pH measurement (Mannion et al. 1993). Briefly, ∼3 mg of freeze-dried muscle was homogenized to a dilution of 5.0 mg ml−1 in a solution of 145 mm KCl, 10 mm NaCl and 5 mm NaF. After homogenization, the muscle sample was incubated for 5 min in a hot water bath at 37°C and an initial pH measurement was obtained using a microelectrode (MI-415, Microelectrodes, Inc., Bedford, NH, USA) connected to a pH meter (Denver Instruments, Denver, CO, USA). If the initial pH was below 7.10, it was adjusted to 7.10 with 10 mm NaOH (Marlin & Harris, 1991; Mannion et al. 1993). The sample was then titrated across a physiological pH range from 7.10 to 6.50 by the addition of 2.5 μl aliquots of 10 mm HCl. Muscle buffering capacity was subsequently calculated in μmol H+ (g dry muscle)−1 pH unit−1.
Muscle glycogen
Briefly, ∼2 mg of freeze-dried muscle was incubated in 2.0 n HCl and heated for 2 h at 100°C to hydrolyse the glycogen to glucosyl units. The solution was subsequently neutralized with an equal volume of 2.0 n NaOH and analysed for glucose using an enzymatic assay adapted for fluorometry (Passoneau & Lowry, 1993).
Statistical analyses
All data were analysed using a 2-factor analysis of variance, with one between factor (group; SIT versus ET) and one within factor (time; pre-training versus post-training). Analyses of mRNA data were performed by comparing the difference between the target and reference CT values (delta CT) as previously described (Livak & Schmittgen, 2001; Mahoney et al. 2004). The differences in the CT values are expressed numerically using the equation mRNA = 2−deltaCT. The level of significance for all analyses was set at P = 0.05. All data are presented as means ± s.e.m. based on n = 8 subjects per group, except for glycogen where n = 7 for the SIT group only.
Results
Exercise performance
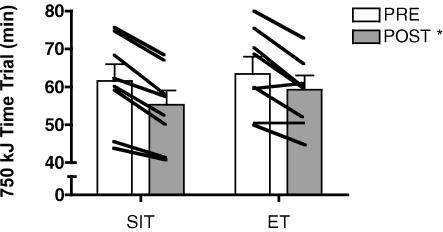
The time required to complete the 750 kJ cycling test decreased after training by 10.1% and 7.5% in the SIT and ET groups, respectively, with no difference between groups (main effect for time, P < 0.001) (Fig. 1). Following training, there was a corresponding increase in mean power output during the 750 kJ time trial from 212 ± 17 to 234 ± 16 W in the SIT group and from 199 ± 13 to 212 ± 12 W in the ET group (main effect for time, P < 0.001). Although the magnitude of improvement was smaller, the time required to complete the 50 kJ test also decreased after training by 4.1% in the SIT group (Post: 113 ± 6 versus Pre: 117 ± 6 s) and 3.5% in the ET group (Post: 122 ± 10 versus Pre: 115 ± 9 s) (main effect for time, P = 0.02). Following training, mean power output during the 50 kJ time trial increased from 435 ± 23 to 453 ± 25 W in the SIT group and 416 ± 39 to 433 ± 40 W in the ET group (main effect for time, P = 0.02).
Figure 1. 750 kJ cycling time trial performance before (PRE) and after (POST) 6 sessions of sprint interval training (SIT) or endurance training (ET) over 2 weeks.
*P ≤ 0.05 versus pre-training (main effect for time). Lines denote individual data for 8 subjects in each group.
Muscle oxidative capacity
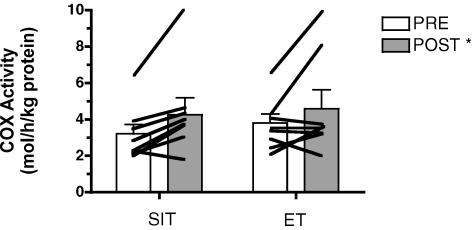
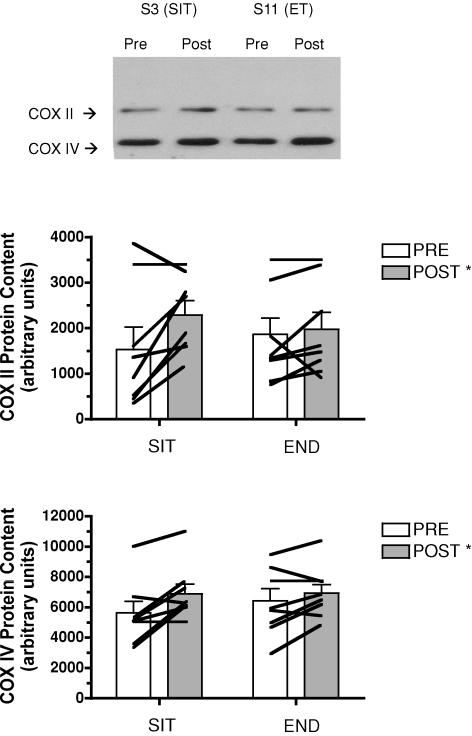
The maximal activity of COX increased after training (main effect for time, P = 0.04), but there was no difference between groups (Fig. 2). Similarly, there were training-induced increases in COX II and COX IV protein contents (main effects for time, P = 0.03 and 0.04, respectively), but no difference between groups (Fig. 3). COX II and COX IV mRNAs were unchanged (P > 0.05) after training in both groups (Table 4).
Figure 2. Maximal activity of COX measured in resting muscle biopsy samples obtained before (PRE) and after (POST) 6 sessions of sprint interval training (SIT) or endurance training (ET) over 2 weeks.
*P ≤ 0.05 versus pre-training (main effect for time). Lines denote individual data for 8 subjects in each group.
Figure 3. Protein content of COX subunit II (middle panel) and IV (bottom panel) measured in resting muscle biopsy samples obtained before (PRE) and after (POST) 6 sessions of sprint interval training (SIT) or endurance training (ET) over 2 weeks.
*P ≤ 0.05 versus pre-training (main effect for time). A representative Western blot (top panel) based on one subject from each group is also presented for each subunit. Lines denote individual data for 8 subjects in each group.
Table 4.
mRNA data
| mRNA | SIT group | ET group | ||
|---|---|---|---|---|
| Pre-TR | Post-TR | Pre-TR | Post-TR | |
| COX II | 0.84 ± 0.06 | 0.89 ± 0.04 | 0.79 ± 0.05 | 0.79 ± 0.04 |
| COX IV | 0.0053 ± 0.0004 | 0.0072 ± 0.0010 | 0.0061 ± 0.0009 | 0.0048 ± 0.0008 |
SIT, sprint interval training; ET, endurance training; COX, cytochrome oxidase.
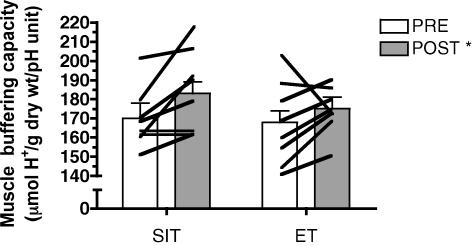
Muscle buffering capacity
Muscle buffering capacity increased after training by 7.6 and 4.2% for the SIT and ET groups, respectively, with no difference between groups (main effect for time, P = 0.03) (Fig. 4).
Figure 4. Skeletal muscle buffering capacity measured in resting muscle biopsy samples before (PRE) and after (POST) 6 sessions of sprint interval training (SIT) or endurance training (ET) over 2 weeks.
*P ≤ 0.05 versus pre-training (main effect for time). Lines denote individual data for 8 subjects in each group.
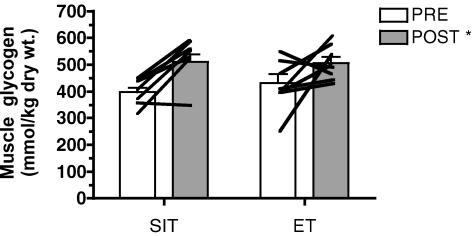
Muscle glycogen content
Resting muscle glycogen content increased after training by 28 and 17% for the SIT and ET groups, respectively, with no difference between groups (main effect for time, P = 0.006) (Fig. 5).
Figure 5. Resting muscle glycogen content before (PRE) and after (POST) 6 sessions of sprint interval training (SIT) or endurance training (ET) over 2 weeks.
*P ≤ 0.05 versus pre-training (main effect for time). Lines denote individual data for 7 subjects in SIT group and 8 subjects in ET group.
Discussion
The major novel finding from the present study was that six sessions of either low volume SIT or traditional high volume ET induced similar improvements in muscle oxidative capacity, muscle buffering capacity and exercise performance. To our knowledge this is the first study to directly compare interval versus continuous training using a research design that matched groups with respect to exercise mode (cycling), training frequency (3 × per week) and training duration (2 weeks), but differed in terms of total training volume and time commitment. Several previous studies have examined muscle metabolic and/or performance adaptations to interval versus continuous training (Henriksson & Reitman, 1976; Saltin et al. 1976; Eddy et al. 1977; Fournier et al. 1982; Gorostiaga et al. 1991; Edge et al. 2006), but the data are equivocal and in all cases the total volume of work was similar between groups. The present study was unique because, by design, the total training volume for the SIT group was only ∼10% that of the ET group (i.e. 630 versus 6500 kJ). In addition, the total training time commitment over 2 weeks was ∼2.5 h for the SIT group (including the work intervals and the recovery periods between intervals), whereas the ET group performed continuous exercise each training day for a total of ∼10.5 h. Thus, while previously speculated by others (Coyle, 2005), to our knowledge this is the first study to demonstrate that SIT is indeed a very ‘time efficient’ training strategy.
Effect of short-term sprint or endurance training on exercise performance
We are aware of only one previous study that examined changes in volitional exercise performance after continuous or interval training. Eddy et al. (1977) had subjects perform cycle exercise training, 4 days per week for 7 weeks, using either a continuous (70% of 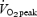 ) or interval method (repeated 1 min bouts at 100%
) or interval method (repeated 1 min bouts at 100%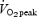 followed by 1 min of rest). The daily workload was matched between groups and increased progressively from ∼100 kJ per session during week 1 to ∼275 kJ per session during week 7. After training, subjects in both groups showed similar improvements during a matched-work exercise test, such that cycling time to exhaustion at 90% of
followed by 1 min of rest). The daily workload was matched between groups and increased progressively from ∼100 kJ per session during week 1 to ∼275 kJ per session during week 7. After training, subjects in both groups showed similar improvements during a matched-work exercise test, such that cycling time to exhaustion at 90% of 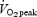 increased by an almost identical amount (∼26 min) in both the continuous and interval groups. In the present study, subjects performed 50 and 750 kJ cycling time trials, which demanded work intensities equivalent to ∼120 and ∼65% of peak power output elicited during the
increased by an almost identical amount (∼26 min) in both the continuous and interval groups. In the present study, subjects performed 50 and 750 kJ cycling time trials, which demanded work intensities equivalent to ∼120 and ∼65% of peak power output elicited during the 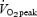 tests. Consistent with the work of Eddy et al. (1977), subjects in the interval and continuous training groups showed remarkably similar improvements in exercise performance. However, whereas Eddy et al. (1977) employed matched-work training protocols, the SIT group in the present study performed only 2–3 min of intense exercise per training session (which lasted 18–27 min in total, including recovery periods between intervals), whereas the ET group performed 90–120 min of continuous exercise per session. While there was no control group in the present study, recent work from our laboratory has shown that control subjects drawn from the same population show no change in cycle endurance capacity (Burgomaster et al. 2005) or time trial performance (Burgomaster et al. 2006) when tested ∼2 weeks apart with no training intervention.
tests. Consistent with the work of Eddy et al. (1977), subjects in the interval and continuous training groups showed remarkably similar improvements in exercise performance. However, whereas Eddy et al. (1977) employed matched-work training protocols, the SIT group in the present study performed only 2–3 min of intense exercise per training session (which lasted 18–27 min in total, including recovery periods between intervals), whereas the ET group performed 90–120 min of continuous exercise per session. While there was no control group in the present study, recent work from our laboratory has shown that control subjects drawn from the same population show no change in cycle endurance capacity (Burgomaster et al. 2005) or time trial performance (Burgomaster et al. 2006) when tested ∼2 weeks apart with no training intervention.
Rapid muscle adaptations induced by sprint or endurance training
Obviously, the factors responsible for training-induced improvements in exercise capacity are extremely complex and determined by numerous physiological (e.g. cardiovascular, muscle metabolic, neural, respiratory, thermoregulatory) and psychological attributes (e.g. mood, motivation, perception of effort). We assessed changes in two parameters – muscle oxidative capacity and muscle buffering capacity – that are related to exercise tolerance (Hawley, 2002) and thus may have contributed to the observed improvement in time trial performance. Surprisingly, only a few previous studies have directly compared changes in mitochondrial capacity after interval or continuous training, and all employed matched-work training protocols that lasted several weeks (Henriksson & Reitman, 1976; Saltin et al. 1976; Fournier et al. 1982; Gorostiaga et al. 1991). The results from these studies are equivocal, with two studies reporting similar increases in the maximal activities of mitochondrial enzymes after interval and continuous training (Henriksson & Reitman, 1976; Saltin et al. 1976), while two others reported increases after continuous training only (Fournier et al. 1982; Gorostiaga et al. 1991). The present study is the first to directly compare changes in muscle oxidative capacity after low-volume SIT and high-volume ET.
In accordance with one of our hypotheses, we observed a training-induced increase in the maximal activity of COX and the protein contents of COX subunits II and IV, but there were no differences between groups despite the marked differences in training volume. The present findings are consistent with recent work from our laboratory that showed muscle oxidative capacity was increased after a SIT protocol similar to that used in the present study, or ∼15 min of intense exercise over six training sessions in 2 weeks (Burgomaster et al. 2005, 2006). While the time course for mitochondrial adaptations after short-term aerobic exercise training is equivocal (Green et al. 1992; Putman et al. 1998), our results are consistent with data from many laboratories showing increases in oxidative enzymes after six to seven sessions of prolonged moderate intensity exercise (Spina et al. 1996; Chesley et al. 1996; Green et al. 1999; Starrit et al. 1999; Youngren et al. 2001). Finally, while the design of the present human study was unique, our data are supported by previous work on rats that examined muscle adaptations to various forms of exercise training (Dudley et al. 1982; Terada et al. 2001). Dudley et al. (1982) reported similar increases in COX maximal activity after 6 weeks of training with either short bouts of intense running or prolonged periods of continuous running at lower work intensities. Given the large difference in training volume between groups, the authors concluded: ‘the typical endurance-training response of a biochemical change in mitochondrial content can be achieved at relatively intense exercise (i.e. exceeding 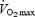 ) maintained for relatively short durations … for the same adaptive response, the length of daily exercise necessary to bring about the change becomes less as the intensity of training increases’ (Dudley et al. 1982). More recently, Terada et al. (2001) showed that 8 days of high-intensity, intermittent swim training (lasting < 5 min per day) increased citrate synthase maximal activity in rat skeletal muscle to a level similar to that induced by 6 h of daily low-intensity training.
) maintained for relatively short durations … for the same adaptive response, the length of daily exercise necessary to bring about the change becomes less as the intensity of training increases’ (Dudley et al. 1982). More recently, Terada et al. (2001) showed that 8 days of high-intensity, intermittent swim training (lasting < 5 min per day) increased citrate synthase maximal activity in rat skeletal muscle to a level similar to that induced by 6 h of daily low-intensity training.
In spite of a significant increase in both COX enzyme activity and protein content for COX subunits encoded by nuclear (COX IV) and mitochondrial (COX II) DNA, we did not find a corresponding increase in mRNA content. Previously, some groups have reported increased mRNA abundance for components of the electron transport chain and/or fatty acid metabolism following endurance training in humans (Tunstall et al. 2002; Schmitt et al. 2003; Timmons et al. 2005). There is also evidence that both the intensity and duration of exercise affect the transcriptional regulation of metabolic genes in a fibre type-specific manner, possibly reflecting the relative stress imposed by the exercise bout (Hildebrandt et al. 2003). Various factors including the specific mRNA species being studied, the duration of the training program, as well as the time of the biopsy following the last bout of exercise can influence skeletal muscle mRNA content. For example, after 9 days of endurance training, baseline mRNA content for fatty acid translocase (FAT/CD36) and carnitine palmitoyltransferase 1(CPT1) was elevated, yet plasma membrane associated FA-binding protein (FABPpm) and β-hydroxyacyl-CoA dehydrogenase (β-HAD) mRNA content was unchanged (Tunstall et al. 2002). Schmitt et al. (2003) observed higher CPTI mRNA content in endurance trained athletes compared with sedentary controls and Short et al. (2003) reported a 66% increase in COX IV mRNA abundance after 16 weeks of endurance exercise training, which suggests longer term training can influence mRNA content. Finally, our laboratory has previously found that the mRNA for several proteins involved in mitochondrial biogenesis and metabolism were elevated 3 h after an acute bout of endurance exercise, but not at 48 h (Mahoney et al. 2005). These data are consistent with the concept that initial signalling events associated with acute exercise induce transient pulses of increased mRNA abundance that eventually lead to an increase in protein content and enzyme activity, and that with longer term training there are increases in mRNA abundance for some but not all mitochondrial proteins (Hood, 2001; Mahoney & Tarnopolsky, 2005; Hawley et al. 2006).
The potency of SIT to elicit rapid changes in oxidative capacity comparable to ET is no doubt related to its high level of muscle fibre recruitment, and the potential to stress type II fibres in particular (Gollnick et al. 1973; Dudley et al. 1982). Contraction-induced metabolic disturbances in muscle activate several kinases and phosphatases involved in signal transduction; in particular, the adenosine monophosphate-activated protein kinase (AMPK), calcium–calmodulin-dependent protein kinase (CAPK), and mitogen-activated protein kinase (MAPK) cascades have been shown to play a role in promoting specific co-activators involved in mitochondrial biogenesis and metabolism (Hood, 2001; Hawley et al. 2006; Koulmann & Bigard, 2006). Studies in animals have shown that different exercise or stimulation protocols result in selective activation of specific intracellular signalling pathways, which may determine the specific adaptations induced by different forms of exercise training (Nader & Esser, 2001; Lee et al. 2002; Atherton et al. 2005; Terada et al. 2005). Recently, Terada et al. (2005) examined the effect of exercise intensity on exercise-induced expression of peroxisome proliferator-activated receptor γ co-activator-1 (PGC-1α) protein in rat skeletal muscle. PGC-1α has emerged as a critical factor coordinating the activation of metabolic genes required for substrate utilization and mitochondrial biogenesis, possibly via its interaction with several DNA binding transcription factors (Knutti & Kralli, 2001). Terada et al. (2005) measured PGC-1α protein content after a single session of exercise that consisted of either high intensity swimming (14 × 20 s intervals while carrying a load equivalent to 14% of body mass, with 10 s of rest between intervals), or low intensity swimming (2 × 3 h with no load, separated by 45 min of rest). Epitrochlearis muscle samples harvested after 2, 6 and 18 h following exercise revealed that PGC-1α increased to a similar extent in both groups (by 126–140% in the high intensity group and 67–95% in the low intensity group) compared with resting control muscle. Given their previous observation that 8 days of swim training using either the high-intensity or low-intensity protocol produced similar increased in muscle oxidative capacity (Terada et al. 2001), the authors concluded ‘high-intensity exercise is a potent tool to increase mitochondrial biogenesis, probably through enhancing PGC-1α expression in rat skeletal muscle.’ With regards to human muscle, several groups have described changes in the expression of PGC-1α and other metabolic transcriptional co-activators and transcription factors after acute exercise (Pilegaard et al. 2003; Russell et al. 2005; Coffey et al. 2006); however, no studies have directly compared adaptations induced by different types of exercise training.
With respect to our other marker of exercise tolerance, both the SIT and ET protocols induced similar increases in skeletal muscle buffering capacity, which was in contrast to one of our hypotheses. Previous studies have yielded equivocal data with respect to exercise training and buffering capacity (Nevill et al. 1989; Bell & Wenger, 1988; Weston et al. 1997) and to our knowledge only one group has specifically examined the issue of training intensity (Edge et al. 2006). Recently, Edge et al. (2006) studied the effect of 5 weeks of interval (4–10 repeats × 2 min at ∼90–100%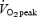 ) or continuous training (∼20 min at ∼60–75%
) or continuous training (∼20 min at ∼60–75%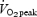 ) on muscle buffering capacity in recreationally active women. Following training, buffering capacity in the interval training group increased by ∼25% but there was no change in the group that performed continuous exercise. While these data (Edge et al. 2006) are in contrast to the present results, there are marked differences between studies in terms of the training stimulus. Edge et al. (2006) matched the interval and continuous training groups in terms of the total work performed during each session, which meant that the continuous training group performed only 20 min of moderate intensity exercise per session. The training stimulus in that study (Edge et al. 2006) was therefore modest compared with the exercise bouts performed by the ET group in the present study (90–120 min of cycling at ∼65%
) on muscle buffering capacity in recreationally active women. Following training, buffering capacity in the interval training group increased by ∼25% but there was no change in the group that performed continuous exercise. While these data (Edge et al. 2006) are in contrast to the present results, there are marked differences between studies in terms of the training stimulus. Edge et al. (2006) matched the interval and continuous training groups in terms of the total work performed during each session, which meant that the continuous training group performed only 20 min of moderate intensity exercise per session. The training stimulus in that study (Edge et al. 2006) was therefore modest compared with the exercise bouts performed by the ET group in the present study (90–120 min of cycling at ∼65%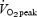 per session). The present study is the first to report improvements in muscle buffering capacity after short-term exercise training (<4 week) and we speculate that changes in this parameter might represent a relatively rapid muscle adaptation that contributes to the observed improvement in exercise capacity. It should be noted that the in vitro method employed in this study isolates for the physico-chemical buffering capacity of skeletal muscle, which is dominated by cytosolic phosphates and proteins/peptides. Histidine-related compounds appear to be the most important determinant of muscle buffering capacity, particularly across the physiological pH range (Abe, 2000), and thus in theory any protein or peptide with histidine residues that are exposed to the cytosol could contribute to buffering capacity.
per session). The present study is the first to report improvements in muscle buffering capacity after short-term exercise training (<4 week) and we speculate that changes in this parameter might represent a relatively rapid muscle adaptation that contributes to the observed improvement in exercise capacity. It should be noted that the in vitro method employed in this study isolates for the physico-chemical buffering capacity of skeletal muscle, which is dominated by cytosolic phosphates and proteins/peptides. Histidine-related compounds appear to be the most important determinant of muscle buffering capacity, particularly across the physiological pH range (Abe, 2000), and thus in theory any protein or peptide with histidine residues that are exposed to the cytosol could contribute to buffering capacity.
Perspective: limitations and implications of the present work
The adaptive response to physical training is obviously influenced by a multitude of complex molecular, cellular and physiological changes, and the present data should not be interpreted to suggest that SIT is necessarily adequate preparation for prolonged endurance-type activities. The duration of the training program in the present study was relatively short (6 sessions over 2 weeks) and it remains to be determined whether similar adaptations are manifest after many weeks or months of interval and continuous training. It is possible that the time course for physiological adjustments differs between training protocols; the very intense nature of the SIT protocol may stimulate rapid changes, whereas the adaptations induced by lower intensity ET may occur more slowly. The present study examined only a few specific muscle parameters, and future studies should examine whether low volume SIT induces other physiological adjustments typically associated with high volume ET (e.g. increased maximal capacity for lipid oxidation, improvements in cardiorespiratory function, changes in blood health status markers, potential for weight loss, etc.). Although pulmonary oxygen uptake 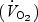 remains high during recovery between intervals, energy expenditure during a 20–30 min SIT session is nonetheless lower than a 90–120 min bout of endurance exercise. We estimate, based on calculations of heart rate reserve, that
remains high during recovery between intervals, energy expenditure during a 20–30 min SIT session is nonetheless lower than a 90–120 min bout of endurance exercise. We estimate, based on calculations of heart rate reserve, that 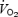 during Wingate-based interval training averages ∼65–70% of
during Wingate-based interval training averages ∼65–70% of 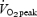 over the course of each 30 s sprint and subsequent 4 min recovery period. Thus, based on the average duration of each training session in the present study, absolute energy expenditure in the SIT group was ≤25% of the ET group. A detailed discussion of the potential health benefits of various training strategies is beyond the scope of this paper; however, obesity experts have recognized a role for high intensity exercise in body weight management (Hunter et al. 1998). There is also a growing appreciation of the potential for intense, interval-based training to stimulate cardiovascular and muscular adaptations in various populations, including disease states (e.g. Rognmo et al. 2004; Vogiatzis et al. 2005).
over the course of each 30 s sprint and subsequent 4 min recovery period. Thus, based on the average duration of each training session in the present study, absolute energy expenditure in the SIT group was ≤25% of the ET group. A detailed discussion of the potential health benefits of various training strategies is beyond the scope of this paper; however, obesity experts have recognized a role for high intensity exercise in body weight management (Hunter et al. 1998). There is also a growing appreciation of the potential for intense, interval-based training to stimulate cardiovascular and muscular adaptations in various populations, including disease states (e.g. Rognmo et al. 2004; Vogiatzis et al. 2005).
In conclusion, the most striking finding from the present study was that two very diverse forms of training induced remarkably similar changes in exercise capacity and selected muscle adaptations that are related to exercise tolerance. Given the markedly lower training volume in the SIT group, our results suggest that intense interval training is indeed a time-efficient strategy to induce rapid muscle and performance adaptations comparable to traditional endurance training. Additional research is warranted to clarify the effect of different acute exercise ‘impulses’ on molecular signalling events in human skeletal muscle, and the precise time course and mechanisms responsible for the contraction-induced changes that facilitate the training adaptation.
Acknowledgments
We thank our subjects for their time and effort, and John Moroz, Todd Prior and Heath D'Sa for technical assistance. This project was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). J.P.L. received an Undergraduate Summer Research Award from the Faculty of Social Sciences, McMaster University. M.V.E. held an Ontario Graduate Scholarship and K.A.B. was supported by a NSERC Canada Graduate Scholarship.
References
- Abe H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Moscow) 2000;65:757–765. [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Bell GJ, Wenger HA. The effect of one-legged sprint training on intramuscular pH and nonbicarbonate buffering capacity. Eur J Appl Physiol. 1988;58:158–164. doi: 10.1007/BF00636620. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Heigenhauser GJF, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time trial performance. J Appl Physiol. 2006;100:2041–2047. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Hughes SC, Heigenhauser GJF, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. 2005;98:1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky M. Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol. 2001;79:386–392. [PubMed] [Google Scholar]
- Chesley A, Heigenhauser GJ, Spriet LL. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Am J Physiol Endocrinol Metab. 1996;270:E328–E235. doi: 10.1152/ajpendo.1996.270.2.E328. [DOI] [PubMed] [Google Scholar]
- Clark SA, Chen ZP, Murphy KT, Aughey RJ, McKenna MJ, Kemp BE, Hawley JA. Intensified exercise training does not alter AMPK signaling in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E737–E743. doi: 10.1152/ajpendo.00462.2003. [DOI] [PubMed] [Google Scholar]
- Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20:190–192. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- Coyle EF. Integration of the physiological factors determining endurance performance ability. Exerc Sport Sci Rev. 1995;23:25–63. [PubMed] [Google Scholar]
- Coyle EF. Very intense exercise-training is extremely potent and time efficient: a reminder. J Appl Physiol. 2005;98:1983–1984. doi: 10.1152/japplphysiol.00215.2005. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Coggan AR, Hopper MK, Walters TJ. Determinants of endurance in well-trained cyclists. J Appl Physiol. 1988;64:2622–2630. doi: 10.1152/jappl.1988.64.6.2622. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53:844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- Eddy DO, Sparks KL, Adelizi DA. The effects of continuous and interval training in women and men. Eur J Appl Physiol Occup Physiol. 1977;37:83–92. doi: 10.1007/BF00421694. [DOI] [PubMed] [Google Scholar]
- Edge J, Bishop D, Goodman C. The effects of training intensity on muscle buffer capacity in females. Eur J Appl Physiol. 2006;96:97–105. doi: 10.1007/s00421-005-0068-6. [DOI] [PubMed] [Google Scholar]
- Fournier M, Ricci J, Taylor AW, Ferguson RJ, Montpetit RR, Chaitman BR. Skeletal muscle adaptation in adolescent boys: sprint and endurance training and detraining. Med Sci Sports Exerc. 1982;14:453–456. doi: 10.1249/00005768-198206000-00008. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Biochemistry of Exercise. In: Maughan RJ, editor. The Encyclopedia of Sports Medicine, VII, Nutrition in Sport. Oxford, UK: Blackwell Science; 2000. pp. 17–38. [Google Scholar]
- Gollnick PD, Armstrong RB, Saltin B, Saubert CW, 4th, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fibre composition of human skeletal muscle. J Appl Physiol. 1973;34:107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- Gorostiaga EM, Walter CB, Foster C, Hickson RC. Uniqueness of interval and continuous training at the same maintained exercise intensity. Eur J Appl Physiol Occup Physiol. 1991;63:101–107. doi: 10.1007/BF00235177. [DOI] [PubMed] [Google Scholar]
- Green HJ, Ball-Burnett M, Symon S, Grant S, Jamieson G. Short-term training, muscle glycogen, and cycle endurance. Can J Appl Physiol. 1995;20:315–324. doi: 10.1139/h95-024. [DOI] [PubMed] [Google Scholar]
- Green H, Grant S, Bombardier E, Ranney D. Initial aerobic power does not alter muscle metabolic adaptations to short-term training. Am J Physiol Endocrinol Metab. 1999;277:E39–E48. doi: 10.1152/ajpendo.1999.277.1.E39. [DOI] [PubMed] [Google Scholar]
- Green HJ, Helyar R, Ball-Burnett M, Kowalchuk N, Symon S, Farrance B. Metabolic adaptations to training precede changes in muscle mitochondrial capacity. J Appl Physiol. 1992;72:484–491. doi: 10.1152/jappl.1992.72.2.484. [DOI] [PubMed] [Google Scholar]
- Harmer AR, McKenna MJ, Sutton JR, Snow RJ, Ruell PA, Booth J, Thompson MW, Mackay NA, Stathis CG, Crameri RM, Carey MF, Enger DM. Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol. 2000;89:1793–1803. doi: 10.1152/jappl.2000.89.5.1793. [DOI] [PubMed] [Google Scholar]
- Hawley JA. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol. 2002;29:218–222. doi: 10.1046/j.1440-1681.2002.03623.x. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Tipton KD, Millard-Stafford ML. Promoting training adaptations through nutritional interventions. J Sports Sci. 2006;24:1–13. doi: 10.1080/02640410500482727. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Reitman JS. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand. 1976;97:392–397. doi: 10.1111/j.1748-1716.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Pilegaard H, Neufer PD. Differential transcriptional activation of select metabolic genes in response to variations in exercise intensity and duration. Am J Physiol Endocrinol Metab. 2003;285:E1021–E1027. doi: 10.1152/ajpendo.00234.2003. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance training and their metabolic consequences. J Appl Physiol. 1984;70:2032–2038. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Hood DA. Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Weinsier RL, Bamman MM, Larson DE. A role for high intensity exercise on energy balance and weight control. Int J Obes Relat Metab Disord. 1998;22:489–493. doi: 10.1038/sj.ijo.0800629. [DOI] [PubMed] [Google Scholar]
- Knutti D, Kralli A. PGC-1, a versatile coactivator. Trends Endocrinol Metab. 2001;12:360–365. doi: 10.1016/s1043-2760(01)00457-x. [DOI] [PubMed] [Google Scholar]
- Koulmann N, Bigard AX. Interaction between signalling pathways involved in skeletal muscle responses to endurance exercise. Pflugers Arch. 2006;452:125–139. doi: 10.1007/s00424-005-0030-9. [DOI] [PubMed] [Google Scholar]
- Kubukeli ZN, Noakes TD, Dennis SD. Training techniques to improve endurance exercise performances. Sports Med. 2002;32:489–509. doi: 10.2165/00007256-200232080-00002. [DOI] [PubMed] [Google Scholar]
- Lee JS, Bruce CR, Spurrell BE, Hawley JA. Effect of training on activation of extracellular signal-related kinase 1/2 and p38 mitogen-activated protein kinase pathways in rat skeletal muscle. Clin Exp Pharmacol Physiol. 2002;29:655–660. doi: 10.1046/j.1440-1681.2002.03713.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mahoney DJ, Carey K, Fu MH, Snow R, Cameron-Smith D, Parise G, Tarnopolsky MA. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics. 2004;18:226–231. doi: 10.1152/physiolgenomics.00067.2004. [DOI] [PubMed] [Google Scholar]
- Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19:1498–1500. doi: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- Mahoney DJ, Tarnopolsky MA. Understanding skeletal muscle adaptation to exercise training in humans: contributions from microarray studies. Phys Med Rehabil Clin N Am. 2005;16:859–873. doi: 10.1016/j.pmr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Mannion AF, Jakeman PM, Willan PL. Determination of human skeletal muscle buffer value by homogenate technique: methods of measurement. J Appl Physiol. 1993;75:1412–1418. doi: 10.1152/jappl.1993.75.3.1412. [DOI] [PubMed] [Google Scholar]
- Marlin DJ, Harris RC. Titrimetric determination of muscle buffering capacity (Bm titr) in biopsy samples. Equine Vet J. 1991;23:193–197. doi: 10.1111/j.2042-3306.1991.tb02753.x. [DOI] [PubMed] [Google Scholar]
- Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol. 2001;90:1936–1342. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- Nevill ME, Boobis LH, Brooks S, Williams C. Effect of training on muscle metabolism during treadmill sprinting. J Appl Physiol. 1989;67:2376–2382. doi: 10.1152/jappl.1989.67.6.2376. [DOI] [PubMed] [Google Scholar]
- Passoneau JV, Lowry OH. Enzymatic Analysis: a Practical Guide. Totowa, NJ, USA: Humana Press; 1993. [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Jones NL, Hultman E, Hollidge-Horvat MG, Bonen A, McConachie DR, Heigenhauser GJ. Effects of short-term submaximal training in humans on muscle metabolism in exercise. Am J Physiol Endocrinol Metab. 1998;275:E132–E139. doi: 10.1152/ajpendo.1998.275.1.E132. [DOI] [PubMed] [Google Scholar]
- Raha S, Myint T, Johnstone L, Robinson BH. Control of oxygen free radical formation from mitochondrial complex I: Roles for protein kinase A and pyruvate dehydrogenase kinase. Free Rad Biol Med. 2002;32:421–430. doi: 10.1016/s0891-5849(01)00816-4. [DOI] [PubMed] [Google Scholar]
- Rognmo O, Hetland E, Helgerud J, Hoff J, Slordahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11:216–222. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J. 2005;19:986–988. doi: 10.1096/fj.04-3168fje. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gollnick PD. Skeletal muscle adaptability. Significance for metabolism and performance. In: Peachey LD, editor. Handbook of Physiology, Skeletal Muscle. Bethesda, MD, USA: American Physiological Society; 1983. pp. 555–631. [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B, Gollnick D. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96:289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]
- Schmitt B, Fluck M, Decombaz J, Kreis R, Boesch C, Wittwer M, Graber F, Vogt M, Howald H, Hoppeler H. Transcriptional adaptations of lipid metabolism in tibialis anterior muscle of endurance-trained athletes. Physiol Genomics. 2003;15:148–157. doi: 10.1152/physiolgenomics.00089.2003. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol. 1996;80:2250–2254. doi: 10.1152/jappl.1996.80.6.2250. [DOI] [PubMed] [Google Scholar]
- Starritt EC, Angus D, Hargreaves M. Effect of short-term training on mitochondrial ATP production rate in human skeletal muscle. J Appl Physiol. 1999;86:450–454. doi: 10.1152/jappl.1999.86.2.450. [DOI] [PubMed] [Google Scholar]
- Terada S, Kawanaka K, Goto M, Shimokawa T, Tabata I. Effects of high-intensity intermittent swimming on PGC-1α protein expression in rat skeletal muscle. Acta Physiol Scand. 2005;184:59–65. doi: 10.1111/j.1365-201X.2005.01423.x. [DOI] [PubMed] [Google Scholar]
- Terada S, Yokozeki T, Kawanaka K, Ogawa K, Higuchi M, Ezaki O, Tabata I. Effects of high-intensity swimming training on GLUT-4 and glucose transport activity in rat skeletal muscle. J Appl Physiol. 2001;90:2019–2024. doi: 10.1152/jappl.2001.90.6.2019. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Larsson O, Jansson E, Fischer H, Gustafsson T, Greenhaff PL, Ridden J, Rachman J, Peyrard-Janvid M, Wahlestedt C, Sundberg CJ. Human muscle gene expression responses to endurance training provide a novel perspective on Duchenne muscular dystrophy. FASEB J. 2005;19:750–760. doi: 10.1096/fj.04-1980com. [DOI] [PubMed] [Google Scholar]
- Tunstall RJ, Mehan KA, Wadley GD, Collier GR, Bonen A, Hargreaves M, Cameron-Smith D. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E66–E72. doi: 10.1152/ajpendo.00475.2001. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Terzis G, Nanas S, Stratakos G, Simoes DC, Georgiadou O, Zakynthinos S, Roussos C. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest. 2005;128:3838–3845. doi: 10.1378/chest.128.6.3838. [DOI] [PubMed] [Google Scholar]
- Weston AR, Myburgh KH, Lindsay FH, Dennis SC, Noakes TD, Hawley JA. Skeletal muscle buffering capacity and endurance performance after high-intensity interval training by well-trained cyclists. Eur J Appl Physiol Occup Physiol. 1997;75:7–13. doi: 10.1007/s004210050119. [DOI] [PubMed] [Google Scholar]
- Youngren JF, Keen S, Kulp JL, Tanner CJ, Houmard JA, Goldfine ID. Enhanced muscle insulin receptor autophosphorylation with short-term aerobic exercise training. Am J Physiol Endocrinol Metab. 2001;280:E528–E533. doi: 10.1152/ajpendo.2001.280.3.E528. [DOI] [PubMed] [Google Scholar]