Abstract
Centrosomes are microtubule-organizing centers and play a dominant role in assembly of the microtubule spindle apparatus at mitosis. Although the individual binding steps in centrosome maturation are largely unknown, Centrosomin (Cnn) is an essential mitotic centrosome component required for assembly of all other known pericentriolar matrix (PCM) proteins to achieve microtubule-organizing activity at mitosis in Drosophila. We have identified a conserved motif (Motif 1) near the amino terminus of Cnn that is essential for its function in vivo. Cnn Motif 1 is necessary for proper recruitment of γ-tubulin, D-TACC (the homolog of vertebrate transforming acidic coiled-coil proteins [TACC]), and Minispindles (Msps) to embryonic centrosomes but is not required for assembly of other centrosome components including Aurora A kinase and CP60. Centrosome separation and centrosomal satellite formation are severely disrupted in Cnn Motif 1 mutant embryos. However, actin organization into pseudocleavage furrows, though aberrant, remains partially intact. These data show that Motif 1 is necessary for some but not all of the activities conferred on centrosome function by intact Cnn.
INTRODUCTION
Centrosomes are comprised of a pair of centrioles surrounded by a pericentriolar matrix (PCM) and represent the major microtubule-organizing centers (MTOC) in most animal cells. Microtubules are nucleated at centrosomes primarily from the γ-tubulin (γ-Tub) complexes that are bound to the PCM (Gunawardane et al., 2000; Oakley, 2000). γ-Tub associates as a tetramer with Dgrip84 and Dgrip91 in a stoichiometric ration of 2:1:1 into a structure called the γ-Tub small complex (γ-TuSC; Gunawardane et al., 2000). γ-TuSC, together with Dgp71WD, Dgrip75, Dgrip128, and Dgrip163, assemble into a larger 25-nm-diameter ring complex (γ-TuRC), which nucleates microtubules (Gunawardane et al., 2000). γ-TuSC appears to be sufficient for centrosome microtubule assembly (Verollet et al., 2006). Microtubules are assembled less efficiently from centrosomes upon γ-Tub depletion (Strome et al., 2001; Hannak et al., 2002). The microtubule-stabilizing protein Minispindles (Msps), the Drosophila homolog of the XMAP215/TOG family, and its binding partner D-TACC (homolog of vertebrate transforming acidic coiled-coil proteins [TACC]) are also required for efficient microtubule assembly at centrosomes (Cullen et al., 1999; Gergely et al., 2000). TACC and TOG family members act together through direct binding and coordinate with γ-Tub to regulate microtubule assembly at the centrosome (Cullen and Ohkura, 2001; Lee et al., 2001; Bellanger and Gonczy, 2003; Le Bot et al., 2003; Srayko et al., 2003; Kinoshita et al., 2005; Peset et al., 2005). In addition, Aurora A kinase is required for centrosomal microtubule assembly and activates D-TACC by phosphorylation (Giet et al., 2002; Barros et al., 2005; Kinoshita et al., 2005; Peset et al., 2005). Downstream of Aurora A, a proposed role for Msps/D-TACC is the stabilization of microtubules nucleated by γ-Tub at centrosomes (Lee et al., 2001; Popov et al., 2002).
Centrosomin (Cnn) is a PCM protein required in Drosophila for assembly of the centrosome into a functional MTOC (Megraw et al., 1999; Vaizel-Ohayon and Schejter, 1999; Megraw et al., 2001). In cnn mutant embryos the centrosomal proteins CP60 and CP190 fail to localize at centrosomes and γ-Tub accumulates at lower levels at spindle poles, yet astral microtubules are detected at some spindle poles (Megraw et al., 1999; Vaizel-Ohayon and Schejter, 1999). In contrast to embryos, cnn mutant neuroblasts and S2 cells depleted of Cnn by RNA interference (RNAi) lack any detectable γ-Tub signal at mitotic centrosomes, and astral microtubules are not assembled at a detectable level (Megraw et al., 2001). In these Cnn-deficient cells the spindle is assembled primarily from microtubules that appear to originate at the chromosomes, employing a meiotic-like anastral mechanism (Megraw et al., 2001; Mahoney et al., 2006). Remarkably, in a cnn null mutant, zygotic development is achieved efficiently without functional mitotic centrosomes, producing adult flies using the “anastral” or “centrosome-free” pathway described above (Megraw et al., 2001). The Schizosaccharomyces pombe Cnn homolog Mto1p is required for the assembly of microtubules from all three types of MTOCs in fission yeast (Sawin et al., 2004; Venkatram et al., 2004; Zimmerman and Chang, 2005). In both systems, Cnn and Mto1p are required for the recruitment of γ-Tub and the associated γ-TuRC proteins to spindle poles (Megraw et al., 2001; Sawin et al., 2004; Venkatram et al., 2004).
After fertilization, development of the Drosophila early embryo begins with 13 rapid, synchronous cleavage cycles 8–12 min long, with little or no gap phases, that occur within a syncytium (Foe et al., 1993; Sullivan and Theurkauf, 1995). The first 8–9 cycles occur deep within the embryo followed by migration of the nuclei to the embryo cortex, where cycles 10–13 occur in a uniform monolayer at high density at the cell cortex. During the cortical cycles centrosomes organize actin into furrows that surround each spindle apparatus at mitosis. The embryo cellularizes at cycle 14 to form a cellular blastoderm.
In cnn maternal effect mutant embryos the cortical cleavage cycles are highly disorganized and progressively aneuploid as a consequence of fused spindles at metaphase and colliding nuclei at telophase (Megraw et al., 1999; Vaizel-Ohayon and Schejter, 1999; and unpublished data). At the first cortical division, cycle 10, the metaphase spindles are appropriately spaced and γ-Tub resides at spindle poles, though at a reduced level compared with wild-type embryos. At telophase of cycle 10, and in subsequent cleavage cycles, neighboring nuclei collide and exhibit excessive lateral movement at the cortex (unpublished data). This is consistent with a lack of organized cortical actin microfilaments (Zalokar et al., 1975; Foe et al., 1993), a characteristic of cnn mutant embryos. In later cycles (after cycle 10) spindle poles lack detectable γ-Tub and astral microtubules, apparently because of centriole loss (see below). cnn maternal effect mutant embryos fail to develop beyond late cleavage cycles and do not cellularize.
Apparent homologues of cnn are found in most eukaryotes, including the fission yeast S. pombe and humans, but appear absent in plants, which lack centrosomes (Megraw et al., 2001; Sawin et al., 2004). There are two putative human Cnn homologues, CDK5RAP2 and Myomegalin (Figure 1A; Wang et al., 2000; Verde et al., 2001). The functions of these proteins have not been characterized, but CDK5RAP2 mutations in humans cause microcephaly due to severe reduction in the size of the cerebral cortex (Bond et al., 2005). Because mutations in cnn affect spindle orientation in neuroblasts (Megraw et al., 2001), one hypothesis is that the reduced number of cortical neurons in CDK5RAP2 mutant individuals results from defective asymmetric division of neural precursors due to centrosome defects (Bond et al., 2005).
Figure 1.
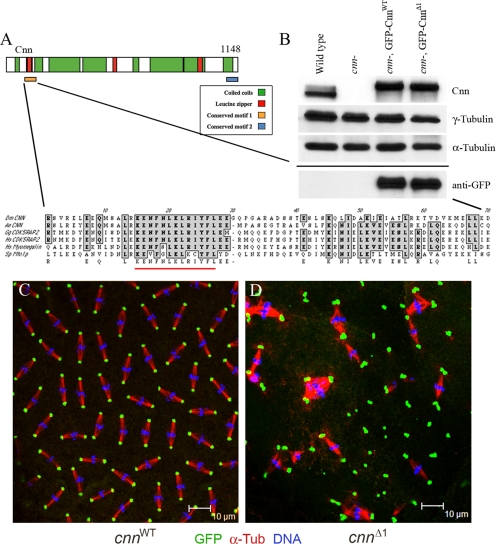
Motif 1 of Cnn is essential. (A) Schematic of Cnn protein showing the two conserved motifs at the amino and carboxyl termini. An expanded view of Motif 1 is shown with the 13 amino acids that are deleted in CnnΔ1 underlined in red. The alignments include the putative Cnn homologues in Aedes egypti (mosquito, A.e.), CDK5RAP2 from Gallus gallus (chicken, G.g.) and human (H.s.), Myomegalin/PDE4DIP from human, and Mto1p from S. pombe (S.p.). (B) Western blot showing the expression of GFP-CnnWT and GFP-CnnΔ1 in a cnn null mutant (cnnhk21/cnn25cn1) background (labeled “cnn-” above the lanes). The samples were loaded onto two gels and blotted, and one gel was probed with anti-Cnn, anti-γ-Tub, and anti-α-Tub (loading control) antibodies as indicated. The second blot, shown below the solid line, was probed with anti-GFP antibody. Immunostaining of cnn null embryos expressing GFP-CnnWT (C) and GFP-CnnΔ1 (D) shows localization to centrosomes and mutant phenotype. Anti-GFP signal is green, anti-α-Tub signal is red, and DNA is blue. The erratic spacing of mitotic figures, linked spindles, and aneuploid nuclei in D are all characteristic of cnn mutant embryos, showing that GFP-CnnΔ1 did not rescue. Note the abundance of centrosome pairs or clusters in the cnnΔ1 mutant embryo.
Although Cnn is essential for centrosome maturation and appears to act early in this process, how Cnn functions at the molecular level is unknown. Here we show that Motif 1, a conserved domain near the amino terminus of Cnn, is essential in vivo and is required to recruit a suite of proteins that are necessary for microtubule assembly at the centrosome including γ-Tub, D-TACC, and Msps. Moreover, despite defects in microtubule-dependent activities from Cnn Motif 1 mutant centrosomes, another function of the centrosome in early embryos, actin organization into pseudocleavage furrows, is partially restored upon expression of Motif 1 mutant Cnn in cnn null embryos.
MATERIALS AND METHODS
Plasmids and Fly Stocks
cnn cDNA sequences were isolated from pBluescriptCnn (Heuer et al., 1995) by digestion with KpnI and SalI, ligated to KpnI-BamHI and SalI-XbaI linkers (KBK and SXS, Supplementary Table S1), restriction-digested with BamHI and XbaI, and then subcloned into pBluescriptII KS−. The KpnI site near the amino terminus of the cnn coding sequence was utilized for subcloning convenience, to shuttle the open reading frame between plasmids for mutagenesis and construction of the transgenesis plasmid. This results in deletion of the amino terminal 17 amino acids of the maternal transcript cnn-RA (see Flybase for nomenclature). The cnnWT construct rescued the mutant, indicating that the amino-terminal 17 aa peptide is not essential. Evidence from rescue experiments in testis indicates that the amino terminal 77 amino acids are not essential for in vivo function (unpublished data). Motif 1 begins approximately at amino acid 98 in cnn-PA. Mutagenesis was performed using the Stratagene quickchange kit (La Jolla, CA; primer sequences are listed in Supplementary Table S1). The cnn wild-type sequences were then subcloned using the BamHI and XbaI sites into pUASpEGFP (Megraw et al., 2002) to generate pUASpEGFP-CNNBX. The wild-type Motif 1 sequence was replaced with Δ1 mutant sequence by subcloning the 879-base pair KpnI-SphI mutant fragment into the KpnI-SphI site of pUASpEGFP-CNNBX. Transgenic flies were generated by standard methods.
pUASpEGFP-CnnWT and pUASpEGFP-CnnΔ1 were expressed in early embryos using the nos-GAL4VP16 driver at 25°C for all experiments. For expression in larvae, HS-GAL4, elav-GAL4, and Tub-GAL4 drivers were used. The GAL4 driver stocks were acquired from the Bloomington Drosophila Stock Center.
cnnhk21 is a null allele with a nonsense mutation that truncates the protein at amino acid 106 (Megraw et al., 1999). The cnn25cn1 allele is a deletion of most of the cnn gene. The deletion spans from the first intron to 1.6 kb downstream of the end of exon 7 (the last exon of cnn). The first exon of the maternal transcript is retained, which encodes the first 62 amino acids of Cnn. The deletion removes the first small exon of the next gene proximal to cnn, CG17034. cnn25cn1 expresses no detectable Cnn protein by Western blotting or immunostaining and is considered a null allele. cnn25cn1 was generated by using a P element in the first intron of cnn (cnnScim, Dobie et al., 2001) and selecting for proximal deletions on chromosome arm 2R by the “male recombination” method using cn1 as a marker (Preston et al., 1996). This allele retains the P element. cnn25cn1 is homozygous viable, but male sterile and maternal effect lethal. The original allele, cnnScim, is maternal effect lethal but male fertile. Throughout this report, cnn null refers to cnnhk21/cnn25cn1. Unless otherwise indicated, cnnWT and cnnΔ1 refer to embryos that were collected from cnn null mothers that express the respective enhanced green fluorescent protein (eGFP)-fusion protein from its transgene.
Immunofluorescence Staining and Antibodies
Embryos and third instar larval brains were immunostained as described previously (Megraw et al., 1999, 2001). Antibodies to amino acids 1–574 of Cnn were raised in rabbits and guinea pigs by Cocalico Biologicals (Reamstown, PA). Primary antibodies used were rabbit anti-Cnn (1:1000; Heuer et al., 1995), guinea pig anti-Cnn (1:1000), rabbit anti-Aurora A (1:200), rabbit anti-D-TACC (1:400), rabbit anti-Phospho (P)-D-TACC (1:500; Barros et al., 2005), mouse anti-γ-tubulin clone GTU88 (Sigma, St. Louis, MO; 1:500), mouse anti-α-tubulin clone DM1A (Sigma, 1:500), Rb anti-Msps (1:1000), Rb anti-Nek2 (1:1000), DM1A-FITC (Sigma, 1:200), mouse anti-phosphotyrosine (1:500, Santa Cruz Biotechnology, Santa Cruz, CA) and Alexa546-Phalloidin (Molecular Probes, Eugene, OR; 1:80). DNA was stained with DRAQ5 (Axxora, San Diego, CA) at 1:800 dilution. Fluorescent secondary antibodies were highly cross-absorbed goat conjugates to Alexa488, 546, or 633 (Invitrogen, Carlsbad, CA) used at 1:400 dilution.
Images were captured on a Leica TCS SP2 confocal microscope (Deerfield, IL) using a 63×/NA1.4 oil immersion lens objective. Live imaging of Drosophila embryos was performed at room temp (23–24°C) with the pinhole set to three airy units. Frames were captured every 3 s, and .avi files were generated with a frame rate of 15 frames per second. Movie files were compressed and saved as QuickTime movies (Apple, Cupertino, CA) using ImageJ software. For colocalization analysis, the ImageJ Colocalization plug-in was used with the threshold pixel intensities set to 150–255 for the Cnn and D-TACC 8-bit raw images. Quantification of the centrosomal immunofluorescence of γ-Tub was performed on maximum projections of image stacks of metaphase cycles 10 and 11 embryos using the Leica TCS SP2 quantification tools.
RESULTS
Cnn Motif 1 Is Essential In Vivo
Cnn proteins have diverged much during evolution, even among the dipterans. The level of overall sequence identity after ClustalW alignment is 28% between Drosophila melanogaster and Anopheles gambiae (mosquito) and only 19% between D. melanogaster and Apis mellifera (honeybee). Two domains, each 60–70 amino acids in length, are more highly conserved among Cnn proteins (Figure 1A). We have designated these Cnn Motif 1 and Cnn Motif 2. Motif 1 has a higher degree of sequence conservation (40% identity/49% similarity) between Cnn and human CDK5RAP2 and is present in all homologues from S. pombe to human. Motif 2, at the extreme carboxyl-terminus of Cnn, shows 29% identity and 20% similarity between fly Cnn and human CDK5RAP2, yet appears conserved only among metazoan Cnn homologues. Besides these two conserved sequence domains, Cnn proteins are structurally similar with extensive coiled-coil domains that separate the two conserved regions. Here we focus on the role of Cnn Motif 1.
Motif 1 of Drosophila Cnn was mutated by deleting 13 core amino acids in the conserved sequences (Figure 1A). The Motif 1 mutant version of Cnn (CnnΔ1) and control “wild-type” Cnn (amino acids 17–1148, CnnWT) were expressed in cnn null embryos as GFP fusion proteins at levels similar to the endogenous wild-type protein (Figure 1B). The control protein (GFP-CnnWT) localized to centrosomes and fully rescued the lethality of cnn null embryos (Figure 1C and Table 1) and therefore appears functional as previously reported for a full-length Cnn fusion to GFP (Megraw et al., 2002). GFP-CnnΔ1 also localized to centrosomes (Figure 1D) but was nonfunctional for rescue in vivo (Table 1). Motif 1 of Cnn is therefore essential for its function in vivo.
Table 1.
Embryonic rescue with wild-type and Motif 1 mutant cnn transgenes
| Genotypea | Percent hatch rate |
|---|---|
| Wild type (w1118) | 94 (n = 272) |
| cnnhk21/cnn25cn1 | 0 (n = 311) |
| cnnhk21/cnn25cn1, UASpGFP-CnnWT | 94 (n = 275) |
| cnnhk21/cnn25cn1, UASpGFP-CnnΔ1 | 0 (n = 145) |
| Dominant effects | |
| Wild-type, UASplacZ | 97 (n = 261) |
| Wild-type, UASpGFP-CnnWT | 95 (n = 189) |
| Wild-type, UASpGFP-CnnΔ1 | 0 (n = 294) |
a All the UASp constructs were expressed using nos-GAL4VP16 at 25°C. cnnhk21 and cnn25cn1 are cnn null mutants.
The phenotype of cnn null embryos replaced with GFP-CnnΔ1 (hereafter referred to as cnnΔ1 mutant embryos) is similar but distinct from cnn null mutants. Like the cnn null mutant, the mitotic figures in late cleavage stage cnnΔ1 mutant embryos are not distributed evenly and exhibit a high degree of aneuploidy (Figure 1D). Cleavage cycles progress to late syncytial blastoderm stages in cnnΔ1 embryos, but embryos fail to undergo cellularization, which normally occurs at cleavage cycle 14. However, in contrast to cnn null mutants, cnnΔ1 mutant centrosomes are retained at spindle poles and are predominantly in pairs that failed to separate. Moreover, some centrosomal proteins are recruited to cnnΔ1 mutant centrosomes that are not found at cnn null spindle poles (see below).
cnnΔ1 Embryos Are Severely Deficient in Centrosome Separation
In fixed and stained preparations the centrosomes in cnnΔ1 embryos were predominantly found in pairs or sets of pairs that failed to separate after duplication (Figure 1D). Because the Cnn proteins expressed in cnnWT and cnnΔ1 embryos were GFP-fusion proteins, live imaging of early embryos was used to track the centrosome cycle (Figure 2 and Supplementary Movies 1 and 2).
Figure 2.
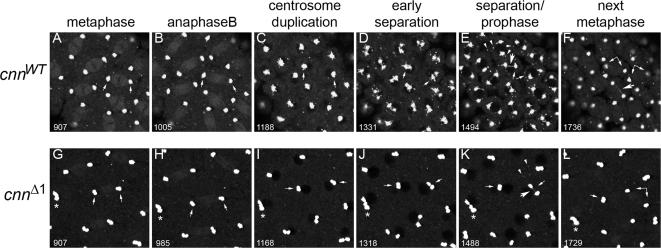
Centrosome separation and satellite movement require Motif 1 of Cnn. Live imaging of cnn null embryos expressing GFP-CnnWT (cnnWT, A–F) or GFP-CnnΔ1 (cnnΔ1, G–L). Cropped stills of movies at metaphase (A and G), anaphase B (B and H), centrosome duplication/telophase (C and I), early centrosome separation (D and J), separation/prophase (E and K), and metaphase of the next cycle (F and L). The time code of these stills, taken from Supplementary Movie 1 (A–F) and Supplementary Movie 2 (G–L), are shown in the lower left in seconds. Small arrows point to centrosomes beginning at one metaphase spindle (A and G) and follows them through one cleavage cycle (Supplementary Movie 1 progresses through three cycles, and Supplementary Movie 2 through four cycles). Each of the two centrosomes indicated with arrows in the cnnWT frames divides once (C) and separates (E), and the nascent pair contributes to a bipolar spindle in the ensuing metaphase (F). In contrast, the cnnΔ1 centrosomes indicated with arrows in G are two pairs at each pole of a metaphase spindle that failed to separate in the previous cycle. These pairs divide, producing two clusters of four centrosomes (J) that fail to separate (K). One pair of centrosome pairs did separate, whereas the other remained as a cluster of four in the next metaphase (L). The asterisk (*) in G indicates two pairs of attached centrosomes that divide to produce a cluster of eight centrosomes that failed to separate (K). More centrosome clusters form in subsequent cycles (see Supplementary Movie 2). The arrowheads in E and K point to centrosomal satellites, which are abundant in cnnWT embryos but scarce in cnnΔ1 embryos. The large arrows in E, F, and K indicate the intracentrosomal fibers that connect separating centrosomes.
In control cnnWT embryos, cleavage appeared normal (Figure 2, A–F, and Movie 1). Figure 2 shows stills of Supplementary Movies 1 and 2 at six stages in one cleavage cycle (Supplementary Movie 1 shows three cycles, and Supplementary Movie 2 show four consecutive cycles). Centrosomes in cnnWT embryos duplicate at telophase, and then centrosome separation ensues during prophase of the next cycle (Figure 2, D and E) as normally occurs in wild-type embryos (Rothwell and Sullivan, 2000). GFP-CnnWT decorates fibers that connect the two centrosomes during separation (large arrows in Figure 2, E and F), and these fibers, which are likely intercentrosomal microtubules, can persist into late anaphase (large arrow in Figure 2F and Supplementary Movie 1). Although Cnn is prominent at centrosomes, it is also localized weakly to spindle microtubules (Figure 2, A, B, G, and H), but is more highly enriched on these presumed intercentrosomal microtubules (Supplementary Movies 1 and 2 and Figure 2, E, F, and K).
Centrosome duplication appears normal in cnnΔ1 embryos, yet separation fails (compare Figure 2, J and K to D and E, and see Supplementary Movie 2). This results in pairs of centrosomes that participate in spindle assembly in the subsequent cycle, resulting in spindles with two centrosomes at one pole and none at the other, or with multiple centrosomes at each pole due to nuclear loss or fusion in prior cleavage divisions (see Figures 3D and 4, D, H, and L). When cnnΔ1 centrosomes did separate, GFP-CnnΔ1 decorated intercentrosomal fibers, as did GFP-CnnWT (Figure 2, J and K).
Figure 3.
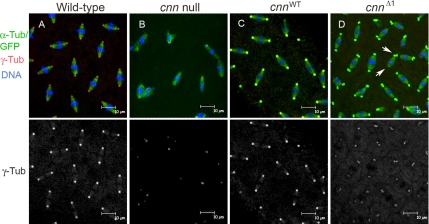
Cnn motif 1 recruits γ-tubulin to centrosomes. Wild-type (A), cnn null (B), and cnn null embryos with GFP-CnnWT (cnnWT, C) and GFP-CnnΔ1 (cnnΔ1, D) at early syncytial blastoderm cleavage stained for γ-Tub (separate channel shown below merged images). The centrosome signals in C and D are yellow compared with the signal for wild-type (A) because of the green signal from GFP-Cnn fusion proteins (red plus green overlap is yellow). The level of γ-Tub localized at cnnΔ1 mutant centrosomes is reduced, yet similar to the levels seen at cnn null embryo spindle poles. Note the unseparated centrosomes in the cnnΔ1 embryo on one spindle pole and none on the opposite spindle pole (arrows in D).
Figure 4.
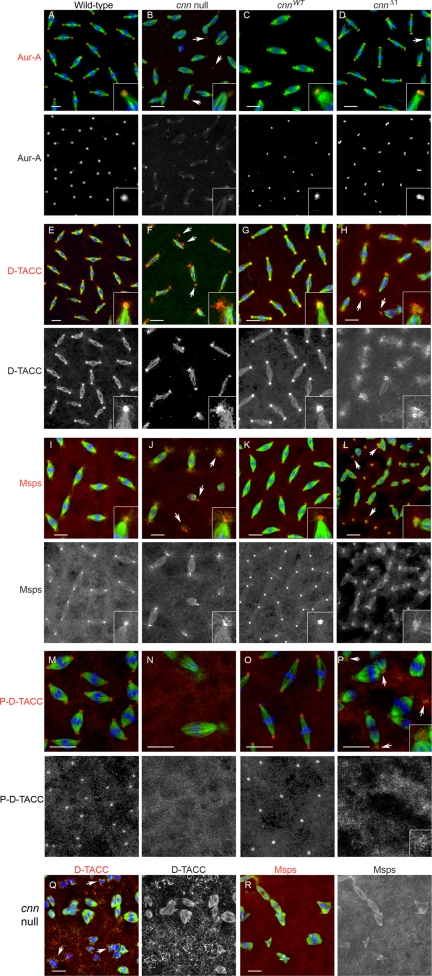
D-TACC and Msps are localized improperly at cnnΔ1 centrosomes. Wild-type (A, E, I, and M), cnn null (B, F, J, and N), cnnWT (C, G, and K) and cnnΔ1 (D, H, L, and P) embryos at early syncytial blastoderm cleavage stage stained for Aurora A (A–D), D-TACC (E–H), Msps (I–L) or P-D-TACC (M–P). All samples were counterstained for microtubules (green) and DNA (blue). Below each row is displayed the channel for Aurora A (Aur-A), D-TACC, Msps and P-D-TACC (all are red in the merged images). Insets are shown to highlight the localization of Aurora A, D-TACC, and Msps at spindle poles. Arrows in (F, H, J, and L) point to centrioles/centrosomes that are free or displaced from the spindle pole. The arrows in P indicate centrosomes with P-D-TACC signal at the centrosome periphery. All embryos shown are in cycles 10–12 except for (Q and R), which appear to be at later cycles. In cnn null mutant embryos the cycle number is unclear after cycle 10 or 11 because of the high degree of nuclear loss, disorder, and aneuploidy. In early cortical cycles (cycles 10 and 11) centrioles are detected at cnn null mutant spindle poles. In later cycles (Q and R), where centrioles are lost from spindle poles, D-TACC accumulates in particles that localize near microtubule bundles in the proximity of mitotic chromosomes (arrows in Q). Bar, 10 μm.
Live imaging revealed that pairs of cnnΔ1 centrosomes that failed to separate in the previous cycle duplicated to produce four conjoined centrosomes that would also fail to separate or do so inefficiently. Often, four centrosomes pulled apart as two pairs (pairs of newly duplicated centrosomes, with the old connection being broken; see Figure 2K and Supplementary Movie 2). We also observed centrosome pairs arranged in long strings that form because of multiple rounds of centrosome duplication accompanied by failed separation (Figure 2K and Supplementary Movie 2).
Cnn Motif 1 Is Required for Satellite Production
Another striking feature of cnnΔ1 mutant embryos is the near absence of satellites (compare Figure 2, D and E to J and K, and Supplementary Movies 1 and 2). Centrosomal satellites are a common feature of eukaryotic cells and have been characterized in vertebrate, Drosophila, and S. pombe cells (Kubo et al., 1999; Dammermann and Merdes, 2002; Megraw et al., 2002; Sawin et al., 2004; Venkatram et al., 2004). They are 70–100-nm particles of PCM proteins that traffic to and from centrosomes. In Drosophila, satellites contain Cnn, D-TACC, and Msps (Lee et al., 2001; Megraw et al., 2002). In vertebrates, PCM1, pericentrin, centrin, ninein, BBS4, and Nek2 kinase are satellite components (Kubo et al., 1999; Dammermann and Merdes, 2002; Kim et al., 2004; Hames et al., 2005). In S. pombe, satellites contain Mto1p, γ-Tub, alp4p (Dgrip84 homolog), and alp6p (Dgrip91 homolog; Sawin et al., 2004; Venkatram et al., 2004; Zimmerman and Chang, 2005). The function of satellites is not clear, but in S. pombe satellites are small MTOCs that nucleate microtubules along existing microtubule tracks, leading to efficient antiparallel bundle formation (Janson et al., 2005). In all these systems, satellite movement is microtubule-dependent (Kubo et al., 1999; Dammermann and Merdes, 2002; Megraw et al., 2002; Sawin et al., 2004; Venkatram et al., 2004; Hames et al., 2005).
Live imaging showed that satellites are produced in abundance in cnnWT embryos and traffic back and forth from centrosomes as previously reported for GFP-Cnn (Megraw et al., 2002). However, in cnnΔ1 embryos satellites are rare, with <1 satellite associated with each centrosome at interphase (when they are most abundant), compared with 6–7 per centrosome in the wild-type (Figure 2 and Supplementary Movies 1 and 2).
Our live imaging analysis of cnnΔ1 embryos revealed defects in centrosome separation, a microtubule-dependent process, and the poorly understood process of satellite formation. This led us to examine recruitment of PCM proteins to cnnΔ1 centrosomes, including molecules with established functions in microtubule assembly from the centrosome.
Cnn Motif 1 Recruits γ-Tub to the Centrosome
cnn null embryos have reduced signal of γ-Tub immunofluorescence at centrosomes compared with wild-type embryos (18.9 ± 6.4%, p = 0.002, Student's t test; Figure 3, A and B; Megraw et al., 1999). cnnΔ1 embryos also have reduced γ-Tub signal at centrosomes compared with wild-type (30.1 ± 10.6%, p = 0.008), indicating that Motif 1 is required to fully recruit the levels of γ-Tub seen at wild-type centrosomes (Figure 3, B and D; note that the centrosome signals in Figure 3, C and D, are yellow compared with red in Figure 3A, due to green fluorescence from the GFP-Cnn fusion proteins showing colocalization with γ-Tub). Immunostaining against Dgrip84 showed a similar reduction in signal at cnnΔ1 centrosomes (data not shown), indicating that the γ-TuSC is not recruited to centrosomes at wild-type levels. The reduced localization of γ-Tub is not due to reduced overall levels of γ-Tub protein in embryos (Figure 1B). These data show that there is a Cnn-independent pool of γ-Tub at embryonic centrosomes, but the major fraction of centrosomal γ-Tub is dependent on Cnn Motif 1 for its localization.
Although the decreased localization of γ-Tub at cnnΔ1 centrosomes is consistent with defects in microtubule-dependent processes, we also examined the recruitment of D-TACC/Msps, which are also required for efficient microtubule assembly at centrosomes, and Aurora A kinase, a regulator of this complex.
Cnn Motif 1 Is Required to Anchor D-TACC/Msps at Centrosomes
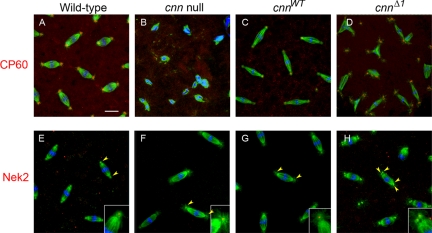
In cnn null embryos the centrosomal proteins CP60 and CP190 do not localize to centrosomes (Megraw et al., 1999; Vaizel-Ohayon and Schejter, 1999). Here we show that Aurora A, D-TACC, and Msps are also mislocalized in cnn null embryos (Figure 4, B, F, J, M, and N). Aurora A is localized at low levels to small dots at spindle poles in cnn null embryos, possibly reflecting localization to centrioles, but nevertheless a loss of the normal contribution of Aurora A that is seen at wild-type centrosomes. The Aurora A signal at the centrosome remnant in cnn null embryos is progressively lost from spindle poles in later (after cycle 10) cleavage cycles (data not shown), indicating that centrioles are lost during late cleavage cycles. We also observed a loss of signal for γ-Tub and Nek2 (a centriolar protein) in later cleavage cycles (Figure 3B and data not shown) that we attribute to centriole loss from spindle poles in cnn null embryos. This loss of the centrosome remnant in cnn null embryos is likely due to detachment from the spindle. Centriole detachment from spindle poles is a feature of cnn null embryos (see Figure 4, B, F, and J), in agreement with recent observations using live imaging of GFP-labeled centrioles (Lucas and Raff, personal communication, 2007).
Several studies have established that D-TACC and Msps (and their counterparts in Xenopus and Caenorhabditis elegans) form a complex through direct interaction (Lee et al., 2001; Bellanger and Gonczy, 2003; Le Bot et al., 2003; Srayko et al., 2003; Kinoshita et al., 2005; Peset et al., 2005). Msps localization depends on D-TACC and vice versa (Cullen and Ohkura, 2001; Lee et al., 2001). D-TACC and Msps localized to centrosomes and to spindle microtubules in wild-type and cnnWT embryos (Figure 4, E, G, I, and K), consistent with previous reports (Cullen et al., 1999; Gergely et al., 2000; Lee et al., 2001; Barros et al., 2005). In cnn null embryo early cortical divisions (cycles 10 and 11) D-TACC and Msps are recruited to spindle poles (Figure 4, F and J), but the localization is more dispersed compared with wild-type or cnnWT embryos (Figure 4, E, G, I, and K). Thus, D-TACC and Msps can be partially recruited to the centrosome remnant present in cnn null mutants, but cannot be fully anchored or maintained in the absence of Cnn. In later cleavage cycles when the centrioles are lost, spindle microtubules are decorated with Msps and D-TACC but spindle poles lack the signal (Figure 4, Q and R). Instead, D-TACC accumulates into particles that localize near the mitotic chromosomes (Figure 4Q).
In cnnWT embryos γ-Tub, CP60, Aurora A, D-TACC, and Msps localization to centrosomes is restored (Figures 3C, 4, C, G, and K, and see Figure 6C). In contrast, D-TACC and Msps are incompletely recruited to cnnΔ1 centrosomes (Figure 4, H and L). D-TACC and Msps are loosely associated near centrosomes in cnnΔ1 mutant embryos, similar to cnn null embryos. Although D-TACC and Msps are recruited to the vicinity of centrosomes, they accumulate at the centrosome periphery in cnnΔ1 embryos (see below). Despite these defects in D-TACC and Msps recruitment to centrosomes, the localization of Aurora A kinase, which regulates D-TACC, appears normal in cnnΔ1 embryos (Figure 4D). This indicates that PCM architecture is not globally disrupted by the mutation in Motif 1. Moreover, in contrast to the cnn null, cnnΔ1 embryos retain centrosomes at spindle poles into late cortical cycles (albeit in clusters of 2 or more, see Figure 2 and Supplementary Movie 2). Furthermore, the association of D-TACC and Msps with cnnΔ1 centrosomes, though aberrant, persists into later cleavage cycles in cnnΔ1 embryos.
Figure 6.
Localization of CP60 and Nek2 to centrosomes does not require Cnn Motif1. Wild-type, cnn null, cnnWT, and cnnΔ1 embryos were stained for α-tubulin (green), DNA (blue), and CP60 (top row, red) or Nek2 (bottom row, red). Arrowheads in bottom row point to the dot of Nek2 signal at the centrioles. For cnn null embryos, the Nek2 signal is present in early syncytial blastoderm embryos shown here, but is lost at later cleavage stage embryos due to loss of centrioles (not shown). Bar, 10 μm.
Because Aurora A kinase is required for D-TACC localization, and activates D-TACC through phosphorylation at Ser863, we examined D-TACC phosphorylation with a phospho-specific antibody (Barros et al., 2005). P-D-TACC is found at centrosomes but not on spindle microtubules in wild-type embryos as reported previously (Figure 4M). In cnn null mutant embryos P-D-TACC was not detected at spindle poles (Figure 4N). D-TACC Ser863 phosphorylation was restored at cnnWT centrosomes (Figure 4O), yet showed weak localization at the centrosome periphery in cnnΔ1 embryos similar to the accumulation observed with anti- D-TACC antibodies (compare Figure 4, H and P).
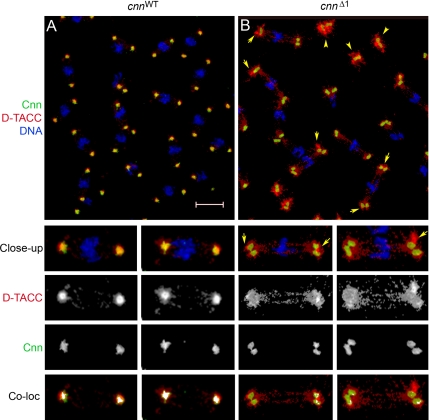
Colocalization analysis of D-TACC with GFP-CnnWT and GFP-CnnΔ1 (Figure 5) shows reduced colocalization of D-TACC with CnnΔ1 at the PCM compared with CnnWT. Instead, the major pool of centrosomal D-TACC accumulates at the immediate periphery of the PCM (arrows in Figure 5B), and only partial recruitment of D-TACC within the central mass of the PCM is achieved at cnnΔ1 mutant centrosomes. Msps was similarly relegated to the centrosome periphery with poor recruitment or maintenance at cnnΔ1 centrosomes (Figure 4L). These data are consistent with an interdependence of D-TACC and Msps for centrosomal localization (Cullen and Ohkura, 2001; Lee et al., 2001).
Figure 5.
Cnn and D-TACC colocalization at centrosomes. cnnWT (A) and cnnΔ1 (B) embryos were stained for Cnn (green), D-TACC (red), and DNA (blue). Two close-up views of each are shown below, with the separate channels for Cnn and D-TACC signals. In the cnnΔ1 mutant D-TACC localized at and near centrosomes, but with higher levels of accumulation at the immediate periphery of the centrosome (arrows in B and in close-up images). There is also a higher level of D-TACC associated with free centrosomes (arrowheads in B). The “Coloc” images show colocalized Cnn and D-TACC signals. The white signal in each image represents colocalized signals above a pixel intensity threshold of 150 (see Materials and Methods). Bar, 10 μm.
In cnnΔ1 mutant embryos defective centrosome separation is likely the result of reduced γ-Tub localization and/or improper assembly of D-TACC/Msps. Thus, the perturbed assembly of these components likely alters the mechanics of microtubule assembly at cnnΔ1 centrosomes. Nevertheless, microtubule asters are produced at cnnΔ1 centrosomes (e.g., see insets in Figure 6).
Some Centrosome Components Localize Normally to CnnΔ1 Centrosomes
Because γ-Tub, D-TACC, and Msps are aberrantly distributed in cnnΔ1 embryos, we tested whether the cnnΔ1 mutant had a global affect on the PCM architecture. We found that Motif 1 is not required for recruitment of other centrosome proteins, including Aurora A kinase, CP60, and Nek2 kinase (Figures 4 and 6), indicating that only specific aspects of PCM assembly are controlled by Cnn Motif 1. Nek2 kinase is a centriolar protein (Prigent et al., 2005), and in embryonic centrosomes there is a single, sharp signal at the center of the spindle pole (Figure 6E). The presence of centrioles at cnn maternal effect mutant spindle poles (at early cortical cycles but lost in later cycles; see above) is consistent with our previous report of centrioles in cnn mutant imaginal disk cells (Megraw et al., 2001). Together, the restored localization of Aurora A and CP60 to cnnΔ1 mutant centrosomes (compared with the cnn null mutant; Figures 4D and 6D) and the retention of centrioles into late cleavage suggests that PCM structure may not be severely disrupted at cnnΔ1 centrosomes. Furthermore, the ability to partially assemble actin into cleavage furrows (see below) shows that aspects of centrosome function are intact in the cnnΔ1 mutant.
cnnΔ1 Is a Dominant Mutation
Overexpression of GFP-CnnWT in a wild-type cnn background produces only minor defects in cleavage, and embryos hatch at wild-type percentages (Figure 7, A and C, and Table 1). The hatch rate is zero when we overexpress GFP-CnnΔ1 in a wild-type background (Table 1). The overexpression phenotype is indistinguishable from the expression of GFP-CnnΔ1 in the cnnhk21/cnn25cn1 (null) background, including centrosome separation defects, reduced satellites, and reduced γ-Tub localization at centrosomes (Figure 7, B and D). GFP-CnnΔ1 is not lethal zygotically, however, when expressed ubiquitously from late embryogenesis onward using a Tub-GAL4 driver.
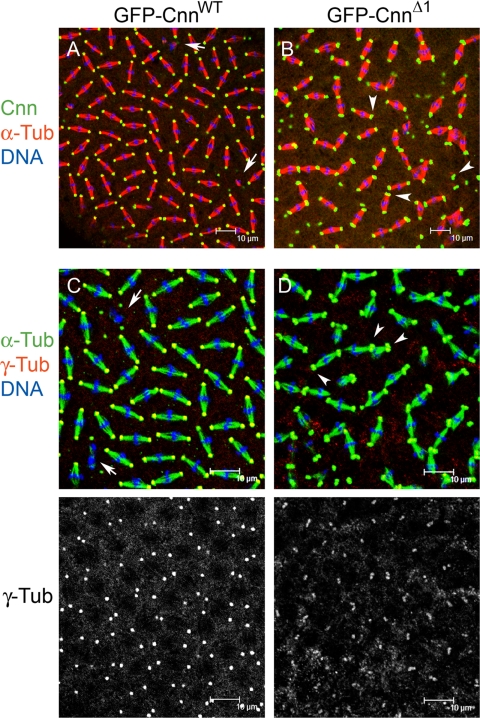
Figure 7.
cnnΔ1 is a dominant mutation. Overexpression of GFP-CnnΔ1 in a wild-type cnn background (B and D) has dominant phenotypes compared with GFP-CnnWT overexpression (A and C). Only mild cleavage failure defects were seen with GFP-CnnWT overexpression (arrows in A and C), which had little bearing on embryo viability (see Table 1). In wild-type embryos where GFP-CnnΔ1 was expressed, most centrosomes were in pairs at metaphase (arrowheads in B and D) due to centrosome separation failure. As was the case for expression in a cnn null mutant background, expression of GFP-CnnΔ1 in a wild-type background resulted in reduced recruitment of γ-Tub to centrosomes (compare γ-Tub channel in C to D).
Third instar larval brains expressing GFP-CnnΔ1 in wild-type or cnn null backgrounds showed only occasional unseparated centrosomes among cells in metaphase (data not shown). This indicates that Motif 1 has a more stringent requirement in the early embryo for centrosome separation.
cnnΔ1 Mutant Embryos Assemble Aberrant Pseudocleavage Furrows
In view of the fact that centrosomes organize actin into cleavage furrows, we examined the ability of cnnΔ1 mutant centrosomes to assemble pseudocleavage furrows. Cortical actin normally undergoes concerted cleavage cycle–dependent rearrangements from actin caps at interphase, to mitotic furrows that surround and separate each syncytial nucleus into its own pseudocleavage furrow at metaphase (Figure 8, A and C; Foe et al., 1993; Sullivan and Theurkauf, 1995). Previous studies showed that centrosomes direct actin organization in the syncytial blastoderm and at cellularization (Raff and Glover, 1989; Rothwell and Sullivan, 2000). cnn null embryos show no organization of F-actin into caps at interphase or to furrows at mitosis (Figure 8B; Vaizel-Ohayon and Schejter, 1999). cnnΔ1 mutant centrosomes, however, are able to assemble actin into pseudocleavage furrows. Staining for F-actin showed robust but defective furrow formation in cnnΔ1 embryos (Figure 8D). In contrast to wild-type and control cnnWT embryos, the furrows assembled in the cnnΔ1 mutant are varied in size and frequently incomplete (Figure 8D). The variability in furrow size or diameter is likely due to the failure of centrosomes to separate and the abundant free centrosomes in cnnΔ1 embryos. Consistent with these findings, free centrosomes not associated with nuclei have previously been shown to produce smaller furrows in early embryos (Raff and Glover, 1989; Rothwell and Sullivan, 2000). Staining for phosphotyrosine epitopes, which are enriched at the furrowed membranes, revealed defects similar to the actin organization phenotypes (data not shown).
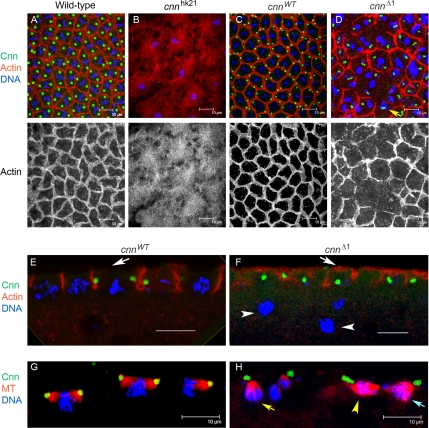
Figure 8.
Cleavage furrow assembly from cnnΔ1 mutant centrosomes. Actin is organized aberrantly into pseudocleavage furrows in cnnΔ1 mutant embryos. Wild-type (A), cnn null (B), and cnn null cleavage stage embryos with GFP-CnnWT (cnnWT, C, E, and G) and GFP-CnnΔ1 (cnnΔ1, D, F, and H) at metaphase stained for filamentous actin, Cnn, and DNA. The furrow organization is highly irregular in the cnnΔ1 mutant, with variable integrity and size of furrows. Incomplete furrows are frequently formed (D). The depth of the furrow ingression is variable in the cnnΔ1 mutant, but can achieve the same depth as the cnnWT control (compare E and F). Arrows in E and F highlight the difference in cortical actin distribution in cnnWT and cnnΔ1 embryos at metaphase. The white arrowheads in F show nuclei that have dropped from the cortex. Embryos in G and H were stained for Cnn, microtubules, and DNA. Yellow arrows in D and H indicate mitotic figures that are rotated 90° from the normal parallel orientation to the embryo cortex. The yellow arrow in H indicates a misoriented half spindle, nearby to one that is oriented correctly (yellow arrowhead) and another that is splayed parallel and perpendicular to the cortex (blue arrow). Note that one of the spindles in G is at an oblique angle, such that the posterior half of the spindle is not included in the image stack. Bar, 10 μm.
The depth of furrow ingression in the cnnΔ1 mutant was more variable than cnnWT, but achieved similar lengths of 4–8 μm (Figure 8, E and F). The sagittal views of cnnΔ1 embryos shown in Figure 8F also revealed prominent actin accumulation or retention at the cortex in the cnnΔ1 mutant embryos at metaphase compared with cnnWT. In wild-type embryos actin caps form over the nuclei in interphase in a centrosome-dependent manner, and the actin spreads at prophase and is predominantly localized in furrows at metaphase (Raff and Glover, 1989; Foe et al., 1993; Sullivan and Theurkauf, 1995). The substantial amount of actin that remains at the cortex in cnnΔ1 mutants at metaphase (Figure 8F) indicates that efficient spreading of actin from caps into furrows is dependent on Cnn Motif 1.
Our analyses also revealed a high frequency of nuclear fallout (arrowheads in Figure 8F) and mitotic spindles that are rotated 90° from their wild-type orientation, where the spindle axis is parallel relative to the embryo cortex (compare mitotic figures in Figure 8G to misoriented one in Figure 8H, yellow arrow). The yellow arrow in Figure 8D shows an example of a rotated spindle from an en face view, looking down the spindle axis. This suggests that mitotic spindles are not anchored properly at the cortex or fail to assemble along the cortical axis.
Here we show that, although cortical actin organization is highly erratic in cnnΔ1 mutant embryos, the furrow defects are not as severe as the cnn null mutant. This partial restoration of centrosome function suggests that Cnn domains other than Motif 1 exert control over the actin-organizing function of the centrosome.
DISCUSSION
Previous studies showed that Cnn is required for centrosome assembly/maturation, for microtubule assembly from the centrosome at mitosis, and to organize actin into pseudocleavage furrows in the early embryo. Here we show that Motif 1 of Cnn is required for specific and essential aspects of centrosome function. Centrosomes assembled in cnnΔ1 embryos recruit some PCM components and are partially proficient to organize actin into pseudocleavage furrows, but do not properly recruit or maintain proteins with an established role in microtubule assembly: γ-tubulin, D-TACC, and Msps. Thus, although astral microtubules are produced at cnnΔ1 mutant centrosomes, centrosome separation, a microtubule-dependent process, is severely affected. In addition, the less-understood process of satellite formation is inhibited at cnnΔ1 centrosomes.
Anchoring of γ-Tub, D-TACC, and Msps to Centrosomes Requires Cnn Motif1
Microtubule assembly at centrosomes is regulated by nucleation, where γ-Tub plays a key role, and by microtubule growth, which depends on a host of factors including Aurora A, D-TACC, and Msps, that promote stability. How these proteins are assembled and regulated is still largely unknown. Here we show that Cnn Motif 1 controls assembly of PCM proteins that are required for MTOC activity at centrosomes.
γ-Tub is an essential component of MTOCs in eukaryotes for microtubule assembly (Wiese and Zheng, 2006). In cnn null mutant neuroblasts, imaginal disk cells, and cells depleted of Cnn by RNAi, neither γ-Tub nor astral microtubules are detected at centrosomes (Megraw et al., 2001; Mahoney et al., 2006). However, in contrast to the above cell types, a Cnn-independent pool of γ-Tub is at the centrosome remnant in cnn null mutant early embryonic spindle poles (Megraw et al., 1999). The small, sharp signal for γ-Tub at cnn null spindle poles implicates a centriolar pool of γ-Tub that is unique to the rapid divisions of early embryos. The level of γ-Tub at cnnΔ1 mutant centrosomes is similar to the cnn null mutant, indicating that Motif 1 is required for recruitment of the Cnn-dependent pool of γ-Tub to the PCM in embryos. Drosophila Cnn and the S. pombe homolog Mto1p have been reported to coIP with γ-Tub (Terada et al., 2003; Sawin et al., 2004; Venkatram et al., 2004), but a direct interaction with γ-Tub or any of the γ-TuRC proteins has not been demonstrated.
D-TACC and Msps, and their counterparts in Xenopus (TACC3/maskin and XMAP215) and C. elegans (TAC-1 and ZYG-9) are direct binding partners required for centrosome-dependent growth of long microtubules (Gergely et al., 2000; Bellanger and Gonczy, 2003; Le Bot et al., 2003; Srayko et al., 2003; Kinoshita et al., 2005; Peset et al., 2005). Mutation or depletion of D-TACC or its homologues does not affect γ-Tub localization to centrosomes, but rather appears to function with Msps in the stability of microtubules that are nucleated by γ-Tub (Lee et al., 2001; Popov et al., 2002). D-TACC and Msps are partially recruited to centrosomes in cnn null and cnnΔ1 mutants, accumulating at the centrosome periphery in cnnΔ1 embryos. This incomplete assembly suggests that recruitment of D-TACC and Msps to centrosomes normally involves at least two steps and that Motif 1 of Cnn is required for a secondary step in the process subsequent to docking of D-TACC at the periphery of the centrosome. Thus, Cnn Motif 1 may be required for a later phase of recruitment to the centrosome or have a role in maintaining D-TACC and Msps once they are recruited.
Aurora A kinase is required to localize D-TACC to centrosomes (Giet et al., 2002) and directly phosphorylates D-TACC at Ser863 to activate its microtubule-stabilizing activity (Barros et al., 2005). The reduced recruitment of Aurora A to cnn null centrosomes further highlights the requirement for Cnn in PCM assembly. However, Aurora A localization did not appear affected in cnnΔ1 embryos, indicating that, although Aurora A is necessary to recruit D-TACC/Msps, its localization at centrosomes is not sufficient to accomplish this. Aurora A binds directly to the C-terminal half of Cnn (Terada et al., 2003), which remains intact in the cnnΔ1 mutant. Moreover, D-TACC is phosphorylated by Aurora A in cnnΔ1 embryos; however, this activated pool of D-TACC is exiled to the centrosome periphery with the bulk pool of centrosomal D-TACC. This indicates that Motif 1 of Cnn is required for anchoring or maintaining D-TACC at centrosomes subsequent to its regulatory phosphorylation by Aurora A. Alternatively, because the immunofluorescence signal for P-D-TACC was weak and we did not quantify P-D-TACC levels, we cannot exclude an affect by cnnΔ1 on Aurora A activity toward D-TACC.
In cnnΔ1 and cnn null embryos microtubule asters are present, particularly at early cortical cycles (cycles 10 and 11). At later cycles asters are not detected at spindle poles in cnn null embryos, coinciding with centriole loss, which is evident from the absence of Nek2 signal. Centriole displacement from the spindle poles in cnn null embryos leads to centriole loss, resulting in anastral spindle poles (Lucas and Raff, personal communication, 2007). By comparison to cnn null embryos, PCM integrity is restored to cnnΔ1 mutant centrosomes, enough to retain centrosomes at spindle poles into later cleavage cycles and with retained ability to assemble astral microtubules. Nevertheless, centrosome separation failure indicates that microtubule-dependent processes are impaired at cnnΔ1 centrosomes.
Centrosome Separation
Centrosome separation is a microtubule-dependent process that is coordinated by pushing forces from interpolar microtubules and forces supplied by molecular motors that include kinesin-5, kinesin-14 (Ncd), and dynein/Lis1/dynactin (Gonczy et al., 1999; Mountain et al., 1999; Robinson et al., 1999; Sharp et al., 1999; Cytrynbaum et al., 2003; Cockell et al., 2004; Siller et al., 2005). The relative contributions of motor proteins and the pushing forces generated from the assembly of interpolar centrosomal microtubules have not been determined.
A necessary role for microtubules in centrosome separation was shown using microtubule-depolymerizing drugs in cell culture and in early Drosophila embryos (Stevenson et al., 2001; Uzbekov et al., 2002). Interpolar centrosomal microtubules may represent a specialized class of microtubules, an idea supported by the recent discovery of an α-tubulin variant, α4-tubulin, which is associated with faster-growing microtubules and is enriched in interpolar microtubules. α4-tubulin is required for centrosome separation in early embryos (Venkei et al., 2006). Cnn localized more strongly to interpolar fibers compared with spindle microtubules, suggesting that Cnn Motif 1 may regulate the organization of interpolar centrosomal microtubules to promote centrosome separation. In instances when cnnΔ1 centrosomes separated, interpolar fibers formed, suggesting that interpolar fibers are obligatory to centrosome separation. Although we favor the proposal that Motif 1 regulates microtubule assembly to achieve centrosome separation, we cannot rule out a role for Motif 1 in regulating molecular motors that are involved in this process. However, localization of the kinesin-5/Eg5 family member Klp61F to spindle poles and spindle microtubules was no different in cnnWT and cnnΔ1 embryos (data not shown).
Consistent with a role for γ-Tub and D-TACC recruitment to centrosomes by Cnn Motif 1 in centrosome separation, depletion or mutation of γ-Tub, γ-TuSC proteins, and D-TACC also perturbed centrosome separation (Barbosa et al., 2000; Gergely et al., 2000; Sampaio et al., 2001; Colombie et al., 2006; Verollet et al., 2006). Thus, although we can detect γ-Tub at reduced levels and also astral microtubules, embryonic cnnΔ1 centrosomes have insufficient or inappropriate microtubule assembly activity to achieve centrosome separation.
Satellite Activity
We previously showed by live imaging of GFP-Cnn embryos that centrosomal satellites are highly dynamic structures that traffic in a microtubule-dependent and an actin-independent manner (Megraw et al., 2002). Satellites, or “flares,” emerge from the PCM and move bidirectionally at speeds of 4–20 μm min−1 and are produced at highest numbers at telophase/interphase, coincident with the relative intensity of astral microtubules during the cleavage cycle (Megraw et al., 2002). cnnΔ1 mutant embryos produce significantly fewer satellites. Even incipient satellites, which are apparent on cnnWT centrosomes (Figure 6C and Supplementary Movie 1) and are present at colchicine-treated centrosomes (Megraw et al., 2002), were nearly absent at cnnΔ1 centrosomes (see Figure 6I). Satellite assembly may be an intrinsic function for Motif 1. Alternatively, fewer satellites may arise as a secondary consequence of altered MTOC activity at cnnΔ1 centrosomes. Currently, we cannot distinguish between these two possibilities.
Cnn and Pseudocleavage Furrow Formation
The organization of actin into pseudocleavage furrows, an activity conveyed by centrosomes, is highly aberrant yet partially restored in cnnΔ1 mutant embryos. This is in sharp contrast to cnn null embryos, where no apparent organization of cortical actin occurs. Although some studies have indicated that microtubules are required for cortical actin organization in the early Drosophila embryo (reviewed in Rothwell and Sullivan, 2000), other evidence suggests that centrosomes organize actin and cortical polarity independent of microtubules (Stevenson et al., 2001; Cowan and Hyman, 2004). Because microtubule-dependent processes are disrupted in cnnΔ1 embryos, our data support the model that centrosomes can organize actin independent of microtubules, but we cannot exclude the possibility that cnnΔ1 centrosomes produce sufficient astral microtubules to coordinate with actin in the assembly of furrows.
In summary, Motif 1, conserved among Cnn family members, is required for centrosome function in early embryos through the recruitment and anchoring of γ-Tub, D-TACC, and Msps, key factors in MTOC function in all eukaryotes where they have been examined. PCM architecture is partially restored in the cnnΔ1 mutant compared with the cnn null, as shown by the normal distribution of CP60 and Aurora A. In addition, conspicuous yet aberrant pseudocleavage furrows assemble in cnnΔ1 embryos but not in the cnn null, evidence that organization of actin by centrosomes is partially restored to cnnΔ1 mutant centrosomes. This suggests that the activity to direct actin organization into cleavage furrows resides in another domain of Cnn. Identification of the direct binding partner for Cnn Motif 1 will be an important step toward understanding the relationship between Motif 1 and the MTOC functions that it governs.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Joel Goodman, Sally Commerford, and members of the lab for their helpful comments on the manuscript. We thank Jurgen Knoblich (IMBA, Vienna, Austria) for the anti-Aur A antibodies, Bill Theurkauf (University of Massachusetts Medical Center, Worcester, MA) for anti-D-TACC, Hiro Ohkura (University of Edinburgh, Edinburgh, United Kingdom) for anti-Msps, Jordan Raff (Gurdon Institute, Cambridge, United Kingdom) for anti-P-D-TACC, Regis Giet (Université de Rennes, Rennes, France) for anti-Nek2, and Jon Scholey (University of California Davis, Davis, CA) for anti-Klp61f. The mAb anti-Myc 9E10 developed by J. Michael Bishop was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa. This project was supported by National Institutes of Health Grant GM-068756 and Welch Foundation grant I-1610 (T.L.M).
Abbreviations used:
- cnn
centrosomin
- MTOC
microtubule organizing center
- PCM
pericentriolar material
- γ-Tub
gamma-tubulin
- D-TACC
Drosophila transforming acidic coiled coil protein
- Msps
minispindles.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0474) on August 1, 2007.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Barbosa V., Yamamoto R. R., Henderson D. S., Glover D. M. Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 2000;14:3126–3139. doi: 10.1101/gad.182800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros T. P., Kinoshita K., Hyman A. A., Raff J. W. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J. Cell Biol. 2005;170:1039–1046. doi: 10.1083/jcb.200504097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger J. M., Gonczy P. TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr. Biol. 2003;13:1488–1498. doi: 10.1016/s0960-9822(03)00582-7. [DOI] [PubMed] [Google Scholar]
- Bond J., et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat. Genet. 2005;37:353–355. doi: 10.1038/ng1539. [DOI] [PubMed] [Google Scholar]
- Cockell M. M., Baumer K., Gonczy P. lis-1 is required for dynein-dependent cell division processes in C. elegans embryos. J. Cell Sci. 2004;117:4571–4582. doi: 10.1242/jcs.01344. [DOI] [PubMed] [Google Scholar]
- Colombie N., Verollet C., Sampaio P., Moisand A., Sunkel C., Bourbon H. M., Wright M., Raynaud-Messina B. The Drosophila gamma-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus. Mol. Biol. Cell. 2006;17:272–282. doi: 10.1091/mbc.E05-08-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C. R., Hyman A. A. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature. 2004;431:92–96. doi: 10.1038/nature02825. [DOI] [PubMed] [Google Scholar]
- Cullen C. F., Deak P., Glover D. M., Ohkura H. mini spindles: A gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J. Cell Biol. 1999;146:1005–1018. doi: 10.1083/jcb.146.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen C. F., Ohkura H. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 2001;3:637–642. doi: 10.1038/35083025. [DOI] [PubMed] [Google Scholar]
- Cytrynbaum E. N., Scholey J. M., Mogilner A. A force balance model of early spindle pole separation in Drosophila embryos. Biophys. J. 2003;84:757–769. doi: 10.1016/S0006-3495(03)74895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A., Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 2002;159:255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie K. W., Kennedy C. D., Velasco V. M., McGrath T. L., Weko J., Patterson R. W., Karpen G. H. Identification of chromosome inheritance modifiers in Drosophila melanogaster. Genetics. 2001;157:1623–1637. doi: 10.1093/genetics/157.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V. E., Odell G. M., Edgar B. A. Mitosis and morphogenesis in the Drosophila embryo: point and counterpoint. In: Bate M., Martinez-Arias A., editors. The Development of Drosophila melanogaster. Vol. 1. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1993. pp. 149–300. [Google Scholar]
- Gergely F., Kidd D., Jeffers K., Wakefield J. G., Raff J. W. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 2000;19:241–252. doi: 10.1093/emboj/19.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R., McLean D., Descamps S., Lee M. J., Raff J. W., Prigent C., Glover D. M. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 2002;156:437–451. doi: 10.1083/jcb.200108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P., Pichler S., Kirkham M., Hyman A. A. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J. Cell Biol. 1999;147:135–150. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane R. N., Lizarraga S. B., Wiese C., Wilde A., Zheng Y. gamma-Tubulin complexes and their role in microtubule nucleation. Curr. Top. Dev. Biol. 2000;49:55–73. doi: 10.1016/s0070-2153(99)49004-0. [DOI] [PubMed] [Google Scholar]
- Hames R. S., Crookes R. E., Straatman K. R., Merdes A., Hayes M. J., Faragher A. J., Fry A. M. Dynamic recruitment of Nek2 kinase to the centrosome involves microtubules, PCM-1, and localized proteasomal degradation. Mol. Biol. Cell. 2005;16:1711–1724. doi: 10.1091/mbc.E04-08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak E., Oegema K., Kirkham M., Gonczy P., Habermann B., Hyman A. A. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is gamma-tubulin dependent. J. Cell Biol. 2002;157:591–602. doi: 10.1083/jcb.200202047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer J. G., Li K., Kaufman T. C. The Drosophila homeotic target gene centrosomin (cnn) encodes a novel centrosomal protein with leucine zippers and maps to a genomic region required for midgut morphogenesis. Development. 1995;121:3861–3876. doi: 10.1242/dev.121.11.3861. [DOI] [PubMed] [Google Scholar]
- Janson M. E., Setty T. G., Paoletti A., Tran P. T. Efficient formation of bipolar microtubule bundles requires microtubule-bound gamma-tubulin complexes. J. Cell Biol. 2005;169:297–308. doi: 10.1083/jcb.200410119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. C., et al. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat. Genet. 2004;36:462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- Kinoshita K., Noetzel T. L., Pelletier L., Mechtler K., Drechsel D. N., Schwager A., Lee M., Raff J. W., Hyman A. A. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J. Cell Biol. 2005;170:1047–1055. doi: 10.1083/jcb.200503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A., Sasaki H., Yuba-Kubo A., Tsukita S., Shiina N. Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J. Cell Biol. 1999;147:969–980. doi: 10.1083/jcb.147.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bot N., Tsai M. C., Andrews R. K., Ahringer J. TAC-1, a regulator of microtubule length in the C. elegans embryo. Curr. Biol. 2003;13:1499–1505. doi: 10.1016/s0960-9822(03)00577-3. [DOI] [PubMed] [Google Scholar]
- Lee M. J., Gergely F., Jeffers K., Peak-Chew S. Y., Raff J. W. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat. Cell Biol. 2001;3:643–649. doi: 10.1038/35083033. [DOI] [PubMed] [Google Scholar]
- Mahoney N. M., Goshima G., Douglass A. D., Vale R. D. Making microtubules and mitotic spindles in cells without functional centrosomes. Curr. Biol. 2006;16:564–569. doi: 10.1016/j.cub.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Megraw T. L., Kao L. R., Kaufman T. C. Zygotic development without functional mitotic centrosomes. Curr. Biol. 2001;11:116–120. doi: 10.1016/s0960-9822(01)00017-3. [DOI] [PubMed] [Google Scholar]
- Megraw T. L., Kilaru S., Turner F. R., Kaufman T. C. The centrosome is a dynamic structure that ejects PCM flares. J. Cell Sci. 2002;115:4707–4718. doi: 10.1242/jcs.00134. [DOI] [PubMed] [Google Scholar]
- Megraw T. L., Li K., Kao L. R., Kaufman T. C. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development. 1999;126:2829–2839. doi: 10.1242/dev.126.13.2829. [DOI] [PubMed] [Google Scholar]
- Mountain V., Simerly C., Howard L., Ando A., Schatten G., Compton D. A. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 1999;147:351–366. doi: 10.1083/jcb.147.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R. gamma-Tubulin. Curr. Top. Dev. Biol. 2000;49:27–54. doi: 10.1016/s0070-2153(99)49003-9. [DOI] [PubMed] [Google Scholar]
- Peset I., Seiler J., Sardon T., Bejarano L. A., Rybina S., Vernos I. Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis. J. Cell Biol. 2005;170:1057–1066. doi: 10.1083/jcb.200504037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov A. V., Severin F., Karsenti E. XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr. Biol. 2002;12:1326–1330. doi: 10.1016/s0960-9822(02)01033-3. [DOI] [PubMed] [Google Scholar]
- Preston C. R., Sved J. A., Engels W. R. Flanking duplications and deletions associated with P-induced male recombination in Drosophila. Genetics. 1996;144:1623–1638. doi: 10.1093/genetics/144.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent C., Glover D. M., Giet R. Drosophila Nek2 protein kinase knockdown leads to centrosome maturation defects while overexpression causes centrosome fragmentation and cytokinesis failure. Exp. Cell Res. 2005;303:1–13. doi: 10.1016/j.yexcr.2004.04.052. [DOI] [PubMed] [Google Scholar]
- Raff J. W., Glover D. M. Centrosomes, and not nuclei, initiate pole cell formation in Drosophila embryos. Cell. 1989;57:611–619. doi: 10.1016/0092-8674(89)90130-x. [DOI] [PubMed] [Google Scholar]
- Robinson J. T., Wojcik E. J., Sanders M. A., McGrail M., Hays T. S. Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J. Cell Biol. 1999;146:597–608. doi: 10.1083/jcb.146.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell W. F., Sullivan W. The centrosome in early Drosophila embryogenesis. Curr. Top. Dev. Biol. 2000;49:409–447. doi: 10.1016/s0070-2153(99)49020-9. [DOI] [PubMed] [Google Scholar]
- Sampaio P., Rebollo E., Varmark H., Sunkel C. E., Gonzalez C. Organized microtubule arrays in gamma-tubulin-depleted Drosophila spermatocytes. Curr. Biol. 2001;11:1788–1793. doi: 10.1016/s0960-9822(01)00561-9. [DOI] [PubMed] [Google Scholar]
- Sawin K. E., Lourenco P. C., Snaith H. A. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr. Biol. 2004;14:763–775. doi: 10.1016/j.cub.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Sharp D. J., Yu K. R., Sisson J. C., Sullivan W., Scholey J. M. Antagonistic microtubule-sliding motors position mitotic centrosomes in Drosophila early embryos. Nat. Cell Biol. 1999;1:51–54. doi: 10.1038/9025. [DOI] [PubMed] [Google Scholar]
- Siller K. H., Serr M., Steward R., Hays T. S., Doe C. Q. Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol. Biol. Cell. 2005;16:5127–5140. doi: 10.1091/mbc.E05-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srayko M., Quintin S., Schwager A., Hyman A. A. Caenorhabditis elegans TAC-1 and ZYG-9 form a complex that is essential for long astral and spindle microtubules. Curr. Biol. 2003;13:1506–1511. doi: 10.1016/s0960-9822(03)00597-9. [DOI] [PubMed] [Google Scholar]
- Stevenson V. A., Kramer J., Kuhn J., Theurkauf W. E. Centrosomes and the Scrambled protein coordinate microtubule-independent actin reorganization. Nat. Cell Biol. 2001;3:68–75. doi: 10.1038/35050579. [DOI] [PubMed] [Google Scholar]
- Strome S., Powers J., Dunn M., Reese K., Malone C. J., White J., Seydoux G., Saxton W. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell. 2001;12:1751–1764. doi: 10.1091/mbc.12.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan W., Theurkauf W. E. The cytoskeleton and morphogenesis of the early Drosophila embryo. Curr. Opin. Cell Biol. 1995;7:18–22. doi: 10.1016/0955-0674(95)80040-9. [DOI] [PubMed] [Google Scholar]
- Terada Y., Uetake Y., Kuriyama R. Interaction of Aurora-A and centrosomin at the microtubule-nucleating site in Drosophila and mammalian cells. J. Cell Biol. 2003;162:757–763. doi: 10.1083/jcb.200305048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbekov R., Kireyev I., Prigent C. Centrosome separation: respective role of microtubules and actin filaments. Biol. Cell. 2002;94:275–288. doi: 10.1016/s0248-4900(02)01202-9. [DOI] [PubMed] [Google Scholar]
- Vaizel-Ohayon D., Schejter E. D. Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr. Biol. 1999;9:889–898. doi: 10.1016/s0960-9822(99)80393-5. [DOI] [PubMed] [Google Scholar]
- Venkatram S., Tasto J. J., Feoktistova A., Jennings J. L., Link A. J., Gould K. L. Identification and characterization of two novel proteins affecting fission yeast gamma-tubulin complex function. Mol. Biol. Cell. 2004;15:2287–2301. doi: 10.1091/mbc.E03-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkei Z., Gaspar I., Toth G., Szabad J. alpha4-Tubulin is involved in rapid formation of long microtubules to push apart the daughter centrosomes during early Drosophila embryogenesis. J. Cell Sci. 2006;119:3238–3248. doi: 10.1242/jcs.03039. [DOI] [PubMed] [Google Scholar]
- Verde I., Pahlke G., Salanova M., Zhang G., Wang S., Coletti D., Onuffer J., Jin S. L., Conti M. Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J. Biol. Chem. 2001;276:11189–11198. doi: 10.1074/jbc.M006546200. [DOI] [PubMed] [Google Scholar]
- Verollet C., Colombie N., Daubon T., Bourbon H. M., Wright M., Raynaud-Messina B. Drosophila melanogaster gamma-TuRC is dispensable for targeting gamma-tubulin to the centrosome and microtubule nucleation. J. Cell Biol. 2006;172:517–528. doi: 10.1083/jcb.200511071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ching Y. P., Lam W. H., Qi Z., Zhang M., Wang J. H. Identification of a common protein association region in the neuronal Cdk5 activator. J. Biol. Chem. 2000;275:31763–31769. doi: 10.1074/jbc.M004358200. [DOI] [PubMed] [Google Scholar]
- Wiese C., Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J. Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- Zalokar M., Audit C., Erk I. Developmental defects of female-sterile mutants of Drosophila melanogaster. Dev. Biol. 1975;47:419–432. doi: 10.1016/0012-1606(75)90295-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman S., Chang F. Effects of {gamma}-tubulin complex proteins on microtubule nucleation and catastrophe in fission yeast. Mol. Biol. Cell. 2005;16:2719–2733. doi: 10.1091/mbc.E04-08-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.