Abstract
Historically, an increase in intracellular H+ (decrease in cell pH) was thought to contribute to muscle fatigue by direct inhibition of the cross-bridge leading to a reduction in velocity and force. More recently, due to the observation that the effects were less at temperatures closer to those observed in vivo, the importance of H+ as a fatigue agent has been questioned. The purpose of this work was to re-evaluate the role of H+ in muscle fatigue by studying the effect of low pH (6.2) on force, velocity and peak power in rat fast- and slow-twitch muscle fibres at 15°C and 30°C. Skinned fast type IIa and slow type I fibres were prepared from the gastrocnemius and soleus, respectively, mounted between a force transducer and position motor, and studied at 15°C and 30°C and pH 7.0 and 6.2, and fibre force (P0), unloaded shortening velocity (V0), force–velocity, and force–power relationships determined. Consistent with previous observations, low pH depressed the P0 of both fast and slow fibres, less at 30°C (4–12%) than at 15°C (30%). However, the low pH-induced depressions in slow type I fibre V0 and peak power were both significantly greater at 30°C (25% versus 9% for V0 and 34% versus 17% for peak power). For the fast type IIa fibre type, the inhibitory effect of low pH on V0 was unaltered by temperature, while for peak power the inhibition was reduced at 30°C (37% versus 18%). The curvature of the force–velocity relationship was temperature sensitive, and showed a higher a/P0 ratio (less curvature) at 30°C. Importantly, at 30°C low pH significantly depressed the ratio of the slow type I fibre, leading to less force and velocity at peak power. These data demonstrate that the direct effect of low pH on peak power in both slow- and fast-twitch fibres at near-in vivo temperatures (30°C) is greater than would be predicted based on changes in P0, and that the fatigue-inducing effects of low pH on cross-bridge function are still substantial and important at temperatures approaching those observed in vivo.
Intense muscular contraction is associated with high rates of ATP hydrolysis and a resulting increase in ADP, inorganic phosphate (Pi), and the hydrogen ion (H+). While the latter two have both been implicated in the development of muscle fatigue (Fitts, 1994), the focus of this work is on the role of cell pH. The intracellular pH of resting skeletal muscle is approximately 7.0, but with fatigue induced by high-intensity stimulation, pH has been reported to decline to values as low as 6.2 in amphibians (Thompson et al. 1992), 6.3 in rats (Metzger & Fitts, 1987) and 6.4 in humans (Hermansen & Osnes, 1972; Sahlin et al. 1976). The decrease in pH and its recovery correlate with changes in muscle force, implicating pH as causative in fatigue (Fitts, 1994). There are many steps in the skeletal muscle excitation–contraction pathway where a reduced pH may alter function, but attention has primarily been directed at the role of pH on the interaction between the muscle contractile proteins, actin and myosin.
The skinned muscle fibre preparation has been widely used to study contractile function under pH conditions that mimic those observed in fatigue fibres. The primary observations were that low pH decreased fibre force and slowed muscle-shortening velocity in a fibre-type-dependent manner (Chase & Kushmerick, 1988; Cooke et al. 1988; Donaldson & Hermansen, 1978; Metzger & Moss, 1987a; Seow & Ford, 1993). In slow fibres, the pH-induced decline in force appeared to be mediated by less force per cross-bridge, while in fast fibres there was an additional effect caused by a reduction in the number of bridges in the high-force state (Metzger & Moss, 1990a). In order to maximize the stability of the preparation, these results were obtained at temperatures ≤15°C. A concern is that this temperature is far below in vivo muscle temperature. More recently, a temperature jump technique was developed whereby single skin fibres were isolated at cold temperatures (10–15°C) and then rapidly jumped into a 30°C chamber for determination of contractile function (Pate et al. 1995). With this technique, it was possible to assess the effects of low pH at temperatures close to physiological ones for mammalian muscles. Utilizing this procedure, investigators have shown that acidosis-induced decrements in skinned fibre force and shortening velocity are temperature dependent, and that the detrimental effects of H+ on these contractile functions are reduced at physiological temperatures (Pate et al. 1995; Wiseman et al. 1996). Additionally, Westerblad et al. (1997) have shown similar results utilizing living fast-twitch fibres. Single mouse fibres were acidified (0.5 pH units) by increasing the CO2 content of the bath from 5% to 30%. The acidification caused a 30% decline in peak tetanic force at 12°C, but this was reduced to only 10% at 32°C. Correspondingly, acidification reduced the maximal shortening velocity (V0) by ∼20% at 12°C, while there was no significant change at 32°C. These studies led to the conclusion that acidification is not a major factor in muscle fatigue at physiological temperatures.
From a performance perspective, the important functional property is not peak tetanic force but rather muscle power, a property that is dependent on both force and velocity, and generally elicited at loads between 25% and 35% of maximal fibre force (P0). To date no studies have evaluated how pH and temperature affect the peak power of individual slow and fast fibres. Consequently, the purpose of this study was to re-evaluate the effects of low intracellular pH on force and velocity at 15°C and 30°C, and determine the extent to which the force–power relationship and peak power are altered by pH and temperature in both slow type I and fast type II fibres. A subset of these data was previously presented in abstract form (Knuth & Fitts, 1999).
Methods
Muscle preparation
Fourteen male Sprague-Dawley rats were obtained from Sasco (Madison, WI, USA) and maintained on a diet of Purina rodent chow and water. Rats averaging 380 ± 23 g were anaesthetized with pentobarbital sodium (50 mg (kg body weight)−1i.p.), and the soleus (source of type I fibres), and the deep region of the lateral head of the gastrocnemius (source of type I and IIa fibres) were removed and placed in cold (4°C) relaxing solution as previously described (Debold et al. 2004). Each muscle was dissected into small bundles (∼1 mm in width) and tied to glass capillary tubing at approximately in situ length. Rats were killed by exsanguination. All muscle bundles were immediately stored in skinning solution maintained at 4°C. After 24 h, the bundles were placed in fresh skinning solution and stored at −20°C. All contractile measurements on fibres from a given muscle were performed within 4 weeks of the initial bundle isolation.
Solutions
The composition of the relaxing (pCa 9.0) and activating (pCa 4.5) solutions for each temperature and pH were derived with an iterative computer program (Fabiato, 1988) using the stability constants reported by Fabiato & Fabiato (1979). All solutions contained (mm) 20 imidazole, 7 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 14.5 creatine phosphate, 4.4 free ATP, and 1 free Mg2+. Calcium was added as CaCl2, and ATP as a disodium salt. Each solution had an ionic strength of 180 mm, which was controlled by varying the amount of KCl added. KOH was used to adjust the pH of the solution to either 7.0 or 6.2. Creatine phosphokinase (CPK) was not included in these solutions as Metzger & Moss (1987b) had previously shown that there was adequate endogenous activity of this enzyme in skinned fibre preparations. Since their experiments were conducted at 15°C, we performed a subset of experiments at 30°C with 140 U of CPK. In addition, to prevent an increase in ADP or decline in ATP, we changed the activating solution after every two contractions. The activating and relaxing solutions were made fresh each week and stored at 4°C. The skinning solution was composed of 50% relaxing solution and 50% glycerol (v/v).
Single fibre preparation
On the day of an experiment a muscle bundle was removed from the skinning solution and transferred to a dissection chamber containing pH 7.0 relaxing solution (4°C). An individual fibre was gently isolated from the bundle and transferred to an ∼500-μl glass-bottomed chamber milled in a stainless steel plate, and containing either pH 7.0 or pH 6.2 relaxing solution. The plate was modified to incorporate a chamber that was cooled to 15°C by Peltier cells at one end and a second chamber at the opposite end, which was heated with an electrically powered heat-controlling unit to 30°C. The chambers were separated by a 3-cm plastic insulator to maintain the temperature differential. For a subset of the soleus fibres, a traditional plate was used in which both chambers were held at either 15°C or 30°C, and the temperature change made by cooling or heating with the Peltier cells. In these experiments, the fibres remained at 30°C for the duration of the tests at that temperature. For slow type I fibres, there were no significant differences in the results obtained by the two techniques (temperature jump plate versus traditional plate). However, due to fibre deterioration the conventional chamber could not be used for the study of fast type II fibres. Regardless of the chamber used, the ends of the fibre were carefully mounted and attached between a force transducer (Cambridge model 400A; Cambridge Technology, Watertown, MA, USA) and servo-controlled direct-current position motor (Cambridge model 300B, Cambridge Technology) exactly as previously described (Widrick et al. 1999). The position and speed of the motor were controlled by custom-designed software running on a microcomputer interfaced with a Lab Master DMA input–output board (Scientific Solutions, Solon, OH, USA). To disrupt any remaining intact membranes, the fibre was submerged into a relaxing solution containing Brij 58 (50 mg/10 ml) for 30 s, after which the fibre bath was exchange twice with relaxing solution.
The experimental chamber was mounted on the stage of an inverted microscope. Sarcomere length was adjusted to 2.5 μm using an eyepiece micrometer (800×), and the length of the fibre was recorded. A polaroid photo was taken of the fibre while it was briefly suspended in air. Fibre width was determined at three points along the length of the photo, and fibre cross-sectional area (CSA) calculated from the mean width measurement, assuming the fibre forms a circular cross-section when suspended in air.
Experimental design
The contractile properties measured in slow type I and fast type II fibres included peak tension (P0), the maximal unloaded shortening velocity (V0), and force–velocity and force–power relationships at 15° and 30°C. A subset of the type I fibres (n = 8) were studied with a repeated-measures design such that each fibre was subjected to both temperatures and both pH conditions. The order of conditions was balanced such that an equal number of fibres were initially exposed to pH 6.2 and 7.0. This method controlled for potential order effects. In all experiments, the fibre was exposed to the 15°C condition first. The fast type II fibres were not as stable as the type I fibres at 30°C, thus a subset of slow fibres and all of the fast fibres were studied with a mixed design. One group of fibres was subjected to pH 6.2 and pH 7.0 at 15°C, while a separate set of fibres was studied at both pHs at 30°C. Fibres were excluded from the analysis if the maximal isometric force dropped below 90% of the initial P0 (Fig. 2). In all experiments, the solution temperature was monitored continuously by a micro-temperature probe.
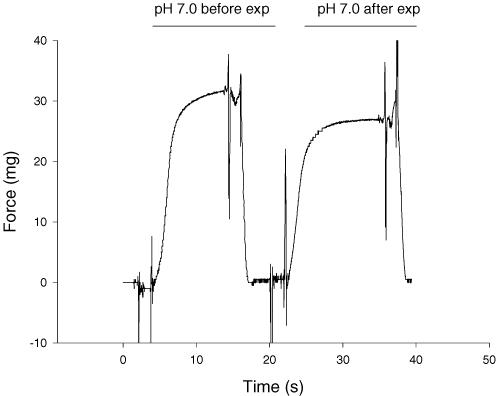
Figure 2. Force traces for a slow type I fibre.
The left trace is a force recording at the beginning of the experiment (exp) under control conditions (pH 7.0 and 15°C), while the right trace is under the same conditions but following the 30°C experiments carried out at pH 6.2 and 7.0. This representative fibre had maintained 93% of its initial peak force (P0). The large deflections at the beginning and end of each force record are artifacts caused during movement of the fibre between the relaxing and activating solutions.
Contractile tests
Peak force was calculated as the difference between the force recorded when the fibre was in relaxing solution and the force attained during maximal Ca2+ activation. Peak tension was calculated as the peak force/fibre CSA.
The unloaded shortening velocity (V0) was measured at 15°C and 30°C by the slack test method as previously described (Widrick et al. 1997, 1999). Briefly, the fibre was maximally activated in pCa 4.5 solution, allowed to reach peak isometric force, and slacked to a predetermined length, which caused tension to drop to zero. The time it took the fibre to take up the slack and initiate the redevelopment of tension was measured. The fibre was then returned to relaxing solution (15°C) and re-extended to its original fibre length. Each fibre was subjected to five different slack steps and fibre V0 (fl s−1) determined from the slope of the least squares regression line of the plot of slack distance versus the time required for the redevelopment of force. At 30°C the fibre had an increased rate of contraction, and consequently data acquisition was increased from 3.33 kHz to 20 kHz. Slack length changes never exceeded 15% of fibre length (mean fibre length = 2.09 mm).
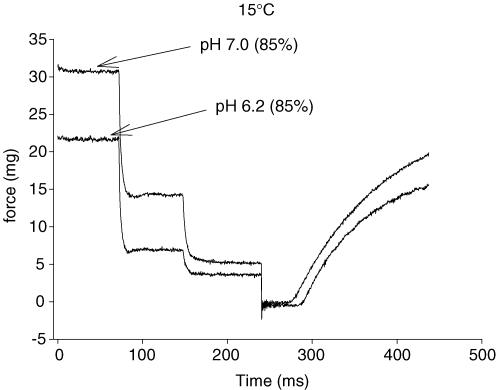
The single-fibre force–velocity parameters were determined by maximally activating the fibre and then (after attainment of steady force) stepping it to three submaximal isotonic loads (Fig. 1). Each load was maintained for a predetermined time. At 15°C the time was either 80 or 100 ms, while at 30°C the time was decreased to 40 ms to prevent the fibre from over shortening. Each fibre was contracted 4–6 times, thus between 12 and 18 data points were obtained for each force–velocity curve. A computer program determined fibre force and shortening velocity over the last half of the force and position records, respectively. The force (as a percentage) and the corresponding shortening velocity were fit to the Hill equation (Hill, 1938) with the use of an iterative non-linear curve-fitting procedure (Marquardt-Levenberg algorithm) as previously described (Widrick et al. 1996). Peak fibre power was calculated with the fitted parameters of the force-velocity curve and P0 (Widrick et al. 1996). Composite force–velocity and force–power curves were constructed by summating velocities or power values from 0 to 100% of P0 in increments of 1%.
Figure 1. Representative force traces for a fast type IIa fibre for three isotonic loads at pH 7.0 and 6.2 each followed by a slack step to zero load.
The highest load for each condition represented 85% of P0.
Myosin heavy chain composition
After the contractile tests, the fibre was removed from the experimental set-up and solubilized in 10 μl of sodium dodecyl sulphate (SDS) sample buffer (6 mg ml−1 EDTA, 0.06 m tris(hydroxymethyl)aminomethane, 1% SDS, 2 mg ml−1 bromophenol blue, 15% glycerol, 5%β-mercaptoethanol). The myosin heavy chain profile of each fibre was identified by loading 2.5 μl of sample buffer on a 3% (w/v) acrylamide stacking gel with a 5% (w/v) acrylamide separating gel (Widrick et al. 1999). All gels were silver stained using the techniques described by Giulian et al. (1983), and fibres identified as slow type I, fast type IIa, fast type IIx or fast type IIb.
Statistical analysis
For the fibres studied with a repeated-measures design, the data were analysed with an ANOVA with repeated factors (temperature and pH). The entire data set was analysed with an ANOVA with a group/independent factor (15°C and 30°C) and a repeated factor (pH). A Tukey's post hoc test was used, and statistical significance accepted at P < 0.05.
Results
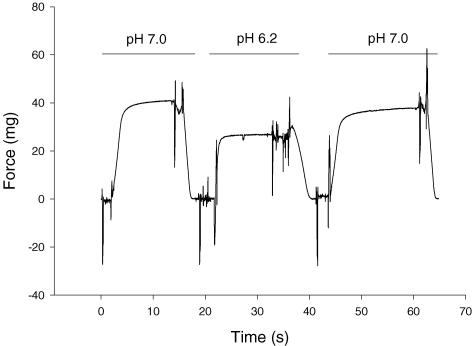
For the slow type I fibres, peak force was well maintained throughout the experiment at both pHs, and temperatures (Fig. 2). Fast fibres were less stable, but we were able to maintain their viability for the number of contractions required to study both pH conditions at one temperature (usually 12 contractions). Figure 3 shows representative force traces for a fast type IIa fibre at pH 7.0, pH 6.2, and once again at pH 7.0. The force at the end of the experiment was 93% of the initial force. The effects of temperature and pH on peak force (P0) for slow type I and fast type II fibres are shown in Tables 1 and 2, respectively. The region of the gastrocnemius sampled contains primarily fast type IIa fibres, thus all of the data in Tables 2 and 4 are from fast type IIa fibres. In both slow and fast fibres, peak force increased with temperature, while the inhibitory effect of low pH on force (both mN and kN m−2) was higher at 15°C than 30°C (Tables 1 and 2). At 15°C, low pH induced an ∼30% decline in peak force in both fast and slow fibres. However at 30°C, the inhibitory effect of low pH was only 12% in slow fibres and between 4% and 11% in fast fibres (Tables 1 and 2).
Figure 3. Representative force traces for a fast type IIa fibre at pH 7.0, pH 6.2, and once again at pH 7.0.
For all conditions the temperature was 15°C. The force at the end of the experiment was 93% of the initial force.
Table 1.
Effect of pH and temperature on type I fibre force and velocity
| P0 | |||||
|---|---|---|---|---|---|
| Muscle | n | Diameter (μm) | (mN) | (kN m−2) | V0 (fl s−1) |
| 15°C pH 7 | 44 | 72 ± 2 | 0.47 ± 0.02 | 120 ± 4 | 1.16 ± 0.04 |
| 15°C pH 6.2 | 44 | 72 ± 2 | 0.33 ± 0.01* | 83 ± 3* | 1.05 ± 0.02* |
| % change | −30 | −31 | −9 | ||
| 30°C pH 7 | 40 | 65 ± 2 | 0.50 ± 0.02 | 153 ± 5 | 9.97 ± 0.38 |
| 30°C pH 6.2 | 40 | 65 ± 2 | 0.44 ± 0.02* | 135 ± 5* | 7.47 ± 0.27* |
| % change | −12 | −12 | −25 | ||
| 30°C pH 7 w/CPK | 7 | 60 ± 2 | 0.44 ± 0.03 | 158 ± 3 | 10.55 ± 0.37 |
| 30°C pH 6.2 w/CPK | 7 | 60 ± 2 | 0.39 ± 0.02 | 139 ± 3* | 7.60 ± 0.35* |
| % change | −11 | −12 | −28 | ||
Values are means ±s.e.m.; n, no. of fibres studied. P0, peak isometric force; V0, maximal shortening velocity; w/CPK, solutions contained 140 U of creatine phosphokinase.
Significantly different from pH 7, P < 0.05. At both pHs, the P0 and V0 values are significantly higher at 30°C, compared to 15°C.
Table 2.
Effect of pH and temperature on type II fibre force and velocity
| P0 | |||||
|---|---|---|---|---|---|
| Muscle | n | Diameter (μm) | (mN) | (kN m−2) | V0 (fl s−1) |
| 15°C pH 7 | 17 | 59 ± 2 | 0.39 ± 0.02 | 143 ± 5 | 3.70 ± 0.21 |
| 15°C pH 6.2 | 17 | 59 ± 2 | 0.26 ± 0.02* | 98 ± 5* | 2.71 ± 0.14* |
| % Change | −33 | −31 | −27 | ||
| 30°C pH 7 | 12 | 62 ± 2 | 0.55 ± 0.03 | 184 ± 10 | 19.93 ± 1.15 |
| 30°C pH 6.2 | 13 | 61 ± 2 | 0.52 ± 0.03 | 176 ± 8 | 13.46 ± 0.81* |
| % Change | −5 | −4 | −32 | ||
| 30°C pH 7 w/CPK | 7 | 51 ± 4 | 0.36 ± 0.04 | 175 ± 5 | 18.86 ± 0.37 |
| 30°C pH 6.2 w/CPK | 6 | 51 ± 4 | 0.32 ± 0.04 | 156 ± 7* | 12.95 ± 0.27* |
| % Change | −11 | −11 | −31 | ||
Values are means ±s.e.m.; n, no. of fibres studied. P0, peak isometric force; V0, maximal shortening velocity; w/CPK, solutions contained 140 U of creatine phosphokinase.
Significantly different from pH 7, P < 0.05. At both pHs, the P0 and V0 values are significantly higher at 30°C, compared to 15°C.
Table 4.
Effect of pH and temperature on type II fibre force–velocity–power parameters
| Peak power | ||||||||
|---|---|---|---|---|---|---|---|---|
| Muscle | n | P0 (kN m−2) | Vmax (fl s−1) | a/P0 | (μN fl s−1) | (W l−1) | Velocity at peak power (fl s−1) | Force at peak power (kN m−2) |
| 15°C pH 7 | 19 | 141 ± 5 | 1.77 ± 0.06 | 0.19 ± 0.01 | 66.0 ± 5.2 | 20.0 ± 1.1 | 0.50 ± 0.01 | 38.9 ± 1.4 |
| 15°C pH 6.2 | 19 | 96 ± 4* | 1.45 ± 0.06* | 0.24 ± 0.02* | 42.4 ± 4.1* | 12.6 ± 0.8* | 0.43 ± 0.02* | 28.0 ± 1.0* |
| % Change | −32 | −18 | 26 | −36 | −37 | −14 | −28 | |
| 30°C pH 7 | 19 | 180 ± 5 | 7.14 ± 0.42 | 0.58 ± 0.07 | 512.1 ± 28.8 | 166.0 ± 8.9 | 2.53 ± 0.09 | 63.1 ± 3.1 |
| 30°C pH 6.2 | 18 | 161 ± 5* | 5.99 ± 0.36* | 0.65 ± 0.06 | 461.1 ± 38.8 | 135.3 ± 7.2* | 2.24 ± 0.11* | 58.0 ± 2.2 |
| % Change | −11 | −16 | 12 | −10 | −18 | −11 | −8 | |
| 30°C pH 7 w/CPK | 6 | 166 ± 7 | 7.09 ± 0.19 | 0.36 ± 0.02 | 337.3 ± 21.5 | 134.1 ± 2.2 | 2.40 ± 0.06 | 56.1 ± 1.9 |
| 30°C pH 6.2 w/CPK | 6 | 145 ± 6* | 5.76 ± 0.17* | 0.33 ± 0.04 | 222.6 ± 14.7* | 90.0 ± 3.5* | 1.89 ± 0.04* | 47.6 ± 1.8* |
| % Change | −13 | −19 | −8 | −34 | −33 | −21 | −15 | |
Values are means ±s.e.m.; n, no. of fibres studied. Vmax, maximal unloaded shortening velocity determined from the Hill plot test; w/CPK, solutions contained 140 U of creatine phosphokinase; a/P0, unitless parameter describing curvature of the force–velocity relationship. The relative power unit of watts/litre (W l−1) is equivalent of kN m−2 fl s−1.
Significantly different from pH 7, P < 0.05. At both pHs, all values are significantly higher at 30°C, compared to 15°C.
Low pH significantly decreased V0 in slow and fast fibre types and at both temperatures (Tables 1 and 2), but interestingly this effect was less pronounced in type I fibres at 15°C (9% decline) than in the other three conditions (25–30% decline).
For the type I fibre, the repeated measures design showed a statistically significant interaction (P < 0.05) between temperature and pH for both peak force and maximal velocity. At the near-physiological temperature of 30°C, peak force (mN and kN m−2) and V0 were less and more depressed by low pH, respectively, compared to 15°C.
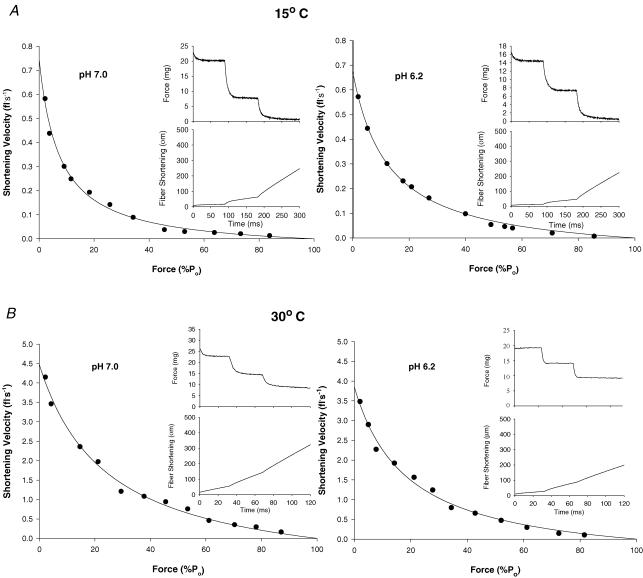
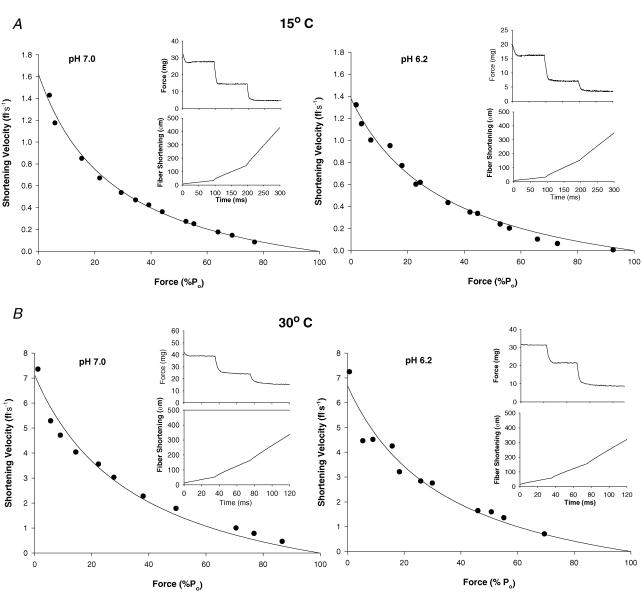
Figure 4A shows representative force–velocity curves for a single slow type I fibre at 15°C and pH 7.0 and 6.2, while Fig. 4B shows similar curves for a slow fibre studied at 30°C. Force and position traces for three isotonic contractions for each condition are shown in the insets. Figure 5A and B shows representative force–velocity curves at 15°C and 30°C and pH 7.0 and 6.2 for the fast type IIa fibre type. The mean force–velocity relationship for slow type I and fast type IIa fibres are shown in Figs 6 and 7, respectively. As with V0, The maximal shortening velocity of the slow fibre type determined from the force–velocity plot (Vmax) was depressed by low pH to a greater extent at 30°C than at 15°C. For both fibre types the pH-induced decline in Vmax was less than that observed for unloaded shortening velocity (V0). The fast-fibre force–velocity relationship exhibited less curvature as evidenced by the higher a/P0 ratio compared to the slow fibre type (Tables 3 and 4). For both fibre types, the a/P0 ratio increased with the increase in temperature (Tables 3 and 4). The effect of low pH on the curvature of the force–velocity relationship was temperature dependent. Importantly, low pH at 30°C significantly depressed the a/P0 ratio in slow type I fibre type (Fig. 6 and Table 3), while at 15°C low pH increased the ratio in both fibre types (Tables 3 and 4).
Figure 4. Force and velocity data points from 12 to 15 contractions from a representative slow-twitch fibre at 15°C and pH 7.0 and 6.2 (A) and 30°C and pH 7.0 and 6.2 (B) were plotted and fitted to the Hill equation.
Insets depict sample traces of force (top) and shortening (bottom) during a maximal contraction in which the fibre was switched from P0 (not shown) to three isotonic loads. For pH 7.0 and 6.2, respectively, the contractile parameters for fibre in A were Vmax = 0.75 and 0.68 fl s−1, P0 = 0.45 mN and 0.30 mN or 110 kN m−2 and 83 kN m−2, and the a/P0 = 0.073 and 0.123, and for the fibre in BVmax = 4.47 fl s−1 and 3.85 fl s−1, P0 = 0.51 mN and 0.44 mN or 151 kN m−2 and 121 kN m−2, and the a/P0 = 0.238 and 0.186.
Figure 5. Force and velocity data points from 11 to 15 contractions from representative fast-twitch fibres at 15°C and pH 7.0 and 6.2 (A) and 30°C and pH 7.0 and 6.2 (B) were plotted and fitted to the Hill equation.
Insets depict sample traces of force (top) and shortening (bottom) during a maximal contraction in which the fibre was switched from P0 (not shown) to three isotonic loads. For pH 7.0 and 6.2, respectively, the contractile parameters for fibre in A were Vmax = 1.62 fl s−1 and 1.38 fl s−1, P0 = 0.58 mN and 0.37 mN or 125 kN m−2 and 80 kN m−2, and the a/P0 = 0.266 and 0.304, and for the fibre in B, Vmax = 7.13 fl s−1 and 6.69 fl s−1, P0 = 0.46 mN and 0.48 mN or 169 kN m−2 and 148 kN m−2, and the a/P0 = 0.356 and 0.337.
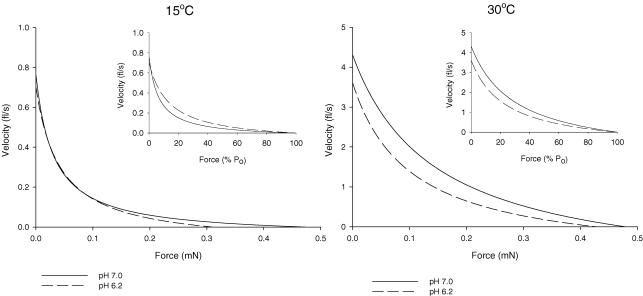
Figure 6. Composite force–velocity curves at 15° and 30°C for soleus type I fibres.
Shortening velocity is plotted as a function of force in mN (main graphs) and as a function of percentage P0 (insets).
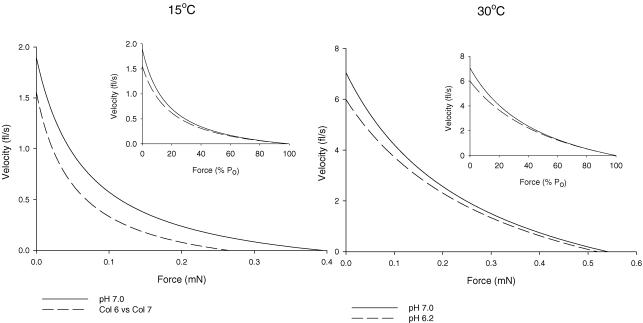
Figure 7. Composite force–velocity curves at 15 and 30°C for gastrocnemius type IIa fibres.
Shortening velocity is plotted as a function of force in mN (main graphs) and as a function of percentage P0 (insets).
Table 3.
Effect of pH and temperature on type I fibre force–velocity–power parameters
| Peak power | ||||||||
|---|---|---|---|---|---|---|---|---|
| Muscle | n | P0 (kN m−2) | Vmax (fl s−1) | a/P0 | (μN fl s−1) | (W l−1) | Velocity at peak power (fl s−1) | Force at peak power (kN m−2) |
| 15°C pH 7 | 43 | 118 ± 4 | 0.77 ± 0.04 | 0.07 ± 0.00 | 14.4 ± 0.7 | 3.5 ± 0.3 | 0.15 ± 0.01 | 24.0 ± 0.9 |
| 15°C pH 6.2 | 44 | 80 ± 3* | 0.71 ± 0.03 | 0.10 ± 0.00* | 11.1 ± 0.6* | 2.9 ± 0.2* | 0.16 ± 0.01 | 17.9 ± 0.7* |
| % Change | −32 | −8 | 43 | −23 | −17 | 7 | −25 | |
| 30°C pH 7 | 39 | 147 ± 5 | 4.31 ± 0.15 | 0.33 ± 0.03 | 217.6 ± 13.4 | 65.5 ± 2.9 | 1.38 ± 0.04 | 47.3 ± 2.1 |
| 30°C pH 6.2 | 39 | 131 ± 5* | 3.61 ± 0.16* | 0.25 ± 0.02* | 143.1 ± 9.8* | 43.0 ± 2.3* | 1.08 ± 0.04* | 39.6 ± 1.8* |
| % Change | −11 | −16 | −24 | −34 | −34 | −22 | −16 | |
| 30°C pH 7 w/CPK | 7 | 155 ± 4 | 4.78 ± 0.22 | 0.50 ± 0.03 | 273.8 ± 15.1 | 98.7 ± 5.7 | 1.74 ± 0.07 | 56.5 ± 1.3 |
| 30°C pH 6.2 w/CPK | 7 | 139 ± 4* | 3.21 ± 0.19* | 0.38 ± 0.02* | 147.9 ± 14.2* | 53.0 ± 4.1* | 1.10 ± 0.05* | 47.7 ± 1.6* |
| % Change | −10 | −33 | −24 | −46 | −46 | −37 | −16 | |
Values are means ±s.e.m.; n, no. of fibres studied; Vmax, maximal unloaded shortening velocity determined from the Hill plot test; w/CPK, solutions contained 140 U of creatine phosphokinase; a/P0, unitless parameter describing curvature of the force–velocity relationship. The relative power unit of watts/litre (W l−1) is equivalent of kN m−2 fl s−1.
Significantly different from pH 7, P < 0.05. At both pHs, all values are significantly higher at 30°C, compared to 15°C.
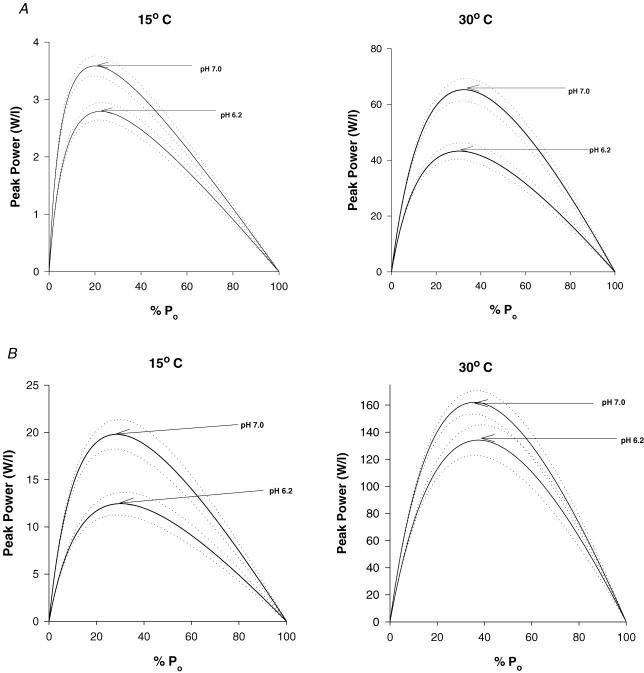
Temperature significantly increased peak power and the velocity and force at peak power in both slow and fast fibres (Tables 3 and 4, and Fig. 8). The effects of low pH were temperature and fibre-type dependent. In the slow type I fibre, peak power was depressed more by low pH at the higher temperature (34%) than at 15°C (17%). The opposite was true in the fast type IIa fibre, as low pH induced less inhibition of peak power at 30°C (18%) than at 15°C (37%). For the slow fibre type, the greater effect of low pH in inhibiting peak power at 30°C compared to 15°C was caused by a decline in both velocity and force at peak power, while at 15°C only force was depressed (Table 3). The reverse was true for the type IIa fibre type, where both velocity and force at peak power were depressed at 15°C but not 30°C. The addition of CPK to the 30°C solutions had no effect on the pH 6.2-induced depression of P0 and V0 (Tables 1 and 2), while peak power was depressed to a greater extent then without CPK (Tables 3 and 4). This difference was not attributed to the presence of the applied CPK, as fibres from this muscle without CPK in the solutions showed a similar response (unpublished observation).
Figure 8. Composite force–power curves at 15 and 30°C and pH 7.0 and 6.2 for soleus type I fibres (A) and gastrocnemius type IIa fibres (B).
The dotted lines represent ±1 standard error of estimate.
Discussion
Historically, intracellular acidosis has been thought to play an important role in the development of muscle fatigue (Fitts, 1994). Intense exercise induces high rates of ATP hydrolysis and glycolysis, and corresponding increases in cell H+, where pH may reach values as low as 6.2 (Metzger & Fitts, 1987; Thompson et al. 1992). Furthermore, in vitro experiments performed on isolated muscle fibres at relatively cold temperatures (10–15°C) have shown low pH significantly decreases P0 and V0 (Fabiato & Fabiato, 1978; Metzger & Moss, 1987a; Metzger & Moss, 1990a). More recently there have been some reports that the effects of low pH on both P0 and V0 are greatly reduced at temperatures above 30°C, suggesting that the fatigue-inducing effects of low pH acting directly at the cross-bridge may be less important than originally thought (Pate et al. 1995; Westerblad et al. 1997; Wiseman et al. 1996). However, these experiments did not establish the fibre type of the fibre population studied, or report the effect of pH on peak power. The work described here was the first to evaluate the effects of low cell pH on the contractile properties of identified slow- and fast-twitch fibres at low (15°C) and near-in vivo (30°C) temperatures. The novel and most important observation was that while the inhibiting effects of low pH on fibre force were reduced in both slow- and fast-twitch fibres at the near in vivo temperature of 30°C, peak power was depressed to a greater extent in the slow type I fibre at 30°C (34%) compared to 15°C (17%). Additionally, while the loss in peak power for the fast-twitch fibre was less at the high temperature (18% versus 37%), it was still considerably greater then the decline in P0. These data illustrate that the fatigue-inducing effects of low pH on cross-bridge function are still substantial and important at temperatures approaching those observed in vivo.
Effect of pH on P0
At 30°C, acidification decreased P0 by only 12% in slow fibres and somewhat less that that in fast fibres, compared to an ∼30% decrease in both fibre types at 15°C. This is similar to the results of Pate et al. (1995) who reported that acidification decreased P0 18% at 30°C, and Westerblad et al. (1997) who saw a 10% decrease in P0 at 32°C. The fibres were not typed in either study, but both studied fibres from primarily fast-twitch muscles. These results suggest that at in vivo muscle temperatures (30–38°C) and saturating levels of Ca2+, acidosis would not be a primary cause of the fatigue-induced decrease in force production. This conclusion is supported by the work of Adams et al. (1991) who observed no reduction in peak tetanic force after perfusing the cat biceps brachii and soleus with 70% CO2, reducing intracellular pH from 7.1 to 6.4. However, the reduced force in fatigued muscle fibres is generally associated with and thought to be in part caused by a decline in the amplitude of the Ca2+ transient (Fitts, 1994). Since low pH is known to shift the pCa–force relationship to the right (more Ca2+ required for a given force), the fatigue-inducing effect of low pH would be exaggerated in fibres with a less than saturating level of free Ca2+. While the low pH effect on the pCa–force relationship has not been determined at near-physiological temperatures, we have recently observed the right shift induced by inorganic phosphate to be greater at 30 compared to 15°C (Debold et al. 2006). If pH were to show similar temperature dependence, the force-inhibiting effect of low pH at suboptimal Ca2+ concentrations might be greater at 30°C compared to 15°C.
The mechanism for attenuation of acidosis effect on P0 at physiological temperature is unknown. We observed similar temperature dependence for the inorganic phosphate (Pi) depression of P0 (Debold et al. 2004). Increases in intracellular Pi are thought to reduce force by accelerating the reverse rate constant for the transition of the cross-bridge from the low- to the high-force state: AM−ADP−Pi (weak binding, low force) ↔ AM′−ADP + Pi (strong binding, high force), while the inhibition of force by H+ at least in fast fibres is thought to be in part caused by a depressant effect on the forward rate constant of the force-generating step (Metzger & Moss, 1990b). The reduced effect of these ions on P0 at high compared to low temperature may result from the forward rate constant of the force-generating step being more temperature sensitive than the reverse rate constant (Debold et al. 2004; Zhao & Kawai, 1994). Studies using sinusoidal analysis have shown the forward rate constant for force development to have a high Q10 and to be greatly accelerated as temperature increases (Zhao & Kawai, 1994). As a consequence, the cross-bridge would spend a relatively greater proportion of the cycle strongly bound to actin. This effect would explain why P0 increases with temperature, and the reduced effect of low pH at in vivo muscle temperatures. It would also explain why the low pH effect is reduced more in fast compared to slow fibres as a portion of the effect in fast fibres has been attributed to inhibition of the forward rate constant of force generation (Metzger & Moss, 1987b, 1990a, 1990b). It is also possible that the force per cross-bridge is depressed by low pH, an effect that is partially offset as temperature rises. The suggestion that low pH depresses the force per cross-bridge is supported by the observation of Metzger & Moss (1990a) of a linear relationship between relative tension and relative stiffness, and a depression of the slope of this relationship in both fast and slow fibres at pH 6.2 compared to pH 7.0. The authors concluded that if stiffness is a measure of the number of cross-bridge attachments to actin, that the force per cross-bridge was similarly depressed in fast- and slow-twitch fibres at low pH. At maximal Ca2+ activation, they found stiffness to be depressed by 22% in fast-twitch fibres but unaltered in slow-twitch fibres. This result was interpreted as a low pH-induced reduction in the number of high-force bridges in fast- but not slow-twitch fibres. This finding was consistent with an earlier report by Metzger & Moss (1987b) that low pH depressed P0 to a greater extent in fast- compared to slow-twitch fibres. At 15°C, our results did not show a fibre type-dependent effect of low pH on P0. This discrepancy may be attributed to the fact that the fast fibres in this study were fast type IIa, while in the study of Metzger & Moss (1987b) the fibres were not typed. It maybe that the fibre type difference in the P0 susceptibility to low pH is between fast type IIb/IIx and slow type I fibres.
Effect of pH on shortening velocity
Our observation that low pH depressed V0 at both 15°C and 30°C conflicts with the 6% increase in Vmax observed by Pate et al. (1995) at 30°C and pH 6.2. Additionally the magnitude of the depression in the V0 of the fast-twitch fibres in this study (∼30%) was considerably greater than the non-significant change observed by Westerblad et al. (1997) in living fibres at pH 6.7 and 32°C. Direct comparisons between our results and those of Westerblad et al. (1997) are difficult because they used living fibres and lowered the pH by bubbling the bath solution with 30% CO2–70% O2. If they were able to decrease pH to 6.2 they may have observed a significant depression in fibre V0.
Compared to V0, we observed Vmax of fast and slow fibres to underestimate maximal shortening velocity and to be ∼50% less influenced by low pH at both 15°C and 30°C. The lower Vmaxversus V0 is a consistent observation by us and others (Widrick et al. 1996), and has been attributed to sarcomere non-uniformity during loaded contractions (Widrick et al. 1996). Additionally, Vmax is an extrapolated value that is influenced by the accuracy of the curve-fitting procedure, while V0 is determined directly from the unloaded shortening data. V0 is a more sensitive indicator of shortening velocity and can clearly distinguish between fast type IIx and IIa fibres, while Vmax cannot (Bottinelli et al. 1991; Widrick et al. 1996). Thus it seems likely that the primary reason for the discrepancy between our results and those of Pate et al. (1995) relates to the lack of sensitivity in the Vmax measurement. The skinned fibre solutions of Pate et al. (1995) differ from our solutions by an increase in ionic strength (200 versus 180 mm), and Pi content (5 mm Piversus no Pi added). Other differences include the pH buffer, Mes or Mops in their study, and imidazole in this study. It seems unlikely that the small difference in ionic strength between studies could explain the contrasting results. The discrepancy between studies could not be due to the different Pi concentration, as Pi has been shown to have no effect on fibre velocity in any fibre type (Cooke et al. 1988; Debold et al. 2004). The low pH-induced decline in fibre V0 was not caused by ATP depletion and/or ADP build-up, as force remained constant during a contraction and the bath solution was changed every few contractions.
It is not clear how an increase in free H+ (low pH) depressed velocity, but possibilities include a direct inhibition of the myofibrillar ATPase and/or an acid-induced decrease in the myofilament lattice spacing (Umazume et al. 1986). Cell pH 6.0 was shown to decrease lattice spacing by 5–7%, and a reduction in filament spacing with high-molecular weight polymers was shown to reduce fibre V0 (Metzger & Moss, 1987b; Umazume et al. 1986).
Effect of temperature and pH on a/P0 and peak power
The peak power recorded for the slow-twitch fibres in this study at 15°C and pH 7.0 agreed well with the data of Debold et al. (2004), while at 30°C our power values were somewhat lower. The reduced type I fibre power relative to the study of Debold et al. (2004) was related to lower velocity and force at peak power. The peak power of fast fibres at pH 7.0 and 15°C is the same as the data of Debold et al. (2004), while at 30°C the data reported here were slightly higher (165 versus 136 W l−1). This difference was due to a higher velocity at peak power in this study (2.51 versus 2.20 fl s−1).
Importantly, the a/P0 ratio increased (less curvature in the force–velocity relationship) with temperature in both slow- and fast-twitch fibres. In the slow type I fibre at 15°C, low pH increased the a/P0 ratio, and this allowed the velocity at peak power to be maintained despite a significant decline in the maximal velocity (Ranatunga, 1984). In contrast, at 30°C, low pH significantly reduced the a/P0 ratio in slow fibres. While this probably contributed to the decline in velocity at peak power, the greater inhibition of peak power by low pH in this fibre type was caused by the combined effects of a reduced force and velocity. While for the fast fibre type, the low pH-induced decline in peak power was less at 30°C compared to 15°C, it still showed an 18% depression. In the study of Pate et al. (1995) peak power was not calculated. However from the force–velocity curves presented in Fig. 2 of that paper, it is apparent that at 30°C, pH 6.2 and in the range where peak power is generated (between 25% and 35% of P0), peak power of the rabbit psoas fibres was depressed by >20%. Although the fibres in the Pate et al. (1995) study were not typed, the psoas is composed almost entirely of fast-twitch fibres.
In summary, these data demonstrate that the direct effect of low pH on peak power in both slow- and fast-twitch fibres at near-in vivo temperatures (30°C) is greater than would be predicted based on changes in P0. For example, at 30°C, slow-fibre P0 showed only a 12% decline at pH 6.2, while peak power (W l−1) fell by 34%. For fast fibres, the change with low pH at 30°C was 11% and 18% for P0 and peak power, respectively. Since the important parameter for performance is peak power and not isometric force, these results demonstrate that the development of cell acidosis with intense exercise does make an important direct contribution to the development of muscle fatigue (i.e. loss of peak power) at low (15°C) and near-in vivo (30°C) temperatures. Furthermore during intense exercise, the importance of an increased H+ in contributing to fatigue would be exacerbated by a non-H+ ion-mediated decline in the amplitude of the intracellular Ca2+ transient to suboptimal levels. Since H+ shifts the pCa–force curve to the right, the force and power developed at a given suboptimal Ca2+ content would be further reduced. Thus when evaluating the importance of low pH in the aetiology of muscle fatigue, it is important to consider the reduction in peak power as well as indirect effects on an increased H+ such as inhibition of the sarcolemma Na+–K+-ATPase, and the sarcoplasmic reticulum pump ATPase.
Acknowledgments
We thank Janell Romatowski for her help in performing the myosin heavy chain isoform analyses and in the preparation of the figures and tables, and Carla Langenthal for her assistance in some of the single-fibre studies. This research was supported in part by National Aeronautics and Space Administration Grants NAG 9-1156 and NCC9-116 to R.H. Fitts, and by the American Heart Association Wisconsin Affiliate Predoctoral Fellowships (#96-F-11 and #97-F-30), and Marquette University Schmitt and Jobling Fellowships to S.T. Knuth.
References
- Adams GR, Fisher MJ, Meyer RA. Hypercapnic acidosis and increased H2PO4 concentration do not decrease force in cat skeletal muscle. Am J Physiol. 1991;260:C805–C812. doi: 10.1152/ajpcell.1991.260.4.C805. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C. Force–velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscles. J Physiol. 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase PB, Kushmerick MJ. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988;53:935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Franks K, Luciani G, Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debold EP, Dave H, Fitts RH. Fiber type and temperature dependence of inorganic phosphate: implications for fatigue. Am J Physiol Cell Physiol. 2004;287:C673–C681. doi: 10.1152/ajpcell.00044.2004. [DOI] [PubMed] [Google Scholar]
- Debold EP, Romatowski J, Fitts RH. The depressive effect of Pi on the force–calcium relationship in skinned single muscle fibres is temperature dependent. Am J Physiol Cell Physiol. 2006;290:C1041–C1050. doi: 10.1152/ajpcell.00342.2005. [DOI] [PubMed] [Google Scholar]
- Donaldson SK, Hermansen L. Differential, direct effects of H+ on Ca2+-activated force of skinned fibers from the soleus, cardiac and addutor magnus muscles of rabbits. Pflugers Arch. 1978;376:55–65. doi: 10.1007/BF00585248. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Meth Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscles. J Physiol. 1978;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Giulian GG, Moss RL, Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimesnional silver-stained slab gels. Anal Biochem. 1983;129:277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Hermansen L, Osnes J. Blood and muscle pH after maximal exercise in man. J Appl Physiol. 1972;32:304–308. doi: 10.1152/jappl.1972.32.3.304. [DOI] [PubMed] [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci. 1938;126:136–195. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Knuth ST, Fitts RH. Effect of pH and temperature on the contractile properties of slow skinned skeletal muscle fibers. Med Sci Sports Exercise. 1999;31:S222. [Google Scholar]
- Metzger JM, Fitts RH. Role of intracellular pH in muscle fatigue. J Appl Physiol. 1987;62:1392–1397. doi: 10.1152/jappl.1987.62.4.1392. [DOI] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Shortening velocity in skinned muscle fibers: influence of filament lattice spacing. Biophys J. 1987a;52:127–131. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987b;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. Effects on tension and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal muscle fibres. J Physiol. 1990a;428:737–750. doi: 10.1113/jphysiol.1990.sp018238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Moss RL. pH modulation of the kinetics of a Ca2+-sensitive cross-bridge state transition in mammalian single skeletal muscle fibres. J Physiol. 1990b;428:751–764. doi: 10.1113/jphysiol.1990.sp018239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga KW. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle samples obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Seow C, Ford L. High ionic strength and low pH detain activated skinned rabbit skeletal muscle crossbridges in a low force state. J Gen Physiol. 1993;101:487–511. doi: 10.1085/jgp.101.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LV, Balog EM, Fitts RH. Muscle fatigue in frog semitendinosus: role of intracellular pH. Am J Physiol Cell Physiol. 1992;262:C1507–C1512. doi: 10.1152/ajpcell.1992.262.6.C1507. [DOI] [PubMed] [Google Scholar]
- Umazume Y, Onodera S, Higuchi H. Width and lattice spacing in radiaclly compressed frog skinned muscle fibres at various pH values, magnesium ion concentrations, and ionic strengths. J Muscle Res Cell Motility. 1986;7:251–258. doi: 10.1007/BF01753558. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton J, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JLW, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17-day spaceflight on contractile properties of human soleus fibers. J Physiol. 1999;516:915–930. doi: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widrick JJ, Romatowski JG, Karhanek M, Fitts RH. Contractile properties of rat, rhesus monkey, and human type I muscle fibers. Am J Physiol. 1997;272:R34–R42. doi: 10.1152/ajpregu.1997.272.1.R34. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Trappe SW, Costill DL, Fitts RH. Force-velocity and force-power properties of single muscle fibers from elite master runners and sedentary men. Am J Physiol Cell Physiol. 1996;271:C676–C683. doi: 10.1152/ajpcell.1996.271.2.C676. [DOI] [PubMed] [Google Scholar]
- Wiseman RW, Beck TW, Chase PB. Effect of intracellular pH on force development depends on temperature in intact skeletal muscle from mouse. Am J Physiol Cell Physiol. 1996;271:C878–C886. doi: 10.1152/ajpcell.1996.271.3.C878. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kawai M. Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J. 1994;67:1655–1668. doi: 10.1016/S0006-3495(94)80638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]