Abstract
Proteins share peptidic sequences, such as a nuclear localization signal (NLS), which guide them to particular membrane-bound compartments. Similarities have also been observed within different classes of signals that target proteins to membrane-less subnuclear compartments. Common localization signals affect spatial and temporal subcellular organization and are thought to allow the coordinated response of different molecular networks to a given signaling cue. Here we identify a higher-order and predictive code, {[RR(I/L)X3r](n, n≥1)+[L(φ/N)(V/L)](n,n>1)}, that establishes high-affinity interactions between a group of proteins and the nucleolus in response to a specific signal. This position-independent code is referred to as a nucleolar detention signal regulated by H+ (NoDSH+) and the class of proteins includes the cIAP2 apoptotic regulator, VHL ubiquitylation factor, HSC70 heat shock protein and RNF8 transcription regulator. By identifying a common subnuclear targeting consensus sequence, our work reveals rules governing the dynamics of subnuclear organization and ascribes new modes of regulation to several proteins with diverse steady-state distributions and dynamic properties.
INTRODUCTION
Biochemical processes occurring in the nucleus contribute to its compartmentalization, which facilitates the control of molecular networks (Chubb and Bickmore, 2003). Unlike the cytoplasm, compartmentalization in the nucleus does not rely on the concentration of molecules behind membranes to enhance biochemical reactions. Similar gene loci and regulatory proteins are concentrated within specific membrane-less nuclear substructures such as speckles, PML bodies, Cajal bodies, and nucleoli (Misteli, 2001, 2004; Chubb and Bickmore, 2003; Isogai and Tjian, 2003; Zimber et al., 2004). The temporal and spatial precision of intranuclear dynamics of nucleic acids and polypeptides is central to the proper control of the cell cycle, transcription, apoptosis, ubiquitylation, ribosomal biogenesis, and several other pathways (Shou et al., 1999; Visintin et al., 1999; Weber et al., 1999; Dundr et al., 2000, 2004; Barseguian et al., 2002; Wong et al., 2002; Isogai and Tjian, 2003; Leung et al., 2004; Zaidi et al., 2005). For example, silent gene loci at the nuclear periphery can move centripetally when activated to be repositioned away from repressive factors and closer to activators concentrated within particular subnuclear compartments (Kosak and Groudine, 2004; Misteli, 2004).
Several molecules rely on common peptidic sequences to localize to a given membrane-bound compartment. This includes nuclear localization/export signals (NLS or NES, respectively; Conti and Izaurralde, 2001; Kutay and Guttinger, 2005; Lee et al., 2006) and cell membrane localization signals (Shikano et al., 2005). Identification of such sequences has been instrumental in the functional characterization of a very large number of proteins. Some similarities have also been observed within each class of subnuclear targeting signal. This has been the case for the nucleolus, a major nuclear substructure that coordinates many cellular activities including ribosomal production (Lam et al., 2005; Shaw and Doonan, 2005), cell cycle control (Shou et al., 1999, 2001; Visintin et al., 1999; Azzam et al., 2004), DNA damage repair (van den Boom et al., 2004), and tRNA processing (Paushkin et al., 2004). However, signals mediating the localization of proteins to the nucleolus (nucleolar localization signal [NoLS]) can range from a few to over a hundred amino acids (Weber et al., 2000; Catez et al., 2002; Hiscox, 2002). Subnuclear localization signals also include nucleolar retention signals (NoRS; Tsai and McKay, 2005; Reed et al., 2006), and nuclear matrix targeting signals (NMTS; Zeng et al., 1997; Barseguian et al., 2002; Chatterjee and Fisher, 2002; Zimber et al., 2004), as well as signals targeting proteins to splicing speckles (Caceres et al., 1997). Shared localization signals are thought to allow the coordinated response of different molecular networks to a given signaling cue.
We had previously reported that the von Hippel-Lindau (VHL) tumor suppressor is targeted for static detention (i.e., change in steady-state distribution coupled to a loss of dynamic properties) by nucleoli in response to increases in the environmental concentration of hydrogen ions (Mekhail et al., 2004a, 2005, 2006). The relocation of VHL to the nucleolus switches the tumor suppressor from hypoxia-inducible gene-silencing to rRNA gene (rDNA)-restrictive molecular networks. This results in an increase in the production and a decrease in the consumption of energy under low oxygen tension (hypoxia; Mekhail et al., 2004a, 2005, 2006).
Initial mapping analysis revealed that a VHL fragment constituted of 30 amino acids is capable of targeting a green fluorescent protein (GFP) reporter protein for static detention in the nucleolus after an increase in extracellular hydrogen ion concentration (Mekhail et al., 2005). We named this new type of protein localization sequence NoDSH+ (nucleolar detention signal regulated by H+). NoDSH+ is inactivated after a return to neutral pH conditions, causing rapid release of detained VHL proteins into the nucleoplasm where they resume their dynamic profile. It is primarily the extremely high affinity of NoDSH+ toward nucleoli that makes this subnuclear localization signal considerably different from NoLS and NoRS. However, some similarities do exist as all of these three types of nucleolar targeting sequences contain arginine residues.
Therefore, we reasoned that unraveling the details of the NoDSH+ of VHL could provide an explanation for this unusually high affinity for nucleoli. We envisioned that these details could also be used to predict the subnuclear coordinates, dynamic properties, and novel modes of regulation of proteins harboring similar signals. More importantly, identification of similar peptidic codes in other proteins would provide insight into the rules governing subnuclear organization, general protein dynamics, and the role of hydrogen ions in basic cellular metabolism. Consistent with the hypothesis that nucleolar sequestration may be a general phenomenon is the observation that the nucleolus can capture and release several proteins in response to different cellular cues (Andersen et al., 2005).
Mutagenesis studies coupled to steady-state and protein dynamic analyses allowed us to identify and test common characteristics hidden within the highly complex and modular NoDSH+ subnuclear targeting sequence of VHL. In addition, we find that, unlike some previously reported subnuclear targeting sequences, NoDSH+ does not harbor NLS-like activity. These findings allowed us to identify many proteins that harbor a NoDSH+ subnuclear targeting signal. Overall, six of the six proteins studied were targeted to the nucleolus under acidosis regardless of the initial steady state distribution before signal activation. By identifying a common subnuclear targeting consensus sequence, our work reveals rules governing the dynamics of subnuclear organization and ascribes new modes of regulation to several proteins.
MATERIALS AND METHODS
Cells and Materials
MCF7 cells were obtained from ATCC (Manassas, VA). The generation of 786-0 cells stably expressing HA-VHL was previously described (Iliopoulos et al., 1995).
Cell Culture
Normoxic cells were incubated at 37°C under 5% CO2 environment. Hypoxia was achieved by incubation in a hypoxic chamber at 37°C under a 1% O2, 5% CO2, and N2–balanced atmosphere. Acidosis experiments were conducted as previously described (Mekhail et al., 2004a, 2005). For standard (SD) or acidosis-permissive (AP) conditions, buffer-free medium (DMEM; Invitrogen, Carlsbad, CA) was freshly prepared and supplemented with 5% (vol/vol) fetal bovine serum (FBS) and 1% (vol/vol) penicillin-streptomycin. Unless otherwise indicated, NaHCO3 was added at 44 mM (or 18 mM for normoxia experiments), and the pH was adjusted to 7.2 (SD) or 6.3 (AP) with HCl. Air was bubbled into both media at 22°C, which stabilized the pH at 7.2. The AP medium slowly reverted to its original pH (6.3) under hypoxia, whereas the SD medium remained at pH 7.2. Transfected or adenovirus-infected cells were grown for 24 h under standard conditions before any treatment.
Plasmids and Adenovirus Construction
VHL and VHL constructs were cloned between a Flag-tag and a C-terminal GFP and into pcDNA3.1, as previously described (Bonicalzi et al., 2001; Groulx and Lee, 2002). RNF8, cIAP2, HSC70, and their predicted NoDSH+, as well as PBK1 and HSP110, were cloned in the same manner as VHL. Cells were transiently transfected using Effectene Transfection Reagent (Qiagen, Mississauga, ONT, Canada).
Triton Solubility Assay
Cells were harvested in transport buffer containing 20 mM HEPES (pH 7.3), 110 mM potassium acetate, 5 mM sodium acetate, 2 mM magnesium acetate, 1 mM EGTA, 2 mM dithiothreitol (DTT), and a cocktail of protease inhibitors added shortly before use (leupeptin, 2 μg/ml; aprotinin, 2 μg/ml; and pepstatin A,1 μg/ml). Cells were left on ice to equilibrate for 1 min, and Triton X-100 was added (1% vol/vol). Permeabilization was monitored by fluorescence microscopy with Hoechst stain 33258 (Sigma, St. Louis, MO), which only stained nuclei of permeabilized cells. Cells were then centrifuged, to separate triton-insoluble from soluble material, and lysed at a final concentration of 5% SDS in a manner to maintain equal final volume for both fractions.
Nucleolar Isolation by Sucrose Gradient
Nucleoli of MCF-7 cells were essentially isolated as previously described (Andersen et al., 2002). Briefly, ∼8 × 107 cells were collected by trypsinization, incubated with a hypotonic solution (10 mM HEPES, 10 mM KCl, 1.2 mM MgCl2, and 0.5 mM DTT in water) and homogenized using a Dounce tissue homogenizer until ∼90% of cells were lysed, but nuclei remained intact. Lysates were centrifuged at 300 relative centrifugal force (rcf) for 7 min, and the pellet was resuspended in 0.25 M sucrose, 10 mM MgCl2 solution (S1 solution) and layered over a 0.35 M sucrose, 0.5 mM MgCl2 solution (S2 solution) before centrifuging at 2000 rcf for 7 min at 4°C. Pure nuclei obtained were resuspended in S2 solution and sonicated for 6-8 25-s bursts (with 15-s intervals) using a 60 Sonic dismembrator (Fisher Scientific, Pittsburgh, PA) set at power 6 (0.7 W). Sonicated material was layered over a 0.88 M sucrose, 0.5 mM MgCl2 solution (S3 solution) and centrifuged 15 min at 3100 rcf at 4°C, and the nucleolar pellet was washed with S2 solution, centrifuged 7 min at 2000 rcf and resuspended in 0.5 ml of S2 solution for storage at −80°C. The purity of isolated nucleoli was assessed both by light microscopy and by Western blot using antibodies against either nucleolar (fibrillarin) or cytoplasmic (LDH) proteins.
Western Blot Analysis
Samples were prepared and Western blots were performed as described (Mekhail et al., 2004a). Primary monoclonal antibodies recognize hemagglutinin (HA), Flag-M2, and lactate dehydrogenase (LDH; Sigma) and p125 and HSC70 (Abcam, Cambridge, MA). Primary polyclonal antibodies detecting RNF8, cIAP2 (Abcam) and fibrillarin (Santa Cruz Biotechnology, Santa Cruz, CA) were also used. A secondary antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA) was used and detected by Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer-Cetus, Boston, MA).
Immunofluorescence
Cells were seeded onto coverslips and fixed with prechilled (to −20°C) methanol for 10 min followed by acetone for 1 min. Anti-HSC70 (Santa Cruz Biotechnology), anti-cIAP2 and p125 (Abcam), and anti-B23 (Sigma) monoclonal antibodies were used. Cells were incubated for 1 h with a primary antibody solution containing 10% FBS and 1% Triton X-100 (vol/vol). Cells were washed several times in phosphate-buffered saline before 1-h incubation with a secondary Texas Red–labeled antibody (Jackson ImmunoResearch). Hoechst stain 33342 (Sigma) was added to visualize nuclei and coverslips were mounted using Fluoromount G (EMS, Hatfield, PA).
Photobleaching and Microscopy
As described by Mekhail et al. (2005), cells were cultured into 35-mm dishes with coverslip bottoms and visualized with a confocal microscope (MRC 1024; Bio-Rad Laboratories, Richmond, CA). A 60× plan Apo oil immersion lens with a 1.4 NA was used for bleaching and imaging. Indicated areas were exposed to five rapid pulses of a 488-nm argon laser at 100% power, and image acquisition was conducted at 1% of full laser power. For fluorescence recovery after photobleaching (FRAP) experiments, images were collected at 10-s intervals (or 1 s for highly mobile proteins). Recovery of the fluorescent signal within a bleached region was calculated as described by Mekhail et al. (2005). For fluorescence loss in photobleaching (FLIP) experiments, cells were repeatedly bleached and imaged at 5-s intervals, and fluorescence loss in unbleached areas was calculated to account for any losses in fluorescence by normalizing the fluorescence in the cell of interest to that of a neighboring cell according to Irel =(I(t)/I(0)) * (N(0)/N(t)), where I(t) is the average intensity of the unbleached nucleus at time point t, I(0) is the average prebleach intensity of the nucleus of interest, and N(0) and N(t) are the average total cellular fluorescence intensities of a neighboring cell in the same field of vision at prebleach or at time point t, respectively. For all bleaching experiments, at least three datasets were analyzed for each result. Average pixel intensities were normalized for background fluorescence. Images of living cells from experiments that do not implicate bleaching or of immunofluorescence experiments were captured with a microscope (Zeiss Axiovert S100TV; Carl Zeiss MicroImaging, Thornwood, NY) equipped with a 40× C-Apochromat water immersion objective with a 1.2 NA using a digital charged-coupled device camera (Empix, Mississauga, ONT, Canada). Pseudocoloring and software packages used to capture images, analyze data, and generate graphs were previously described (Mekhail et al., 2005).
Bioinformatic Analyses
Searches for candidate proteins with the subnuclear targeting consensus sequence (with up to 110 residues separating the arginine from the first three-residue hydrophobic repeat) were conducted on the UniProtKB/SwissProt database (Bairoch et al., 2004) with the SynaREX function of My Genomics Resource Centre (MGRC) using motifs R-R-I/L-X3-R-X0-100-L-φ-V/L, L-φ-V/L-X0-100-R-R-I/L-X3-R, R-R-I/L-X0-110-L-φ-V/L, L-φ-V/L-X0-110-R- R-I/L, where φ symbolizes any hydrophobic residue. Retrieved entries were then filtered for low arginine domain disorder using the DisEMBL program (Linding et al., 2003). Transmembrane proteins containing the consensus sequences were not eliminated from the list as acidosis could thus signal the nucleolar sequestration of these proteins.
RESULTS
Initial Dissection of a Subnuclear Targeting Signal Reveals Three Criteria
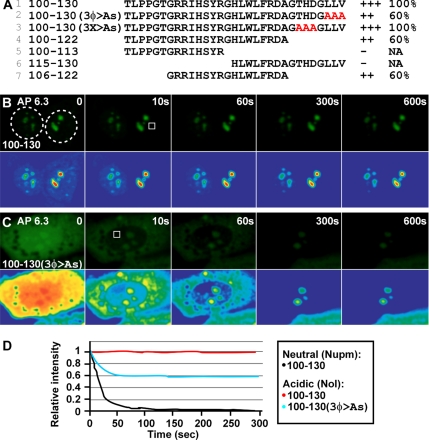
To study the activity of subnuclear targeting sequences within VHL, we incubated MCF7 cells in SD medium, which prevents fluctuations in pH, or in AP medium, which is prepared to enable cells to naturally acidify their extracellular milieu (see Materials and Methods; Mekhail et al., 2004a, 2005, 2006). GFP-tagged VHL or a fragment constituted of its amino acids 100-130 completely relocate from a diffuse nucleocytoplasmic distribution to nucleoli only upon the establishment of acidosis (Figure 1A). We first set out to assess the requirement for residues 100-130 in the subnuclear targeting of the VHL tumor-suppressor protein. Deletion of residues 100-130 or of small sections within these residues did not abrogate targeting to nucleoli suggesting that other residues within VHL harbor similar targeting capacity (Figure 1B, lines 1–6 and 16). Stepwise mapping of VHL from the C-terminus revealed that a second nucleolar targeting domain may reside within amino-acids 152-186 (Figure 1B, lines 8–16). Closer examination of these VHL polypeptide sequences revealed three striking patterns (Figure 1B and Supplementary Figure S1). First, positive polypeptides contained at least one of two homologous arginine-rich segments constituted of residues 107-RRIHSYR-113 or 176-RRLDIVR-182, with a putative consensus sequence RR(I/L)X3R (Figure 1, B and C, and Supplementary Figure S1, A and B). Second, positive fragments contain at least one or two three-residue hydrophobic repeats located between the two arginine domains with each repeat starting with a leucine and ending with either a valine or leucine (LWL, LLV, LFV, LQV; Figure 1, B and C, and Supplementary Figure S1, A and B). Third, both arginine domains are located in the wild-type VHL protein within regions with low probability for structural disorder as calculated by the DisEMBL program (Figure 1C and Supplementary Figure S1C; Linding et al., 2003). The arginine domains are located at regions of disorder probabilities of <0.03, 0.2, and 0.32 compared with threshold levels for disorder of 0.09, 0.5, and 0.43, following the predictors Hot-Loops, Remark-465, and Loops or Coil, respectively (Supplementary Figure S1C; Linding et al., 2003). Agreement between the different predictors supports a role for these arginine-rich sequences in high-affinity interactions. Sequences implicated in hit-and-run interactions such as NLSs are often characterized with high disorder (Lee et al., 2006). No disorder pattern was observed for the three-residue hydrophobic repeats. Taken together, these observations suggest that this subnuclear targeting signal might conform to three main criteria (Figure 1C) to achieve high-affinity interactions with nucleoli: 1) At least one arginine domain (RR(I/L)X3r; hereinafter referred to as subnuclear targeting arginine domain [STAD]; data below will explain lower case r); 2) A number of three-residue hydrophobic repeats (referred to as subnuclear targeting hydrophobic domain [STHD]); and 3) Low disorder character of STADs might contribute to its subnuclear targeting activity.
Figure 1.
Initial mapping of VHL reveals high-order and multipartite character of its pH-dependent nucleolar targeting signal. (A) Steady-state distribution of wild-type VHL and VHL(100-130) under neutral or acidotic conditions. MCF7 cells were cultured in standard media (SD) and transiently transfected to express the indicated GFP-tagged polypeptides. Cells were replenished with either fresh SD medium (pH 7.2) or acidification permissive medium (AP, initial pH 7.2) that allows maximal extracellular acidification to pH 6.3 (see Materials and Methods). Cells were transferred to hypoxia (1% O2) for 18 h. Extracellular pH at the end point is indicated on each panel. (B) VHL mapping analysis. MCF7 cells transfected to transiently express indicated GFP-tagged proteins were incubated in AP medium in hypoxia. Schematic of VHL exons and derived amino acid domains are shown. Nucleolar localization was scored according to representative images (bottom). For A and B, insets show Hoechst staining of DNA; scale bars, 10 μm. (C) Shared characteristics of VHL fragments displaying nucleolar detention under acidosis. Subnuclear targeting arginine domains (STADs) as well as subnuclear targeting hydrophobic domain (STHD) composed of three-residue hydrophobic repeats are shown.
Validation of NoDSH+ Subnuclear Targeting Criteria
To achieve higher sensitivity of detection of the effects of different mutations, we decided to test how they affect both the steady-state distribution as well as the dynamic profile under signal-activating conditions. The affinity of fluorescent protein chimeras for the nucleolus was determined by FLIP experiments (Lippincott-Schwartz et al., 2003). In FLIP, a living cell is repeatedly hit with a laser beam in the same region. Loss of fluorescence in an area outside the bleached spot is reflective of protein mobility between that area and the bleached spot. Both VHL-GFP and 100-130-GFP, which are highly dynamic under neutral conditions, become statically detained by the nucleolar architecture under acidosis (Figure 2, A, line 1, B, D, and Supplementary Figure S2A; Mekhail et al., 2005). In stark contrast, the resident nucleolar protein B23 rapidly dissociates from the nucleolus in hypoxic-acidotic cells (Supplementary Figure S2B; Mekhail et al., 2005). The 100-130 segment of VHL harbors two of the three-residue hydrophobic repeats of the wild-type protein (Figure 2A, line 1, and Supplementary Figure S1A, line 16). Substitution of the three hydrophobic residues 128-LLV-130 to alanines (Figure 2A, line 2) or deletion of a section spanning these residues (mutant 100-122; Figure 2A, line 4) prevented complete relocation to the nucleolus but nucleolar accumulation was still easily detectable under acidosis (++ steady-state score), suggesting a reduction in nucleolar binding affinity. Consistent with these observations, only 60% of the total protein pool of each of these fragments was still associated with nucleoli after 5- and 10-min FLIP experiments (Figure 2, A, lines 2 and 4, C, and D). In contrast, no change was observed when three nonhydrophobic residues were substituted to alanines (Figure 2A, line 3). Although fragments 100-113 and 115-130 failed to display any activity, 106-122 displayed a 10-min nucleolar affinity score similar to that of 100-122 (Figure 2A, lines 5–7, 3B, and Supplementary Figure S2C), providing a large range of retained activity for analysis by saturated mutagenesis.
Figure 2.
Analysis of a minimal subnuclear targeting signal at the level of steady-state and dynamic phenotypes implicates arginine-rich segments and a stretch of three hydrophobic residues. (A) Mutational analysis of VHL's NoDSH+ confirms the importance of hydrophobic repeats and involvement of arginines in subnuclear targeting. MCF7 cells expressing indicated GFP-tagged proteins were incubated in AP medium in hypoxia. Steady-state nucleolar distribution (+/− rating) of VHL constructs and their affinity with the nucleolar architecture (represented by percentage of statically detained fractions as revealed by FLIP analysis) were scored. NA, not applicable. (B and C) MCF7 cells were transfected to express low levels of GFP-tagged VHL(100-130) without (B) or with a hydrophobic repeat substituted by alanines (C). Cells were then submitted to hypoxic treatment under AP conditions and FLIP analysis was performed. Bleached nuclear regions (squares) are shown and pseudocolored panels are included to better illustrate subtle changes in fluorescence intensity. See Supplementary Figure S2, A and B, for controls. (D) Quantitation of FLIP experiments shown in B and C and in Supplementary Figure S2A. Nupm, nucleoplasm; Nol, nucleolus.
Glycine and arginine residues have been randomly observed in NoLS signals (Weber et al., 2000; Catez et al., 2002; Hiscox, 2002). Fragment 106-122 harbors two glycine and four arginine residues (Figure 3A, line 1). Substitution of glycines (Figure 3A, lines 2 and 3) or R120 (Figure 3A, line 7) to alanine failed to affect localization or affinity scores. In contrast, single substitution of each of the other arginine residues 107, 108, and 113 to alanine did not have a detectable effect on steady-state distribution but lowered affinity scores from 60 to 45, 43, and 55%, respectively (Figures 3, A, lines 1, and 4–6, B). Similar substitution of F119 or Y112 essentially had no effect on activity (Figure 3A, lines 7 and 16). Concomitant alteration of R107 and R108 rendered it difficult to distinguish nucleolar from nucleoplasmic signal and further lowered the affinity score to 30% (Figure 3A, line 9). Complete loss of nucleolar targeting was observed when all arginines were substituted to alanines, to the negatively charged glutamic acid, or to the positively charged lysines (Figure 3A, lines 13–15), suggesting that arginines cooperate in mediating nucleolar localization in a manner that is not recapitulated by charged amino acids. Histidine residues have been implicated in responses to changes in environmental parameters, including pH and oxygen (Ludwig et al., 2003; Murakami et al., 2004; Stewart et al., 2004; Ishikawa et al., 2005; Lee and Helmann, 2006; Ramsey et al., 2006). Substitution of H110, both alone (data not shown) or in conjunction with R108 (Figure 3A, line 8) did not alter activity. Considering the importance of LLV to this subnuclear targeting sequence (Figure 2, A, line 2, C, and D), we considered a role for another three-residue hydrophobic cluster that also starts with a leucine, 116-LWL-118. Substitution of L116 alone or of all three hydrophobic residues LWL to alanines (Figure 3A, lines 10 and 11) rendered it difficult to distinguish nucleolar from nucleoplasmic signal and completely abolished static detention capability (Figure 3, A, lines 10 and 11, B, and Supplementary Figure S2, C and D), suggesting that three-residue hydrophobic repeats play a key role in the observed phenomenon. Interestingly, concomitant mutation of LWL and only R107 seems to render the sequence completely insensitive to changes in pH levels as nucleoli remain black after the establishment of acidosis (Figure 3A, line 12), suggesting a cooperation between arginine residues within the N-terminal STAD (N-STAD) and hydrophobic residues in subnuclear targeting.
Figure 3.
Saturated mutagenesis analysis of fragment 106-122 of VHL highlights three arginines and implicates multiple hydrophobic residues. (A) Effects of mutation of arginine, glycine, and hydrophobic residues within VHL 106-122 on nucleolar steady-state distribution and affinity scores. MCF7 cells expressing GFP-tagged VHL 106-122, or this segment with different mutations, were incubated in AP medium (pH 6.3). Steady-state nucleolar distribution (+/− rating) of VHL constructs and their affinity for the nucleolar architecture (represented by percentage of statically detained fractions as revealed by FLIP analysis) are shown. NA, not applicable. (B) Quantitation of FLIP experiments of the wild type or some mutant 106-122 segments shown in A. WT, wild-type sequence.
A fragment composed of VHL residues 162-186, which contains the three-residue hydrophobic cluster 163-LQV-165 and the C-terminal STAD (C-STAD), exhibited a double-plus nucleolar redistribution under acidosis and a 60% affinity score by FLIP (Figure 4A, line 1). Similar to the effect of mutating LWL in fragment 106-122 (Figure 3A, line 11), substitution of LQV to alanines in fragment 162-186 rendered it difficult to distinguish nucleolar from nucleoplasmic signal and reduced the 10-min FLIP affinity score to nil (Figure 4A, line 2). It is important to note that this nonfunctional fragment still contains a section where the sequence VRSLVK is present (Figure 4A, line 2). This suggests that a two-residue leucine-containing hydrophobic cluster cannot compensate for the loss of LQV. Similar results were obtained for fragment 106-122(L116A), which still harbors a 117-WLF-119 hydrophobic cluster (Figure 3A, line 10).
Figure 4.
Wild-type NoDSH+ of VHL conforms to a set of rules and is composed of two subnuclear targeting arginine-rich domains and multiple hydrophobic repeats with precise clustering features. (A) Effects of amino acid replacement of a three-residue hydrophobic repeat within VHL 162-186 on the nucleolar distribution and affinity of this segment. (B) Analysis of the positioning of hydrophobic clusters relative to the STAD for nucleolar targeting and static detention. (C) Importance of arginine and isoleucine residues within the N-STAD. (D) Effects of disorder and spacing between arginine residues and hydrophobic repeats within the fusion construct 107-113-HD1 on nucleolar steady-state distribution and affinity. (E) Importance of arginine and leucine/isoleucine residues within the C-STAD. (F) VHL's NoDSH+ does not have an NLS activity. VHL 107-113-HD1 was fused to the diffusion-incompatible GFP-tagged pyruvate kinase, with or without the SV40 NLS sequence, and localization under neutral or acidic condition was monitored. Insets show Hoechst staining of DNA; scale bars, 10 μm. (G) Schematic of wt-NoDSH+ of the VHL tumor suppressor protein.
The herein analyzed subnuclear targeting signal thus seems to be built with strict components that are organized within a flexible framework. The fragment constituted of residues 100-113, which contain the N-STAD, fails to exhibit any nucleolar targeting activity (Figure 2A, line 5, 4B, line 1). A relatively downstream fragment composed of residues 128-137 (hereon called hydrophobic domain 1 or HD1), which contains two hydrophobic clusters but no arginine residues, is also completely insensitive to changes in extracellular pH levels (Figure 4B, line 2). Thus, we predicted and found that fusion proteins 100-113-HD1 and HD1-100-113 exhibit significant activity, as reflected by a double-plus steady-state distribution pattern and a 76% nucleolar affinity score (Figure 4B, lines 3 and 4, and Supplementary Figure S2E). Similar results (75% affinity score) were obtained for the fusion protein 107-113-HD1 (Figure 4C, lines 1–3), further supporting data indicating that G106 is not important for subnuclear targeting (Figure 3A, lines 2 and 3). Single substitutions of arginine to alanine within 107-113-HD1 confirmed a more prominent role for R107 and R108 relative to R113 (Figure 4C, lines 4, 5, and 7, compared with 3A, lines 4–6). Interestingly, I109 seems to be as important as R108 in establishing high-affinity interactions with the nucleolus (Figure 4C, lines 5 and 6). However, removal of I109 along with 110-HSY-112 completely abolished nucleolar targeting capacity, irrespective of the spacing between the arginine residues (Figure 4D, lines 1–3). DisEMBL-mediated analyses revealed that while single or combined substitutions of the arginine or isoleucine residues to alanine or cysteine do not affect disorder levels of the 107-113-HD1 fragment or the wild-type VHL protein, combined substitutions of 110-HSY-112 to alanines introduce above threshold disorder levels within that region (Figure 4D, line 4, and Supplementary Figure S3, line 2; data not shown; Linding et al., 2003). Consistent with this prediction, substitution of HSY within 107-113-HD1 to alanines decreased affinity to nucleoli from a detention score of 75% (Figure 2C, line 3) to 58% (Figure 4D, line 4). This suggests that residues embedded between the key arginines cooperate in the maintenance of low disorder, as expected for sequences involved in the establishment of high-affinity interactions (Lee et al., 2006). Interestingly, as predicted by DisEMBL, artificial reduction of disorder below threshold levels via reduction of the spacing between I109 and R113 from three to two alanines increases affinity from 58 to 66% (Figure 4D, lines 4 and 5, and Supplementary Figure S3, lines 2 and 3). Alteration of spacing between the three-residue hydrophobic repeats increased or decreased their level of disorder but had no detectable effect on nucleolar affinity scores (Figure 4D, lines 6–8; data not shown), supporting the prediction that low disorder enhances the function of STADs but not the hydrophobic repeats (Supplementary Figure S1C). Mapping analysis of the C-STAD of VHL revealed that L178 behaves similarly to I109 of the N-STAD (Figure 4, E, lines 1–5, and C, line 6). Similar to previous reports, reduction of the temperature from 37 to 22°C did not have any significant effect on the kinetics or extent of recovery of any of the tested proteins in the nucleus or cytoplasm (data not shown and see Phair and Misteli, 2000). A chimeric protein containing the 107-113-HD1 segment of VHL fused to the nuclear diffusion incompatible GFP-tagged pyruvate kinase (PK-GFP) failed to exhibit any nuclear or nucleolar accumulation after a shift to acidosis (Figure 4F). Introduction of an NLS signal into the protein chimera resulted in nucleolar accumulation under acidosis, suggesting that NoDSH+ does not harbor NLS-like activity under either neutral or acidic conditions (Figure 4F). PK-GFP-NLS alone does not exhibit any nucleolar targeting activity under neutral or acidic conditions (data not shown). Interestingly, we noticed that both arginine domains are positioned on one side of the VHL molecule, whereas the three-residue leucine-containing hydrophobic repeats are clustered on the other face of the molecule (data not shown). Although several of our small constructs are efficiently targeted to nucleoli (Figures 1–4), we cannot completely rule out the possibility that this differential positioning of NoDSH+ components does not contribute in any way to the function of the sequence within the setting of the wild-type VHL protein. Taken together, these findings suggest that the analyzed subnuclear targeting signal (Figure 4G) generally conforms to three rules: 1) At least one STAD [RR(I/L)X3r] (where r reflects a more accessory role for the last arginine); 2) Preferably, two or more three-residue hydrophobic repeats [Lφ(V/L)]; and 3) STADs are preferably positioned within low disorder regions.
Rules Help Identify Additional Proteins Targeted to Nucleoli in Acidosis
We thus suspected that proteins that abide by these rules would be targeted for high-affinity interactions with the nucleolar architecture after signal activation. A search of human proteins in the SwissProt database (Bairoch et al., 2004) using the SynaRex program of MGRC followed by filtering for low structure disorder of STADs with the DisEMBL program was performed (Supplementary Figure S4; data not shown; Linding et al., 2003). This allowed us to generate a list of candidate proteins (Supplementary Table S1). We chose three of these—RING finger protein ubiquitin ligase/transcription regulator RNF8 (SwissProt entry O76064), inhibitor of apoptosis cIAP2 (Q13489), and DNA polymerase delta catalytic subunit p125 (P28340)—at random for analysis of the full-length proteins and their predicted subnuclear targeting sequences. As indicated in the SwissProt database, GFP-tagged wild-type RNF8 and cIAP2 displayed nucleoplasmic and nucleocytoplasmic distribution under standard growth conditions, respectively (Figure 5A). Both the GFP-tagged versions of RNF8 and cIAP2 as well as their predicted subnuclear targeting sequences alone exhibited a complete relocation to nucleoli upon acidosis as revealed by fluorescence microscopy as well as immunofluorescence colocalization studies with the resident nucleolar protein B23 (Figure 5, A, B, and G; data not shown). Similar relocation of endogenous cIAP2 and p125 was detected by immunofluorescence microscopy (Figure 5, C and D). Because of their inherent density, pure nucleoli can be isolated from cell culture using a combination of sonication and sucrose density centrifugation (see Materials and Methods; Andersen et al., 2002). Endogenous RNF8, cIAP2, and p125 proteins behaved similar to VHL and were detected in nucleoli isolated from acidotic cells only (Figure 5E). In addition, detergent-based biochemical fractionation revealed that acidosis triggered a complete shift of endogenous cIAP2 and p125 from the triton-soluble to the triton-insoluble cellular fraction, which is enriched in resident nucleolar proteins such as fibrillarin (Figure 5F; Mekhail et al., 2004a).
Figure 5.
Confirmation of predicted H+-responsiveness of RNF8, cIAP2, and p125. (A) Steady-state distribution of GFP-tagged RNF8 and cIAP2 under SD or AP conditions in either normoxia (21% O2) or hypoxia (1% O2). (B) Acidosis-dependent colocalization of RNF8-GFP with endogenous B23 protein detected by immunofluorescence microscopy. (C) pH-dependent nucleolar localization of endogenous cIAP2 as revealed by immunofluorescence microscopy. (D) Endogenous p125 relocates to nucleoli in response to acidosis. MCF7 cells transiently transfected to express B23-GFP were cultured under indicated conditions and submitted to immunofluorescence analysis using an antibody specific to p125. (E) Western blot analysis of nucleoli isolated from MCF7 cells expressing adenovirus-introduced Flag-tagged VHL-GFP through a combination of sucrose-gradient centrifugation and sonication (see Materials and Methods) show that endogenous NoDSH+-containing proteins accumulate in nucleoli under acidotic conditions. W.C., whole cell extract. (F) 786-0 cells stably expressing HA-VHL were incubated under SD or AP conditions and fractionated based on Triton X-100 solubility. Lysates were immunoblotted for HA, cIAP2, p125, nucleolar fibrillarin, and cytoplasmic LDH. W.C., whole cell extract; Sol., solubility. (G) The NoDSH+ sequences of RNF8 and cIAP2 are sufficient to target a reporter GFP protein to nucleoli under acidosis. For each protein, a fusion of wild-type STAD and two three-residue hydrophobic repeats was GFP-tagged. MCF7 cells transiently transfected to express low levels of the fusion proteins were incubated under the indicated conditions and monitored by fluorescence microscopy. Insets show Hoechst staining of DNA. Scale bars, 10 μm.
We next used FRAP to assess the kinetic properties of RNF8-GFP and cIAP2-GFP (Lippincott-Schwartz et al., 2003). Specific cellular regions expressing fusion proteins were bleached with the use of a laser pulse that irreversibly quenches the GFP signal, and the recovery of signal in the bleached area was recorded by time-lapse confocal microscopy. The kinetics and extent of recovery of fluorescence in a cellular region after bleaching are reflective of the dynamics of the studied fluorescent chimeras. As previously reported, the resident nucleolar protein B23 retained a dynamic interaction with the nucleolar architecture under acidotic conditions as revealed by a rapid and full recovery of fluorescence after bleaching (Supplementary Figure S5C). Similar to VHL (Supplementary Figure S5, A and B; Mekhail et al., 2005), both RNF8 (Figure 6, A and B) and cIAP2 (Figure 6, C and D) abandoned a highly dynamic nucleoplasmic or nucleocytoplasmic profile, respectively, and became statically detained by nucleoli in response to acidosis.
Figure 6.
FRAP analysis confirms predicted changes to the dynamic character of RNF8 and cIAP2 upon signal activation. MCF7 cells, transiently transfected to express low levels of RNF8-GFP (A and B) or cIAP2-GFP (C and D), were incubated under indicated conditions and imaged before and after bleaching of the square-marked regions. Time after bleaching is indicated in seconds, and pseudocolored panels are included to better illustrate minimal changes in fluorescence. Fluorescence recovery value R is shown. See Supplementary Figure S5, A–C, for VHL-GFP and B23-GFP controls.
We next evaluated the dynamics of RNF8 within the nucleolar space. RNF8 did not exhibit any fluorescence recovery after bleaching an area within the nucleolus (Supplementary Figure S5D). In contrast, B23 retains its highly mobile subnucleolar properties within our experimental settings (Supplementary Figure S2B and S5C; data not shown; Mekhail et al., 2005). We next examined whether interaction with the nucleolar architecture is required for acidosis-mediated modification of protein dynamics. We transfected cells to express higher levels of RNF8-GFP, saturating nucleolar-binding sites and preventing the full redistribution of the fluorescent protein chimera to the nucleolus after acidification in hypoxia (Figure S5E). This establishes two different protein pools in the cell: nucleoplasmic and nucleolar. Although repetitive bleaching of a small nucleoplasmic region in a FLIP assay resulted in the complete loss of nucleoplasmic fluorescence, nucleolar RNF8-GFP signal remained constant over the course of the experiment (Supplementary Figure S5E). We next asked whether this process is reversible and if RNF8 can be released from nucleoli to recover its highly mobile state. After the reinstatement of neutral pH conditions, RNF8 rapidly reverted to a dynamic nucleoplasmic profile (Supplementary Figure S5F). Cullin-2 assembles within the VHL ubiquitin ligase complex that targets HIF-1α for degradation. Cullin-2 and HIF-1α provide two examples that do not follow the subnuclear targeting rules and do not accumulate in nucleoli of VHL-deficient cells under acidosis (Supplementary Table S2; Mekhail et al., 2004a, 2005). Taken together, these results indicate that the subnuclear targeting rules are predictive in terms of both the targeting to a particular subnuclear compartment and the degree of affinity involved in these interactions.
Next, we decided to investigate the functional implications of nucleolar sequestration of RNF8. We have previously shown that static detention of the VHL tumor suppressor protein results in the stabilization of its main target, the alpha subunit of the hypoxia inducible factor (HIF-α; Mekhail et al., 2004a). The RING finger protein RNF8 is known to interact with the retinoid X receptor alpha (RXRα) to enhance its transcription-stimulating activity, as demonstrated by the assessment of transcription of the RXR responsive element (RER)-containing cytosolic retinol binding protein II gene (CRBPII gene; Takano et al., 2004). As expected, CRBPII mRNA levels were greatly reduced only when cells were incubated under acidification-permissive conditions (Supplementary Figure S5G). We next transfected cells to express high levels of RNF8-GFP to create a pool of dynamic nuclear molecules (Supplementary Figure S5E). This rescued CRBPII transcript levels in hypoxic-acidotic cells (Supplementary Figure S5G), as expected. Taken together, these findings reveal how the herein described rules and subnuclear targeting sequences can help uncover new modes of regulation of protein function.
Reverse Correlation of pH-responsive Proteins to the Rules
We next isolated nucleoli from cells incubated under either neutral or acidic conditions (Figure 7A). After the separation of purified nucleolar proteins by SDS-PAGE, a prominent ∼70-kDa band that appeared only in nucleoli isolated from cells incubated under acidic conditions was sliced from the gel (Figure 7B). Analysis of the protein content of this band using MALDI-MS after trypsin digestion identified the HSC70 (P11142) heat-shock protein as its major constituent (Figure 7B). Unlike HSC70, other proteins detected by MALDI-MS in this band were also found in a parallel area of the gel cut from the neutral lane (data not shown). Nucleolar targeting of HSP70, an inducible homologue of HSC70, was previously reported to contribute to the recovery of nucleolar morphology after heat shock (Pelham, 1984). Endogenous HSC70, similar to VHL, was detected by immunofluorescence analysis performed on cells exposed to low pH conditions (Figure 7C) or by immunoblotting only in pure nucleoli isolated from acidotic cells (Figure 7D). Examination of amino acid sequences revealed the presence of leucine-containing three-residue hydrophobic repeats (although here some of these repeats had the middle residue as N) and an arginine domain (composed of residues RRL) that was located within a low disorder region of the wild-type protein (Figure 7E and Supplementary Figure S6A). RRL still matches the consensus sequence RR(I/L)X3r, as the last arginine of the STAD sequence seems to play a more accessory role in subnuclear targeting (Figures 3 and 4). Further examination of the amino acid sequence of VHL revealed the presence of three more LXVs (two LPVs and one LNV). Therefore, we tested the response of GFP-tagged HSC70 and its predicted subnuclear targeting sequence (harboring RRL and the LNV and LLL hydrophobic repeats) to acidosis. We compared that response to that of another heat-shock protein, HSP110 (Q92598), which contains a double arginine that is not followed by a leucine or an isoleucine (Figure 7E) and also contains several three-residue hydrophobic repeats that do not match our consensus sequence (e.g., VVG, VVF, FQV, FVV). As predicted, acidosis triggered the relocation of HSC70 and its predicted domain alone, but not HSP110, to nucleoli (Figure 7, F and G), suggesting the possible existence of variants of the three residue hydrophobic repeats where the middle residue might be substituted for specific nonhydrophobic amino acids (e.g., LNV and LPV). This is supported by our mapping analysis of VHL since if we take the LPVs and LNV repeats of VHL into account, all triple-plus mutants would now have at least two hydrophobic repeats (Figures 1–4, and Supplementary Figure S1A). In addition, LNV, and LPVs also cluster with the other hydrophobic repeats on the surface of the VHL macromolecule (data not shown). HSC70 became statically detained by nucleoli under acidosis (Figure 7, H and I), but HSP110 retained its dynamic nucleocytoplasmic profile (Supplementary Figure S6, A and B) as revealed by FRAP analysis. These findings establish a reverse correlation between a pH-responsive protein and the subnuclear targeting criteria. In addition, this indicates the potential for the expansion of the list of proteins harboring this flexible but resilient subnuclear targeting signal.
Figure 7.
Blind identification of the pH-responsive subnuclear targeting signal after the isolation and characterization of a protein enriched in acidotic nucleoli. (A) Nucleoli were isolated from MCF7 cells by sucrose gradient centrifugation as in Figure 5E. (B) Nucleoli of cells incubated under either neutral (SD) or acidification-permissive (AP) conditions were submitted to SDS-PAGE/silver staining, and bands of interest were excised and sequenced by MALDI MS/MS. Three peptides that are unique to HSC70 were identified from a band running at ∼70 kDa. (C) Steady-state distribution of endogenous HSC70 as revealed by immunofluorescence analysis. Primary antibody exclusion (−1r Ab) control is also shown. (D) Western blot analysis of nucleoli isolated from MCF7 cells reveals that endogenous HSC70 accumulates with endogenous VHL in nucleoli of acidotic cells. Anti-LDH immunoblotting was used to control for cytoplasmic contamination. (E) Sequence comparison between HSC70 and HSP110 showing both arginine repeats and the number of hydrophobic clusters conforming to the motif [L-(φ/N)-(L/V)], where φ symbolizes any hydrophobic residue. (F) Steady-state distribution of GFP-tagged HSC70 and HSP110 under SD or AP conditions. Insets show Hoechst staining of DNA. (G) The NoDSH+ sequence of HSC70 is sufficient to target a reporter GFP protein to nucleoli under acidosis. A fusion of wild-type STAD and two three-residue hydrophobic repeats of HSC70 was GFP-tagged. MCF7 cells transiently transfected to express low levels of the fusion protein were incubated under the indicated conditions and monitored by fluorescence microscopy. (H and I) FRAP analysis reveals that HSC70 becomes statically detained by the nucleolus under acidosis. MCF7 cells expressing low levels of HSC70-GFP were incubated under SD (H) or AP (I) conditions and imaged before and after bleaching the square-marked regions. Time after bleaching is indicated in seconds, and pseudocolored panels with arrows are included to better illustrate minimal changes in fluorescence. Fluorescence recovery value R is shown. Scale bars, 10 μm.
Common Signal Reveals Subnuclear Targeting Gradients
Serial SwissProt-SynaREX-DisEMBL searches, where the third arginine of the STAD domain was not considered, resulted in the expansion of the list of candidate proteins (Supplementary Table S3 and Supplementary Figure S7). We were intrigued by the fact that one of the proteins on the list—Ribosomal L1 domain–containing PBK1 (O76021)—was reported to display a steady-state nucleolar distribution (Andersen et al., 2005; Tong et al., 2005). The steady-state distribution of GFP-tagged PBK1 displayed a strictly nucleolar pattern irrespective of hydrogen ion concentration (Figure 8A), as expected. Full fluorescence recovery was observed after bleaching a single nucleolus in a cell expressing GFP-tagged PBK1 and was cultured under neutral conditions (Figure 8B). In stark contrast, no recovery was observed when cells were cultured under acidic conditions, as predicted (Figure 8C). These results add another layer of complexity to the herein identified subnuclear targeting signal and support a model where subnuclear targeting is a relative term, the extent of which is determined by the strength of interactions with nuclear substructures. In addition, this reveals that proteins can share regulated subnuclear targeting sequences irrespective of their steady-state distribution in the absence of signal activation.
Figure 8.
Validation of predicted alterations to the dynamic character of a NoDSH+-containing resident nucleolar protein. (A) Steady-state distribution of GFP-tagged PBK1 transiently expressed in MCF7 cells incubated under indicated conditions. Hoechst staining of DNA is shown. Scale bar, 10 μm. (B and C) FRAP analysis reveals that the nucleolar protein PBK1 is dynamic under neutral conditions but adopts a static detention profile in response to acidosis. MCF7 cells transiently transfected to express GFP-tagged PBK1 were incubated under indicated conditions and imaged before and after photobleaching of the arrow-marked nucleoli. Time after bleaching is indicated in seconds, and pseudocolored panels are included to better illustrate minimal changes in fluorescence. Fluorescence recovery value R is shown. (D) NoDSH+ consensus sequence R, critical arginines; r, less critical arginine; X, any amino acid; n, number of sequence elements; shading, preferably low intrinsic disorder.
DISCUSSION
Our analysis of the VHL tumor suppressor has revealed a set of rules governing its subnuclear targeting in response to an increase in extracellular hydrogen ion concentration. In principle, these rules are sequence-based and require the presence of at least one arginine domain (named STAD) and hydrophobic repeats (named STHD) as presented in the consensus sequence {[RR(I/L)X3r](n,n≥1)+[L(φ/N)(V/L)](n,n>1)]}. In combination, these criteria provide substantial restrictions that constitute a robust filter in sequence space. This allowed us to uncover the regulated subnuclear targeting of a number of proteins and their subnuclear targeting domains. In addition, we reverse correlated the rules to a protein blindly isolated from nucleoli of acidotic cells. Interestingly, an in silico–identified protein, which displays a steady-state nucleolar distribution under standard conditions, increases its affinity of interaction with the nucleolar architecture after signal activation. This highlights the sharing of regulated subnuclear targeting sequences between proteins irrespective of their steady-state distribution in the absence of signal activation. It is important to mention that proteins that do not follow some or all of these rules might still be targeted to nucleoli in response to the same environmental cues. For example, although we have tested the effect of changing specific amino acids to alanines in an effort to change the composition or disorder level of the sequences reported here, it is still possible that some of the key residues identified here can be replaced by other specific ones in different proteins. Also, although we have tested the effect of disorder on nucleolar targeting using different mutations, the absolute requirement for low disorder within the wild-type protein setting awaits further characterization. Nonetheless, 1) we have shown that six randomly selected proteins of six that abide by these rules are responsive; 2) we have yet to stumble on a protein that harbors NoDSH+ but fails to undergo nucleolar sequestration in acidotic cells; 3) a protein that is enriched in nucleoli of acidotic cells follows the rules; and 4) five of five randomly selected proteins that do not encode a NoDSH+ did not localize to the nucleolus under acidosis. We expect that future work by us and others will help refine and possibly expand the consensus sequence presented here (Figure 8D). By identifying a common subnuclear targeting consensus sequence, our work reveals rules governing the dynamics of subnuclear organization and ascribes new modes of regulation to several proteins.
Proteins identified by the NoDSH+ rules include the antiapoptotic cIAP2, transcriptional regulator RNF8, the heat shock protein HSC70, and ribosomal L1 domain-containing protein PBK1. The functional relevance of nucleolar detention of the VHL and MDM2 ubiquitin ligases is already known because this regulates their HIF/rDNA- and p53-regulatory functions, respectively (Tao and Levine, 1999; Weber et al., 1999; Lohrum et al., 2003; Mekhail et al., 2004a, 2005, 2006). In addition, our findings suggest that H+-dependent nucleolar detention of RNF8 prevents it from acting as an enhancer of the transcriptional-stimulating activity of RXRα (Figure 5 and Supplementary Figure 5S; Takano et al., 2004). Thus, we propose that reversible nucleolar detention is a general mechanism of regulation of protein function. For example, inactivation of the ATP-dependent chaperone HSC70 by nucleolar detention could allow the cell to rely more on the energy-independent HSP110, which does not localize to nucleoli under acidosis (Figure 7), when facing limited energy supply under hypoxia (Bukau and Horwich, 1998; Easton et al., 2000). We had previously reported that acidosis and perturbations to ribosomal biogenesis target VHL and MDM2 to the nucleolus, respectively (Mekhail et al., 2005). Here, we uncover that the same signal—acidosis—can specifically target several different proteins for static nucleolar detention. In addition to our previous work, findings presented here therefore suggest the existence of a potential complex pattern of regulation of molecular networks. pH-dependent static nucleolar detention of several proteins such as VHL and RNF8 eliminates key interactions they perform within certain molecular networks (Figure 5 and Supplementary Figure 5S; see Mekhail et al., 2004a,b, 2005, 2006). This could also be the case for some of the proteins that exhibit a steady-state localization to the endoplasmic reticulum under standard growth conditions but harbor an NoDSH+ (Supplementary Table S1). Identification of putative NoDSH+ sequences within these proteins supports the existence of broader cellular programs that remodel various molecular networks in response to environmental cues. We also know that pH-dependent nucleolar targeting of VHL, for example, introduces it within a molecular network that restricts rRNA biogenesis (Mekhail et al., 2006). It is also possible that the other herein identified pH-responsive proteins also participate with VHL in the restriction of rRNA biogenesis. Nucleolar targeting of HSP70, an inducible homologue of HSC70, was previously reported to contribute to the recovery of nucleolar morphology after heat shock (Pelham, 1984). Therefore, further characterization of the sequences involved in the nucleolar targeting of these proteins in response to different environmental conditions could uncover general stress response sequence elements. Taken together, these findings support the proposal that the nucleolar proteome is dynamic and constantly changes its entity in response to environmental conditions (Andersen et al., 2002, 2005).
We find that the high-affinity character of nucleolus-NoDSH+ interactions provided us with a large window where saturated mutagenesis was capable of revealing the relative contribution of different amino acids before complete loss of activity was observed. In addition, the regulated nature of the system provided us with an additional layer for the assessment of specificity. Different scenarios can be envisioned for the nature of biochemical interactions mediating static nucleolar detention. We previously reported that VHL interacts with the intergenic spacer of rRNA genes (rDNA) under acidosis (Mekhail et al., 2006). Our data here cannot uncouple nucleolar localization and detention activities suggesting that STADs and STHDs cooperate in mediating the herein analyzed high-affinity interactions. Thus, one possibility is that different combinations of hydrophobic repeats confer features required for arginine domains to physically associate with their nucleolar binding sites. Another possibility is that the presence of arginine domains allows the hydrophobic repeats to act as anticodon-like (three residues per single repeat) structures that physically associate with specific regions of rDNA, depending on the nature of distribution of hydrophobic repeat recognition sites on the chromatin lattice. Although several of our smaller VHL fragments are efficiently targeted to nucleoli, we cannot completely rule out the possibility that the differential positioning of NoDSH+ components does not contribute in any way to the function or the regulation of the sequence within the setting of the wild-type VHL protein. Arginine/lysine-rich cryptic NoLS sequences have been identified in several proteins including HDM2, Coilin, and Survivin (Hebert and Matera, 2000; Lohrum et al., 2000; Weber et al., 2000; Catez et al., 2002; Hiscox, 2002; Song and Wu, 2005). Therefore, it is possible that acidosis induces a conformational change in the wild-type VHL protein that changes the positioning of the STHD relative to the STADs to reveal the functional “cryptic” NoDSH+ sequence. Identification of potential posttranslational modifications within VHL could help uncover such a mechanism.
In conclusion, dissection of the NoDSH+ of VHL allowed us to identify a common subnuclear targeting consensus sequence. Our work thus provides insight into the rules governing subnuclear organization/dynamics and ascribes new modes of regulation to several proteins. We thus propose that proteins with diverse steady-state distribution share the higher order code NoDSH+, which determines their subnuclear coordinates under specific environmental cues once they enter the nucleus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mark Olson (University of Mississippi Medical Center, Jackson, Mississippi) and Tom Misteli (National Cancer Institute, National Institutes of Health, Bethesda, MD) for kindly providing plasmids. This work is supported by grants from the Canadian Institutes of Health Research (CIHR) of the National Cancer Institute of Canada (NCIC). S.L. is the recipient of the NCIC Harold E. Johns Award. K.M. is supported by a Canada Graduate Scholarship (CGS-D) from the Natural Science and Engineering Research Council of Canada (NSERC) and by a CIHR Institute of Aging Postdoctoral Fellowship. L. R-L. is supported by an Ontario Graduate Scholarship (OGS).
Abbreviations used:
- AP
acidification-permissive
- B23
rRNA processing factor nucleophosmin
- cIAP2
inhibitor of apoptosis 1
- E3
ubiquitin ligase
- FLIP
fluorescence loss in photobleaching
- FRAP
fluorescence recovery after photobleaching
- HIF
hypoxia-inducible factor
- HSC70
heat-shock cognate 71-kDa protein
- NES
nuclear export signal
- NLS
nuclear localization signal
- NoDSH+
nucleolar detention signal regulated by [H+]
- NoLS
nucleolar localization signal
- NoRS
nucleolar retention signal
- PBK1
ribosomal L1 domain–containing protein 1
- RNF8
RING finger protein 8 ubiquitin ligase
- SD medium
standard (medium)
- VHL
von Hippel-Lindau.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-03-0295) on July 25, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Andersen J. S., Lam Y. W., Leung A. K., Ong S. E., Lyon C. E., Lamond A. I., Mann M. Nucleolar proteome dynamics. Nature. 2005;433:77–83. doi: 10.1038/nature03207. [DOI] [PubMed] [Google Scholar]
- Andersen J. S., Lyon C. E., Fox A. H., Leung A. K., Lam Y. W., Steen H., Mann M., Lamond A. I. Directed proteomic analysis of the human nucleolus. Curr. Biol. 2002;12:1–11. doi: 10.1016/s0960-9822(01)00650-9. [DOI] [PubMed] [Google Scholar]
- Azzam R., Chen S. L., Shou W., Mah A. S., Alexandru G., Nasmyth K., Annan R. S., Carr S. A., Deshaies R. J. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science. 2004;305:516–519. doi: 10.1126/science.1099402. [DOI] [PubMed] [Google Scholar]
- Bairoch A., Boeckmann B., Ferro S., Gasteiger E. Swiss-Prot: juggling between evolution and stability. Brief Bioinform. 2004;5:39–55. doi: 10.1093/bib/5.1.39. [DOI] [PubMed] [Google Scholar]
- Barseguian K., Lutterbach B., Hiebert S. W., Nickerson J., Lian J. B., Stein J. L., van Wijnen A. J., Stein G. S. Multiple subnuclear targeting signals of the leukemia-related AML1/ETO and ETO repressor proteins. Proc. Natl. Acad. Sci. USA. 2002;99:15434–15439. doi: 10.1073/pnas.242588499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonicalzi M. E., Groulx I., de Paulsen N., Lee S. Role of exon 2-encoded beta -domain of the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 2001;276:1407–1416. doi: 10.1074/jbc.M008295200. [DOI] [PubMed] [Google Scholar]
- Bukau B., Horwich A. L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Caceres J. F., Misteli T., Screaton G. R., Spector D. L., Krainer A. R. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J. Cell Biol. 1997;138:225–238. doi: 10.1083/jcb.138.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catez F., Erard M., Schaerer-Uthurralt N., Kindbeiter K., Madjar J. J., Diaz J. J. Unique motif for nucleolar retention and nuclear export regulated by phosphorylation. Mol. Cell. Biol. 2002;22:1126–1139. doi: 10.1128/MCB.22.4.1126-1139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee T. K., Fisher R. A. RGS12TS-S localizes at nuclear matrix-associated subnuclear structures and represses transcription: structural requirements for subnuclear targeting and transcriptional repression. Mol. Cell. Biol. 2002;22:4334–4345. doi: 10.1128/MCB.22.12.4334-4345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb J. R., Bickmore W. A. Considering nuclear compartmentalization in the light of nuclear dynamics. Cell. 2003;112:403–406. doi: 10.1016/s0092-8674(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Conti E., Izaurralde E. Nucleocytoplasmic transport enters the atomic age. Curr. Opin. Cell Biol. 2001;13:310–319. doi: 10.1016/s0955-0674(00)00213-1. [DOI] [PubMed] [Google Scholar]
- Dundr M., Hebert M. D., Karpova T. S., Stanek D., Xu H., Shpargel K. B., Meier U. T., Neugebauer K. M., Matera A. G., Misteli T. In vivo kinetics of Cajal body components. J. Cell Biol. 2004;164:831–842. doi: 10.1083/jcb.200311121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr M., Misteli T., Olson M. O. The dynamics of postmitotic reassembly of the nucleolus. J. Cell Biol. 2000;150:433–446. doi: 10.1083/jcb.150.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton D. P., Kaneko Y., Subjeck J. R. The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones. 2000;5:276–290. doi: 10.1379/1466-1268(2000)005<0276:thagsp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groulx I., Lee S. Oxygen-dependent ubiquitination and degradation of hypoxia-inducible factor requires nuclear-cytoplasmic trafficking of the von Hippel-Lindau tumor suppressor protein. Mol. Cell. Biol. 2002;22:5319. doi: 10.1128/MCB.22.15.5319-5336.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert M. D., Matera A. G. Self-association of coilin reveals a common theme in nuclear body localization. Mol. Biol. Cell. 2000;11:4159–4171. doi: 10.1091/mbc.11.12.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox J. A. The nucleolus—a gateway to viral infection? Arch Virol. 2002;147:1077–1089. doi: 10.1007/s00705-001-0792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos O., Kibel A., Gray S., Kaelin W. G., Jr Tumour suppression by the human von Hippel-Lindau gene product. Nat. Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Kato M., Hori H., Ishimori K., Kirisako T., Tokunaga F., Iwai K. Involvement of heme regulatory motif in heme-mediated ubiquitination and degradation of IRP2. Mol. Cell. 2005;19:171–181. doi: 10.1016/j.molcel.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Isogai Y., Tjian R. Targeting genes and transcription factors to segregated nuclear compartments. Curr. Opin. Cell Biol. 2003;15:296–303. doi: 10.1016/s0955-0674(03)00052-8. [DOI] [PubMed] [Google Scholar]
- Kosak S. T., Groudine M. Form follows function: the genomic organization of cellular differentiation. Genes Dev. 2004;18:1371–1384. doi: 10.1101/gad.1209304. [DOI] [PubMed] [Google Scholar]
- Kutay U., Guttinger S. Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 2005;15:121–124. doi: 10.1016/j.tcb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Lam Y. W., Trinkle-Mulcahy L., Lamond A. I. The nucleolus. J. Cell Sci. 2005;118:1335–1337. doi: 10.1242/jcs.01736. [DOI] [PubMed] [Google Scholar]
- Lee B. J., Cansizoglu A. E., Suel K. E., Louis T. H., Zhang Z., Chook Y. M. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell. 2006;126:543–558. doi: 10.1016/j.cell.2006.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Helmann J. D. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- Leung A. K., Gerlich D., Miller G., Lyon C., Lam Y. W., Lleres D., Daigle N., Zomerdijk J., Ellenberg J., Lamond A. I. Quantitative kinetic analysis of nucleolar breakdown and reassembly during mitosis in live human cells. J. Cell Biol. 2004;166:787–800. doi: 10.1083/jcb.200405013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linding R., Jensen L. J., Diella F., Bork P., Gibson T. J., Russell R. B. Protein disorder prediction: implications for structural proteomics. Structure. 2003;11:1453–1459. doi: 10.1016/j.str.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Altan-Bonnet N., Patterson G. H. Photobleaching and photoactivation: following protein dynamics in living cells. Nat. Cell Biol. 2003;(Suppl):S7–S14. [PubMed] [Google Scholar]
- Lohrum M. A., Ashcroft M., Kubbutat M. H., Vousden K. H. Identification of a cryptic nucleolar-localization signal in MDM2. Nat. Cell Biol. 2000;2:179–181. doi: 10.1038/35004057. [DOI] [PubMed] [Google Scholar]
- Lohrum M. A., Ludwig R. L., Kubbutat M. H., Hanlon M., Vousden K. H. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3:577–587. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- Ludwig M. G., Vanek M., Guerini D., Gasser J. A., Jones C. E., Junker U., Hofstetter H., Wolf R. M., Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- Mekhail K., Gunaratnam L., Bonicalzi M. E., Lee S. HIF activation by pH-dependent nucleolar sequestration of VHL. Nat. Cell Biol. 2004a;6:642–647. doi: 10.1038/ncb1144. [DOI] [PubMed] [Google Scholar]
- Mekhail K., Khacho M., Carrigan A., Hache R. R., Gunaratnam L., Lee S. Regulation of ubiquitin ligase dynamics by the nucleolus. J. Cell Biol. 2005;170:733–744. doi: 10.1083/jcb.200506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhail K., Khacho M., Gunaratnam L., Lee S. Oxygen sensing by H+: implications for HIF and hypoxic cell memory. Cell Cycle. 2004b;3:1027–1029. [PubMed] [Google Scholar]
- Mekhail K., Rivero-Lopez L., Khacho M., Lee S. Restriction of rRNA synthesis by VHL maintains energy equilibrium under hypoxia. Cell Cycle. 2006;5:2401–2413. doi: 10.4161/cc.5.20.3387. [DOI] [PubMed] [Google Scholar]
- Misteli T. Protein dynamics: implications for nuclear architecture and gene expression. Science. 2001;291:843–847. doi: 10.1126/science.291.5505.843. [DOI] [PubMed] [Google Scholar]
- Misteli T. Spatial positioning; a new dimension in genome function. Cell. 2004;119:153–156. doi: 10.1016/j.cell.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Murakami N., Yokomizo T., Okuno T., Shimizu T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 2004;279:42484–42491. doi: 10.1074/jbc.M406561200. [DOI] [PubMed] [Google Scholar]
- Paushkin S. V., Patel M., Furia B. S., Peltz S. W., Trotta C. R. Identification of a human endonuclease complex reveals a link between tRNA splicing and pre-mRNA 3′ end formation. Cell. 2004;117:311–321. doi: 10.1016/s0092-8674(04)00342-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984;3:3095–3100. doi: 10.1002/j.1460-2075.1984.tb02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair R. D., Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature. 2000;404:604. doi: 10.1038/35007077. [DOI] [PubMed] [Google Scholar]
- Ramsey I. S., Moran M. M., Chong J. A., Clapham D. E. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. L., Dove B. K., Jackson R. M., Collins R., Brooks G., Hiscox J. A. Delineation and modelling of a nucleolar retention signal in the coronavirus nucleocapsid protein. Traffic. 2006;7:833–848. doi: 10.1111/j.1600-0854.2006.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Doonan J. The nucleolus. Playing by different rules? Cell Cycle. 2005;4:102–105. doi: 10.4161/cc.4.1.1467. [DOI] [PubMed] [Google Scholar]
- Shikano S., Coblitz B., Sun H., Li M. Genetic isolation of transport signals directing cell surface expression. Nat. Cell Biol. 2005;7:985–992. doi: 10.1038/ncb1297. [DOI] [PubMed] [Google Scholar]
- Shou W., et al. Net1 stimulates RNA polymerase I transcription and regulates nucleolar structure independently of controlling mitotic exit. Mol. Cell. 2001;8:45–55. doi: 10.1016/s1097-2765(01)00291-x. [DOI] [PubMed] [Google Scholar]
- Shou W., Seol J. H., Shevchenko A., Baskerville C., Moazed D., Chen Z. W., Jang J., Charbonneau H., Deshaies R. J. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 1999;97:233–244. doi: 10.1016/s0092-8674(00)80733-3. [DOI] [PubMed] [Google Scholar]
- Song Z., Wu M. Identification of a novel nucleolar localization signal and a degradation signal in Survivin-deltaEx3, a potential link between nucleolus and protein degradation. Oncogene. 2005;24:2723–2734. doi: 10.1038/sj.onc.1208097. [DOI] [PubMed] [Google Scholar]
- Stewart A. K., Kerr N., Chernova M. N., Alper S. L., Vaughan-Jones R. D. Acute pH-dependent regulation of AE2-mediated anion exchange involves discrete local surfaces of the NH2-terminal cytoplasmic domain. J. Biol. Chem. 2004;279:52664–52676. doi: 10.1074/jbc.M408108200. [DOI] [PubMed] [Google Scholar]
- Takano Y., et al. The RING finger protein, RNF8, interacts with retinoid X receptor alpha and enhances its transcription-stimulating activity. J. Biol. Chem. 2004;279:18926–18934. doi: 10.1074/jbc.M309148200. [DOI] [PubMed] [Google Scholar]
- Tao W., Levine A. J. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl. Acad. Sci. USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C., Tan L., Li P., Zhu Y. S. Identification of a novel nucleus protein involved in the regulation of urokinase in 95D cells. Acta Biochim. Biophys. Sin. 2005;37:303–309. doi: 10.1111/j.1745-7270.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- Tsai R. Y., McKay R. D. A multistep, GTP-driven mechanism controlling the dynamic cycling of nucleostemin. J. Cell Biol. 2005;168:179–184. doi: 10.1083/jcb.200409053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom V., Citterio E., Hoogstraten D., Zotter A., Egly J. M., van Cappellen W. A., Hoeijmakers J. H., Houtsmuller A. B., Vermeulen W. DNA damage stabilizes interaction of CSB with the transcription elongation machinery. J. Cell Biol. 2004;166:27–36. doi: 10.1083/jcb.200401056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R., Hwang E. S., Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823. doi: 10.1038/19775. [DOI] [PubMed] [Google Scholar]
- Weber J. D., Kuo M. L., Bothner B., DiGiammarino E. L., Kriwacki R. W., Roussel M. F., Sherr C. J. Cooperative signals governing ARF-mdm2 interaction and nucleolar localization of the complex. Mol. Cell. Biol. 2000;20:2517–2528. doi: 10.1128/mcb.20.7.2517-2528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. D., Taylor L. J., Roussel M. F., Sherr C. J., Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- Wong J. M., Kusdra L., Collins K. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat. Cell Biol. 2002;4:731–736. doi: 10.1038/ncb846. [DOI] [PubMed] [Google Scholar]
- Zaidi S. K., Young D. W., Choi J. Y., Pratap J., Javed A., Montecino M., Stein J. L., van Wijnen A. J., Lian J. B., Stein G. S. The dynamic organization of gene-regulatory machinery in nuclear microenvironments. EMBO Rep. 2005;6:128–133. doi: 10.1038/sj.embor.7400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C., van Wijnen A. J., Stein J. L., Meyers S., Sun W., Shopland L., Lawrence J. B., Penman S., Lian J. B., Stein G. S., Hiebert S. W. Identification of a nuclear matrix targeting signal in the leukemia and bone-related AML/CBF-alpha transcription factors. Proc. Natl. Acad. Sci. USA. 1997;94:6746–6751. doi: 10.1073/pnas.94.13.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimber A., Nguyen Q. D., Gespach C. Nuclear bodies and compartments: functional roles and cellular signalling in health and disease. Cell Signal. 2004;16:1085–1104. doi: 10.1016/j.cellsig.2004.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.