Abstract
Given the large increase in cutaneous vascular conductance (CVC) during whole-body heat stress, this vascular bed is important in the regulation of blood pressure during orthostatic stress. In this thermal state, changes in CVC are reported to be due to withdrawal of active vasodilator activity. The purpose of this study was to identify, contrary to the current line of thinking, whether cutaneous vasoconstrictor neural activity is enhanced and capable of contributing to reductions in CVC during an orthostatic challenge of heat-stressed individuals. Healthy normotensive subjects were pretreated, subcutaneously, with botulinum toxin A (BTX-A) to inhibit the release of neurotransmitters from cutaneous active vasodilator nerves. On the experimental day, microdialysis probes were placed in the BTX-A-treated site and in an adjacent untreated site. In protocol 1, internal temperature was elevated ∼0.7°C, followed by the application of lower body negative pressure (LBNP; −30 mmHg). LBNP reduced CVC at the BTX-A-treated sites (Δ4.2 ± 2.9%max), as well as at the control site (Δ9.8 ± 4.1%max). In protocol 2, after confirming the absence of cutaneous vasodilatation at the BTX-A-treated site during whole-body heating, CVC at this site was elevated to a similar level relative to the control site (55.4 ± 13.4 versus 60.7 ± 10.4%max, respectively) via intradermal administration of isoproterenol prior to LBNP. Similarly, when flow was matched between sites, LBNP reduced CVC at both the BTX-A-treated (Δ15.3 ± 4.6%max) and the control sites (Δ8.8 ± 5.6%max). These data suggest that the cutaneous vasoconstrictor system is engaged and is capable of decreasing CVC during an orthostatic challenge in heat-stressed individuals.
Heat stress greatly reduces orthostatic tolerance in humans relative to normothermic conditions, and this response is not simply due to pooling of blood in the lower limbs (Lind et al. 1968). Pronounced heat stress increases cardiac output to up to 13 l min−1, with 50% or more of that value being distributed to skin (Rowell, 1986; Rowell et al. 1969). In these conditions, control of cutaneous vascular conductance (CVC) is a critical factor in the maintenance of blood pressure and thus orthostatic tolerance.
The cutaneous circulation is controlled by two neural systems; a sympathetic adrenergic vasoconstrictor system, and a separate sympathetic non-adrenergic active vasodilator system (Edholm et al. 1957; Kellogg et al. 1993). During heat stress, the active vasodilator system accounts for 85–95% of the overall cutaneous vasodilator response (Grant & Holling, 1938; Kellogg et al. 1995). During orthostasis of a heat-stressed individual, reductions in CVC could occur by engagement of the vasoconstrictor system and/or by withdrawal of the active vasodilator system. Kellogg et al. (1990) addressed this question via bretylium-induced blockade of the cutaneous vasoconstrictor system, and concluded that reductions in CVC during combined heat and orthostatic stress were primarily mediated through withdrawal of the cutaneous active vasodilator system. This conclusion was based upon the observation of similar reductions in CVC at both the control and the bretylium-treated sites during lower body negative pressure (LBNP).
Wilson et al. (2002) found that cutaneous vasoconstrictor responsiveness to local administration of noradrenaline was reduced when subjects were heat stressed relative to normothermic conditions. This observation raises a possibility that factors associated with heat stress may attenuate vasoconstrictor responses, perhaps secondary to engagement of the active vasodilator system. Attenuated cutaneous vasoconstrictor responsiveness may partially explain Kellogg's findings (Kellogg et al. 1990) of a relative absence of a contribution of the cutaneous vasoconstrictor system in decreasing CVC during LBNP of heat-stressed individuals.
A method to identify the potential contribution of the cutaneous vasoconstrictor system in modulating CVC during an orthostatic challenge of heat-stressed subjects would be to evaluate CVC responses during these stresses at a site where the active vasodilator system is blocked. Subcutaneous injection of botulinum toxin (BTX-A) abolishes both sweating and cutaneous vasodilatation during heat stress (Kellogg et al. 1995), by blocking neurotransmitter release from cholinergic nerves (Simpson, 1981). Using this agent to block the cutaneous active vasodilator system, we tested the hypothesis that reductions in CVC during combined orthostatic and heat stresses can occur through engagement of the sympathetic vasoconstrictor system.
Methods
Six healthy normotensive subjects participated in protocol 1. Their age, height, and weight were 33 ± 6 years, 173.2 ± 4.6 cm, and 71.7 ± 10.2 kg, respectively. In protocol 2, seven subjects participated (31 ± 7 years, 167.1 ± 9.9 cm, and 65.8 ± 6.2 kg). Subjects were informed of the purpose and risks of this study before providing their written consent. The consent form was approved by the University of Texas Southwestern Medical Center at Dallas and Presbyterian Hospital of Dallas. All subjects refrained from caffeine, alcohol, and exercise for 24 h before the study. The protocol was performed in accordance with the Declaration of Helsinki.
Protocol 1
At least 3 days prior to experimentation, BTX-A (10 units in 0.15 ml) was administered in dorsal forearm skin by intradermal injection to locally abolish cutaneous active vasodilatation (Kellogg et al. 1995). On the day of experimentation, each subject was instrumented for the measurement of mean skin temperature from the weighted average of six thermocouples attached to the skin (Taylor et al. 1989) and the ECG. The subject was then dressed in a tube-lined water perfusion suit that permitted the control of skin and internal temperatures by changing the temperature of the water perfusing the suit. The suit covered the entire body surface except for the head, feet, hands and a forearm.
Each subject was then placed in the supine position in an LBNP device that included a bicycle seat for support. The device was sealed to the subject at the level of the iliac crest. A two-piece water-perfused suit was used such that the LBNP device was sealed directly to the subject's skin, thereby minimizing/eliminating cooling associated with LBNP. Simulation of orthostatic stress was accomplished by applying a negative pressure inside the box, which causes blood to pool in the lower extremities. Subjects rested in the supine position while normothermic water (34°C) circulated through the water-perfused suit. Ambient temperature in the laboratory was controlled at 24–25°C. A thermistor was placed in the sublingual sulcus to provide an index of internal temperature. Heart rate (HR) was continuously obtained from the ECG (SpaceLabs) with the signal interfaced with a cardiotachometer (CWE). Arterial blood pressure was measured via auscultation of the brachial artery (Suntech). Mean arterial pressure (MAP) was calculated as one-third pulse pressure plus diastolic pressure.
Two microdialysis probes were placed in the dermal space of dorsal forearm skin. One microdialysis probe was placed within the BTX-A-treated area, while the other probe was placed in an adjacent untreated site. After placement, the probes were perfused with Ringer's solution at a rate of 2 μl min−1. A multifibre laser-Doppler probe was placed over each microdialysis membrane to monitor cutaneous blood flow using laser Doppler flowmetry (Perimed). Cutaneous vascular conductance (CVC) was calculated from the ratio of skin blood flow to MAP. Skin blood flow was normalized to its maximum value, identified by local administration of 50 mM sodium nitroprusside upon completion of each protocol. Approximately 90–120 min after microdialysis membrane placement, once the hyperaemic response associated with membrane placement subsided, the experimental tests were performed.
Vasoconstrictor responsiveness at both sites was assessed by performing a 3-min cold stress in which water at 5°C water perfused through the suit. After this cold stress and subsequent return of skin blood flow to pre-cold stress levels, a heat stress ensued by perfusing 46°C water through the suit. An absence of substantial cutaneous vasodilatation at the BTX-A-treated site, relative to the control site, confirmed an effective block. Following confirmation of an effective block at the BTX-A-treated site, baseline data were obtained followed by 3 min of −30 mmHg LBNP. Skin and internal temperatures were then returned to preheating levels by perfusing cooler water through the suit. This was followed by local administration of sodium nitroprusside as outlined above.
Protocol 2
In protocol 1, prior to LBNP CVC would be substantially higher at the control site relative to the BTX-A sites, given that BTX-A would block heat-induced active vasodilatation. Protocol 2 was performed to identify CVC responses to LBNP when CVC at the BTX-A site was elevated to the same level relative to the control site. For this protocol, similar procedures were performed relative to protocol 1; however, after confirmation of an effective blockade at the BTX-A-treated site during the heat stress, the β-adrenergic agonist isoproterenol (dosing range 8.1 × 10−6 to 8.1 × 10−5m isoproterenol) was infused through the microdialysis membrane at that site to increase skin blood flow to a similar level relative to the control site. Ringer's solution continued to be perfused through the adjacent control site. Once skin blood flow at the BTX-A-treated was elevated and stable, −30 mmHg of LBNP was applied for 3 min. LBNP was then released, allowing skin blood flow and blood pressure to return to pre-LBNP levels. To achieve a greater level of reduction in CVC relative to protocol 1, subjects were also exposed to 3 min of −40 mmHg LBNP or until the onset of presyncopal symptoms. After this final LBNP stage, subjects were cooled by perfusing cool water through the suit, and maximal skin blood flow was identified as described above.
Statistical analysis
Data were recorded at 50 Hz (Biopac) and reduced to 20 s averages for each stage (i.e. pre-cold stress baseline, cold stress, pre-LBNP, LBNP). Two of seven subjects in protocol 2 showed presyncopal symptoms early during −40 mmHg LBNP (i.e. within the first ∼30 s). For these subjects, data were averaged from the final 20 s of −30 mmHg LBNP protocol. For those subjects who experienced presyncopal symptoms near the end of the −40 mmHg LBNP stage, data were reduced during the 20 s period before LBNP was turned off. Thus, for both protocols the endpoint was either the occurrence of presyncopal symptoms or the completion of the prescribed time at the highest level of LBNP. The effects of heat stress, relative to preheating baseline, on thermal and haemodynamic variables were compared by Students paired t test. To compare the reduction (i.e. Δ) in CVC due to either the cold stress or LBNP between the control and the BTX-A-treated sites, a Student's paired t test was also used. All data are expressed as means ± s.d. The level of statistical significance was set at P < 0.05.
Results
Protocol 1
Approximately 90–120 min following microdialysis probe placement, baseline CVC was similar between BTX-A pretreated and untreated sites. Cold stress decreased CVC at all sites, but the magnitude of the reduction in CVC during cold stress was not different between sites (see Table 1).
Table 1.
Heart rate (HR), mean arterial pressure (MAP) and cutaneous vascular conductance (CVC) at the control and BTX-A-pretreated sites during cold stress.
| Protocol 1 | Protocol 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| CVC | CVC | |||||||
| HR (beats min−1) | MAP (mmHg) | Control (%max) | BTX-A (%max) | HR (beats min−1) | MAP (mmHg) | Control (%max) | BTX-A (%max) | |
| Baseline | 59.9 ± 3.7 | 90.2 ± 10.6 | 20.8 ± 12.7 | 16.8 ± 10.2 | 63.1 ± 16.2 | 84.3 ± 6.5 | 11.3 ± 3.3 | 10.9 ± 3.1 |
| Cold stress | 54.1 ± 5.5* | 95.7 ± 10.3* | 13.1 ± 12.0* | 10.5 ± 7.5* | 55.5 ± 9.4* | 90.7 ± 11.3* | 6.0 ± 3.6* | 7.3 ± 2.5* |
Different relative to baseline, P < 0.05.
Prior to application of LBNP, whole-body heating elevated internal temperature (36.8 ± 0.3 to 37.5 ± 0.3°C, P < 0.05), resulting in an average increase in this variable of 0.8 ± 0.1°C. As shown in Fig. 1, CVC at the BTX-A-treated site was slightly increased during heat stress (1.9 ± 0.5 times baseline), perhaps due to withdrawal of tonic vasoconstrictor activity, but this increase was much less relative to the control site, which increased 4.7 ± 3.0 times baseline.
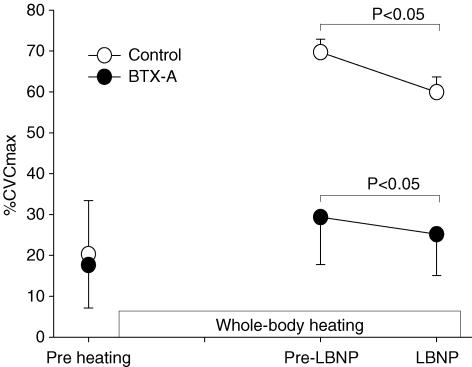
Figure 1. Cutaneous vascular conductance (CVC) responses during whole-body heat stress followed by lower body negative pressure (LBNP) at the control (○) and the BTX-A-treated (•) sites for protocol 1.
Whole-body heating significantly increased CVC at the control site, whereas CVC at the BTX-A-treated site was only slightly increased. LBNP significantly reduced CVC at both sites.
In one subject there was no decrease in CVC during LBNP at either the control or BTX-A-treated sites. Given the lack of change in CVC at the control site, data from this subject were excluded from the analysis. For the remaining subjects, application of LBNP significantly reduced CVC at both the control and BTX-A-treated sites (Fig. 1). The reductions in CVC at the control and the BTX-A-treated sites were 9.8 ± 4.1 and 4.2 ± 2.9%max, respectively. LBNP did not significantly change skin (37.7 ± 0.5 to 37.5 ± 0.4°C, P = 0.07) or internal (37.5 ± 0.3 to 37.6 ± 0.3°C, P = 0.19) temperatures.
Protocol 2
The procedures performed for this protocol are outlined in Fig. 2. Cold stress decreased CVC at all sites and the magnitude of the reduction in CVC during cold stress was not different between sites (see Table 1).
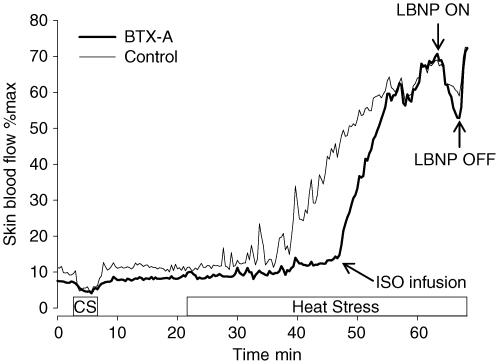
Figure 2. Depiction of the methods used for protocol 2.
Skin blood flow was assessed at both a control and BTX-A-treated site. Subjects were first exposed to a cold stress (CS) to confirm an intact vasoconstrictor system at both sites. Subjects were then exposed to whole-body heating. Upon identification of a successful blockade at the BTX-A-treated site, isoproterenol (ISO) was administered at this site to increase skin blood flow to a level similar relative to the control site. Subjects were then exposed to lower body negative pressure (LBNP).
Internal temperature was significantly increased during whole-body heating (36.3 ± 0.4 to 37.5 ± 0.4°C, P < 0.05). Prior to isoproterenol infusion, an ∼4-fold increase in CVC (from 11.3 ± 3.3 to 43.9 ± 10.3%max; P < 0.05) was observed at the control site, while at this same time point CVC at the BTX-A-treated site was only slightly increased (10.9 ± 3.1 to 13.1 ± 3.3%max; P = 0.06). After this confirmation of an effective blockade at the BTX-A-treated site, isoproterenol was administered through the microdialysis probe at this site to increase CVC to similar levels relative to the adjacent control site (control: 60.7 ± 10.4%max, BTX-A + isoproterenol: 55.4 ± 13.4%max, P = 0.30; see Fig. 3).
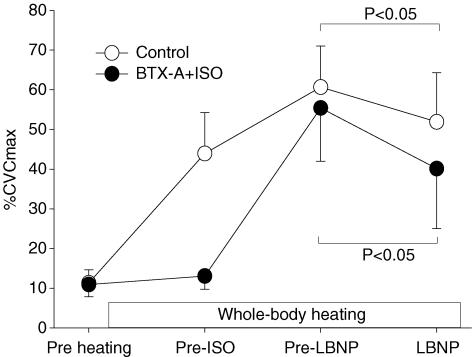
Figure 3. Cutaneous vascular conductance (CVC) responses during the procedures outlined in protocol 2.
Upon identification of an effective blockade at the BTX-A-treated site (labelled pre-ISO), the β-adrenergic agonist isoproterenol (ISO) was infused through the microdialysis membrane at that site to increase skin blood flow to a similar level relative to the control site (labelled pre-LBNP). Subjects were then exposed to lower body negative pressure (LBNP). LBNP significantly decreased CVC at both sites.
Despite blockade of the active vasodilator system, and consistent with protocol 1, LBNP decreased CVC at both the control and BTX-A-treated sites (Figs 3 and 4). Interestingly, the magnitude of the reduction in CVC was significantly greater at the BTX-A + isoproterenol site relative to the control site (15.3 ± 4.6%max versus 8.8 ± 5.6%max, P < 0.05). LBNP did not significantly alter either mean skin temperature (37.9 ± 0.4 to 37.7 ± 0.5°C, P = 0.18) or internal temperature (37.5 ± 0.4 to 37.4 ± 0.6°C, P = 0.41).
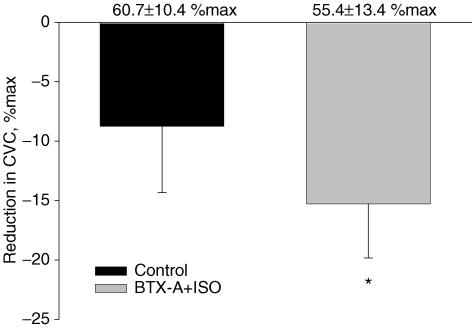
Figure 4. Changes in cutaneous vascular conductance (CVC) during lower body negative pressure (LBNP) for protocol 2.
CVC decreased at the control and BTX-A + isoproterenol-treated sites, however, the reduction in CVC was significantly greater at the BTX-A + isoproterenol-treated site. Baseline values prior to LBNP are shown at the top of each column. There were no significant differences between baseline values (i.e. prior to LBNP). *Significantly different relative to the control site, P < 0.05.
Discussion
The present data are the first to demonstrate that the cutaneous vasoconstrictor system is capable of contributing to reductions in CVC during an orthostatic challenge of heat-stressed individuals. This finding is supported by the observed reduction in CVC at the site where the cutaneous active vasodilator system was blocked via BTX-A administration under conditions when flow at the BTX-A site was, and was not, matched relative to the control site (see Figs 1 and 3).
In protocol 1, during heat stress but before the application of LBNP, CVC at the BTX-A-treated site was significantly lower relative to the control site. This is an expected finding given the known effect of BTX-A in blocking cutaneous active vasodilatation (Kellogg et al. 1995). LBNP decreased CVC at the both the control and BTX-A-treated sites. This observation suggests that cutaneous vasoconstrictor activity can be engaged, and thus is capable of contributing to the reduction in CVC during LBNP in heat-stressed individuals. However, given differences in CVC prior to LBNP between control and BTX-A-treated sites for protocol 1, it was unclear whether the magnitude of the reduction in CVC due to LBNP was affected by blockade of the active vasodilator system. To address this issue, in protocol 2 the β-adrenergic agonist isoproterenol was infused via microdialysis at the BTX-A-treated site to increase CVC to levels comparable to the control site. Consistent with protocol 1, significant reductions in CVC were observed at both sites during LBNP (Figs 2 and 3). Further analysis revealed that the magnitude of the reduction in CVC due to LBNP was significantly greater at the BTX-A + isoproterenol-treated site (Fig. 4).
In heat-stressed humans, with the exception of the onset of dynamic exercise (Kellogg et al. 1991), it has classically been thought that skin blood flow is primarily controlled through modulation of the cutaneous active vasodilator system during perturbations that reduce CVC. This conclusion has been drawn from a variety of studies in which the cutaneous vasoconstrictor system was blocked via bretylium treatment and the responsiveness to a perturbation, such as orthostatic stress or isometric exercise, was similar relative to an adjacent control site (Kellogg et al. 1989; Kellogg et al. 1991; Crandall et al. 1995; Kenney et al. 1997; Mack et al. 2001). Given that the vasoconstrictor system was blocked, these investigators concluded that modulation of skin blood flow by these perturbations occurred through modulation of cutaneous active vasodilatation. However, as shown in the present study, similar responses between control and bretylium-treated sites do not eliminate the possibility that the vasoconstrictor system may be engaged by a perturbation. What remains unclear is, under these and similar circumstances, whether the vasoconstrictor system is always engaged but its effects are masked by withdrawal of the active vasodilator system, or whether the vasoconstrictor system is engaged only when the active vasodilator system is blocked.
An important component of the present study is the observation of normal vasoconstrictor responses at the BTX-A-treated site during the cold stress. This observation is critical because it demonstrates that BTX-A does not alter the effectiveness of the cutaneous vasoconstrictor system. In contrast, and as expected, BTX-A abolished active cutaneous vasodilatation during the whole-body heat stress at a time when CVC at the adjacent control site was elevated greater than four-fold. Although slight elevations in CVC were observed at the BTX-A-treated site at the onset of heating, this observation was probably due to withdrawal of tonic vasoconstrictor tone. To confirm this suspicion, skin blood flow and sweat rate were simultaneously monitored at a BTX-A-pretreated site and at an adjacent site during whole-body heat stress in two subjects. For both subjects, CVC slightly increased during heating at the BTX-A site (13.4 to 22.6%max), however, sweating was completely abolished. In contrast, at the adjacent control site skin blood flow increased flow: 16.7 to 67.4%max, while sweat rate increased greater than 0.2 mg cm−2 min−1. The absence of sweating at the BTX-A-treated site confirms the effectiveness of the blockade, and provides strong evidence that slight elevations in CVC at this site are not due to inadequate blockade, but rather due to withdrawal of tonic vasoconstrictor tone.
Johnson et al. (1973) assessed reductions in forearm vascular conductance during LBNP of heat-stressed subjects. It is interesting to note that leading up to and at presyncope, CVC remained substantially elevated relative to preheat stress levels. An explanation for this observation could be the result of a balance between thermoregulatory-mediated vasodilatation and baroreflex mediated vasoconstriction, with baroreflex-mediated reductions in CVC unable to overcome an influence of thermoregulatory-mediated cutaneous vasodilatation even under conditions of extreme hypotension. Findings illustrated in Fig. 4 suggest that without the influence of the active vasodilator system, cutaneous vasoconstrictor responses are enhanced to a challenge such as LBNP. This observation raises a second hypothesis to explain what appears to be an inadequate reduction in CVC in the aforementioned findings by Johnson et al. (1973). That is, attenuated vasoconstrictor responses in this setting could be due to inhibition of the cutaneous vasoconstrictor system by the active vasodilator system. Such a hypothesis is speculative and warrants further investigation. Regardless of the mechanisms, given the importance of the control of CVC in the regulation of blood pressure in heat-stressed individuals (Rowell et al. 1969; Rowell, 1986), it may be that attenuated reductions in CVC contribute to reduced orthostatic tolerance in heat-stressed subjects.
The conclusion that the cutaneous vasoconstrictor limb can be engaged during an orthostatic challenge of heat-stressed individuals is based upon the observation of a significant reduction in CVC at the BTX-A-treated site in response to LBNP during whole-body heating. However, such a conclusion is reliant on the premise that baroreflex modulation of the cutaneous active vasodilator and vasoconstrictor limbs are the only mechanisms available by which changes in CVC under these conditions can occur. Therefore, by removing the active vasodilator limb (i.e. at the BTX-A-treated site), residual changes in CVC at that site are proposed to occur through engagement of the cutaneous vasoconstrictor system. That said, it remains possible that unknown factor(s), which are not related to modulation of either the cutaneous vasoconstrictor or active vasodilator systems, may be responsible for the observed reduction in CVC at the BTX-A-treated site. Such supposition warrants further investigation.
Previously, Stjarne & Brundin (1975, 1976) reported that isoproterenol is capable of facilitating nerve stimulation-induced release of noradrenaline. Since in protocol 2 isoproterenol was used to elevate skin blood flow at the BTX-A site to levels similar relative to the control site, it could be argued that reductions in CVC at the BTX-A + isoproterenol site could due to an indirect effect of isoproterenol leading to an enhanced release of noradrenaline. A couple of points argue against this being a limitation to the interpretation of the data. First, in protocol 1 there is a clear reduction in CVC at the BTX-A site in the absence of isoproterenol. This alone demonstrates that the vasoconstrictor system can be engaged during combined orthostatic and heat stress. Second we obtained data from two subjects in which adenosine, which does not exhibit the same properties as isoproterenol with respect to facilitating noradrenaline release, was administered instead of isoproterenol. In these subjects we observed similar CVC responses at the BTX-A (decreased CVC 12.2%max) and control (decrease 9.1%max) sites. Together, these observations strongly suggest that the cutaneous vasoconstrictor system can be engaged during combined heat and LBNP stress.
In conclusion, upon blockade of the cutaneous active vasodilator system via local BTX-A administration, the present data provide compelling evidence that reductions in CVC during an orthostatic challenge of heat-stressed humans can occur via the cutaneous adrenergic vasoconstrictor system despite prior findings to the contrary. It remains unclear whether adrenergic-mediated cutaneous vasoconstriction under such conditions only occurs when the cutaneous active vasodilator system is abolished, or whether the cutaneous vasoconstrictor system contributes to the reduction in CVC during an orthostatic challenge in combination with withdrawal of the cutaneous active vasodilator system, albeit to a lesser extent relative to the withdrawal of the cutaneous active vasodilator system. Finally, attenuated responsiveness of the cutaneous vasoconstrictor system during heat stress may be a major contributing factor in the large reductions in orthostatic tolerance known to occur when individuals are in this thermal state.
Acknowledgments
Appreciation is expressed to the subjects for their participation in the study and to Kimberly Williams RN and Marilee Brown RN for their skillful nursing assistance. This research project was funded in part by grant from the National Institutes of Health-National Heart, Lung, and Blood Institute: HL61388 and HL67422, National Institute of General Medical Sciences: GM68865.
References
- Crandall CG, Musick J, Hatch JP, Kellogg DL, Jr, Johnson JM. Cutaneous vascular and sudomotor responses to isometric exercise in humans. J Appl Physiol. 1995;79:1946–1950. doi: 10.1152/jappl.1995.79.6.1946. [DOI] [PubMed] [Google Scholar]
- Edholm OG, Fox RH, MacPherson RK. Vasomotor control of the cutaneous blood vessels in the human forearm. J Physiol. 1957;139:455–465. doi: 10.1113/jphysiol.1957.sp005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RT, Holling HE. Further observations on the vascular responses of the human limb to body warming; evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci Lond. 1938;3:273–285. [Google Scholar]
- Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol. 1973;35:798–803. doi: 10.1152/jappl.1973.35.6.798. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kenney WL, Kosiba WA, Pergola PE. Mechanisms of control of skin blood flow during prolonged exercise in humans. Am J Physiol. 1993;265:H562–H568. doi: 10.1152/ajpheart.1993.265.2.H562. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol. 1989;257:H1599–H1606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Baroreflex control of the cutaneous active vasodilator system in humans. Circ Res. 1990;66:1420–1426. doi: 10.1161/01.res.66.5.1420. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Competition between cutaneous active vasoconstriction and active vasodilation during exercise in humans. Am J Physiol. 1991;261:H1184–H1189. doi: 10.1152/ajpheart.1991.261.4.H1184. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol. 1997;272:H1609–H1614. doi: 10.1152/ajpheart.1997.272.4.H1609. [DOI] [PubMed] [Google Scholar]
- Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- Mack GW, Cordero D, Peters J. Baroreceptor modulation of active cutaneous vasodilation during dynamic exercise in humans. J Appl Physiol. 2001;90:1464–1473. doi: 10.1152/jappl.2001.90.4.1464. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation Regulation During Physical Stress. New York: Oxford University Press; 1986. [Google Scholar]
- Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981;33:155–188. [PubMed] [Google Scholar]
- Stjarne L, Brundin J. Dual adreoceptor-mediated control of noradrenaline secretion from human vasoconstrictor nerves: facilitation by BETA-receptors and inhibitor by alpha-receptors. Acta Physiol Scand. 1975;94:139–141. doi: 10.1111/j.1748-1716.1975.tb05872.x. [DOI] [PubMed] [Google Scholar]
- Stjarne L, Brundin J. Beta2-adrenoceptors facilitating noradrenaline secretion from human vasoconstrictor nerves. Acta Physiol Scand. 1976;97:88–93. doi: 10.1111/j.1748-1716.1976.tb10238.x. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci. 2002;97:122–128. doi: 10.1016/s1566-0702(02)00046-2. [DOI] [PubMed] [Google Scholar]