Abstract
Transforming growth factor (TGF)-β receptors stimulate diverse signaling processes that control a wide range of biological responses. In polarized epithelia, the TGFβ type II receptor (T2R) is localized at the basolateral membranes. Sequential cytoplasmic truncations resulted in receptor missorting to apical surfaces, and they indicated an essential targeting element(s) near the receptor's C terminus. Point mutations in the full-length receptor confirmed this prediction, and a unique basolateral-targeting region was elucidated between residues 529 and 538 (LTAxxVAxxR) that was distinct, but colocalized within a clinically significant signaling domain essential for TGFβ-dependent activation of the Smad2/3 cascade. Transfer of a terminal 84 amino-acid fragment, containing the LTAxxVAxxR element, to the apically sorted influenza hemagglutinin (HA) protein was dominant and directed basolateral HA expression. Although delivery to the basolateral surfaces was direct and independent of any detectable transient apical localization, fluorescence recovery after photobleaching demonstrated similar mobility for the wild-type receptor and a missorted mutant lacking the targeting motif. This latter finding excludes the possibility that the domain acts as a cell membrane retention signal, and it supports the hypothesis that T2R sorting occurs from an intracellular compartment.
INTRODUCTION
Epithelial cells form highly polarized monolayers that generate two morphologically and functionally distinct domains, an apical luminal facing domain and a basolateral domain that separates the epithelia from the underlying mesenchyme. Because the maintenance of cell polarity is dependent upon the asymmetrical distribution of proteins and lipids to precise locales on the cell surface (Rodriguez-Boulan et al., 2005), a number of cis-acting targeting signals have been described mediating protein sorting. For example, basolateral delivery is often regulated by minimal amino acid motifs in the cytoplasmic region of a wide range of membrane proteins, often being localized to juxtamembrane locales (Aroeti et al., 1998; Ikonen and Simons, 1998; Rodriguez-Boulan et al., 2005). Although extensive heterogeneity exists, certain features commonly arise in the amino acid sequences. Specifically, the targeting of many basolateral proteins, including the low-density lipoprotein receptor and vesicular stomatitis virus glycoprotein, have been demonstrated to be regulated by tyrosine-based motifs (Matter et al., 1992; Thomas et al., 1993) and a consensus sequence, YXXφ (where X is any amino acid and φ represents a large hydrophobic residue), has been proposed to be needed for correct trafficking (Hunziker and Mellman, 1991; Matter et al., 1992; Honing and Hunziker, 1995; Aroeti et al., 1998). Moreover, bipartite sorting signals and dihydrophobic residues such as dileucine (LL) motifs have also figured highly as effectors of basolateral trafficking (Hunziker and Fumey, 1994; Miranda et al., 2001). However, for many other reported basolaterally located proteins, the exact nature of the signal(s) is unclear (Okamoto et al., 1992; Aroeti et al., 1993, 1998; Hobert et al., 1997; Le Gall et al., 1997; Odorizzi and Trowbridge, 1997; Simmen et al., 1999). A similar dependence on tyrosine and dileucine motifs has been reported for cargo internalized through clathrin-coated pits (Hunziker and Mellman, 1991; Matter et al., 1992, 1994; Thomas et al., 1993; Hunziker and Fumey, 1994; Thomas and Roth, 1994; Honing and Hunziker, 1995; Bonifacino and Dell'Angelica, 1999; Folsch et al., 1999). However, although the essential nature of each motif seems to be distinct and many basolateral targeting motifs are distal from their internalization domains (Aroeti et al., 1998), the frequency of this colinear existence presents the possibility of functional interplay between the trafficking machinery.
Apically directed membrane trafficking involves distinct sorting mechanisms from that observed for basolaterally destined cargo. Although basolateral transport is achieved almost exclusively by recognition of intracellular amino acid sequences, apical-determining motifs routinely incorporate extracellular acceptor sites for N- or O-glycosylation (Matter, 2000). In addition, glycosyl-phosphatidylinositol (GPI)-anchored proteins have been demonstrated to localize at the apical membrane. This is thought to result from the enrichment of specialized lipids, specifically glycosphingolipids and cholesterol, at the apical surface and their subsequent assembly into specific membrane domains or rafts in the trans-Golgi network (TGN) where apically destined cargo is recruited (Harder and Simons, 1997; Brown and London, 1998; Paladino et al., 2006).
Transforming growth factor-β (TGFβ) is a multifunctional protein involved in a wide range of cellular functions essential for correct growth and development (Blobe et al., 2000). However, the cellular response to TGFβ is greatly dependent on the type of cell involved. Although TGFβ stimulates proliferation in fibroblasts and many other mesenchymal cells, it acts as a potent growth inhibitor in a variety of cell types, including epithelial, hematopoietic, and endothelial cells (Howe et al., 1991; Serini and Gabbiani, 1999; Bissell, 2001; Yue and Mulder, 2001). Three mammalian TGFβ isoforms have been described to date, termed TGFβ-1, -2, and -3, which generally exhibit similar overall effects in vitro, yet they have distinct activities in vivo (Hartsough and Mulder, 1997; Kulkarni et al., 2002). They signal through three distinct mammalian TGFβ binding transmembrane proteins, termed type I, type II, and type III receptors (Laiho et al., 1990, 1991; Lopez-Casillas et al., 1991; Wang et al., 1991; Chen et al., 2003). The type I and type II TGFβ receptors are single-pass, transmembrane serine/threonine kinases of 53 and 75 kDa, respectively (Lin et al., 1992; Bassing et al., 1994). Although homomeric complexes occur on the cell surface, TGFβ signaling is primarily regulated through heteromeric interactions between the type I and type II receptors (Anders and Leof, 1996).
On ligand binding, the constitutively active type II TGFβ receptor recruits and transphosphorylates the type I receptor in the juxtamembrane GS domain (Wrana et al., 1992; Wrana et al., 1994). Once activated, the type I receptor serves as a docking site for the receptor-associated Smads (R-Smads), termed Smad2 and Smad3, which after phosphorylation dissociate from the receptor and complex with the common mediator Smad4. The R-Smad/Smad4 complex subsequently translocates to the nucleus where it can function as a comodulator of transcription (Ten Dijke et al., 2002; Shi and Massagué, 2003). In addition to the aforementioned Smad-dependent pathways, Smad-independent responses have also been documented critical for many aspects of TGFβ signaling, including cell proliferation and morphological transformation (Hocevar et al., 1999; Wilkes et al., 2003).
Although the type I and type II TGFβ receptors show a relatively homogeneous distribution over the cell surface of fibroblasts (Ehrlich et al., 2001; Yao et al., 2002), we have shown that both receptors localize to the basolateral domains in polarized epithelium (Murphy et al., 2004). Because basolateral trafficking for both receptors was independently regulated and mediated by sequences in the cytoplasmic tails of the receptors (Murphy et al., 2004), a further definition of the presumptive cis-acting element(s) was required. The current study documents that basolateral delivery of the T2R is dependent upon a 10 amino-acid sequence between residues 529 and 538. Moreover, this region 1) is essential for correct receptor membrane localization, 2) is dominant to apical targeting elements in the influenza hemagglutinin (HA) protein, and 3) regulates TGFβ receptor activity independent of Smad2 and Smad3 phosphorylation.
MATERIALS AND METHODS
Materials
Human TGFβ was obtained from R&D Systems (Minneapolis, MN), and recombinant granulocyte macrophage–colony-stimulating factor (GM-CSF) was purchased from the Mayo Clinic Pharmacy (Rochester, MN). Cell culture medium was from Invitrogen (Carlsbad, CA) or from Biological Industries (Beit Haemek, Israel). Fetal bovine serum (FBS) was from Summit (Fort Collins, CO) or Biological Industries. Mouse monoclonal immunoglobulin G (IgG) against the hemagglutinin (HA) tag (12CA5; anti-HA) were from Roche Applied Science (Indianapolis, IN); Fab′ fragments were prepared from the anti-HA IgG as described previously (Henis et al., 1994). Alexa546-F(ab′)2 of goat anti-mouse F(ab′)2 were from Invitrogen-Molecular Probes Europe (Leiden, The Netherlands), and they were converted to Alexa546-Fab′ as described previously (Gilboa et al., 1998). Normal goat IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). Transwell 12-mm (#3402) polycarbonate membrane plates were purchased from Corning Life Sciences (Acton, MA). Unless specifically noted, all other reagents were from Sigma (St. Louis, MO).
Cells and Plasmid Constructs
HeLa, DR26, NMuMG, COS7, and Madin-Darby canine kidney (MDCK) cells were maintained in DMEM supplemented with 10% (vol/vol) FBS. Stable MDCK cell lines were generated upon cotransfection of the pCMV5-based vectors with pcDNA3 Hygro, by using the Lipofectamine 2000 transfection reagent (Roche Diagnostics, Indianapolis, IN), and clones were selected in DMEM/10%FBS supplemented with 300 μg/ml hygromycin B (Invitrogen). The selected MDCK cell clones were maintained in DMEM/10%FBS supplemented with 50 μg/ml hygromycin B. Transient transfections of polarized MDCK cells by using Lipofectamine 2000 were performed using 0.1 μg of receptor DNA and 0.4 μg of pCR3.1CMV green fluorescent protein (GFP) as carrier.
The designations αI and βII refer to the extracellular domains of the human GM-CSF receptor α or β subunits coupled to the transmembrane and cytoplasmic domains of the TGFβ type I or type II receptor, respectively (Anders and Leof, 1996). Truncated receptors were generated using a unique BssHII site located at nucleotide 1291 in the GM-CSF β chain and a unique BamHI site located at the termini of the gene, into which truncated receptor fragments were cloned. The truncated fragments were generated by polymerase chain reaction (PCR) by using a common forward primer upstream of the BssHII site (TGGAAGGACAGCAAGACCGAGAC) and specific reverse primers spanning 24 nucleotides of overlapping sequence adjacent to the truncation sites before a stop codon and a BamHI restriction site.
Full-length mutant receptors were cloned by initially creating a BamHI site near the termini of the gene upon a conserved C-to-G point mutation at nucleotide 1527. The point mutations were then introduced as BamHI-flanked DNA fragments engineered by annealing and PCR fill-in of long oligonucleotides (120-mers) with 43-nucleotide central overlapping domains into which the desired point mutations were written. To generate the full-length endogenous receptor mutants, the GM-CSF β extracellular region of the respective βII mutant was replaced with the native extracellular type II receptor (T2R) domain. This was achieved by exchanging a NdeI/Bsa BI 1.5-kb cytomegalovirus (CMV) promoter/type II receptor fragment (NdeI site located centrally to the CMV promoter and BsaBI site at nucleotide 1121 in the T2R), with the equivalent 2.4-kb NdeI/BsaBI chimeric receptor/CMV promoter fragment. The native N-terminally HA-tagged T2R was graciously provided by Dr. Jeff Wrana (University of Toronto).
Construction of the internal deletion mutants was based on a membrane-truncated βII chimeric receptor (Figure 3), βIIΔ198, which incorporated a BamHI site cloned adjacent to Ser198 of the type II receptor followed by a stop site. The terminal 20 or 84 amino-acid fragments of the βII receptor were PCR cloned into this BamHI site by using BamHI-flanked primer pairs from amino acid residues S547 or V484, respectively, to the native stop site. Mutated terminal 84 amino-acid receptor fragments expressing the R528A or A531G point mutations, described above, were PCR cloned using BclI restriction sites. Last, the influenza HA chimeras were prepared by truncating to S552 and adding a BamHI site and stop signal; pHA-ICD (Figure 4). The identical Bam HI-flanked 20 and 84 amino-acid fragments of the βII receptor described above were subsequently cloned into this terminal BamHI site.
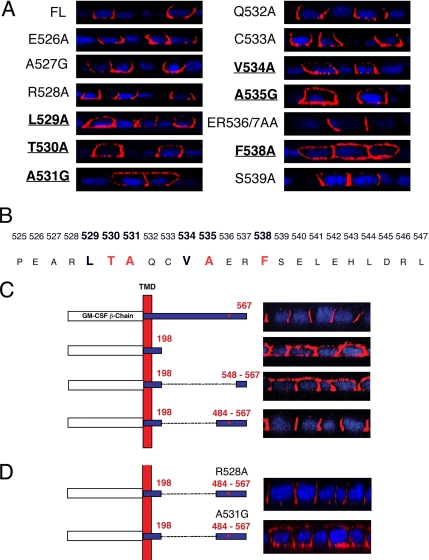
Figure 3.
Basolateral retention of the TGFβ type II receptor is dependent on six residues located between amino acids 529-538. (A) Alanine or glycine point mutations were engineered into the βII receptor between amino acid residues 525-547. Mutant receptors were transiently transfected into fully polarized MDCK monolayers for 6 h before staining with the GM-CSF β antibody. Confocal images are presented for the full-length (FL) T2R or mutants spanning the region 526-539. (B) The predicted basolateral targeting signal motif LTAxxVAxxF is illustrated with red, indicating the four most critical residues. (C) Stable MDCK clones expressing full-length (βII; first construct), membrane-truncated (βIIΔ198; second construct), or internal sequence deletion mutant βII receptors (βIIΔ198-547 or βIIΔ198-483; third and fourth constructs, respectively) or βIIΔ198-483 constructs (D) containing R528A or A531G point mutations were stained as described in Figures 1 and 2. The location of the targeting motif defined in A and B is indicated by the red * in the 484–567 fragment. Nuclei were stained with DAPI and images are represented as perpendicular XZ cross sections.
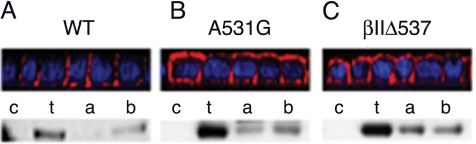
Figure 4.
Biotin T2R labeling at apical or basolateral membrane domains. Stable MDCK cell lines expressing wild type (A), A531G point-mutated (B) or βIIΔ537-truncated (C) βII chimeric receptor constructs were biotin labeled apically (a) or basolaterally (b) as polarized monolayers or as a nonpolarized monolayer for total labeling (t). Biotinylated protein was extracted on streptavidin-agarose beads and Western blotted using a receptor specific antibody. Bottom, control nonlabeled cells are designated c. Top, XZ cross sections of parallel immunofluorescently stained polarized monolayers.
Polarized Monolayer Cell Culture and Transfection
Parental MDCK or MD-1 (MDCK clone expressing chimeric αI and βII TGFβ receptors; Murphy et al., 2004) epithelial cells were plated in 12-mm Costar transwell polycarbonate membrane plates at densities of 0.5 × 105 cells per well, in DMEM/10% FBS. Formation of tight junctions and integrity of the monolayers were determined by serial measurement of transepithelial resistance. Fully polarized monolayers were achieved 72 h after cell plating. The peak transmembrane resistance, corrected for background, was typically in the range of 150–200 Ω/cm2. Transient transfection of cells plated on 12-mm transwells was performed on day 3 fully polarized monolayers by using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Briefly, 0.05–0.2 μg of receptor DNA supplemented with 0.4 μg of empty vector carrier DNA were prepared in 50 μl of Opti-MEM medium (Invitrogen). Lipofectamine 2000 reagent (2 μl) was equilibrated in Opti-MEM medium (50 μl) for 5 min at room temperature (RT) before addition to the DNA solution with vortexing. After 20 min at RT the transfection mix was added to the apical reservoir of the transwell, whose medium was replaced 30 min earlier with 250 μl of fresh Opti-MEM. The cells were then incubated at 37°C for 5–6 h before staining and visualization by immunofluorescence microscopy.
Immunofluorescence Microscopy
For surface receptor staining MDCK cells were initially plated at a density of 0.5 × 105 cells per 12-mm transwell. Polarized MDCK monolayers were washed three times with ice-cold phosphate-buffered saline (PBS), 0.1 mM CaCl2, 1 mM MgCl2, 0.2% bovine serum albumin (BSA), pH 7.4, before addition of primary antibody diluted in PBS, 0.2% BSA, 5% normal donkey serum (NDS) for 1 h on ice. The monolayer containing membranes were subsequently washed with three 10-min incubations in ice-cold PBS, 0.2% BSA before a final 5-min wash with PBS. Cells were fixed for 30 min at room temperature with 2% formaldehyde containing PBS, 0.1 mM CaCl2, and 1 mM MgCl2, washed once with PBS, 0.2% BSA, and treated for 10 min on ice with 50 mM NH4Cl in PBS to block background autofluorescence. For internal staining, after fixation the cells were permeablized for 1 min at room temperature with 0.25% Triton X-100 in PBS. Incubation with primary antibody and blocking was performed as detailed above. Cells were subsequently washed twice with PBS, 0.2% BSA and secondary antibody diluted in PBS, 0.2% BSA, 5% NDS was added for 30 min in the dark. Nuclear staining (blue) was performed by incubation for 10 min in the dark with 300 nM 4,6-diamidino-2-phenylindole (DAPI) diluted in PBS, 0.2% BSA, 5% NDS. Cells were then washed three times with PBS, 0.2% BSA, mounted with Vectasheild, and the membranes were viewed at 40× using an LSM 510 confocal microscope (Carl Zeiss, Jena, Germany). Applied primary antibody concentrations were as follows: human TGFβ type I receptor antibody (sc9048; Santa Cruz Biotechnology, Santa Cruz, CA), 1:20; and GM-CSF receptor antibodies to the α (sc458; Santa Cruz Biotechnology) and β (sc457; Santa Cruz Biotechnology) chains were applied at 1:60. Secondary anti-mouse Cy3 (715-165-150; Jackson ImmunoResearch Laboratories), and anti-rabbit Alexa488 (A-11008; Invitrogen) were each used at concentrations of 1:200. All figures depict representative images from three independent experiments observed in ∼20–30 cells.
Western Blotting
DR26 (Mv1Lu mutants lacking the type II TGFβ receptor) cells were plated in six-well plates at densities of 5 × 105 cells per well. The receptors were transfected using Lipofectamine 2000 reagent as suggested by the supplier. Cell transfections and ligand stimulations were performed in DMEM/0.5% FBS. After ligand stimulation, cells were washed twice with ice-cold PBS at 4°C and carefully scraped from the membranes in 0.5 ml of ice-cold PBS. Cells were pelleted at 5000 × g and lysed in 120 μl of radioimmunoprecipitation assay (RIPA) lysis buffer: 50 mM Tris, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 50 mM NaCl, 1 mM EGTA, 1 mM Na3VO4, 1 mM NaF, and protease Complete inhibitor cocktail (Roche Diagnostics). The cell debris was removed by centrifugation at 21,000 × g, and equivalent supernatant protein was separated on an 8% SDS-polyacrylamide gel electrophoresis (PAGE). Total and phospho-Smad2 antibodies were from Upstate Biotechnology (Lake Placid, NY) (06-654 and 06-829, respectively), and HA antibody was from Roche Applied Science (12CA5).
Golgi Block and Tannic Acid Fixation
MDCK cells plated on 12-mm transwells were transfected with 0.2 μg of receptor DNA as detailed above and incubated at 37°C for 3 h. Cells were then placed at 20°C for 3 h to facilitate Golgi block of newly synthesized receptors. Pre-existing cell-surface receptors were removed by incubation with 0.0025% trypsin in PBS for 15 min at 20°C. After PBS wash and addition of fresh DMEM/10% FBS, the cultures were returned to 37°C and stained for cell-surface receptor expression at the indicated times. For tannic acid fixation, Golgi-blocked cells were incubated with filter sterilized 0.5–5% tannic acid in PBS for 10 min at 20°C, PBS washed, and then placed at 37°C in DMEM/10% FBS for 1 h before immunofluorescent staining.
Biotinylation of Cell-Surface Receptors
MDCK cell clones expressing wild-type or specific point mutant (A531G or R528A) chimeric βII receptors were plated onto 24 mm transwells or six-well plates at 2 × 105 or 4 × 105 cells per well, respectively. The cultures were allowed to polarize over 96 h with daily medium (DMEM/10% FBS) changes. Once fully polarized, the cells were washed twice with ice-cold PBS and once with ice-cold KLH buffer (5 mM KCl, 128 mM NaCl, 1.3 mM CaCl2, 5 mM MgSO4, 50 mM HEPES, pH 7.4). EZ-Link Sulfo-NHS-LC-Biotin (Pierce Chemical, Rockford, IL) was dissolved in ice-cold KLH buffer at a concentration of 1 mg/ml, and it was applied in triplicate to the six-well plate wells (1 ml), apical (0.5 ml) or basolateral (1.0 ml) surfaces. Ice-cold DMEM/10% FBS was placed in the opposite transwell reservoir, and ice-cold KLH buffer was placed in control six-well wells. Plates were rocked at 4°C for 2 h before the biotinylation solution was replaced with fresh solution and rocked at 4°C for a further 4 h. Cells were then washed three times with ice-cold PBS, and scraped and lysed in 500 μl of RIPA buffer with protease inhibitors (as described in Western Blotting). Streptavidin agarose (50 μl; Novagen, Madison, WI) was mixed with 500 μg–1 mg of protein in 1 ml total volume, and the samples were incubated for 2 h at 4°C with agitation. The agarose was washed four times with lysis buffer (1 ml), and the biotin-bound proteins were eluted by boiling for 5 min in Laemmli buffer. Proteins resolved on 8% SDS-PAGE gel were probed with a rabbit anti-GM-CSF β chain antibody (sc-676; Santa Cruz Biotechnology) at 1:800 dilution, and a goat anti-rabbit horseradish peroxidase secondary (sc2004) at 1:2500.
Fluorescence Recovery after Photobleaching (FRAP)
MDCK or COS7 Cells were grown on glass coverslips in 35-mm dishes. Except for stably expressing MDCK cell lines, they were transfected with 1 μg of plasmid DNA encoding the wild-type (WT) type II receptor carrying an extracellular HA tag near the N terminus (HA-T2R-WT) or the truncation mutant ending at amino acid 527 (HA-T2RΔ527). After 48 h, the cells were washed with ice-cold Hank's balanced salt solution (Biological Industries) supplemented with 20 mM HEPES and 2% BSA, pH 7.2 (HBSS/HEPES/BSA). After blocking with normal goat IgG in the same buffer (200 μg/ml for 30 min at 4°C), they were labeled in the cold with monovalent Fab′ anti-HA followed by Alexa546-Fab′ of goat anti-mouse F(ab′)2 (each incubation was with 50 μg/ml Fab′ for 45 min). After three washes, the coverslips were mounted over a chamber containing HBSS/HEPES/BSA, and samples were subjected to FRAP measurements at 16°C, replacing samples within 15 min to minimize internalization during the measurement. FRAP experiments were conducted as described previously (Axelrod et al., 1976; Koppel, 1976; Koppel et al., 1976), by using a monitoring argon ion laser beam (1 μW) at 528.7 nm, focused to a Gaussian radius of 0.85 ± 0.02 μm (63× oil-immersion objective). A 5-mW pulse (for 20 ms) bleached 60–75% of the fluorescence in the illuminated region, and fluorescence recovery was followed by the attenuated monitoring beam. The lateral diffusion coefficient (D) and the mobile fraction (Rf) were extracted from the fluorescence recovery curves by nonlinear regression analysis, fitting to the lateral diffusion equation (Petersen and Elson, 1986).
RESULTS
Basolateral Trafficking of the Type II TGFβ Receptor Occurs Independently of the Type I Receptor and Is Mediated via a C-Terminal Motif
To identify cis-acting elements regulating TGFβ receptor trafficking, we initially used chimeric TGFβ receptors due to the availability of high-specificity antibodies to the extracellular receptor domains, enabling imaging of the receptor's surface localization in the absence of intracellular staining (Murphy et al., 2004). The chimeric receptors consist of the ligand binding domains of GM-CSF α or β receptors (Gearing et al., 1989; Hayashida et al., 1990) fused to the transmembrane and cytoplasmic domains of the type I and type II TGFβ receptors, termed α1 and βII, respectively (Anders and Leof, 1996; Anders et al., 1997, 1998). Because high-affinity GM-CSF binding and subsequent signaling occurs through the formation of α/β heterodimers (in a manner analogous to the endogenous TGFβ receptors), and signals required for basolateral localization have, to date, been solely localized to the cytoplasmic domains in all basolateral proteins studied, the chimeras would be expected to contain all the endogenous signals necessary for basolateral localization.
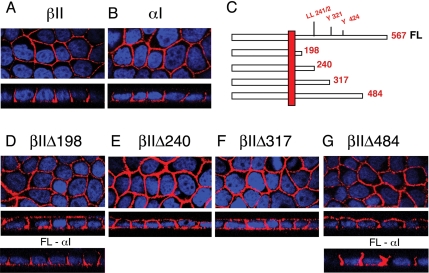
As reported previously (Murphy et al., 2004), both the βII (Figure 1A) and αI (Figure 1B) chimeric receptors localize to the basolateral domains of fully polarized MDCK cells. To address whether the observed basolateral targeting of the T2R was a function of basolateral targeting signals contained in the receptor's cytoplasmic domain, C-terminal truncation mutants were investigated. Initially, four truncated receptors were constructed, introducing stop signals after amino acid residues S198, E240, L317, and V484 in the cytoplasmic domain of the T2R (Figure 1C). These sites were chosen to sequentially eliminate putative dileucine and tyrosine targeting motifs. Specifically, a dileucine is located at amino acid positions 241/2, and possible effector tyrosine residues are located at amino acids 321 and 424 (Figure 1C). As presented in Figure 1, D–G, stable MDCK cell clones expressing the truncated βI1Δ198, βI1Δ240, βI1Δ317, and βI1Δ484 receptors all demonstrated the loss of specific basolateral retention, with significant T2R staining observed on both the apical and basal membrane domains (compare Figure 1A with Figure 1, D–G, middle Z-section panels). However, the coexpressed full-length αI receptor maintained basolateral localization despite βII mislocalization (Figure 1, D and G, bottom Z-section panels) (Murphy et al., 2004).
Figure 1.
The TGFβ type II receptor is trafficked to the basolateral membranes by a mechanism independently of conventional tyrosine or dileucine motifs. MDCK cell clones were plated at 5 × 104 cells/12-mm transwell and allowed to fully polarize over 72 h, as described in Materials and Methods. MD-1 cells were stained for βII (A) or αI chimeric receptors (B) by using primary antibodies to the external GM-CSF β or α chains, respectively, and secondarily tagged with Cy3 (red). (C) Truncated chimeric βII receptors depicting the location of potential trafficking motifs stably expressed in MDCK cells (D–G). MDCK clones βIIΔ198 (D) and βIIΔ484 (G) additionally express the full-length αI chimeric receptor (FL-αI) (D and G, bottom). Images are represented as the horizontal XY flat sections above lower perpendicular XZ cross-sectional images. Nuclei (blue) were stained with DAPI.
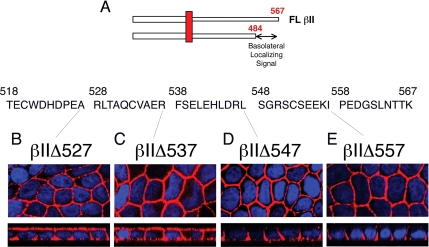
The results from Figure 1 support the independent trafficking of the type I and II receptors and indicate that a basolateral targeting signal is localized between amino acids 484 and 567 of the T2R (Figure 2A). To further define this motif additional truncations were made, sequentially deleting 10 amino acids from the end of the receptor: βI1Δ557, βI1Δ547, βI1Δ537, and βI1Δ527 (Figure 2A). Stable MDCK cell clones expressing the truncated βI1Δ527 (Figure 2B) and βI1Δ537 (Figure 2C) receptors showed both apical and basolateral expression, whereas inclusion of an additional 10 amino acids (538-547) restored specific basolateral staining similar to the full-length receptor (compare Figure 2D with Figure 1A). As expected, addition of 10 amino acids (βI1Δ557 clone) had no discernible effect on the basolateral expression observed in the βI1Δ547 truncation (Figure 2E). Hence, the data support a basolaterally localizing motif in the T2R surrounding amino acids 537-547.
Figure 2.
The basolateral localizing signal of the TGFβ type II receptor is located at the C-terminal in the region of amino acid 537. (A) The basolateral targeting signal is located between amino acids 484 and 567. (B–E) Serial 10 amino acid C-terminal truncation mutants (βIIΔ527, βIIΔ537, βIIΔ547, and βIIΔ557) were stably expressed in MDCK cells and imaged using the GM-CSF β antibody and the Cy3-tagged secondary (red). Nuclei were additionally stained with DAPI. Images are represented as the horizontal XY flat sections above lower perpendicular XZ cross-sectional images of polarized cultures.
Basolateral Targeting of the T2R Is Mediated by a Novel C-Terminal Domain Involving Amino Acids 529-538
Although truncations can facilitate initial mapping of a potential motif, to eliminate complications associated with receptor folding and tertiary structure it is necessary to perform single and/or double point mutations. As such, full-length chimeric receptors with amino acids 525-547 singly or doubly mutated to alanine (alanine residues were mutated to glycines) were constructed, and receptor localization was examined. As shown in Figure 3A, staining of polarized MDCK cells expressing these mutant receptors revealed that a combination of six amino acid residues were involved in the basolateral localization of the receptor. Specifically, leucine 529, threonine 530, and alanine 531 form a sequential three amino-acid motif where point mutations in each of these residues results in the missorting of the mutant receptor to the apical domains (in addition to the basolateral). Three additional amino acid residues, valine 534, alanine 535, and phenylalanine 538, were also deemed essential for correct basolateral retention of the T2R (Figure 3A). The impact of missorting with the T530, A531, A535, and F538 mutant receptors was highly significant, resulting in apical expression in almost every cell image. For mutations at L529 and V534, although imparting significant apical staining, the majority of receptor staining was still basolateral (Figure 3A). Amino acid residues 540-547, as well as amino acid residue 525, were found to have no influence on the basolateral retention of the receptor (data not shown). These results enabled us to report a novel basolateral targeting domain, which includes six essential amino acids located between amino acids 529 and 538, LTAxxVAxxF (Figure 3B), in which individual point mutations in any of the six residues results in mislocalization of the T2R to both the apical and basal membrane domains (Figure 3A).
Although the previous T2R truncations and deletions have determined the LTAxxVAxxF residues to be essential for correct basolateral targeting (Figures 1–3A), they do not eliminate the possibility that other cytoplasmic sequences, potentially the dileucine and tyrosine motifs described in Figure 1C, provide additional function(s). As such, internal deletion mutants were constructed with deletions from amino acid residues 199 to 547 (βIIΔ198-548) and from 199 to 483 (βIIΔ198-484) (Figure 3C). Stable MDCK cell clones expressing the βIIΔ198-548 mutant, which lacks the terminal basolateral motif, were observed to mislocalize the receptor over both apical and basolateral domains, similar to the βIIΔ198 cytoplasmic deletion control (Figure 3C, compare second and third constructs). However, MDCK cell clones expressing the βIIΔ198-484 mutant, which includes the motif (location indicated by *) and additional sequence to ensure correct folding, demonstrated basolateral expression analogous to the full-length receptor (Figure 3C, compare first and last constructs). Additional mutants were constructed with deletions around the 529LTAxxVAxxF538 region: βIIΔ198-508, βIIΔ198-518, βIIΔ198-528, and βIIΔ198-538. Transient transfection of MDCK cells demonstrated that truncations closer to the motif eliminated basolateral targeting, with the βIIΔ198-508 and βIIΔ198-518 receptors efficiently maintaining polarity, although to a less stringent degree than the βIIΔ198-484 construct (data not shown). Subsequent deletions up to (βIIΔ198-528) and including (βIIΔ198-538) residues 529LTAxxVAxxF538 abolished basolateral T2R targeting (data not shown). To further document the essential role of the LTAxxVAxxF sequence, point mutations were introduced at A531 or R528 in the context of the internal βIIΔ198-484 deletion. Consistent with the results of Figure 3, A and C, the A531G point mutation extensively mislocalized to the apical domains, whereas the control R528A mutation was without effect (Figure 3D). Hence, Figures 1–3 demonstrate that the described domain is necessary for basolateral localization of the T2R.
Biotinylation of Cell-Surface Receptors Confirms Apical Mislocalization upon Mutation or Deletion of the C-Terminal Targeting Domain
To further validate the basolateral targeting properties of the TGFβ type II receptor, in addition to the immunofluorescence and signaling data, biotinylation of cell-surface receptors was investigated. MDCK clones expressing either the wild-type βII, A531G point mutant, or βIIΔ537 truncated receptors were exposed to biotin cross-linking reagents from either the apical or basal transwell chambers. As shown in Figure 4, the wild-type βII receptor was seen to selectively biotin label the basolateral membranes, whereas the A531G and βIIΔ537 mutant βII receptors demonstrated extensive apical biotin labeling, paralleling the immunofluorescent staining.
Basolateral Targeting and Signaling Is Identically Regulated in Wild-Type and Chimeric T2Rs
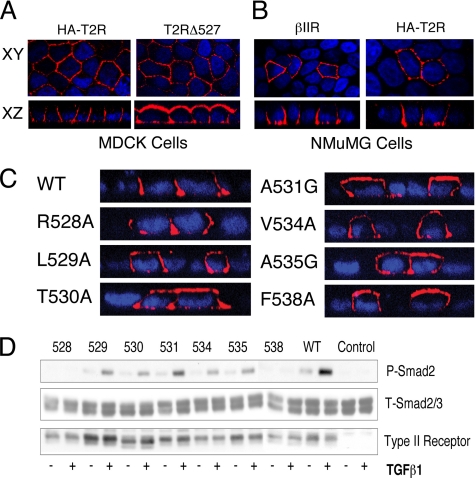
Although initial studies were conducted with the chimeric βII receptor due to the lack of antibodies to the extracellular domains of the wild-type TGFβ type II receptor, the use of a signaling-competent native receptor with an amino terminal HA tag made possible the verification of this targeting data in the context of the wild-type receptor backbone. The HA-tagged full-length human type II receptor (HA-T2R-WT) displayed a staining profile identical to the chimeric βII receptor, localizing specifically to the basolateral membrane domains in both MDCK cells and the alternative NMuMG epithelial cell line (Figure 5, A and B). To investigate the functionality of the C-terminal basolateral targeting domain in the wild-type backbone, a truncation mutant lacking the LTAxxVAxxF region was constructed (HA-T2RΔ527) analogous to βIIΔ527 (Figure 2B). Stable MDCK clones expressing T2RΔ527 yielded significant staining on both the apical and basal membrane domains (Figure 5A), identical to that shown with the chimeric equivalent (βIIΔ527; Figure 2B). To further verify the results obtained with the chimeric mutants, native T2Rs were engineered containing the key point mutations described in Figure 3. As presented in Figure 5C, all the endogenous receptor mutants reflected identical expression profiles as reported for the chimeric equivalents (Figure 3A). Specifically L529, T530, A531, V534, A535, and F538 were observed to mislocalize to the apical domains, whereas the R528 and wild-type receptors correctly localized to the basolateral domains (Figure 5C).
Figure 5.
Chimeric and endogenous type II TGFβ receptors similarly localize and induce Smad phosphorylation. (A) Stable MDCK clones expressing either the HA-tagged wild-type (HA-T2R) or amino acid 527-truncated (T2RΔ527) native T2R were generated and stained using the primary HA antibody (12CA5) and the Cy3-tagged secondary (red). (B) Expression of the chimeric βII (βIIR) and wild-type HA-T2R in fully polarized NMuMG epithelial cells. Six hours after transient transfection cells were stained for chimeric (βIIR) or native (HA-T2R) receptor expression by using the GM-CSF β or HA (12CA5) primary antibodies, respectively, and secondary Cy3 (red). (C) Native WT or containing the indicated point mutation HA-tagged native type II receptors were transiently transfected into fully polarized MDCK cells. Receptor expression was determined 5 h later using 12CA5 as described, and expression is represented as perpendicular XZ cross-sectional images. Nuclei in panels A-C were additionally stained with DAPI. (D) DR26 epithelial cells (do not express T2Rs) were used directly (control) or transfected with the indicated point mutant or wild-type (WT) T2Rs depicted in C. Cultures were left untreated (−) or stimulated (+) with 10 ng/ml TGFβ1 for 45 min before being processed for Western analysis (150 μg). After protein transfer, the membranes were sequentially probed for phospho-Smad2 (top) and type II receptor expression (bottom) before stripping and reprobing with a total-Smad2/3 antibody (middle) to control for protein loading. Identical results are obtained with the analogous chimeric T2R mutants (data not shown).
An important consideration was whether the C-terminal basolateral-targeting domain was coincident or distinct from elements regulating TGFβ signaling. To address that question, DR26 cells (a mink lung epithelial cell line lacking expression of the T2R) were transfected with the receptor point mutants shown to mislocalize receptor expression, and Smad2 phosphorylation was examined (Figure 5D). Because an arginine to alanine point mutation at residue 528 had been reported to lack Smad signaling (Loeys et al., 2005), it was used as a control to study the signaling activity of the other mutants. As expected, the R528 mutation (which shows wild-type trafficking) was unable to induce Smad2 phosphorylation after addition of TGFβ (Figure 5D). However, receptor point mutants at amino acids 529, 530, 531, 534, or 535 (which missort to the apical membrane) all activated Smad2 to a similar degree as wild-type receptors. Of the six critical trafficking residues in the C-terminal targeting domain, only the phenylalanine-to-alanine mutation at amino acid 538 impacted basolateral receptor localization as well as TGFβ-dependent Smad2 and Smad3 signaling (Figure 5; data not shown). Identical signaling activity was observed for the chimeric receptor point mutants (data not shown). These findings suggest that LTAxxVAxxF-directed T2R basolateral trafficking and Smad phosphorylation are independent activities controlled by the TGFβ receptor complex.
The T2R Basolateral Targeting Domain Is Dominant over the Influenza Virus HA Protein Apical-localizing Signal
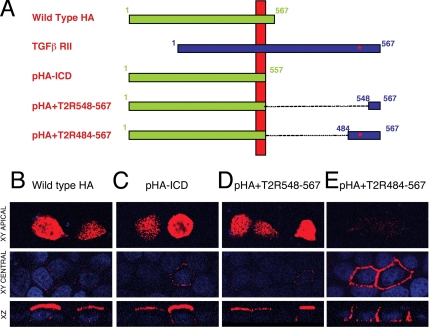
To determine whether the C-terminal region identified in the T2R could provide basolateral targeting to an exogenous protein, the domain was placed at the termini of the influenza HA protein. Because the HA protein localizes specifically to the apical domain of polarized epithelial cells, we fused the T2R 484-567 (contains the targeting motif) and 548-567 (lacks targeting motif) terminal fragments, described in Figure 3C, to the transmembrane domain of the HA protein. The resultant constructs (pHA+T2R484-567 and pHA+T2R548-567, respectively), along with wild-type HA or an HA control lacking the intracellular domain (pHA-ICD) were then transiently expressed in polarized MDCK cells (Figure 6). Wild-type HA or the HA control lacking the intracellular domain (pHA-ICD) were demonstrated to stain exclusively at the apical regions of the plasma membrane (Figure 6, B and C). Similarly, staining for the HA hybrid protein containing the 548–567 T2R sequence (pHA+T2R548-567; which lacks the basolateral targeting domain) was restricted to the apical locale (Figure 6D). However, the HA construct expressing the 484-567 T2R sequence (pHA+T2R484-567; containing the T2R targeting region) was observed to be restricted to the basolateral membranes with a distinct absence of staining at the apical surfaces (Figure 6E). Thus, these data provide evidence that the T2R C-terminal domain is 1) sufficient in directing basolateral localization outside the context of the TGFβ receptor and 2) dominant over the apical targeting functions of the influenza HA protein.
Figure 6.
The TGFβ type II receptor basolateral localizing signal is dominant over the apical targeting signal in the influenza virus HA protein. (A) Influenza virus HA protein chimeras were constructed with terminal fragments of the T2R fused to the HA transmembrane domain. The full-length type II receptor (TGFβ RII) is provided for orientation and the location of the identified targeting sequence is indicated by a red *. (B–E) Fully polarized MDCK cell monolayers were transfected for 5 h with plasmids expressing either wild-type HA, an ICD-deleted HA protein (pHA-ICD), or chimeras consisting of pHA-ICD expressing type II receptor terminal fragments 548-567 or 484-567 (pHA+T2R548-567 and pHA+T2R484-567, respectively). Receptors and nuclei were visualized as described. Images are represented as horizontal XY flat sections focused at either the apical surface (top) or the central nuclei plains (middle), above lower perpendicular XZ cross sections through the plain of the cell.
Basolateral Delivery of the T2R Is Direct and Not Due to Tethering to Basolateral-specific Structures
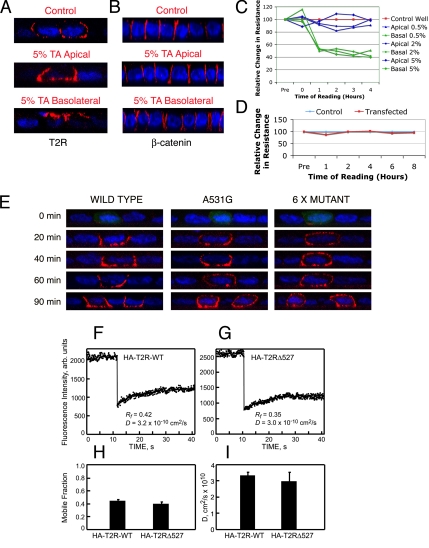
To establish whether receptor targeting to the basolateral surface is direct and does not depend on transient delivery to the apical membrane, tannic acid was selectively applied to either the apical or basolateral transwell reservoirs of polarized MDCK cells. Because tannic acid is a weak cell-impermeable fixative that does not diffuse across tight junctions (Polishchuk et al., 2004; Paladino et al., 2006), this will prevent vesicle docking only at the treated locale in the timeframe used (Paladino et al., 2006). As shown in Figure 7A, application of 5% tannic acid to the apical domains had no effect on receptor localization. However, when tannic acid was applied to the basolateral reservoir, the T2R was observed to relocalize to the apical surfaces, indicative of a default apical trafficking mechanism in the absence of basolateral membrane delivery. Similar results were obtained using 0.5 or 2% tannic acid, and fixation of both apical and basolateral membrane domains eliminated all receptor surface expression (data not shown). Although these findings indicate the absence of a requirement for transient apical T2R delivery before basolateral membrane expression, the effect of tannic acid and transient transfection on monolayer integrity was further examined. Localization of the lateral marker β-catenin was unaffected by the tannic acid treatment, indicating a retention of cellular polarity (Figure 7B). However, similarly to that reported by Paladino et al. (2006), although tannic acid rapidly depolarized MDCK cell monolayers upon addition to the basolateral reservoirs, it had no effect on polarity when applied to apical reservoirs (Figure 7C). An additional concern relating to membrane targeting and integrity was whether the transepithelial resistance was altered after transient transfection. No effect on resistance was observed over an extended 8-h transfection period (Figure 7D).
Figure 7.
The type II TGFβ receptor traffics directly to the basolateral membrane and loss of the targeting signal does not affect lateral membrane diffusion. Fully polarized MDCK cell monolayers on 12-mm transwells were transfected with chimeric βII receptors and incubated at 37°C for 3 h. The cells were then transferred to 18°C for 3 h before incubation with 5% tannic acid (TA) for 10 min in the apical or basolateral reservoirs as labeled. Cells were then washed three times with PBS before returning to 37°C for 1 h in full medium before cell-surface receptor (A) or β-catenin (B) staining as described. Monolayers were additionally monitored for any change in transepithelial resistance after tannic acid (C) or transfection (D) treatments for the times specified. (E) Polarized MDCK cell monolayers transfected with the wild-type, A531G point mutant, or 6 x Mutant βII receptors were stained for cell-surface receptor expression at the indicated times after 37°C release from Golgi block. (F–I) FRAP studies on the lateral diffusion of HA-T2R-WT and HA-T2RΔ527 in MDCK cells. Cells were transfected with expression vectors for the above-mentioned proteins, and the cell-surface receptors were labeled in the cold by fluorescent monovalent Fab′ (see Materials and Methods). FRAP studies were conducted at 16°C in HBSS/HEPES/BSA. (F and G) Typical FRAP curves depicting the lateral diffusion of HA-T2R-WT (F) and HA-T2RΔ527 (G). Solid lines are the best fit to the lateral diffusion equation (Petersen and Elson, 1986). (H and I) Average Rf and D values, respectively, derived from multiple FRAP measurements (mean ± SEM of 30–40 measurements in each case). No significant differences were detected between HA-T2R-WT and HA-T2RΔ527 in either Rf (p > 0.1, Student's t test) or D (p > 0.2).
To further differentiate between initial apical sorting and direct delivery to the basolateral membranes (without the potential complications of chemical treatment), we examined the appearance of the T2R in the different domains at early time points after transfection of the wild-type or a mislocalized chimeric receptor construct. Weak receptor expression was detectable at the cell surface as early as 2 h posttransfection in a small number of cells (data not shown). As such, to control the timing of receptor delivery to the cell surface, transfected cells were initially incubated at 37°C for 3 h before initiating an 18°C Golgi block and removal of any early receptor surface expression by dilute trypsin. This protocol was found to have no effect on monolayer polarity during the time frame tested (data not shown). Cell-surface receptor expression was then evaluated by immunofluorescence staining at various times after 37°C release (Figure 7E). Although the wild-type βII receptor was only observed at the basolateral surfaces (Figure 7E, left), the A531G missorted mutant receptor (Figure 3A) showed significant apical staining at the earliest detectable time point (Figure 7E, middle). In further support of these findings, an additional mutant receptor was constructed with the six reported targeting amino acids mutated: LTAxxVAxxF to AAGxxAGxxA (6 x Mutant). Release of this 6 x Mutant after 2-h Golgi block similarly resulted in mislocalization to the apical domains (Figure 7E, right).
To investigate the mechanism that targets the T2R to the basolateral surface, we used FRAP studies, comparing the lateral diffusion of the wild-type receptor (HA-T2R-WT) and a missorted mutant truncated just before the targeting signal (HA-T2RΔ527) (Figure 5A). This experiment was designed to differentiate between involvement of the targeting signal in intracellular delivery to the basolateral membrane as opposed to a retention role, where the receptor is tethered at the basolateral membrane after its arrival. In the latter case, one expects the lateral diffusion of the mutant lacking the targeting signal to be less restricted. In the experiments shown in Figure 7, F–I, MDCK cells transiently transfected with either HA-T2R-WT or HA-T2RΔ527 were labeled externally in the cold (to avoid internalization) by fluorescent monovalent Fab′ fragments; the cells were then shifted to 16°C, and the lateral diffusion of the labeled receptors in the plasma membrane was measured by FRAP within 15 min. Typical FRAP curves are depicted in Figure 7, F and G, and the average data derived from multiple measurements on different cells are shown in Figure 7, H and I. The results demonstrate similar lateral diffusion parameters (D and Rf) for the wild-type and mutant receptors. Similar results were obtained on MDCK cell lines stably expressing these receptors. Moreover, analogous experiments on COS7 cells transfected with the same receptor constructs (data not shown) revealed no significant differences between HA-T2R-WT and HA-T2RΔ527, which diffused similar to each other albeit with a somewhat higher lateral mobility than in MDCK cells (D ∼4.5 × 10−10 cm2/s; Rf ∼0.60). We conclude that there are no significant differences between the lateral diffusion of the two receptors, suggesting the lack of a significant contribution of interactions with membrane protein coats and assemblies, which would be expected to retard preferentially the lateral diffusion of the wild-type receptor but not of the HA-T2RΔ527 mutant lacking the targeting signal. These findings are in line with the notion that the basolateral sorting of the T2R occurs intracellularly and that it does not depend on “tethering” interactions at the plasma membrane. Together, the results of Figure 7 suggest that 1) T2Rs traffic directly to the basolateral surface, independently of transient apical delivery; 2) the C-terminal targeting domain functions to localize receptors to the basolateral membrane; and 3) receptor overexpression can saturate the cellular targeting machinery and result in overflow to a default apical trafficking pathway.
DISCUSSION
Epithelial cells are tightly controlled by TGFβ with a loss of ligand responses resulting in aberrant growth and potentially malignant transformation. Because epithelial cells contain distinct apical and basolateral domains, correct expression and membrane localization of cell-surface receptors is crucial to appropriately respond to environmental cues. In that regard, we previously demonstrated that both the type I and type II TGFβ receptors localized specifically to the basolateral domains of polarized epithelial cells (Murphy et al., 2004). Because TGFβ receptor(s) mislocalization could result in either the activation or dampening of necessary regulatory signals, the current study was designed to identify the cis-acting elements in the T2R mediating basolateral delivery.
The presence of a basolateral targeting signal(s) in the C-terminal of the T2R was initially indicated by apical mislocalization after receptor truncation to the transmembrane domain (Figure 1D). Inclusion of additional receptor sequence and further truncation analyses indicated that a motif directing basolateral receptor expression was localized near amino acid 537 (Figures 1 and 2). Although truncation studies can provide initial information to address this question, to eliminate confounding effects due to receptor folding, multiple elements, or both, trafficking studies were performed on full-length T2Rs in which single or double point mutations were made spanning residues 525-546. Confocal microscopy showed that a region spanning amino acids 529-538 (i.e., L529, T530, A531, V534, A535, and F538) was essential for correct basolateral localization (Figure 3). Although T530, A531, A535, and F538 mutations resulted in apical receptor mislocalization in almost every cell studied, mutations at L529 and V534 were less pronounced. Together, these findings support a basolateral-targeting domain for the TGFβ type II receptor involving essential amino acids 529LTAxxVAxxF538.
Removal of a basolateral signal often results in selective apical localization, indicating that a silenced determinant is revealed that can direct apical transport in the absence of basolateral signals (Mostov et al., 1992; Matter and Mellman, 1994). Alternatively, loss of such a motif can result in missorting to both domains in a nonpolarized manner (Aroeti et al., 1998), suggestive of a nonpolarized default mechanism. For the T2R studied here, the later scenario seems more apt, because mutation or deletion of the reported basolateral domain resulted in receptor distribution over both apical and basolateral surfaces. Moreover, overexpression of the T2R or tannic acid fixation of the basolateral membranes results in mislocalization to the apical domains (Figure 7A; data not shown). These findings indicate that in the absence of the targeting domain a default mechanism for receptor delivery is used that has little preference for either the apical or basolateral surfaces, and they suggest a model whereby the T2R contacts the basolateral sorting machinery before (presumably in the Golgi) being sorted via a “random-trafficking” mechanism. Receptor overexpression would saturate those factors regulating basolateral delivery and result in expression at both the basolateral and apical membranes.
The basolateral targeting signal that we present in this article (529LTAxxVAxxF538) bears no similarity to any previously reported motif (Aroeti et al., 1998), and canonical tyrosine and dileucine motifs were demonstrated not to be involved (Figure 1). In addition, distribution of the element over 10 amino acids is a feature common to a number of published basolateral trafficking motifs (Aroeti et al., 1998). However, because bipartite basolateral sorting signals are not unusual (Matter et al., 1992; Madrid et al., 2001; Simmen et al., 2002), it was critical to determine whether additional elements more internal in the receptor sequence might function in concert with the 529–538 site. To that end, internal truncation mutants were constructed deleting the majority of the receptor from the transmembrane domain to amino acid 483. This internally truncated receptor (as well as additional internal deletions closer to the 529-538 motif) showed basolateral retention similar to the full-length receptor (Figure 3C; data not shown). Furthermore, introduction of a point mutation at amino acid 531 (A531G), a site shown to be critical for appropriate targeting in the context of the full-length native or chimeric receptor (Figures 3A, 4B, 5C, and 7E), similarly mislocalized the central-deleted receptor (Figure 3D). Together, these results map a basolateral targeting motif centered on the six essential amino acids 529LTAxxVAxxF538. Although mutations spanning residues 525-548 (Figures 3A and 5; data not shown), together with truncations (Figures 1–3) and central deletions (Figure 3C; data not shown) have defined the primary amino acids regulating basolateral T2R delivery, it is still possible that residues adjacent to the mutated region have additional functions. Specifically, although the receptor truncations (Figure 2) effectively map the C-terminal end of the motif, the N-terminal region is less tightly defined. For example, although the βIIΔ198-508 and βIIΔ198-518 central deletion constructs maintained basolateral targeting in the majority of cells, deletion of 10 additional amino acids (βIIΔ198-528) resulted in a T2R similarly expressed on all membrane surfaces (data not shown). This loss of function as central deletions approached the 529-538 region indicates a critical structural and/or spacing requirement. Consistent with that hypothesis is the data provided in Figure 3D where mutation of A531G in βIIΔ198-483 resulted in both apical and basolateral receptor expression. However, it is possible that additional amino acids in the 508–524 region impart other function(s) for appropriate T2R targeting, which requires further investigation.
As an additional means to define the membrane locale of the T2R in polarized epithelial cells, biotinylation of cell-surface receptors was performed (Figure 4). The wild-type chimeric βII receptor was observed to be selectively biotinylated upon exposure of biotin cross-linking reagents to the basolateral domain (Figure 4A, bottom). As observed in the confocal Z-sections (Figure 4A, top), negligible apically localized receptor was detected. However, significant apical labeling was very significant for the A531G point mutants and βIIΔ537 truncation mutants (Figure 4, B and C). The degree of biotinylation for the A531G point-mutant was consistently observed to be less than that for the βIIΔ537 truncation-mutant. Whether this reflects a nonquantitative aspect of biotinylation or a degree of compensation from other residues within the LTAxxVAxF domain is unknown and is currently being investigated. In support of the latter hypothesis is the faster and more extensive population of the apical domains by the 6 x Mutant (i.e., mutations in all effector residues) compared with the A531G point-mutant (Figure 7E; data not shown).
Because no additional sequences necessary for T2R targeting were identified, we next examined the relation of the LTAxxVAxxF domain in the context of an apically directed protein. The hierarchy of trafficking signals is highlighted by the retargeting of some basolateral proteins to the apical membranes upon deletion of basolateral sorting elements (Mostov et al., 1992; Matter and Mellman, 1994). Although this could indicate a dominance of basolateral trafficking mechanisms to those mediating apical delivery, it could also result from cargo first encountering and being sequestered by the basolateral trafficking machinery. Although these possibilities are difficult to distinguish, an additional approach to verify the presence of a basolateral sorting signal is to incorporate the motif into an apically localized transmembrane protein such as the influenza HA protein. Although basolateral signals are not always dominant (Jacob et al., 1999), expression of a hybrid HA protein with the T2R terminal 84 amino acids (to ensure proper folding and containing the LTAxxVAxxF sequence) fused to its transmembrane domain was now efficiently relocated to basolateral membrane surfaces (Figure 6).
The extensive heterogeneity observed in the primary sequence of reported basolateral targeting signals, even for those dependent on identical trafficking machinery, indicates a role for secondary structure in domain function (Aroeti et al., 1998). Two-dimensional NMR analysis of synthetic peptides containing known tyrosine-based clathrin-coated pit-interacting motifs suggests that tyrosine residues often lie within a conserved structural feature, either a tight turn (Vaux et al., 1992) or a surface loop (Ktistakis et al., 1990; Trowbridge et al., 1993). Similarly, tyrosine- and nontyrosine-based basolateral signals are frequently characterized by β turns or loop-like secondary structures such that mutation prevents appropriate interaction with the trafficking machinery (Aroeti et al., 1993; Reich et al., 1996; Aroeti et al., 1998). In that regard, although both Chou-Fasman and Garnier-Osguthorpe-Robson algorithms predict β turn structures within the T2R motif region (which can be affected by base mutations), their significance requires further investigation.
The basolateral targeting domain of the T2R was found to be distinct, but it shows some overlap with a region essential for activation of Smad2 and Smad3 (Figure 5D; data not shown). Specifically, R528 and F538 were determined to be essential for Smad signaling. Further studies demonstrated that E526 and R537 were also necessary for Smad phosphorylation (data not shown). Although the requirement for E526, R528, or R537 has been linked to diseases affecting cardiovascular, craniofacial, neurocognitive, and skeletal development (Tanaka et al., 2000; Mizuguchi et al., 2004; Loeys et al., 2005), a similar role for F538 has not, as yet, been reported. Thus, of the four amino acids (E526, R528, R537, or F538) shown to regulate TGFβ Smad signaling, only one amino acid (F538) was similarly required to direct basolateral receptor targeting. These findings support the proposition that polarized T2R trafficking and Smad protein activation are coupled but uniquely regulated.
Apical tannic acid fixation indicated that T2Rs traffic directly to the basolateral membrane without transient apical localization (Figure 7A). Direct intracellular sorting was also implicated recently in the transport of GPI-anchored proteins in polarized MDCK cells to the other pole, i.e., the apical surface (Paladino et al., 2006). Although apical addition of tannic acid had no effect on cell polarity, basolateral treatment resulted in receptor relocation to apical membrane domains and rapid depolarization of the cell monolayer (Figure 7, A and C). Although monolayer polarity was lost after basolateral tannic acid, diffusion of β-catenin between the apical and basolateral compartments did not occur (Figure 7, B and C). The retention of lateral β-catenin staining in the absence of monolayer polarity differs from that reported for the tight junctional marker Pals1-associated tight junction protein (Paladino et al., 2006), the significance of which is unknown but may reflect differences in the manner by which proteins are tethered at various locales. However, of particular note for the current study, further validation of direct basolateral trafficking was provided by the kinetic analysis of T2R plasma membrane delivery after release from Golgi block (Figure 7E). No evidence for apical membrane expression could be detected, even at the earliest time points (20 min). These findings are highly suggestive of a basolateral trafficking mechanism where the targeting signal on the receptor contacts the basolateral sorting machinery in an intracellular compartment (i.e., the Golgi). In support of this notion, the FRAP studies (Figure 7, F–I), which measure the lateral diffusion of the receptors at the plasma membrane of live cells, seem to exclude a significant contribution of tethering interactions with sorting coats at the plasma membrane. Receptor overexpression, resulting in saturation of the trafficking machinery, or loss of the LTAxxVAxxF targeting domain can redirect the receptor to an even distribution between the apical and basolateral domains.
In summary, we present evidence that the C-terminal domain of the type II TGFβ receptor 1) directs basolateral targeting of endogenous and chimeric T2Rs in MDCK and NMuMG epithelial cells; 2) contains a unique element, 529LTAxxVAxxF538, which is necessary for basolateral expression; 3) is dominant to the apical targeting activity of the influenza HA protein; 4) regulates basolateral delivery intracellularly, before T2R arrival at the cell surface; and 5) overlaps with a signaling motif essential for ligand-mediated Smad signaling. Current studies are focusing on determining whether a similar acting motif functions in the type I receptor as well as defining the interacting cellular machinery.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants GM-54200 and GM-55816 from the National Institutes of General Medical Sciences and the Mayo Foundation (to E.B.L.) and by grants from the Israel Science Foundation (grant 185/05) and the Israel Cancer Research Fund (to Y.I.H.). Y.I.H. is an incumbent of the Zalman Weinberg Chair in Cell Biology.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-10-0930) on July 18, 2007.
REFERENCES
- Anders R. A., Arline S. L., Dore J. J., Leof E. B. Distinct endocytic responses of heteromeric and homomeric transforming growth factor β receptors. Mol. Biol. Cell. 1997;8:2133–2143. doi: 10.1091/mbc.8.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders R. A., Dore J. J., Jr, Arline S. L., Garamszegi N., Leof E. B. Differential requirement for type I and type II transforming growth factor β receptor kinase activity in ligand-mediated receptor endocytosis. J. Biol. Chem. 1998;273:23118–23125. doi: 10.1074/jbc.273.36.23118. [DOI] [PubMed] [Google Scholar]
- Anders R. A., Leof E. B. Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-beta (TGF-β) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-β signaling. J. Biol. Chem. 1996;271:21758–21766. doi: 10.1074/jbc.271.36.21758. [DOI] [PubMed] [Google Scholar]
- Aroeti B., Kosen P. A., Kuntz I. D., Cohen F. E., Mostov K. E. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J. Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B., Okhrimenko H., Reich V., Orzech E. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim. Biophys. Acta. 1998;1376:57–90. doi: 10.1016/s0304-4157(98)00005-7. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassing C. H., Yingling J. M., Wang X. F. Receptors for the TGF-beta ligand family. Vitam. Horm. 1994;48:111–156. doi: 10.1016/s0083-6729(08)60497-5. [DOI] [PubMed] [Google Scholar]
- Bissell D. M. Chronic liver injury, TGF-beta, and cancer. Exp. Mol. Med. 2001;33:179–190. doi: 10.1038/emm.2001.31. [DOI] [PubMed] [Google Scholar]
- Blobe G. C., Schiemann W. P., Lodish H. F. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Dell'Angelica E. C. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Chen W., Kirkbride K. C., How T., Nelson C. D., Mo J., Frederick J. P., Wang X. F., Lefkowitz R. J., Blobe G. C. beta-Arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Shmuely A., Henis Y. I. A single internalization signal from the di-leucine family is critical for constitutive endocytosis of the type II TGF–beta receptor. J. Cell Sci. 2001;114:1777–1786. doi: 10.1242/jcs.114.9.1777. [DOI] [PubMed] [Google Scholar]
- Folsch H., Ohno H., Bonifacino J. S., Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989;8:3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L., Wells R. G., Lodish H. F., Henis Y. I. Oligomeric structure of type I and type II transforming growth factor beta receptors: homodimers form in the ER and persist at the plasma membrane. J. Cell Biol. 1998;140:767–777. doi: 10.1083/jcb.140.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T., Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Hartsough M. T., Mulder K. M. Transforming growth factor-beta signaling in epithelial cells. Pharmacol. Ther. 1997;75:21–41. doi: 10.1016/s0163-7258(97)00020-x. [DOI] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc. Natl. Acad. Sci. USA. 1990;87:9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y. I., Moustakas A., Lin H. Y., Lodish H. F. The types II and III transforming growth factor-beta receptors form homo-oligomers. J. Cell Biol. 1994;126:139–154. doi: 10.1083/jcb.126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert M. E., Kil S. J., Medof M. E., Carlin C. R. The cytoplasmic juxtamembrane domain of the epidermal growth factor receptor contains a novel autonomous basolateral sorting determinant. J. Biol. Chem. 1997;272:32901–32909. doi: 10.1074/jbc.272.52.32901. [DOI] [PubMed] [Google Scholar]
- Hocevar B. A., Brown T. L., Howe P. H. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S., Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J. Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe P. H., Draetta G., Leof E. B. Transforming growth factor beta 1 inhibition of p34cdc2 phosphorylation and histone H1 kinase activity is associated with G1/S-phase growth arrest. Mol. Cell. Biol. 1991;11:1185–1194. doi: 10.1128/mcb.11.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13:2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Mellman I. Relationships between sorting in the exocytic and endocytic pathways of MDCK cells. Semin. Cell Biol. 1991;2:397–410. [PubMed] [Google Scholar]
- Ikonen E., Simons K. Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin. Cell Dev. Biol. 1998;9:503–509. doi: 10.1006/scdb.1998.0258. [DOI] [PubMed] [Google Scholar]
- Jacob R., Preuss U., Panzer P., Alfalah M., Quack S., Roth M. G., Naim H., Naim H. Y. Hierarchy of sorting signals in chimeras of intestinal lactase-phlorizin hydrolase and the influenza virus hemagglutinin. J. Biol. Chem. 1999;274:8061–8067. doi: 10.1074/jbc.274.12.8061. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys. J. 1976;16:1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel H. Quantitative immunoelectrophoresis in routine clinical diagnosis. Wien Med. Wochenschr. Suppl. 1976;35:3–10. [PubMed] [Google Scholar]
- Ktistakis N. T., Thomas D., Roth M. G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J. Cell Biol. 1990;111:1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Thyagarajan T., Letterio J. J. Function of cytokines within the TGF-beta superfamily as determined from transgenic and gene knockout studies in mice. Curr. Mol. Med. 2002;2:303–327. doi: 10.2174/1566524024605699. [DOI] [PubMed] [Google Scholar]
- Laiho M., Weis F. M., Boyd F. T., Ignotz R. A., Massagué J. Responsiveness to transforming growth factor-beta (TGF-β) restored by genetic complementation between cells defective in TGF-β receptors I and II. J. Biol. Chem. 1991;266:9108–9112. [PubMed] [Google Scholar]
- Laiho M., Weis M. B., Massagué J. Concomitant loss of transforming growth factor (TGF)-β receptor types I and II in TGF-β-resistant cell mutants implicates both receptor types in signal transduction. J. Biol. Chem. 1990;265:18518–18524. [PubMed] [Google Scholar]
- Le Gall A. H., Powell S. K., Yeaman C. A., Rodriguez-Boulan E. The neural cell adhesion molecule expresses a tyrosine-independent basolateral sorting signal. J. Biol. Chem. 1997;272:4559–4567. doi: 10.1074/jbc.272.7.4559. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992;68:775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Loeys B. L., et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- Lopez-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massagué J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67:785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Madrid R., Le Maout S., Barrault M. B., Janvier K., Benichou S., Merot J. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J. 2001;20:7008–7021. doi: 10.1093/emboj/20.24.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K. Epithelial polarity: sorting out the sorters. Curr. Biol. 2000;10:R39–R42. doi: 10.1016/s0960-9822(99)00256-0. [DOI] [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K., Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr. Opin. Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Matter K., Yamamoto E. M., Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J. Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K. C., Khromykh T., Christy P., Le T. L., Gottardi C. J., Yap A. S., Stow J. L., Teasdale R. D. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC–PK1 epithelial cells. J. Biol. Chem. 2001;276:22565–22572. doi: 10.1074/jbc.M101907200. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T., et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat. Genet. 2004;36:855–860. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K., Apodaca G., Aroeti B., Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J. Cell Biol. 1992;116:577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. J., Dore J. J., Edens M., Coffey R. J., Barnard J. A., Mitchell H., Wilkes M., Leof E. B. Differential trafficking of transforming growth factor-β receptors and ligand in polarized epithelial cells. Mol. Biol. Cell. 2004;15:2853–2862. doi: 10.1091/mbc.E04-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G., Trowbridge I. S. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J. Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto C. T., Shia S. P., Bird C., Mostov K. E., Roth M. G. The cytoplasmic domain of the polymeric immunoglobulin receptor contains two internalization signals that are distinct from its basolateral sorting signal. J. Biol. Chem. 1992;267:9925–9932. [PubMed] [Google Scholar]
- Paladino S., Pocard T., Catino M. A., Zurzolo C. GPI-anchored proteins are directly targeted to the apical surface in fully polarized MDCK cells. J. Cell Biol. 2006;172:1023–1034. doi: 10.1083/jcb.200507116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O., Elson E. L. Measurement of diffusion and chemical kinetics by fluorescence photobleaching recovery and fluorescence correlation spectroscopy. Methods Enzymol. 1986;130:454–484. doi: 10.1016/0076-6879(86)30021-1. [DOI] [PubMed] [Google Scholar]
- Polishchuk R., Di Pentima A., Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat. Cell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- Reich V., Mostov K., Aroeti B. The basolateral sorting signal of the polymeric immunoglobulin receptor contains two functional domains. J. Cell Sci. 1996;109:2133–2139. doi: 10.1242/jcs.109.8.2133. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Kreitzer G., Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Serini G., Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp. Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- Shi Y., Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Simmen T., Honing S., Icking A., Tikkanen R., Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- Simmen T., Nobile M., Bonifacino J. S., Hunziker W. Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol. Cell. Biol. 1999;19:3136–3144. doi: 10.1128/mcb.19.4.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Mori M., Mafune K., Ohno S., Sugimachi K. A dominant negative mutation of transforming growth factor-beta receptor type II gene in microsatellite stable oesophageal carcinoma. Br. J. Cancer. 2000;82:1557–1560. doi: 10.1054/bjoc.1999.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Dijke P., Goumans M. J., Itoh F., Itoh S. Regulation of cell proliferation by Smad proteins. J. Cell. Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Brewer C. B., Roth M. G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J. Biol. Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- Thomas D. C., Roth M. G. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J. Biol. Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1992;360:372. doi: 10.1038/360372a0. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Lin H. Y., Ng-Eaton E., Downward J., Lodish H. F., Weinberg R. A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991;67:797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Wilkes M. C., Murphy S. J., Garamszegi N., Leof E. B. Cell-type-specific activation of PAK2 by transforming growth factor beta independent of Smad2 and Smad3. Mol. Cell. Biol. 2003;23:8878–8889. doi: 10.1128/MCB.23.23.8878-8889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Carcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Yao D., Ehrlich M., Henis Y. I., Leof E. B. Transforming growth factor-β receptors interact with AP2 by direct binding to β2 subunit. Mol. Biol. Cell. 2002;13:4001–4012. doi: 10.1091/mbc.02-07-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J., Mulder K. M. Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol. Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]