Abstract
The dopamine transporter (DAT) plays an important role in calibrating the duration and intensity of dopamine neurotransmission in the central nervous system. We have used a strain of mice in which the gene for the DAT has been genetically deleted to identify the DAT’s homeostatic role. We find that removal of the DAT dramatically prolongs the lifetime (300 times) of extracellular dopamine. Within the time frame of neurotransmission, no other processes besides diffusion can compensate for the lack of the DAT, and the absence of the DAT produces extensive adaptive changes to control dopamine neurotransmission. Despite the absence of a clearance mechanism, dopamine extracellular levels were only 5 times greater than control animals due to a 95% reduction in content and a 75% reduction in release. Paradoxically, dopamine synthesis rates are doubled despite a decrease of 90% in the levels of tyrosine hydroxylase and degradation is markedly enhanced. Thus, the DAT not only controls the duration of extracellular dopamine signals but also plays a critical role in regulating presynaptic dopamine homeostasis. It is interesting to consider that the switch to a dopamine-deficient, but functionally hyperactive, mode of neurotransmission observed in mice lacking the DAT may represent an extreme example of neuronal plasticity resulting from long-term psychostimulant abuse.
Dopamine is an important regulator of many physiological functions, including control of locomotion, cognition, affect, and neuroendocrine hormone secretion (1, 2). In the central nervous system, dopamine signaling is governed by a balance between the amount released, the duration of effects, and the responsiveness of receptors. The dopamine transporter is thought to play a central role in determining the duration of action of dopamine by rapidly taking up extracellular dopamine into presynaptic terminals after release (3). Differences in the number of uptake sites (4) in different brain regions provides dopamine with different extracellular lifetimes (5), which allows its diffusion to remote receptor sites (6). Furthermore, dopamine uptake rates are lowered by drugs of abuse such as cocaine and amphetamines, and this action is responsible for their stimulatory effects on behavior (7–9). In addition to the dynamic processes that govern dopamine neurotransmission, in the long term, dopamine is eventually inactivated by the degradative enzymes monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT) (10).
The physiological importance of the DAT has been mostly inferred from the behavioral and psychosocial effects of pharmacological agents that interfere with dopamine transport, such as psychostimulants and antidepressants. To examine the importance of the DAT, we created a strain of mice lacking the dopamine tranporter protein using homologous recombination (11). The most obvious phenotype of these genetically modified animals is their marked spontaneous hyperlocomotion, which is similar to animals on high doses of psychostimulants. In this work, we examine the underlying biochemical changes that accompany this genetic alteration. Although the contribution of the DAT to the dynamics of dopamine in the extracellular space is well appreciated, this work reveals the central role of the DAT in the regulation of presynaptic dopamine function. The data show that the DAT not only regulates the lifetime of extracellular dopamine but, rather unexpectedly, is critically involved in maintaining the delicate balance between dopamine synthesis, release, and degradation.
MATERIALS AND METHODS
Animals.
C57BL/129SvJ wild-type mice (DAT+/+) and their littermates heterozygous (DAT+/−) and homozygous (DAT−/−) for DAT deletion were obtained by homologous recombination as described previously(11) and used between the ages of 2–4 months for the experiments described. They were housed in an animal care facility at 23°C on a 12-hour light/12-hour dark cycle and given food and water ad libitum. They were caged with approximately three other littermates of the same sex. Animal care and experimental protocols were in accordance with institutional guidelines.
Cyclic Voltammetry in Brain Striatal Slices.
Mice were decapitated and their brains were rapidly removed. Brain slices (400 μm thick) were prepared with a microtome, placed in a recording chamber, and superfused with an artificial cerebrospinal fluid (12). Carbon-fiber electrodes were prepared as described (13). Cyclic voltammograms (Einit = −0.4 V, Eswitch = 1.0 V, 300 V/s) were repeated every 100 ms. The oxidation current for dopamine (0.5–0.7 V) was converted to concentration by electrode calibration at the end of the experiment. Dopamine release was evoked by single pulse stimulations from an adjacent stimulating electrode (12). The rates of uptake of extracellular dopamine in DAT+/+ and DAT+/− mice were evaluated with the Michaelis–Menten equation (12). Time courses in homozygote (DAT−/−) mice were evaluated as a pseudo first-order rate constant (k). To compare kinetics between types of animals, a rate constant k was calculated by dividing Vmax by Km values in DAT+/+ and DAT+/− mice.
In Vivo Microdialysis.
Brain microdialysis (14) was performed in the striatum of freely moving mice using concentric microdialysis probes (2 mm membrane length; cut off 6,000 Da; CMA-11, CMA/Microdialysis, Solna, Sweden). Stereotaxic coordinates were: AP 0.0; DV −4.4; L 2.5 for DAT+/+ and DAT+/− mice and AP 0.0; DV −3.2; L 1.8 for DAT−/−, relative to bregma (15). Approximately 24 hours after surgery, the dialysis probe was perfused at 0.8 μl/min with artificial cerebral spinal fluid [150 mM Na+/3.0 mM K+/1.4 mM Ca2+/0.8 mM Mg2+/31.0 mM PO4−/155 mM Cl− (ESA, Bedford, MA)/0.25 mM ascorbate (pH 7.3)]. Perfusate samples were collected every 20 min. After basal dopamine levels were established, different dopamine concentrations were perfused through the probe and loss or gain of dopamine was measured (16, 17). Measurements of dopamine and metabolites in microdialysis samples were by HPLC with electrochemical detection (HPLC/EC) with a microbore column (5 μm particles, Unijet C18, 1 × 150 mm, BAS, West Lafayette, IN) and electrochemically detected with a Unijet (3 mm) electrode. The mobile phase contained 50 mM sodium citrate, 10 mM NaH2PO4, 0.5 mM octyl sodium sulfate, 0.1 mM EDTA, and 17% methanol, at pH 3.5.
Biochemical Assays.
Tissue content of catecholamines in striatal homogenates was measured by HPLC/EC (column: Ultremex 3 C18 IP, 100 × 4.6 mm, Phenomenex, Chicago) using the same mobile phase as with microdialysis.
The in vivo rate of dopamine biosynthesis was determined by the accumulation of striatal l-dihydroxyphenylalanine (L-DOPA) 40 min after administration of 3-hydroxybenzylhydrazine (NSD-1015, 100 mg/kg i.p.) (18). L-DOPA was determined by HPLC/EC with the following mobile phase: 50 mM NaH2PO4, 0.2 mM octyl sodium sulfate, 0.1 mM EDTA, and 10% methanol. To inhibit tyrosine hydroxylase (TH), α-methyl-p-tyrosine (α-MPT, 250 mg/kg i.p.) was administered and striatal dopamine content was determined by HPLC/EC 1 hour later. Maximal TH activity was assayed in striatal homogenates by the accumulation of L-DOPA after administration of NSD-1015 in the presence of saturating concentrations of tyrosine and cofactor BH4 (19).
The activity of soluble COMT in mouse striatal homogenates was determined with 3,4-dihydroxybenzoic acid as the substrate at a saturating concentration (400 μM) (20). The O-methylated products of the reaction were determined with HPLC/EC. Maximal in vitro MAO activity was determined in striatal homogenates (21) with 50 mM dopamine as a saturating concentration of substrate. The product, 3,4-dihydroxyphenylacetic acid (DOPAC), was quantitated by HPLC as described. Vesicular uptake of [3H]DA (New England Nuclear) during a 5-min incubation at 37°C (22) and binding of [3H]dihydrotetrabenazine (Amersham) during a 60-min incubation at 25°C were assessed in crude vesicle preparations (23) from at least three animals in each group per assay. No significant differences were found between DAT+/+ and DAT−/− mice.
To determine protein levels of TH, protein extracts (50 μg) from wild-type, heterozygote, and homozygote striata (n = 3 each group) were electrophoresed on 12% SDS/polyacrylamide gels and transferred to nitrocellulose. TH was identified by standard Western blotting techniques using a monoclonal rat antibody from Incstar (Minnesota) and chemiluminescent detection (Amersham). Autoradiograms were scanned with a Howtek scanner at 1200 dpi and images were analyzed using NIH image (Wayne Rasband).
Chemicals.
d-Amphetamine, cocaine, and pargyline were from Sigma. NSD-1015, desipramine, nomifensine, and α-MPT were purchased from RBI (Natick, MA). Ro407592 was a gift from Hoffmann–LaRoche (Nutley, NJ), and fluoxetine was a gift from Eli Lilly (Indianapolis). All drugs were applied to brain slice preparations at a concentration of 10 μM with the exception of desipramine (0.5 μM).
RESULTS AND DISCUSSION
Extracellular Regulation of Dopamine.
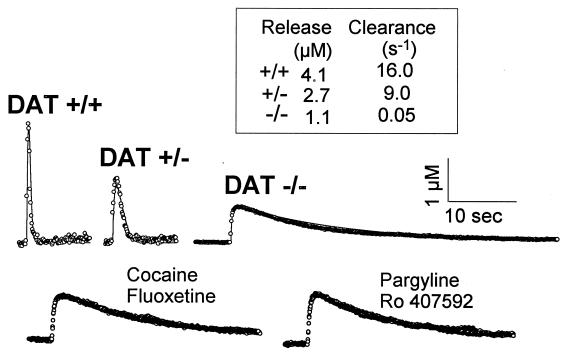
Release and uptake of dopamine were simultaneously assessed by dopamine measurements with fast-scan cyclic voltammetry at carbon-fiber microelectrodes implanted in brain striatal slices. In the genetically modified mice, electrically stimulated dopamine release was decreased by about 75% (Fig. 1). In addition, the rate of dopamine clearance, reported as a rate constant k, was 300 times slower in DAT−/− mice relative to wild-type mice (0.05 s−1 vs. 16 s−1) (Fig. 1). In heterozygote mice, the clearance rate constant was reduced by half (k = 9 s−1), consistent with the roughly 50% reduction in DAT sites (11). The psychostimulants cocaine and amphetamine (2) prolonged the clearance of dopamine in striatal slices from wild-type and heterozygote animals, but these agents were totally devoid of effects in slices from DAT−/− animals (Fig. 1 and Table 1). In DAT−/− mice, but also in wild-type and heterozygote mice, the rate of dopamine clearance was unaltered by inhibitors of the serotonin and norepinephrine transporters [fluoxetine and desipramine, respectively (2); Fig. 1 and Table 1]. Furthermore, selective inhibitors of dopamine degradative enzymes (ref. 24; pargyline and Ro 407592) did not influence dopamine clearance (Fig. 1 and Table 1). Thus, despite the protracted period of time that dopamine persists in the extracellular space, neither transporters for other monoamines nor degradative enzymes can compensate for the absence of the DAT. Indeed, the long time required for removal of a single bolus of dopamine in DAT−/− slices is similar to estimates for diffusion-controlled clearance from a slice (25). Thus, other non-DAT-mediated uptake systems for removal of extracellular dopamine such as uptake into glial cells or other compartments, long speculated and referred to as Uptake-2 (26, 27), do not significantly contribute to dopamine clearance even in the range of high extracellular concentrations.
Figure 1.
Time course of dopamine release and uptake in mouse striatal slices. (Upper) Individual recordings of dopamine efflux evoked by a single electrical pulse in striatal slices from control (DAT+/+), heterozygote (DAT+/−), and homozygote (DAT−/−) mice as measured by cyclic voltammetry (12) (○, data; solid lines, simulations). Pseudo-first order rate constants (k) for uptake are shown in inset. (Lower) Effects of various drugs on stimulated dopamine efflux in striatal slices from DAT−/− animals. Results after drug application are superimposed on the predrug response. All drugs were applied at a concentration of 10 μM for 20 min, and none caused any measurable effect. Results are representative of recordings from at least 4 different animals.
Table 1.
Effects of uptake and degradation enzyme inhibitors, degradation enzyme activity, and 3-MT in normal and mutant mice
| Parameter | DAT+/+ | DAT+/− | DAT−/− |
|---|---|---|---|
| Uptake blockers, kdrug/kcontrol* | |||
| Cocaine (10 μM) | 21 ± 4.0† | 22 ± 7.1† | 1.2 ± 0.4 |
| Amphetamine (10 μM) | 27 ± 5.0† | 17 ± 7.6† | 0.9 ± 0.1 |
| Nomifensine (10 μM) | 78 ± 8.0† | 42 ± 9.0† | 0.9 ± 0.3 |
| Desipramine (0.5 μM) | 1.9 ± 0.8 | 2.2 ± 1.0 | 0.8 ± 0.1 |
| Fluoxetine (10 μM) | 3.5 ± 1.8 | 2.2 ± 1.0 | 1.0 ± 0.1 |
| Degradation enzyme inhibitors, kdrug/kcontrol | |||
| Pargyline (10 μM) | 2.5 ± 0.5 | 1.8 ± 0.7 | 1.2 ± 0.2 |
| Ro 407592 (10 μM) | 3.0 ± 0.6 | 4.2 ± 1.4 | 0.9 ± 0.1 |
| Degradative enzyme activity, nmol/mg tissue/min | |||
| MAO | 32 | 32 | 8‡ |
| COMT | 0.61 | 0.55 | 0.57 |
| Extracellular metabolite, dialysate level | |||
| 3-MT, nM | 1.6 ± 0.1 | 3.0 ± 0.6‡ | 6.0 ± 1.1‡ |
Data represent mean ± SEM of 4–6 experiments.
Values of pseudo-first-order rate constants (k) for uptake were measured by cyclic voltammetry.
Significant difference from predrug value (P < 0.01), Student’s t test.
Significant difference from DAT+/+ value (P < 0.01), Student’s t test.
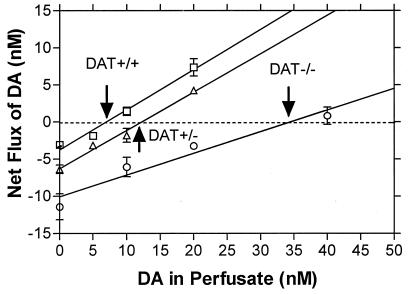
The persistence of dopamine in the extracellular space suggests that resting concentrations should be elevated. Because fast-scan cyclic voltammetry only assesses changes in dopamine concentrations, basal levels were measured using the quantitative technique of “no net flux” microdialysis (16, 17). In freely moving animals, a 5-fold higher concentration of striatal extracellular dopamine in DAT−/− (35 nM) versus wild-type animals (7 nM) was found, and the dopamine concentration in heterozygote animals was almost doubled (12 nM, Fig. 2). These continuously high extracellular dopamine levels are of the same magnitude as those transiently measured in normal mice treated with the psychostimulant cocaine (data not shown). On the basis of the combined results from cyclic voltammetry and microdialysis, the profile of extended dopamine lifetime and increased extracellular concentration is certainly consistent with the spontaneous hyperactivity phenotype of the DAT−/− mice (11).
Figure 2.
Basal extracellular concentrations of dopamine measured in vivo. Concentrations of dopamine in the striatum of freely moving mice determined using a quantitative microdialysis method (16, 17). Data are given as the mean ± SEM of the difference between the concentration of dopamine applied to the dialysis probe in the perfusate and that collected at the probe effluent. The point of zero flux (“no net flux”), extrapolated by linear regression, represents the concentration of dopamine at which diffusion into the probe equals that out of the probe. These values, that provide the basal extracellular concentration of dopamine, were 6.95 ± 0.51 nM for DAT+/+; 12.2 ± 0.79 nM for DAT+/−, and 34.5 ± 3.22 nM for DAT−/− mice. Four animals were measured in each group. The slope of the lines yield the apparent recovery of dopamine from the brain. Recovery is much lower in the DAT−/− (29 ± 5%) than in wild-type (54 ± 5%) or heterozygote (52 ± 4%) animals, in accord with theory for systems without active transport (16).
Intracellular Regulation of Dopamine.
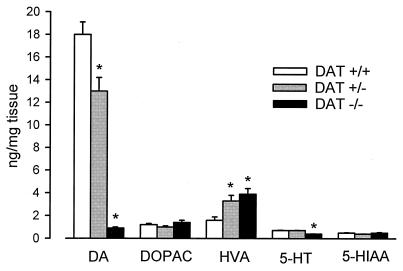
Because of the remarkable changes in extracellular dopamine and the absence of a presynaptic recycling process, we expected that the function of the dopamine terminals might be effected. We document that marked changes in the synthesis, storage, and metabolism of dopamine occur in mice lacking the DAT. The tissue content of dopamine in DAT−/− striatum, as measured by HPLC/EC, was found to be less than 5% of wild-type levels (Fig. 3). However, dopamine terminals in the striatum were intact, ruling out an anatomical abnormality as a cause for the decrease (M.J. et al., unpublished observation). The extremely low tissue content of dopamine was unexpected considering the high basal extracellular levels of dopamine and the behavioral and biochemical hyperactivity of these mice (11). When compared with total tissue content, there is a 100-fold greater ratio of extracellular to total dopamine in the mutant compared with the wild-type mice. These data show that the decrease in the number of DAT results in a large diminution of the intracellular stores of dopamine, implying that the reserve pools of dopamine are acutely dependent on recycled extracellular dopamine. This contention is further strengthened if one considers that rates of synthesis of dopamine are doubled in the mutant relative to normal animals (see below).
Figure 3.
Tissue content of monoamines and metabolites. Amounts of monoamines and metabolites were measured in striatal homogenates. DOPAC/dopamine ratios were 0.06, 0.08, and 1.6 for DAT+/+, DAT+/−, and DAT−/− mice, respectively, and HVA/dopamine ratios were 0.09, 0.3, and 4.3, respectively. Results are presented as mean ± SEM of determinations from 10–12 animals. An asterisk (∗) indicates value is significantly different (P < 0.05) from corresponding value in DAT+/+ mice as determined by Student’s t test.
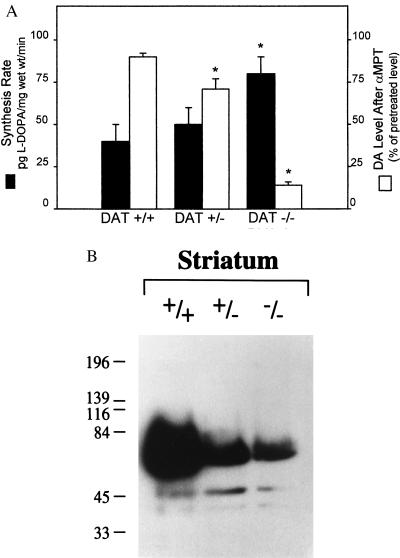
The rate limiting enzyme in dopamine synthesis is TH (2). In vivo assays of TH activity, measured as L-DOPA accumulation after inhibition of L-aromatic acid decarboxylase with NSD-1015 (18), showed a doubling in the rate of dopamine synthesis in DAT−/− vs. wild-type animals (Fig. 4A). Also, a 1 hour in vivo treatment with α-MPT, an inhibitor of TH, produced an almost 90% decrease in the already low tissue levels of dopamine in the DAT−/− mice, as well as similar decreases in DOPAC and homovanillic acid (HVA) (data not shown), whereas the same treatment produced only a marginal effect in wild-type animals (Fig. 4A). The high dependence of DAT−/− mice on ongoing synthesis of dopamine was readily apparent after α-MPT or NSD-1015 administration because both caused much more rapid sedation than in wild-type animals (data not shown).
Figure 4.
(A) Indices of dopamine synthesis. Synthesis rates of dopamine (solid bars) and effect of a tyrosine hydroxylase inhibitor (2) (αMPT) on tissue levels of dopamine (open bars) in normal (DAT+/+), heterozygote (DAT+/−), and homozygote mice (DAT−/−). Results are presented as the mean ± SEM of determinations from 4 to 6 different animals per genotype. (B) Western blot of TH in the striatum of wild-type and mutant mice. TH labeling decreased by 59% and 96% in heterozygote and homozygote striata, respectively, as compared with wild-type values. Immunohistochemistry with a TH antibody demonstrates that the decrease in TH protein levels is not due to the destruction of dopamine neurons as these were present at normal levels (M.J. et al., unpublished observation). An asterisk (∗) indicates value is significantly different (P < 0.05) from corresponding value in DAT+/+ mice as determined by Student’s t test.
In contrast to the elevated synthesis rate, the levels of striatal TH protein in DAT−/− mice were found to be less than 10% of wild-type levels (Fig. 4B) and, consistent with this, the maximal TH activity measured in brain homogenates was 80% lower in the DAT−/− than in the wild type, but unaltered in DAT+/− mice (data not shown). Thus, the few TH molecules present in the DAT−/− mice are very efficient in converting tyrosine to L-DOPA. This result may be partly due to the absence of newly transported dopamine in the cytoplasm that normally inhibits TH activity (28, 29). Additionally, the down-regulation of presynaptic D2 receptors (11) in DAT−/− mice decreases another inhibitory route of TH activity and could result in enhanced synthetic rates (30). The low tissue levels of dopamine and the increased rate of dopamine synthesis, as well as the behavioral and neurochemical observations after synthesis inhibition, establish that dopaminergic activity in the DAT−/− mice is tightly coupled to synthesis.
Metabolism of Dopamine in Genetically Altered Mice.
In the absence of the DAT, metabolism of dopamine is also altered. An extracellular product of COMT degradation of dopamine, 3-methoxytyramine (3-MT), was nearly 400% higher in DAT−/− than in wild-type mice, as measured by in vivo microdialysis (Table 1). Similarly, HVA, also an extracellular COMT product, was increased to 350% of control in striatal homogenates from DAT−/− mice (Fig. 3). Because there is a higher concentration of substrate, COMT produces more metabolites, although the maximal rate of COMT activity is the same as control (Table 1). Thus, in the absence of uptake, a situation where released DA cannot be recycled by neurons, COMT plays a proportionally increased role as a compensatory mechanism to regulate extracellular dopamine levels.
Surprisingly, tissue levels of DOPAC, an intracellular product of MAO action on dopamine, which might be expected to reflect the low intracellular dopamine tissue levels, are the same in all three types of mice (Fig. 3). Because MAO is predominantly intracellular, it has been difficult to distinguish whether its primary source of substrate is dopamine, which is newly uptaken or newly synthesized (2, 31, 32); however, when uptake is removed, the main source of DOPAC must be newly synthesized dopamine. Although these higher-than-expected DOPAC levels are maintained by the enhanced synthesis of dopamine described above, the fact that the maximal in vitro activity of MAO is decreased by about 75% in the DAT−/− mice (Table 1) bolsters the argument for the susceptibility of newly synthesized dopamine to degradation. Impaired vesicular packaging of dopamine does not contribute to the sensitivity of newly synthesized dopamine to degradation, because vesicular transporter binding and tetrabenazine-sensitive uptake of dopamine are both unchanged in the DAT−/− animals (data not shown). The findings concerning synthesis, storage, and metabolism all strongly suggest that in the normal situation recycled rather than newly synthesized dopamine contributes more significantly to the maintenance of vesicular stores of dopamine, a notion not previously appreciated. This could occur if, for example, uptake sites are normally spatially closer to vesicles than to mitochondria, the site of MAO.
Characterization of DAT Heterozygotes.
In the heterozygotes, where uptake is halved, stimulated release is lowered to a similar extent (Fig. 1), maintaining homeostasis of dopamine function. Indeed, behaviorally, the DAT+/− mice appeared normal, with locomotor activity not significantly different from wild-type animals (11). However, the neurochemical adaptations in the DAT+/− mice, which are all intermediate between that of wild-type and homozygote animals, indicates that the neuronal plasticity observed in DAT−/− mice can even occur in the presence of the transporter. These properties suggest the DAT+/− animals may also represent an appropriate model to further study adaptive changes in response to long-term blockade of DAT.
Importance of DAT in Dopamine Homeostasis.
The neurochemical and biochemical changes in response to the lack of DAT have revealed points of control in dopamine dynamics, the importance of which had not been apparent before. These results provide a direct demonstration that the maintenance of normal stores of dopamine are more critically dependent on recycled rather than newly synthesized dopamine. These data also suggest an important role for MAO in the metabolism of newly synthesized dopamine. Thus, in the normal situation, newly synthesized rather than recycled extracellular dopamine may be preferentially degraded by MAO. In contrast to the dramatic changes found in dopamine synthesis, metabolism, storage, release, and clearance, other transporter systems (glial, serotonin, or norepinephrine) did not adapt to compensate for the deleted DAT. In addition, the profound changes observed in the properties of TH in mice lacking the DAT should help in elucidating which of the various postulated mechanisms of regulation (33) contribute to the maintenance of the physiological activity of TH.
The DAT is the primary determinant of the lifetime of extracellular dopamine. In the DAT−/− mice, a situation is created where the volume effected by a single dopamine terminal must be greatly increased. Considerable evidence suggests that dopamine neurotransmission, even in the presence of a fully functional complement of DAT, normally occurs over distances much greater than that delineated by synaptic structures (10–20 μm) (5, 34, 35). In vivo in the DAT−/− mice, within the time required for clearance, dopamine can be predicted to diffuse for millimeters. The new properties of the system resemble the long-distance volume transmission mode (6, 36) postulated for dopamine in the medial prefrontal cortex, a brain region where uptake is relatively inefficient, reserve pools of dopamine are very low, release is tightly coupled to synthesis, and autoreceptor regulation of synthesis is negligible (35, 37). Indeed, regional differences in the expression of the DAT, as found in the striatum when compared with the medial prefrontal cortex or amygdala (35), for example, appear to be the major determinant of differences in intraneuronal homeostasis and mode of neurotransmission, i.e., synaptic or volume transmission.
In the absence of the DAT, adaptive changes of an unexpected diversity and magnitude are concurrently induced in the striatal dopaminergic system. These broad compensatory changes illustrate the importance of the DAT not only in terminating dopamine transmission, but in maintaining normal dopamine homeostasis and function. The switch to a novel mode of dopamine neurotransmission in the mice with impaired dopamine uptake might represent an extreme case of changes accompanying drug addiction. Indeed, recent evidence in human cocaine (38, 39) and methamphetamine (40) abusers suggests that dopamine deficits occur in brain regions such as the striatum that are highly regulated by DAT, while such changes are not seen in medial prefrontal cortex (39). This brain dopamine deficiency has been suggested to result in the long-term consequences of psychostimulant use including extrapyramidal disturbances and dysphoric abstinence symptoms (41, 42). In addition to the well appreciated sensitization theory of addiction, that focuses mainly on postsynaptic mechanisms (43), presynaptic alterations by drugs of abuse deserve consideration. Such adaptive changes might also underlie the delayed therapeutic effects of antidepressants, which also block monoamine transporters. Our findings provide new insights into dopamine regulation and suggest potential avenues for therapeutic intervention.
Acknowledgments
We thank S. Suter for excellent technical help, F. Fumagalli and S. Ferguson for helpful comments on the manuscript, Hoechst Roussel Pharmaceuticals for their gift of nomifensine maleate, and Eli Lilly Co. for their gift of fluoxetine. M.J. was the recipient of a postdoctoral fellowship from European Molecular Biology Organization. R.M.W. is a recepient of Guggenheim Fellowship for sabbatical leave from University of North Carolina and is supported by National Institue on Drug Abuse Grant DA 10900. S.R.J. is supported by National Institutes of Health Grants 5 T32 AG00029-20 and F32 DA05749-01. R.R.G. is a visiting fellow from the Institute of Pharmacology RAMS (Moscow), supported by a Tourette Syndrome Association, Inc. fellowship. M.G.C. is supported by National Institutes of Health Grant NS-19576 and an Unrestricted Neuroscience Grant from Bristol Myers Squibb.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: DAT, dopamine transporter; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; MAO, monoamine oxidase; COMT, catechol-O-methyltransferase; TH, tyrosine hydroxylase.
References
- 1.Amara S G, Kuhar M J. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 2.Feldman R S, Meyer J S, Quenzer L F. Principles of Neuropsycho-Pharmacology. Sunderland, MA: Sinauer; 1997. , Chapt. 8 and 9. [Google Scholar]
- 3.Horn A S. Prog Neurobiol. 1990;34:387–400. doi: 10.1016/0301-0082(90)90033-d. [DOI] [PubMed] [Google Scholar]
- 4.Nirenberg M J, Chan J, Pohorille A, Vaughan R A, Uhl G R, Kuhar M J, Picel V M. J Neurosci. 1997;17:6899–6907. doi: 10.1523/JNEUROSCI.17-18-06899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garris P A, Ciolkowski E L, Pastore P, Wightman R M. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnati L F, Bjelke B, Fuxe K. Med Res Rev. 1995;15:33–45. doi: 10.1002/med.2610150104. [DOI] [PubMed] [Google Scholar]
- 7.Koob G F, Bloom F E. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 8.Ritz M C, Lamb R J, Goldberg S R, Kuhar M J. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 9.Volkow N D, Wang G-J, Fischman M W, Foltin R W, Fowler J S, Abumrad N N, Vitkun S, Logan J, Gatley S J, Pappas N, Hitzemann R, Shea C E. Nature (London) 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- 10.Kopin I J. Pharmacol Rev. 1985;37:333–364. [PubMed] [Google Scholar]
- 11.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 12.Jones S R, Garris P A, Kilts C D, Wightman R M. J Neurochem. 1995;64:2581–2589. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawagoe K T, Zimmerman J B, Wightman R M. J Neurosci Methods. 1993;48:225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- 14.Ungerstedt U. In: Measurement of Neurotransmitter Release in Vivo. Marsden C A, editor. Chicester, U.K.: Wiley; 1984. pp. 81–105. [Google Scholar]
- 15.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 16.Bungay P M, Morrison P F, Dedrick R L. Life Sci. 1990;46:105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- 17.Justice J B., Jr J Neurosci Methods. 1993;48:263–276. doi: 10.1016/0165-0270(93)90097-b. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson A, Davis J N, Kehr W, Lindqvist M, Atack C V. Naunyn Schmeideberg’s Arch Pharmacol. 1972;275:153–168. doi: 10.1007/BF00508904. [DOI] [PubMed] [Google Scholar]
- 19.Blank C L, Pike R. Life Sci. 1976;18:859–865. doi: 10.1016/0024-3205(76)90013-8. [DOI] [PubMed] [Google Scholar]
- 20.Kaakkola S, Mannisto P T, Nissinen E. J Neural Trans. 1987;69:221–228. doi: 10.1007/BF01244343. [DOI] [PubMed] [Google Scholar]
- 21.Freeman K B, Bulawa M C, Zeng Q, Blank C L. Anal Biochem. 1993;208:182–196. doi: 10.1006/abio.1993.1026. [DOI] [PubMed] [Google Scholar]
- 22.Scherman D, Henry J-P. Biochem Pharmacol. 1980;29:1883–1890. doi: 10.1016/0006-2952(80)90098-2. [DOI] [PubMed] [Google Scholar]
- 23.Near J A. Mol Pharmacol. 1986;30:252–257. [PubMed] [Google Scholar]
- 24.Kaakkola S J, Wurtman R J. Brain Res. 1992;587:241–249. doi: 10.1016/0006-8993(92)91003-w. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson C. Biophys J. 1995;68:1699–1715. doi: 10.1016/S0006-3495(95)80348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Near J A, Bigelow J C, Wightman R M. J Pharmacol Exp Ther. 1988;245:921–927. [PubMed] [Google Scholar]
- 27.Pelton E W, Kimelberg H K, Shipherd S V, Bourke R S. Life Sci. 1981;28:1655–1663. doi: 10.1016/0024-3205(81)90322-2. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro P, Wang Y, Citron B A, Kaufman S. Proc Natl Acad Sci USA. 1992;89:9593–9597. doi: 10.1073/pnas.89.20.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spector S, Gordon R, Sjoerdsma A, Udenfriend S. Mol Pharmacol. 1967;3:549–555. [PubMed] [Google Scholar]
- 30.Wolf M E, Roth R H. In: Presynaptic Receptors and the Question of Autoregulation of Neurotransmitter Release. Kalsner S, Westfall T C, editors. Vol. 604. New York: NY Academy of Sciences; 1990. pp. 323–343. [Google Scholar]
- 31.Roth R H, Murrin L C, Walters J R. Eur J Pharmacol. 1976;36:163–171. doi: 10.1016/0014-2999(76)90268-5. [DOI] [PubMed] [Google Scholar]
- 32.Zetterstrom T, Sharp T, Collin A K, Ungerstedt U. Eur J Pharmacol. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein M. In: Psychopharmacology: The Fourth Generation of Progress. Bloom F E, Kupfer D J, editors. New York: Raven; 1995. pp. 189–195. [Google Scholar]
- 34.Groves P M, Linder J C, Young S J. Neuroscience. 1994;58:593–604. doi: 10.1016/0306-4522(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 35.Garris P A, Wightman R M. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuxe K, Agnati L F. In: Advances in Neuroscience. Fuxe K, Agnati L F, editors. Vol. 1. New York: Raven; 1991. pp. 1–10. [Google Scholar]
- 37.Bannon M J, Michaud R L, Roth R H. Mol Pharmacol. 1981;19:270–275. [PubMed] [Google Scholar]
- 38.Volkow N D, Wang G-J, Fowler J S, Logan J, Gatley S J, Hitzemann R, Chen A D, Dewey S L, Pappas N. Nature (London) 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 39.Wilson J M, Levey A I, Bergeron C, Kalaisinsky K, Ang L, Peretti F, Adams V I, Smialek J, Anderson W R, Shannak K, Deck J, Niznik H B, Kish S J. Neurology. 1996;47:718–726. doi: 10.1002/ana.410400312. [DOI] [PubMed] [Google Scholar]
- 40.Wilson J M, Kalaisinsky K, Levey A I, Bergeron C, Reiber G, Anthony R M, Schmunk G A, Shannak K, Haycock J W, Kish S J. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 41.Dackis C A, Gold M S. Neurosci Neurobehav Rev. 1985;9:469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- 42.Gawin F H, Kleber H D. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 43.Robinson T E, Berridge K C. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]