Abstract
The objectives of this study were to evaluate the physiological importance of the flow and shear generated by phasic contractions of lymphatic vessels and the mechanisms responsible for the influences of such shear on lymphatic pumping. Lymphatic segments of the rat thoracic duct were isolated, cannulated and pressurized. The diastolic diameters were measured in phasically non-active segments. The diastolic and systolic diameters, half-relaxation time (HRT), contraction frequency, ejection fraction and fractional pump flow were determined in phasically active segments. Since imposed flow was excluded, flow and shear occurred only as a result of the intrinsic contractions in phasically active segments whereas in phasically non-active segments contraction-generated flow and shear were absent. The influences of incrementally increased transmural pressure (from 1 to 5 cmH2O) were examined in control conditions and after NO synthase blockade (l-NAME 10−4m) or cyclooxygenase blockade (indomethacin 10−5m). The spontaneous phasic contractions produced a flow-dependent diastolic relaxation. This reduction of the lymphatic tone is a regulatory mechanism that maintains pumping in thoracic duct in an energy-saving/efficient mode: it improves diastolic filling (enhanced lusitropy – lowering HRT), makes lymphatic contractions stronger (enhanced inotropy – higher contraction amplitude) and propels more fluid forward during each contraction (elevated ejection fraction) while decreasing contraction frequency (reduced chronotropy). The findings also demonstrated that the NO pathway, not the cyclooxygenase pathway is responsible for this reduction of lymphatic tone and is the prevailing pathway responsible for the self-regulatory adjustment of thoracic duct pumping to changes in lymph flow pattern.
There are several motive forces which drive lymph centripetally. Traditionally these forces are divided into two groups depending on their origin (for review see Bridenbaugh et al. 2003). The term ‘active’ or ‘intrinsic’ lymph pump describes the lymph driving force which is generated by the active spontaneous contractions of the lymphangions. The term ‘passive’ or ‘extrinsic’ lymph pump combines all other forces outside of the lymphatic vessel, which are not dependent on the active contractions of lymphatic wall but may assist lymph flow in different regions of body. These ‘passive’ forces include influences of lymph formation, contractions of adjacent skeletal muscles, vasomotion of adjacent blood vessels, gastrointestinal motility, breathing and fluctuations of central venous pressure. The lymphatic system possesses numerous active pumps – lymphangions (Mislin, 1961), the contraction energy of which is essential for lymph flow in many lymphatic beds. Since the driving force of one such small pump is not enough to propel lymph all the way down to the central end of the lymphatic system, lymphatic vessels are organized in chains of these pumps. Thus lymph flow occurs as a result of complicated combinations of active and passive lymph driving forces. Because the active and passive lymph pumps are often not synchronized, flow patterns in lymphatic networks are extremely variable.
There is no evidence in any regional lymphatic network that contractions of the lymphatic vessels occur simultaneously along all of its length. Contrary to this idea, numerous reports have demonstrated the propagation of contractile waves along lymphatic vessels (McHale & Roddie, 1976; McHale & Meharg, 1992; Zawieja et al. 1993; Crowe et al. 1997). In some regional lymphatic networks (in lower limbs), an interrupted fluid column was demonstrated during the normal contractile activity of lymphangions (Armenio et al. 1981a,b; Gashev et al. 1990). In this situation adjacent lymphangions work in a counter phase fashion: some lymphangions are contracting while the next one or few lymphangions are in diastolic relaxation (Gashev et al. 1990). Thus each lymphangion can be principally described as a short-distance pump whose primary ‘task’ is to drive a bolus of fluid down the next one or few lymphangions. But together, the chain of such pumps is able to maintain an effective long-distance transport of lymph. During the active pumping of lymphangions, coordinated contractions of the lymphatic muscle cells create an increase in intralymphatic pressure and form a local positive pressure gradient near the downstream front of the propagating contracting zone to propel lymph centripetally. At the upstream edge of the contracting zone, a negative pressure gradient always occurs between contracting lymphangions and upstream relaxing lymphangions. This gradient generates the short-lasting localized reversed flow that produces valve closure (McHale & Roddie, 1976; Gashev, 1991). Therefore phasic contractions of lymphangions generate flow and shear in the lymphatics by themselves.
We have already demonstrated that the intrinsic lymphatic pumps are highly sensitive to an imposed flow (Gashev, 2002; Gashev et al. 2002, 2004). Even low imposed steady flows in isolated lymphatics produced an endothelial-dependent inhibition of both phasic and tonic contractile activity. This contractile inhibition was at least in part due to nitric oxide (NO) (Gashev et al. 2002). Others have published a report of flow-dependent lymphatic contractile inhibition in rats that was prostanoid mediated (Koller et al. 1999). However, the experiments with imposed steady flows (Koller et al. 1999; Gashev et al. 2002, 2004) mimic the influences of passive forces on lymphatic pumping (e.g. increased lymph formation or upstream flow generation). Based on the fact that lymphatics are highly sensitive to even small increases in imposed flow (Gashev et al. 2002, 2004), we hypothesized that lymphatic contractions may be also influenced by the flow generated by themselves. In other words, we proposed that the lymph flow that is generated during phasic contractions may itself play an important self-regulatory role in the lymphatic contractile cycle in a shear-dependent manner.
To test this hypothesis we chose thoracic duct, which is known for its sensitivity to an imposed flow and its variable contractile behaviour (Nusbaum et al. 1964; Browse et al. 1971; Campbell & Heath, 1973; Kinnaert, 1973; Orlov et al. 1975; Orlov & Borisova, 1982; Gashev et al. 2004). In many cases contractions may occur in one part of thoracic duct but not propagate between different segments (Nusbaum et al. 1964; Browse et al. 1971; Campbell & Heath, 1973; Kinnaert, 1973; Orlov et al. 1975; Orlov & Borisova, 1982). Our own experience with thoracic duct studies is that contractile waves often do not propagate along this vessel and often the phasic contractions develop locally while adjacent parts of the duct are not contracting (Muthuchamy et al. 2003). We used this feature of the thoracic duct when we designed the experiments to evaluate the importance of flow and shear generated by lymphatic phasic contractions in the regulation of the lymphatic contractile cycle. We used two types of segments of thoracic duct – phasically active segments and phasically non-active segments. We specifically paid close attention to maintenance of the input and output pressures at the same level, thereby excluding any imposed flow. Thus in the phasically active lymphatic segments, flow and shear occurred only as a result of their inherent contractions. In phasically non-active segments flow and shear did not take place. As a result we used experimental conditions that allowed us to investigate the influences of flow and shear, generated solely by the phasic lymphatic pump, on the contractile function without any extra imposed flow.
Methods
Animals and surgery
We examined the contractile activity of thoracic ducts from 34 male Sprague-Dawley rats (weighing between 300 and 400 g). The animal facilities used for these studies have been accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC International) and adhere to the regulations, policies and principles detailed in the Public Health Service Policy for the Humane Care and Use of Laboratory Animals (PHS Policy, 1996) and the United States Department of Agriculture's Animal Welfare Regulations (Animal Welfare Act, AWA, 9CFR, 1985, 1992). All animal procedures performed for this study were reviewed and approved by our institutional animal care and use committee, the Texas A & M University Laboratory Animal Care Committee.
To isolate the thoracic duct, rats were killed with pentobarbital (120 mg (kg body weight)−1i.p.). Then the animal was positioned on its back; the ventral chest wall was opened by lateral incision; the sternum and approximately half of the ribs were excised. The inferior vena cavae was ligated and cut close to the diaphragm. The lungs and heart were set to the left side of animal so as to expose the thoracic duct between the aorta and vertebral column. The thoracic duct was then carefully cleared of all surrounding tissues using a dissecting microscope. Extreme caution was used to avoid grabbing or pinching the thoracic duct at any time, thereby reducing the likelihood of damage. The area of interest was kept moist for the period of dissection using the standard Dulbecco's phosphate-buffered saline (Invitrogen 14040-133). Sections of thoracic duct 1–2 cm long were dissected and used for experiments. Throughout the experiments we measured the diameters of thoracic duct sections used for these studies. At a transmural pressure of 3 cmH2O, the average diastolic diameters were 575 ± 30 μm.
Isolated vessel procedures, experimental techniques and protocol
Once the thoracic duct was exteriorized, the lymphatic segment was transferred to an isolated vessel chamber (modified Living Systems Instrumentation single vessel chamber model CH/1) filled with room temperature albumin–physiological salt solution (APSS) (mm): 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 0.02 EDTA, 3.0 Mops, and 10 g l−1 bovine serum albumin, pH adjusted to 7.36 at 38°C. The isolated thoracic duct segment was cannulated and tied onto two carefully matched glass pipettes (450–550 μm). Great care was used to prepare and select pairs of resistance-matched pipettes for these experiments as described in our previous studies (Gashev et al. 2002, 2004). The inflow and outflow pipettes were connected to independently adjustable pressure reservoirs filled with APSS. Care was taken to ensure that there were no air bubbles in the tubing or the pipettes. Once the vessel was cannulated, a slight positive transmural pressure (2–3 cmH2O) was applied to detect leaks and to ensure that the vessel was undamaged and untwisted. The vessel was set to its approximate in situ length and positioned just above the glass coverslip comprising the chamber bottom. The chamber was transferred to the stage of a microscope. The vessel was set to an equilibration transmural pressure of 3 cmH2O and warmed to 38°C over 15–20 min. Once tone alone or tone and spontaneous contractions were observed, the vessel was allowed to equilibrate at 3 cmH2O for another 30 min. A video camera, hi-resolution monitor and DVD/HDD recorder were used to observe and record the lymphatic segment continuously in all experiments.
Both phasically contracting and non-contracting segments of thoracic duct were used for these experiments. At the beginning of every experiment all lymphatic segments were exposed to a range of transmural pressures: 1, 3 and 5 cmH2O for 5 min at each pressure to evaluate the pressure-induced changes in lymphatic contractile activity. We chose this set of transmural pressures because we have shown that the thoracic duct displays maximal active pumping at 3 cmH2O (Gashev, 2002). Since we have previously shown that flow inhibits the active lymph pump (Gashev, 2002; Gashev et al. 2002, 2004) we constantly monitored the levels of input and output pressure to prevent imposed flow at all levels of transmural pressure. In all experiments the input and output pressure were set equally so flow and shear were only generated by phasic contractions of thoracic duct segments. After completion of the transmural pressure range in APSS (control) we set the transmural pressure at 3 cm H2O and APSS in the chamber was replaced with APSS containing the nitric oxide synthase inhibitor l-NAME (Sigma N5751; 10−4m; Rees et al. 1990; Nakaike et al. 1995; Mizuno et al. 1998; Datte et al. 2005; Wang et al. 2005; Watanabe et al. 2005; Arenas et al. 2006) or with the cyclooxygenase inhibitor, indomethacin (Sigma I7378; 10−5m; Nakaike et al. 1995; Mizuno et al. 1998; Koller et al. 1999; Chan & Von Der Weid, 2003; Tsunemoto et al. 2003; Chan et al. 2004; Kousai et al. 2004).
Both phasically contracting and non-contracting segments of thoracic duct were tested with l-NAME. In another set of experiments with different lymphatic segments, both phasically contracting and non-contracting segments of thoracic duct were tested with indomethacin. After the addition of each tested drug, the vessel was allowed to equilibrate in the presence of the drug at 3 cmH2O for 15 min, and then the range of transmural pressures 1, 3 and 5 cmH2O was repeated. At the end of each experiment in phasically active segments, the passive (relaxed) diameter was measured at each pressure after the vessels were exposed to a nominally calcium-free, EDTA (3.0 mm)-supplemented APSS for 15 min. All phasically non-active segments were tested for their ability to contract to substance P at 10−6m (Sigma S6883) after the l-NAME or indomethacin administration. In these cases we washed out the inhibitors by superfusion of regular APSS for 15 min at a pressure of 3 cmH2O. Then APSS was replaced with APSS including substance P. Substance P is a well-known activator of lymphatic contractility (Foy et al. 1989; Amerini et al. 2004) and caused immediate strong constriction of the lymphatic segments. If we did not observed a substance P-induced constriction in the phasically non-active segments, that experiment was terminated and the data were not used. If the constriction occurred, the vessel was allowed to equilibrate at 3 cmH2O for 15 min, then the range of transmural pressures of 1, 3 and 5 cm H2O was repeated in the presence of substance P. At the end of this procedure all phasically non-active segments were also exposed to calcium-free, EDTA-supplemented APSS as described above to obtain the values of passive diameters at each level of pressure.
Data analysis and statistics
The lymphatic diameters were tracked from the DVD records of experiments using ‘Vessel Track’ software developed and generously provided by Professor Michael J. Davis (Davis, 2005; Davis et al. 2006). Briefly, the outer lymphatic diameters were measured from the DVD record with a tracking frequency of ∼30 times s−1. We used cardiac pump analogies to define systolic and diastolic lymphatic diameters in reference to the lymphatic contractile cycle (Granger et al. 1977; Benoit et al. 1989; Zawieja et al. 1991; Gashev et al. 2004) and the end-diastolic and end-systolic points in the diameter tracings were recorded for each 5 min interval for each set of pressures with or without drugs. From the lymphatic end-diastolic and end-systolic diameters, the following lymph pump parameters were calculated: lymphatic tone index (the difference between the passive lymphatic diameter in Ca-free APSS and end-diastolic diameter expressed as a percentage of the passive lymphatic diameter in Ca-free APSS), contraction amplitude (the difference between the diastolic and systolic diameters), contraction frequency, ejection fraction (the fraction of end-diastolic volume ejected during the single lymphatic contraction) and fractional pump flow (an index of lymph pump flow, calculated as the ejection fraction times the contraction frequency). To compare the changes in diameters during the lymphatic contractile cycle, the diastolic and systolic diameters were normalized to the passive lymphatic diameters in Ca-free APSS at the corresponding transmural pressure because of the anatomical and regional variations between lymphatic vessels. The anatomy and basal contraction frequencies of lymphatic vessels in many species (rat, dog, guinea pig, human, etc.) are quite variable from vessel to vessel, even when similar vessels from age-, weight- and sex-matched animals are observed (Yoffey & Courtice, 1970; Gnepp, 1984; Borisov, 1997). Thus to study lymphatic function effectively, the lymphatic diameters were normalized to the passive diameters. The normalization of lymphatic diameters minimizes the animal-to-animal variability in basal lymphatic parameters and allows for a more sensitive investigation of the controlled parameters by preventing the observed changes in lymphatic function from being superimposed on normal random variations in vessel sizes between normal animals. Normalizations of a similar nature are commonly used in lymphatic studies (Zawieja et al. 1991; von der Weid et al. 1996; von der Weid & Van Helden, 1996; Hollywood et al. 1997; Mizuno et al. 1998; von der Weid, 1998; Koller et al. 1999; Mizuno et al. 1999; Shirasawa et al. 2000; von der Weid et al. 2001; Gashev, 2002; Gashev et al. 2002, 2004) and other microcirculatory studies (Kuo et al. 1988, 1990a,b; Meininger et al. 1991; Hill & Gould, 1997; Hiramatsu et al. 1998; Guibert & Beech, 1999).
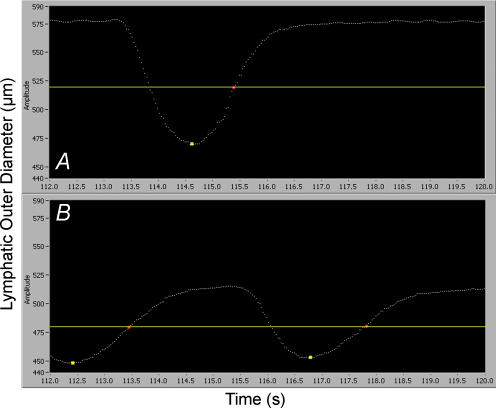
In a subset of studies in phasically active segments of thoracic duct with abluminal administration of l-NAME 10−4m, we determined the time intervals from end-systolic diameter (maximally contracted) to the mid-diastolic diameter (half-relaxed). This time interval was called the half-relaxation time (HRT) and was used as an indicator of the speed of the diastolic relaxation (Goethals et al. 1975; Perez et al. 1993). Figure 1 represents a typical diameter tracing of the thoracic duct segment at a transmural pressure of 3 cmH2O before (Fig. 1A) and after (Fig. 1B) the abluminal administration of L-NAME (the yellow marker points correspond to end-systolic diameter). The yellow separator line and red marker points were set at the positions which correspond to the diameter at half-relaxation. The time interval between the yellow marker point and next red marker point corresponds to the HRT for that lymphatic cycle.
Figure 1. Typical diameter tracings of phasic contractions of the thoracic duct segment at a transmural pressure of 3 cmH2O before (A) and after (B) administration of l-NAME (10−4m).
Yellow marker points were set at the positions that correspond to the end-systolic diameter. The yellow separator line and red marker points were set at the positions which correspond to the half-relaxed diameter. The time interval between the yellow marker point and the next red marker point corresponds to the half-relaxation time (HRT). The X-axis represents time in seconds; the Y-axis represents outer lymphatic diameter in micrometres.
Statistical differences were determined by ANOVA, regression analysis and Student's t test (JMP software version 5.0.1.2. for Windows; SAS, Cary, NC, USA) and considered significant at P ≤ 0.05. In the Results section, the numbers of lymphatic vessels used in the reported data are shown separately for each group of experiments, where n denotes the number of thoracic duct segments used for each experimental protocol. In 30 cases out of 34 only one vessel segment from a single animal was used for experimentation. In the other four cases we used two vessel segments from one single animal to perform experiments in two separate but identical experimental set-ups.
Results
We compared the pressure-induced contractile responses in phasically active (n = 13) and phasically non-active (n = 8) segments of the rat thoracic duct (in different sets of experiments) before and after abluminal administration of l-NAME at 10−4m. We also investigated the differences in the pressure-induced contractile responses in phasically active (n = 8) and phasically non-active (n = 9) segments of the rat thoracic duct before and after abluminal administration of indomethacin at 10−5m. We described the detailed results of these experiments below.
Lymphatic tone index in phasically active and phasically non-active segments of thoracic duct
We found that the indices of lymphatic tone were significantly lower in all phasically active control vessels than those in the phasically non-active control segments at all corresponding levels of transmural pressure (Table 1). In the control groups of phasically active contracting vessels (before l-NAME and before indomethacin), the lymphatic tone index was 5% at all transmural pressures. However in the control groups of phasically non-active contracting vessels (before l-NAME and before indomethacin), the lymphatic tone index was 13% (a tone that is about 160% higher than that in the phasically active controls) at 1 cmH2O of transmural pressure, 11–12% (tone increased about 120% above the phasically active controls) at 3 cmH2O and 10–11% (tone increased about 100% above the phasically active controls) at 5 cmH2O. Figure 2 presents the comparison of lymphatic tone indices for these experiments. The blue columns reflect the values of lymphatic tone in phasically active control vessels while green columns are the lymphatic tone in phasically non-active controls. We tested all phasically non-active vessels for their ability to contract to substance P to determine that such lymphatic segments were not damaged. All vessels in these studies that were qualified as phasically non-active were able to contract normally to substance P after abluminal administration. The average lymphatic tone indices after substance P in these cases were 35, 33 and 29% at 1, 3 and 5 cmH2O transmural pressures respectively.
Table 1.
Influence of transmural pressure on diastolic diameters and lymphatic tone indices in phasically active (C) and phasically non-active (NC) segments of rat thoracic duct after administration of l-NAME (10−4m) or indomethacin (10−5m) (INDO)
| l-NAME treatment group | INDO treatment group | |||||||
|---|---|---|---|---|---|---|---|---|
| Transmural pressure (cm H2O) | Vessel group | n | DD (μm) | LTI (%) | Vessel group | n | DD (μm) | LTI (%) |
| 1 | Control C | 13 | 544 ± 39 | 5 ± 1 | Control C | 8 | 596 ± 40 | 5 ± 1 |
| l-NAME C | 13 | 501 ± 37 | 13 ± 2 | INDO C | 8 | 603 ± 40 | 4 ± 1 | |
| Control NC | 8 | 486 ± 26 | 13 ± 2 | Control NC | 9 | 499 ± 47 | 13 ± 3 | |
| l-NAME NC | 8 | 485 ± 23 | 13 ± 2 | INDO NC | 9 | 487 ± 46 | 14 ± 3 | |
| 3 | Control C | 13 | 583 ± 38 | 5 ± 1 | Control C | 8 | 629 ± 33 | 5 ± 1 |
| l-NAME C | 13 | 543 ± 35 | 12 ± 1 | INDO C | 8 | 632 ± 34 | 4 ± 1 | |
| Control NC | 8 | 526 ± 23 | 11 ± 2 | Control NC | 9 | 557 ± 39 | 12 ± 2 | |
| l-NAME NC | 8 | 534 ± 23 | 11 ± 1 | INDO NC | 9 | 563 ± 38 | 11 ± 2 | |
| 5 | Control C | 13 | 597 ± 38 | 5 ± 1 | Control C | 8 | 640 ± 33 | 5 ± 1 |
| l-NAME C | 13 | 559 ± 35 | 11 ± 1 | INDO C | 8 | 634 ± 32 | 5 ± 2 | |
| Control NC | 8 | 557 ± 23 | 10 ± 2 | Control NC | 9 | 570 ± 37 | 11 ± 2 | |
| l-NAME NC | 8 | 556 ± 26 | 10 ± 3 | INDO NC | 9 | 574 ± 38 | 11 ± 2 | |
Values are means ±s.e.m.; n, number of lymphatic segments; DD, diastolic diameter; LTI, lymphatic tone index.
Figure 2. Pressure-induced changes in the lymphatic tone index of rat thoracic duct after administration of l-NAME (10−4m; A) and indomethacin (10−5m; B).
Experimental conditions: A: Control C – control for contracting segments; l-NAME C –l-NAME administration in contracting segments; Control NC – control for non-contracting segments; l-NAME NC –l-NAME administration in non-contracting segments. B: Control C – control for contracting segments; INDO C – indomethacin administration in contracting segments; Control NC – control for non-contracting segments; INDO NC – indomethacin administration in non-contracting segments. *Significant differences (P ≤ 0.05) between lymphatic tone index in control phasically active segments and lymphatic tone indices for other experimental conditions at each level of transmural pressure; presented separately for A and B.
Influence of l-NAME and indomethacin on lymphatic tone indices in phasically active and phasically non-active segments of thoracic duct
We tested segments of thoracic duct with l-NAME and indomethacin – two well-known modulators of flow-induced relaxation in vascular smooth muscle, to determine the involvement of the NO synthase and cyclooxygenase pathways in this contraction-initiated flow-dependent relaxation. In accordance with the experimental protocol described above, we investigated the effects of abluminal administration of l-NAME (10−4m) or indomethacin (10−5m) on lymphatic tone in phasically active and phasically non-active segments of thoracic duct. We found that l-NAME administration (NO synthase blockade) to phasically active thoracic duct segments increased their lymphatic tone indices 160, 140 and 120% above control at 1, 3 and 5 cm H2O transmural pressures respectively (blue and red columns on Fig. 2A). The values of lymphatic tone indices after NO synthase blockade reached the same values that were observed in phasically non-active vessels (red and green columns in Fig. 2A). Thus we found that the phasic contractions generated a significant reduction of the resting tone in contracting lymphatic segments that was completely dependent on NO-release. As extra evidence of this fact, we found that when we blocked NO synthase in phasically non-active segments, we did not find any differences in lymphatic tone indices before and after NO synthase blockade at all levels of transmural pressure (green and red-hatched columns in Fig. 2A). Furthermore, the administration of indomethacin had no effect on lymphatic tone indices in either the phasically active segments or the phasically non-active segments. Phasically active segments remained relaxed during diastole to the same degree before and after indomethacin (blue and black columns in Fig. 2B). Again, the phasically non-active segments had greater lymphatic tone indices than did the contracting segments irrespective of indomethacin (green and black-hatched columns in Fig. 2B with blue and black columns on the Fig. 2B). Thereby we found that the NO-dependent pathway, but not the cyclooxygenase pathway, is responsible for the pump flow-generated reduction of resting tone in rat thoracic duct. Table 1 summarizes all values of absolute diastolic diameters (non-normalized) and lymphatic tone indices for all phasically active and non-active segments of thoracic duct before and after administration of l-NAME and indomethacin.
Influence of l-NAME and indomethacin on parameters of active pumping in phasically active segments of thoracic duct
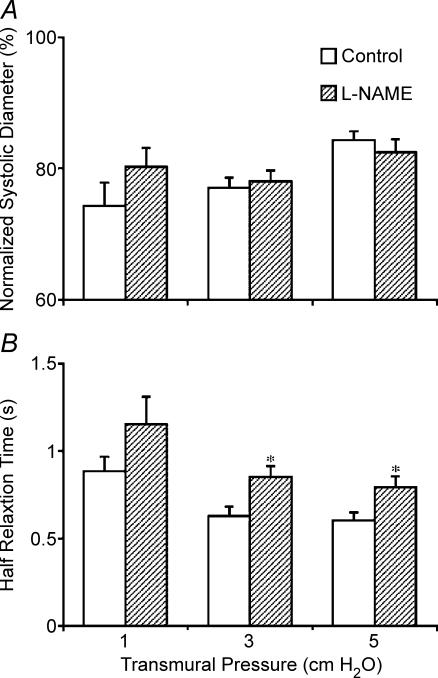
In phasically active segments we analysed other parameters of the active lymph pump as well as the lymphatic tone indices. Contrary to the changes in lymphatic tone indices, the systolic diameters did not change significantly after NO synthase blockade in the phasically contracting vessels (Fig. 3A). As a combined result of changes in lymphatic tone and lymphatic systolic diameters, the lymphatic contraction amplitude was always significantly lower after NO synthase blockade (Fig. 4). This fact is also clearly visible in Fig. 1, which presents the typical diameter tracings of thoracic duct segment at a transmural pressure of 3 cmH2O before and after NO synthase blockade. Thus we found that NO, released by the flow/shear generated by the phasic contractions of the thoracic duct, maintains a more effective contraction amplitude. Since the normalized systolic diameter wasn't profoundly altered by NO synthase blockade, we also analysed diastolic function in the phasically contracting vessels. We evaluated diastolic filling by measuring the time of half-relaxation before and during NO-synthase inhibition. We found that NO synthase blockade caused notable increases in the half-relaxation time (HRT) at all tested levels of transmural pressure. The HRT values were (before and after NO synthase blockade accordingly): 0.88 ± 0.08 s and 1.15 ± 0.15 s at a transmural pressure of 1 cmH2O; 0.63 ± 0.05 s and 0.85 ± 0.06 s at 3 cmH2O; and 0.60 ± 0.04 s and 0.79 ± 0.06 s at 5 cmH2O (Fig. 3B). This is direct evidence that NO release due to the flow and wall shear stress generated by the contractions of the thoracic duct eases the diastolic lymphatic filling. We also found that the ejection fraction was elevated (Fig. 4) in the presence of inherently generated NO. Figure 4 also demonstrates that blockade of NO-synthase (l-NAME administration) caused a positive chronotropic effect (increase in contraction frequency) in thoracic duct segments. Because of this chronotropic effect, the decrease in contraction amplitude after blockade of NO synthase was essentially completely compensated since fractional pump flow did not significant change during the NO synthase blockade (Fig. 4). Figure 5 demonstrates that we did not find any significant changes in any parameters of active pumping before and after indomethacin administration in phasically active segments of thoracic duct.
Figure 3. Pressure-induced changes in normalized systolic diameter (A) and half-relaxation time (B) in phasically active segments of rat thoracic duct after administration of l-NAME (10−4m).
*Significant differences (P ≤ 0.05) between half-relaxation times before and after l-NAME administration for each level of transmural pressure.
Figure 4. Pressure-induced changes in the active lymph pump parameters in phasically active segments of rat thoracic duct after administration of l-NAME (10−4m).
*Significant differences (P ≤ 0.05) between pumping parameters before and after l-NAME administration for each level of transmural pressure.
Figure 5. Pressure-induced changes in the active lymph pump parameters in phasically active segments of rat thoracic duct before and after administration of indomethacin (10−5m).
Discussion
Physiological importance of the pump-generated relaxation in thoracic duct
Lymphatic resting tone in phasically active segments of the thoracic duct was 2–2.6 times lower than in phasically non-active segments. The only difference between the experimental conditions of the active and non-active segments was the existence of actively generated flow in the contracting lymphatic segments. Hence we discovered a relaxation in the thoracic duct, which was connected exclusively with its spontaneous phasic pumping activity. Investigating the possible mechanisms of this relaxation, we found that blockade of NO synthase by l-NAME completely abolished this difference in lymphatic tone between the phasically active and non-active segments. Therefore this evidence indicates that the contraction-generated reduction of lymphatic tone in the thoracic duct is mediated only by NO. Based on this fact we determined what happened with active lymphatic pumping if we completely blocked the mechanism of the contraction-generated reduction of tone. We found that the reduction of tone in lymphatic segments generated by the phasic contractions improves their diastolic filling (enhanced lusitropy – lowering HRT), makes lymphatic contractions stronger (enhanced inotropy – higher contraction amplitude) and propels more fluid forward during each contraction (elevated ejection fraction) while decreasing contraction frequency (reduced chronotropy). After NO synthase blockade, the lymphatic segment must contract more often (higher contraction frequency) to maintain the minute productivity (fractional pump flow) appropriate to the existing level of preload (transmural pressure). We conclude that the reduction in lymphatic tone due to the flow/shear generated by phasic contractions is a regulatory mechanism that maintains lymphatic pumping in an energy-saving efficient mode (stronger, but fewer contractions per minute).
Importantly, the flow-mediated relaxation can exist in any phasically contracting lymphatic. But, the lymph flow profile is a complicated and variable sum of different forces, not only the result of phasic lymphatic contractions. When discussing the flow conditions in a single lymphangion, it is reasonable to divide the flow pattern into two components: ‘intrinsic flow’ (meaning the flow which is a result of the contractions of that lymphangion) and ‘extrinsic flow’ (meaning the flow which is a result of all influences from outside that single lymphangion, predominantly influences from upstream of that single lymphangion but some influences from downstream as well). From the experiments with imposed flow we know that as the imposed flow was increased, the degree of inhibition of lymphatic pumping increased (Gashev, 2002; Gashev et al. 2002, 2004). In the thoracic duct we observed (Gashev et al. 2004) that during periods of high imposed flow (a transaxial gradient of 5 cmH2O) normalized diastolic diameter increased, resulting in a 57% reduction in resting tone (in comparison to the absence of imposed flow at the same transmural pressure level). On the other hand at this level of imposed flow, the spontaneous contractions of the thoracic duct were almost completely abolished (Gashev et al. 2004). That led us to the conclusion that in situ, where the extrinsic flow varies dramatically and is dependent on many factors, the lymphangions are constantly operating under a combination of intrinsic and extrinsic flows. When extrinsic flow is not enough to move lymph downstream, the maintenance of low lymphatic tone by the extrinsic flow (demonstrated in Gashev, 2002; Gashev et al. 2002, 2004) is replaced by the reduction in lymphatic tone mediated by intrinsic flow during the pumping-effective phasic contractions (demonstrated in this study). When extrinsic flow is high enough to propel lymph by itself, spontaneous contractions may be inhibited to save energy and only the lowering of tone by the extrinsic flow will occur.
Regulatory pathways of flow-mediated changes in lymphatic pumping
Involvement of the NO synthase and cyclooxygenase pathways in the regulation of flow-mediated responses in blood vasculature is well known (Fleming & Busse, 1999; Vanhoutte, 2000; Davidge, 2001; Andrews et al. 2002; Griffith, 2002). Related knowledge in the physiology of lymph pumping is still quite limited. The importance of nitric oxide in the endothelium-dependent modulation of the lymphatic contractile cycle was demonstrated both in vitro and in vitro (Yokoyama & Ohhashi, 1993; Ferguson & DeFilippi, 1994; von der Weid et al. 1996, 2001; Mizuno et al. 1998; Shirasawa et al. 2000; Gashev et al. 2002). Some investigations argued against the involvement of the cyclooxygenase pathway in the endothelium-dependent control of lymphatic pumping (Ohhashi & Takahashi, 1991). Our data concerning the involvement of NO synthase and cyclooxygenase pathways in the flow-mediated regulation of the lymphatic pumping correlate well with findings reported by Tsunemoto et al. (2003) who demonstrated the release of NO but not prostaglandin or other vasoactive prostanoids during imposed flow when using a cascade bioassay technique on canine thoracic duct.
The data described above as well as the current data support the concept that the NO pathway, not the cyclooxygenase pathway, is responsible for the contraction-generated, flow-dependent lowering of tone in the thoracic duct. This is contrary to the data presented in a single report by Koller et al. (1999) that stated that prostanoids, not NO, regulated the imposed flow-mediated responses in isolated and perfused rat iliac lymphatic vessels. We discussed this difference in our previous report on the involvement of nitric oxide in the control of the imposed-flow responses in rat mesenteric lymphatics (Gashev et al. 2002). The current data provide further evidence of the importance of NO for flow-dependent regulation of lymphatic pumping: this time in another vessel type – rat thoracic duct with a different flow pattern (with only contraction-generated flow but without imposed flow) and a different NO inhibitor (l-NAME). Of course it is possible that the mechanisms responsible for flow-/shear-mediated changes in the lymphatic contractile responses are different in different tissues, as has been described in the endothelium-dependent responses of blood vessels (Vanhoutte, 2000). This may be particularly true given our recent findings of the regional heterogeneity of pumping behaviour and contractile apparatus in the lymphatic system (Muthuchamy et al. 2003; Gashev et al. 2004). But one may also note that of the five groups of rat lymphatic tissues studied thus far, lymphatics from four of these tissues (femoral, mesenteric, cervical lymphatics and thoracic duct) demonstrated a decrease in tone and contraction frequency (Gashev et al. 2004) in response to the increased imposed flow, whereas the iliac regional lymphatics behaved in an opposite fashion (Koller et al. 1999) to increases of imposed flow. One may ask what the potential physiological benefit is of having two linked lymphatic networks, which decrease their resistances during periods of increased flow (the femoral lymphatics and thoracic duct), and a lymphatic network physically between them, the iliac lymphatics, which will increase it's resistance at the same time. This question requires further investigation. However in the study demonstrating the behaviour of iliac lymphatics to increases in imposed flow, Koller et al. (1999) indicated that before the actual diameter recordings ‘each level of flow was maintained for 5–10 min to allow the vessels to exhibit stable and spontaneous diameter oscillations’ (Koller et al. 1999). This experimental approach had preset limitations due to the exclusion of the immediate maximal effects of increased flow and time-dependent phenomenon of adaptation to the increased flow in lymphatics which was demonstrated in later studies (Gashev et al. 2002). Moreover and perhaps more importantly, the iliac report (Koller et al. 1999) did not mention whether or not they prepared and selected pairs of resistance-matched pipettes for experiments with controlled imposed flow. It is important that the pairs of pipettes have carefully matched tip diameters and lengths in isolated microvessel experiments to study the effects of flow/shear that are independent of transmural pressure. Normally we and others have tested to ensure that the difference between the measured electrical resistances of the pipettes did not exceed 10% (Kuo et al. 1990b). This is even more critical in studies of isolated lymphatics since they are exceptionally sensitive at very low pressures (1–10 cmH2O). If this procedure was not performed in studies by Koller et al. (1999) it is possible that hydraulic resistance of outflow micropipettes was higher than resistance of inflow pipettes and/or too high by itself. In such cases when the resistance of the outflow pipettes is too high, the intravascular pressure will increase during the periods of imposed flow. As input pressure is increased to create the higher imposed flow, the higher will be the increase in transmural pressure. This could be a likely explanation of the authors' observation that ‘higher perfusion rates have smaller effects on Dmax and Dmin and frequency of oscillations’ (Koller et al. 1999). The effects of increases in perfusion (pressure gradient) on the diameters of lymphatics and the frequency of lymphatic vasomotions that the authors demonstrated in Fig. 2 (Koller et al. 1999) looks practically identical in shape to the pressure-induced responses we observed in rat thoracic duct (Figs 1 and 2 of Gashev et al. 2004). Taking these issues into account, the preponderance of data published by us and others, as well as the data presented in this paper, support the hypothesis that NO-dependent regulation is the prevailing pathway responsible for the adjustment of lymphatic pumping in response to changes in lymph flow.
We propose that contractile activity of the transporting lymphatics in situ constantly adjusts to the local ‘need’ to propel variable volumes of lymph by a continuous interplay between the influences of extrinsic and intrinsic flows. At low levels of inflow in the transporting lymphatics, the influences of intrinsic flow will dominate and NO release due to the phasic contractions will maintain the effective energy-saving lymphatic pumping patterns. As soon as the levels of lymph formation and accordingly inflow are increased in the transporting lymphatics, the influences of extrinsic flow will dominate, leading to a large NO release which will temporarily inhibit the intrinsic contractility of transporting lymphatics. In future studies, direct measurements of the nitric oxide concentrations in lymphatics will be able to provide more detailed information on this matter. However we believe that our current findings strongly support the idea that flow-/shear-dependent self-regulatory mechanisms in the lymphangions continuously adjust the lymphatic tone and phasic contractions to the physiologically variable preloads and outflow resistances.
In conclusion, using the unique contractile characteristics of the thoracic duct, we performed evaluations of contractile activity in phasically active and phasically non-active segments. Because imposed flow was excluded, flow and shear in phasically active segments occurred only as a result of their own contractions, whereas in phasically non-active segments contraction-generated flow/shear did not take place. As a result, we discovered that relaxation of the thoracic duct produced enhanced diastolic filling and increased lymph pump efficiency, which resulted exclusively from the flow/shear generated from its spontaneous phasic activity. The results of this study indicate that the reduction of lymphatic tone due to the flow generated by phasic contractions is a regulatory mechanism that maintains pumping in the thoracic duct in an efficient mode. The findings presented in this manuscript demonstrated that the NO pathway not the cyclooxygenase pathway plays the key role in the regulation of the contraction-generated reduction of lymphatic tone in the thoracic duct. We believe that NO-dependent regulation is the prevailing pathway responsible for the self-regulatory adjustment of lymphatic pumping to the changes in lymph flow pattern.
Acknowledgments
This work was supported by National Institutes of Health grants HL-070308 and HL-075199. The authors thank Elizabeth Wink for her help in primary data analysis and Professor Michael Davis for the use of his diameter analysis program.
References
- Amerini S, Ziche M, Greiner ST, Zawieja DC. Effects of substance P on mesenteric lymphatic contractility in the rat. Lymphat Res Biol. 2004;2:2–10. doi: 10.1089/1539685041690409. [DOI] [PubMed] [Google Scholar]
- Andrews KL, Triggle CR, Ellis A. NO and the vasculature: where does it come from and what does it do? Heart Fail Rev. 2002;7:423–445. doi: 10.1023/a:1020702215520. [DOI] [PubMed] [Google Scholar]
- Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-α antagonism. Am J Physiol Heart Circ Physiol. 2006;290:H1259–H1263. doi: 10.1152/ajpheart.00990.2005. [DOI] [PubMed] [Google Scholar]
- Armenio S, Cetta F, Tanzini G, Guercia C. Spontaneous contractility in the human lymph vessels. Lymphology. 1981a;14:173–178. [PubMed] [Google Scholar]
- Armenio S, Cetta F, Tanzini G, Guercia C, Burroni F. Spontaneous contractility of the lymphatic vessels in man (in Spanish) Angilogia. 1981b;33:325–327. [PubMed] [Google Scholar]
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol. 1989;257:H2059–H2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- Borisov AV. The theory of the design of the lymphangion (in Russian) Morfologiia. 1997;112:7–17. [PubMed] [Google Scholar]
- Bridenbaugh EA, Gashev AA, Zawieja DC. Lymphatic muscle: a review of contractile function. Lymphatic Res Biol. 2003;1:147–158. doi: 10.1089/153968503321642633. [DOI] [PubMed] [Google Scholar]
- Browse NL, Lord RS, Taylor A. Pressure waves and gradients in the canine thoracic duct. J Physiol. 1971;213:507–524. doi: 10.1113/jphysiol.1971.sp009396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T, Heath T. Intrinsic contractility of lymphatics in sheep and in dogs. Q J Exp Physiol. 1973;58:207–217. doi: 10.1113/expphysiol.1973.sp002209. [DOI] [PubMed] [Google Scholar]
- Chan AK, Vergnolle N, Hollenberg MD, von der Weid PY. Proteinase-activated receptor 2 activation modulates guinea-pig mesenteric lymphatic vessel pacemaker potential and contractile activity. J Physiol. 2004;560:563–576. doi: 10.1113/jphysiol.2004.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AK, von der Weid PY. 5-HT decreases contractile and electrical activities in lymphatic vessels of the guinea-pig mesentery: role of 5-HT7 -receptors. Br J Pharmacol. 2003;139:243–254. doi: 10.1038/sj.bjp.0705264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MJ, von der Weid PY, Brock JA, Van Helden DF. Co-ordination of contractile activity in guinea-pig mesenteric lymphatics. J Physiol. 1997;500:235–244. doi: 10.1113/jphysiol.1997.sp022013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datte JY, Yapo PA, Offoumou MA. Nitric oxide effect on 5-hydroxytryptamine-induced vasoconstrictions of isolated smooth muscle. Pharmacol Rep. 2005;57:113–120. [PubMed] [Google Scholar]
- Davidge ST. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650–660. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- Davis MJ. An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation. 2005;12:361–372. doi: 10.1080/10739680590934772. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol. 2006;4:3–10. doi: 10.1089/lrb.2006.4.3. [DOI] [PubMed] [Google Scholar]
- Ferguson MK, DeFilippi VJ. Nitric oxide and endothelium-dependent relaxation in tracheobronchial lymph vessels. Microvasc Res. 1994;47:308–317. doi: 10.1006/mvre.1994.1024. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol. 1999;31:5–14. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- Foy W, Allen J, McKillop J, Goldsmith J, Johnston C, Buchanan K. Substance P and gastrin releasing peptide in bovine mesenteric lymphatic vessels: chemical characterization and action. Peptides. 1989;10:533–537. doi: 10.1016/0196-9781(89)90138-1. [DOI] [PubMed] [Google Scholar]
- Gashev AA. The mechanism of the formation of a reverse fluid filling in the lymphangions (in Russian) Fiziol Zh SSSR Im I M Sechenova. 1991;77:63–69. [PubMed] [Google Scholar]
- Gashev AA. Physiologic aspects of lymphatic contractile function: current perspectives. Ann N Y Acad Sci. 2002;979:178–187. doi: 10.1111/j.1749-6632.2002.tb04878.x. discussion 188–196. [DOI] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev AA, Orlov RS, Borisov AV, Kliuchin'ski T, Andreevskaya MV, Bubnova NA, Borisova RP, Andreev YA, Erofeev NP, Priklonskaya EG. The mechanisms of lymphangion interaction in the process of the lymph movement (in Russian) Fiziol Zh SSSR Im I M Sechenova. 1990;76:1489–1508. [PubMed] [Google Scholar]
- Gnepp DR. Lymphatics. In: Staub NC, Taylor AE, editors. Edema. New York: Raven Press; 1984. pp. 263–298. [Google Scholar]
- Goethals MA, Adele SM, Brutsaert DL. Contractility in mammalian heart muscle; calcium and osmolality. Circ Res. 1975;36:27–33. doi: 10.1161/01.res.36.1.27. [DOI] [PubMed] [Google Scholar]
- Granger HJ, Kovalcheck S, Zweifach BW, Barnes GE. Proceedings of the VII Summer Computer Simulation Conference. La Jolla, CA, USA: Simulation Council; Quantitative analysis of active lymphatic pumping; pp. 562–565. [Google Scholar]
- Griffith TM. Endothelial control of vascular tone by nitric oxide and gap junctions: a haemodynamic perspective. Biorheology. 2002;39:307–318. [PubMed] [Google Scholar]
- Guibert C, Beech DJ. Positive and negative coupling of the endothelin ETA receptor to Ca2+-permeable channels in rabbit cerebral cortex arterioles. J Physiol. 1999;514:843–856. doi: 10.1111/j.1469-7793.1999.843ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Gould DJ. Pathway-specific effects of calcitonin gene-related peptide on irideal arterioles of the rat. J Physiol. 1997;505:797–809. doi: 10.1111/j.1469-7793.1997.797ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu O, Goto M, Yada T, Kimura A, Chiba Y, Tachibana H, Ogasawara Y, Tsujioka K, Kajiya F. In vivo observations of the intramural arterioles and venules in beating canine hearts. J Physiol. 1998;509:619–628. doi: 10.1111/j.1469-7793.1998.619bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood MA, Cotton KD, Thornbury KD, McHale NG. Tetrodotoxin-sensitive sodium current in sheep lymphatic smooth muscle. J Physiol. 1997;503:13–20. doi: 10.1111/j.1469-7793.1997.013bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaert P. Pressure measurements in the cervical portion of the thoracic duct in man. Br J Surg. 1973;60:558–561. doi: 10.1002/bjs.1800600717. [DOI] [PubMed] [Google Scholar]
- Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol. 1999;277:R1683–R1689. doi: 10.1152/ajpregu.1999.277.6.R1683. [DOI] [PubMed] [Google Scholar]
- Kousai A, Mizuno R, Ikomi F, Ohhashi T. ATP inhibits pump activity of lymph vessels via adenosine A1 receptor-mediated involvement of NO- and ATP-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2004;287:H2585–H2597. doi: 10.1152/ajpheart.01080.2003. [DOI] [PubMed] [Google Scholar]
- Kuo L, Chilian WM, Davis MJ. Coronary arteriolar myogenic response is independent of endothelium. Circ Res. 1990a;66:860–866. doi: 10.1161/01.res.66.3.860. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol. 1988;255:H1558–H1562. doi: 10.1152/ajpheart.1988.255.6.H1558. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990b;259:H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- McHale NG, Meharg MK. Co-ordination of pumping in isolated bovine lymphatic vessels. J Physiol. 1992;450:503–512. doi: 10.1113/jphysiol.1992.sp019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol. 1976;261:255–269. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol. 1991;261:H950–H959. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- Mislin H. Experimental detection of autochthonous automatism of lymph vessels (in German) Experientia. 1961;17:29–30. doi: 10.1007/BF02157935. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol. 1998;274:R790–R796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Ono N, Ohhashi T. Involvement of ATP-sensitive K+ channels in spontaneous activity of isolated lymph microvessels in rats. Am J Physiol. 1999;277:H1453–H1456. doi: 10.1152/ajpheart.1999.277.4.H1453. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920–922. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- Nakaike R, Shimokawa H, Yasutake H, Sumimoto H, Ito A, Numaguchi K, Egashira K, Takeshige K, Takeshita A. Effects of L-arginine analogues on vasomotion of isolated porcine coronary arteries. Am J Physiol. 1995;268:H1966–H1972. doi: 10.1152/ajpheart.1995.268.5.H1966. [DOI] [PubMed] [Google Scholar]
- Nusbaum M, Baum S, Hedges RC, Blakemore WS. Roentgenographic and direct visualization of thoracic duct. Arch Surg. 1964;88:127–135. doi: 10.1001/archsurg.1964.01310190107012. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, Takahashi N. Acetylcholine-induced release of endothelium-derived relaxing factor from lymphatic endothelial cells. Am J Physiol. 1991;260:H1172–H1178. doi: 10.1152/ajpheart.1991.260.4.H1172. [DOI] [PubMed] [Google Scholar]
- Orlov RS, Borisova RP. Contractions of the lymphatic vessels, their regulation and functional role (in Russian) Vestn Akad Med Nauk SSSR. 1982;7:74–83. [PubMed] [Google Scholar]
- Orlov RS, Borisova RP, Mandryko ES. The contractile and electrical activity of the smooth muscles of the major lymph vessels (in Russian) Fiziol Zh SSSR Im I M Sechenova. 1975;61:1045–1053. [PubMed] [Google Scholar]
- Perez NG, Mattiazzi A, Cingolani HE. Lusitropic changes induced by acid base alterations in cat papillary muscles. Arch Int Physiol Biochim Biophys. 1993;101:233–237. doi: 10.3109/13813459309046481. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa Y, Ikomi F, Ohhashi T. Physiological roles of endogenous nitric oxide in lymphatic pump activity of rat mesentery in vivo. Am J Physiol. 2000;278:G551–G556. doi: 10.1152/ajpgi.2000.278.4.G551. [DOI] [PubMed] [Google Scholar]
- Tsunemoto H, Ikomi F, Ohhashi T. Flow-mediated release of nitric oxide from lymphatic endothelial cells of pressurized canine thoracic duct. Jpn J Physiol. 2003;53:157–163. doi: 10.2170/jjphysiol.53.157. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Say NO to ET. J Auton Nerv Syst. 2000;81:271–277. doi: 10.1016/s0165-1838(00)00126-0. [DOI] [PubMed] [Google Scholar]
- Wang A, Nishihashi T, Trandafir CC, Murakami S, Ji X, Shimizu Y, Kurahashi K. Involvement of endothelial cyclo-oxygenase metabolites in noradrenaline-induced contraction of rat coronary artery. Clin Exp Pharmacol Physiol. 2005;32:628–632. doi: 10.1111/j.0305-1870.2005.04242.x. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Yashiro Y, Mizuno R, Ohhashi T. Involvement of NO and EDHF in flow-induced vasodilation in isolated hamster cremasteric arterioles. J Vasc Res. 2005;42:137–147. doi: 10.1159/000083652. [DOI] [PubMed] [Google Scholar]
- von der Weid PY. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and β-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol. 1998;125:17–22. doi: 10.1038/sj.bjp.0702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY, Crowe MJ, Van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol. 1996;493:563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid PY, Van Helden DF. β-Adrenoceptor-mediated hyperpolarization in lymphatic smooth muscle of guinea pig mesentery. Am J Physiol. 1996;270:H1687–H1695. doi: 10.1152/ajpheart.1996.270.5.H1687. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol. 2001;280:H2707–H2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- Yoffey JM, Courtice FC. Lymphatics, Lymph and the Lymphomyeloid Complex. London, New York: Academic Press; 1970. [Google Scholar]
- Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol. 1993;264:H1460–H1464. doi: 10.1152/ajpheart.1993.264.5.H1460. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol. 1993;264:H1283–H1291. doi: 10.1152/ajpheart.1993.264.4.H1283. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. Am J Physiol. 1991;260:H1935–H1943. doi: 10.1152/ajpheart.1991.260.6.H1935. [DOI] [PubMed] [Google Scholar]