Abstract
We have investigated the cellular responses to hydrostatic pressure by using the fission yeast Schizosaccharomyces pombe as a model system. Exposure to sublethal levels of hydrostatic pressure resulted in G2 cell cycle delay. This delay resulted from Cdc2 tyrosine-15 (Y-15) phosphorylation, and it was abrogated by simultaneous disruption of the Cdc2 kinase regulators Cdc25 and Wee1. However, cell cycle delay was independent of the DNA damage, cytokinesis, and cell size checkpoints, suggesting a novel mechanism of Cdc2-Y15 phosphorylation in response to hydrostatic pressure. Spc1/Sty1 mitogen-activated protein (MAP) kinase, a conserved member of the eukaryotic stress-activated p38, mitogen-activated protein (MAP) kinase family, was rapidly activated after pressure stress, and it was required for cell cycle recovery under these conditions, in part through promoting polo kinase (Plo1) phosphorylation on serine 402. Moreover, the Spc1 MAP kinase pathway played a key role in maintaining cell viability under hydrostatic pressure stress through the bZip transcription factor, Atf1. Further analysis revealed that prestressing cells with heat increased barotolerance, suggesting adaptational cross-talk between these stress responses. These findings provide new insight into eukaryotic homeostasis after exposure to pressure stress.
INTRODUCTION
Within the biosphere, hydrostatic pressure (HP) can range over 4 orders of magnitude, from 0.1 MPa at the surface to 110 MPa in the deep-sea Mariana Trench in the Pacific Ocean. Yet, how organisms survive and adapt to pressure stress is largely unknown. Among eukaryotes, it has been found that exposure of the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe to increased HP resulted in reduced growth rates, whereas exposure to higher levels caused loss of viability (Sato et al., 1996). Detailed ultrastructural analysis of S. cerevisiae, Candida tropicalis, and S. pombe revealed the nuclear membranes, microtubules, and microfilaments of these organisms to be susceptible to pressure stress (Sato et al., 1995, 1996). In S. cerevisiae, actin cables disappeared after high HP, and this was associated with a delay in cell budding (Kawarai et al., 2006). Moreover, exposure of both S. cerevisiae and S. pombe to high HP was found to result in polyploidy (Hamada et al., 1992, 1996). Effects of pressure stress on the cell cycle have also been observed in human cells, which could be reversibly arrested in mitosis by high-pressure nitrous oxide (Rao, 1968).
The mechanisms by which the cell cycle can be modulated in response to stress have been extensively studied. In fission yeast, the G2/M transition is the major control point of the cell cycle, and it is regulated by the highly conserved Cdc2 kinase, which initiates mitosis. Cdc2 kinase activity is regulated through inhibitory tyrosine 15 (Y15) phosphorylation by the activities of the Wee1 and Mik1 tyrosine kinases and the antagonistic Cdc25 and Pyp3 tyrosine phosphatases (for review, see MacNeill and Nurse, 1997). These regulators, in turn, integrate signals from multiple cell cycle checkpoint pathways. The G2/M transition can be delayed in response to unreplicated or damaged DNA by the DNA replication and DNA damage checkpoints, respectively (for review, see Carr, 2002). Central to these checkpoints is the highly conserved phosphoinositol 3-kinase–related protein kinase Rad3, whose activation, by damaged or unreplicated DNA, leads to G2/M arrest by both activation of Wee1 and Mik1 kinases and inactivation of Cdc25 through a number of downstream effector molecules, including the checkpoint kinases Chk1 and Cds1 and the 14-3-3 protein Rad24 (Carr, 2002). Entry into mitosis is also regulated by a cell size checkpoint, where large cells advance entry into mitosis in response to reduced nitrogen, whereas increased nitrogen supply increases the size at which cells enter mitosis (Fantes and Nurse, 1977). This size control checkpoint requires Wee1 (Fantes and Nurse, 1978). Disrupting the actin cytoskeleton, by using latrunculin A, also activates a cell cycle delay in cells below a minimal size; however, this mitotic size control was found to target Cdc25 (Rupes et al., 2001). Mitotic entry is also delayed by the cytokinesis checkpoint through the inactivation of Cdc25, and possibly stabilization of Wee1, in response to cytokinetic defects through a process that requires the septation initiation network, the Cdc14 family phosphatase, Clp1/Flp1, and Rad24 (Trautmann et al., 2001; Wolfe and Gould, 2004; Mishra et al., 2005).
In S. pombe, the stress-activated Spc1 mitogen-activated protein (MAP) kinase pathway (homologous to mammalian p38 MAPK) is activated by a wide range of environmental stresses and is crucial for cell survival after stress (for review, see Toone and Jones, 1998). Environmental stress can be sensed by a conserved multistep phospho-relay module consisting of Mak1, Mak2, and Mak3 (Buck et al., 2001). These sensor kinases transmit the stress signal through the phosphotransmitter Mpr1/Spy1 and the response regulators Mcs4 and Prr1 (Shieh et al., 1997; Aoyama et al., 2000; Nguyen et al., 2000; Greenall et al., 2002). Signaling to the MAP kinase kinase kinase (MAPKKK)'s Wak1 (also known as Wis4 or Wik1) and Win1 phosphorylate and activate the MAPK kinase Wis1, which in turn activates the MAP kinase Spc1 (also known as Sty1 and Phh1) (Shiozaki and Russell, 1995; Samejima et al., 1997). The downstream effector Atf1, which is directly phosphorylated and activated by Spc1, and Pap1, which is indirectly regulated by Spc1, are homologues of the mammalian bZip transcription factors ATF-2 and c-Jun, respectively (Shiozaki and Russell, 1996; Wilkinson et al., 1996; Toone et al., 1998). A number of genes have now been identified that are transcriptionally induced by Atf1 or Pap1 and that function in stress remediation responses (Chen et al., 2003; Paredes et al., 2003). The Spc1 MAP kinase pathway can additionally modulate cell cycle control in response to changes in the extracellular environment (Shiozaki and Russell, 1995; Samejima et al., 1997). Recently mitotic entry was found to be regulated through Spc1-dependent activation of Srk1, which directly phosphorylates and inhibits Cdc25 in response to osmotic stress (Lopez-Aviles et al., 2005). Furthermore, Spc1-dependent phosphorylation of polo kinase (Plo1) was found to promote mitotic commitment under normal conditions as well as cell cycle recovery after heat stress (Petersen and Hagan, 2005).
In this report, we have investigated the cellular responses of S. pombe to hydrostatic pressure. We identify a pressure-induced G2 cell cycle delay that requires both Cdc25 and Wee1. Furthermore, we identify key roles for the Spc1 MAP kinase pathway in both facilitating cell cycle recovery, in part through promoting Plo1 phosphorylation, and in maintaining cell viability after exposure to hydrostatic pressure stress.
MATERIALS AND METHODS
Yeast Strains, Media, and General Techniques
The strains of S. pombe used in this study are listed in Supplemental Table 1. Cells were cultured in complete media (YE5S), synthetic minimal media (EMM2), and sporulation (ME) media, at 30°C as described in Moreno et al., 1991.
Stress Experiments
Exponentially growing cultures of S. pombe cells were subjected to osmotic stress (1 M sorbitol) or oxidative stress (0.5 mM H2O2) agents for 30 min by adding appropriate volumes of a concentrated solution to a prewarmed YE5S media containing the cells at 30°C (Chen et al., 2003). For heat stress, cells were rapidly transferred to 39°C for 30 min before collection and analyzed at the time intervals indicated. Cells were subjected to hydrostatic pressure by using a purpose-built Pressure System, designed on the principle of a previously described pressure apparatus, NKK-ABB (Hamada et al., 1992). A 2-ml collapsible polyethylene tube was filled with yeast suspension, tightly stoppered at one end to avoid air bubbles, and immersed in prewarmed YE5S media within the pressure chamber. Cells were subjected to hydrostatic pressure for times indicated, with compression and decompression times of <1 min, by using a manually operated piston pump (Research & Industrial Instruments, London, United Kingdom). The inner chamber pressure was measured using a calibrated mechanical pressure gauge. After treatment, cells were collected for further investigation. For viability assays, samples were plated in triplicate.
Microscopy
4,6-Diamidino-2-phenylindole (DAPI)-stained cells were visualized using 100× oil immersion lens of an Olympus BX51 (Olympus Optical Co. Ltd., Japan) fluorescence microscope with a 100-W Mercury bulb. Images were captured using a charged-coupled-device camera and Genus software (Applied Imaging, Newcastle, United Kingdom). The presence of interphase or mitotic spindles in green fluorescent protein (GFP)-tubulin–encoding cells (TH1561) was determined by visualizing GFP and DAPI fluorescence of live cells by using Bio-Rad Radiance 2100 confocal laser scanning system attached to a Nikon Diaphot inverted microscope (Nikon, Tokyo, Japan).
Protein Analysis
Western blots were performed as described previously (Gaits et al., 1998). The following primary antibodies were used: polyclonal anti-phospho-Cdc2 (Cell Signaling Technology, Danvers, MA) (1/1000), monoclonal anti-Cdc2 (Norbury Lab, Cancer Research UK [CRUK], London, United Kingdom) (1/800), monoclonal anti-α-tubulin (Nurse Lab, CRUK) (1/5000), monoclonal anti-hemagglutinin (HA) (BAbCO, Cambridge, United Kingdom) (1/1000), monoclonal anti-myc antibody (BAbCO, Cambridge, United Kingdom) (1/1000), and polyclonal anti-phospho-p38 (Cell Signaling Technology) (1/1000). Horseradish peroxidase-conjugated anti-mouse (Promega, Madison, WI) (1/2500) or anti-rabbit (Cell Signaling Technology) (1/2000) antibodies were used as secondary antibodies. Membranes were developed by enhanced chemiluminescence (ECL kit; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). To reduce errors resulting from stripping and reprobing, levels of phospho-Cdc2-Y15 were compared with α-tubulin protein levels before stripping and then to Cdc2 levels after stripping the blot. Quantitation of Western blot signals was performed using Bio-Rad Quantity One software. The Spc1 protein was partially purified from cells bearing an integrated tagged version of spc16HisHA(ura4) in a wild-type background (TH172) or in a wis1 mutant background (TH1728), under denaturing conditions (8 M urea, 0.1 M NaHPO4, 50 mM Tris-HCl, pH 8.0, 1 μM okadaic acid, and Roche Complete EDTA-free protease inhibitor cocktail tablet) by using nickel-nitrilotriacetic acid agarose as described previously (Millar et al., 1995). Precipitated proteins were resolved by SDS-polyacrylamide gel electrophoresis and transferred electrophoretically to Immobilon-P transfer membrane (Millipore, Billerica, MA). Membranes were probed with monoclonal antibody to the HA epitope and with the polyclonal antibody to phospho-p38.
Preparation and Analysis of Synchronous Culture
Cells were synchronized by centrifugal elutriation by using a JE-5.0 elutriation rotor (Beckman Coulter, Fullerton, CA) at 2500 rpm (Walker, 1999). All experiments were internally controlled for possible centrifugal stress during the elutriation procedure (Shiozaki et al., 1998). Elutriated samples were divided and subjected to pressure treatment as described above or left untreated. Aliquots were removed at 15-min intervals and fixed in formaldehyde, and the proportion of mitotic or septated cells was scored.
RESULTS
Effect of Hydrostatic Pressure Stress on Wild-Type S. pombe Viability
To analyze the effects of HP on cell viability, exponentially growing wild-type S. pombe cells (TH9) were subjected to a range of hydrostatic pressures, from 11.8 to 354 MPa, for 10 min by using a purpose-built pressure system (see Materials and Methods). Cells were then plated on standard YE5S agar plates under normal growth conditions (at 30°C for 3 d), and then viability was determined. From these experiments, the maximum pressure dose for which there was no loss in viability in wild-type cells was determined to be 70.8MPa, whereas complete loss of viability was observed after exposure to 177 MPa HP (Figure 1A). The effect of increasing the duration of exposure to pressure stress was also examined, and it was found that longer exposure to 70.8 MPa resulted in greater loss of viability compared with the unstressed cells (Figure 1B). Therefore, 10 min was selected as the optimal time for pressure treatment in subsequent experiments.
Figure 1.
Effect of pressure stress on the viability of S. pombe. (A) Viability of wild-type S. pombe (TH9) and spc1-m13 (TH123) cells (as indicated) in response to different doses of hydrostatic pressure. Unstressed cells were used as a reference for 100% viability. These data are representative of at least three separate experiments. SEs, calculated on a Poisson survival distribution are shown. (B) Viability of wild-type S. pombe (TH9) after exposure to 70.8-MPa HP for the times indicated. Unstressed cells were used as a reference for 100% viability. Graph is representative of at least three separate experiments, and data are expressed as mean ± SEM. *p < 0.05.
Exposure to Pressure Stress Results in G2 Cell Cycle Delay
After exposure of asynchronous wild-type cells to the maximal pressure stress for cell survival at 70.8 MPa, analysis of cell number revealed a delay in cell division (our unpublished data). To examine this further, wild-type S. pombe, synchronized in G2 phase of the cell cycle by centrifugal elutriation, was subjected to either 47.2 or 70.8 MPa of HP for 10 min, and cell cycle progression was assessed by determining the percentage of cells undergoing nuclear division and septation. Little effect on the timing of nuclear division and septation was observed after exposure to 47.2-MPa pressure compared with untreated cells (Figure 2, A and B). In contrast, exposure to 70.8-MPa HP resulted in a delay in both nuclear division and septation by ∼75 min in G2-synchronized wild-type S. pombe compared with that of untreated control cells (Figure 2, C and D). These results confirmed that exposure to maximal pressure stress resulted in a cell cycle delay. Because there were delays in both nuclear division and septation in pressure-treated cells, this suggested that the cell cycle delay was either in late G2 or in early mitosis, before nuclear division. To identify the cell cycle stage targeted by pressure stress, the percentage of cells exhibiting mitotic spindles was determined (see Materials and Methods). A strain (TH1561) containing a GFP-tagged tubulin gene tub2+ under the control of the thiamine-repressible nmt promoter (rep3X) was grown in the absence of thiamine to express GFP-Tub2, and synchronized G2 cells were treated with 70.8-MPa HP for 10 min. Cells exposed to pressure stress exhibited a delay in the formation of mitotic spindles compared with untreated controls, suggesting that pressure-induced cell cycle delay was induced at a point before mitosis (Figure 2E).
Figure 2.
Hydrostatic pressure can induce a G2-specific cell cycle delay. G2-synchronized wild-type S. pombe (TH9) were subjected to 47.2-MPa (A and B) or 70.8-MPa HP (C and D) for 10 min, and percentage of binucleate (top) or septated (bottom) cells was determined at times indicated. Untreated (solid squares) or pressure treated (open circles) cells are shown. Each set of data are representative of at least three separate experiments. (E) G2-synchronized S. pombe encoding GFP-tubulin (TH1561) were subjected to 70.8-MPa HP for 10 min (open circles) or untreated (solid squares), and the percentage of cells exhibiting mitotic spindles was determined at the times indicated after treatment. (F) Western blot analysis of Cdc2-Y15 phosphorylation in untreated or pressure treated (70.8-MPa) G2-synchronized wild-type (TH9) S. pombe cells for the times indicated. Western blot was probed with anti-phospho-Cdc2 (Y15) antibody (top), and then anti-α-tubulin (lowest panel), before stripping and reprobing for anti-Cdc2 (middle) (see Materials and Methods). Data are representative of three separate experiments. Significant values (Student's t test) are indicated (*p < 0.05), relative to percentage of Cdc2-Y15 phosphorylation in untreated cells at the start of the analysis.
S. pombe Cdc2 kinase activity is negatively regulated by Y15 phosphorylation during S and G2 phases of the cell cycle (Gould and Nurse, 1989). Thus, to further assess whether pressure stress resulted in a G2 delay, levels of Cdc2-Y15 phosphorylation were also examined. A culture of wild-type S. pombe was elutriated to obtain synchronous G2 cells of which half of the culture was left untreated, and the other half was subjected to 70.8-MPa HP for 10 min. The relative levels of Cdc2-Y15 phosphorylation were then determined over time in both unstressed and stressed cells by Western blot analysis (see Materials and Methods). Untreated cells showed a gradual decline in Cdc2-Y15 phosphorylation compared with constant levels of Cdc2 protein over time, consistent with cells having begun to enter mitosis after 10-min mock treatment (Figure 2F, compare times 0–60 min, top left and middle). In contrast, the levels of Cdc2-Y15 phosphorylation in pressure-treated cells were significantly elevated for at least 15 min posttreatment compared with unstressed controls, consistent with a G2 delay in these cells (Figure 2F, top right and middle). α-Tubulin protein levels served as loading control (Figure 2F, lowest panel).
Pressure-induced Cell Cycle Delay Is Independent of DNA Integrity, Cytokinesis, or Cell Size Checkpoints
The pressure-induced G2 cell cycle delay could have resulted from activation of the DNA integrity checkpoints through pressure-induced DNA damage. To test whether the pressure-induced cell cycle delay was dependent on the DNA integrity checkpoints, the effect of pressure stress on the cell cycle was examined in G2-synchronized rad3Δ cells (TH146) in which the DNA damage and DNA replication checkpoints are abrogated (al-Khodairy and Carr, 1992; Enoch et al., 1992). This strain was synchronized in G2 by centrifugal elutriation and then subjected to 70.8-MPa HP for 10 min. However, a pressure-induced cell cycle delay was observed in rad3Δ cells compared with unstressed controls (Supplemental Figure 1A). Similar results were obtained for strains lacking chk1+ (TH 451) and rad24+ (TH 380) (Supplemental Figure 1, B and C), indicating that pressure-induced cell cycle delay did not result from activation of the DNA integrity checkpoint pathway. Cdc25 is negatively regulated by phosphorylation in response to unreplicated or damaged DNA and in response to environmental stress (for review, see Karlsson-Rosenthal and Millar, 2006). A DNA integrity checkpoint-deficient mutant cdc25-9A, in which nine serine or threonine phosphorylation sites (at positions 99, 148, 178,192, 204, 206, 226, 234, and 359) are mutated to alanine, and which is poorly phosphorylated by Cds1 or Srk1 (Zeng and Piwnica-Worms, 1999; Lopez-Aviles et al., 2005), still exhibited a cell cycle delay after exposure to 70.8-MPa pressure stress compared with that of unstressed controls (Supplemental Figure 1D). This indicated that Cdc25 phosphorylation by the DNA integrity checkpoints or Srk1 was not required for the delay.
A role for Clp1, which regulates the G2/M transition and coordinates cytokinesis with cell cycle progression was also investigated; however, an abrogation of cell cycle delay in clp1Δ cells (TH1995) was not observed, indicating that Clp1 was not required for the pressure-induced cell cycle delay (Supplemental Figure 1E).
Furthermore, a role for the cell size checkpoint was examined, which delays entry into mitosis until a minimal cell size is reached (Fantes and Nurse, 1977). However, pressure-induced cell cycle delay was still observed in elongated cdc25-22 cells compared with untreated cells, after a shift to the restrictive temperature for 4 h. Thus, the pressure-induced cell cycle delay was unlikely to have resulted from a cell size checkpoint in undersized cells (Supplemental Figure 1F; Rupes et al., 2001).
Pressure-dependent Cell Cycle Delay Requires Both Cdc25 and Wee1
Because pressure stress induced a cell cycle delay in G2, it was possible that pressure stress resulted in inactivation of Cdc25 phosphatase independently of the DNA integrity, cytokinesis, and cell size checkpoints, thereby maintaining Cdc2 kinase in an inactive form. The S. pombe mutant cdc2-3w (TH300) is largely insensitive to Cdc25 inactivation, and it is characterized by a “wee” phenotype (Russell and Nurse, 1987). Thus, to test a possible role for Cdc25 in the pressure-induced cell cycle delay, cell cycle progression was examined in G2-synchronized cdc2-3w cells after exposure to pressure stress (70.8 MPa). A cell cycle delay was observed in pressure-treated cdc2-3w cells compared with that of untreated cells (Figure 3A). Because the pressure-induced cell cycle delay was not abrogated in cdc2-3w cells, this suggested that Cdc25 was either not involved in the cell cycle delay or that Cdc25 did not act independently to cause this delay.
Figure 3.
Pressure-induced cell cycle delay requires both Cdc25 and Wee1. The percentage of binucleate cells was determined at times indicated after exposure of G2-synchronized cells to 70.8-MPa HP for 10 min for (A) cdc2-3w (TH300) and (B) cdc2-1w (TH319). The percentage of binucleate and septated cells was determined for cdc25-22 wee1-50 (TH370) (C and D) and wild-type (TH9) (E and F), at times indicated after exposure of G2-synchronized cells to 70.8-MPa HP for 10 min at 35.5°C. Untreated (solid squares) or pressure treated (open circles) cells are shown. Data are representative of at least three separate experiments. (G) Cell viability of wild-type (TH9; gray) and cdc25-22 wee1-50 (TH370; light gray) after exposure to 70.8-MPa HP for 10 min at 35.5°C was compared with unstressed wild-type control (black column). Data are representative of at least three experiments.
The inhibitory Tyr15 phosphorylation on Cdc2 kinase is catalyzed by Wee1 tyrosine kinase, which maintains Cdc2 in an inactive state (for review, see MacNeill and Nurse, 1997). Because pressure stress induced a cell cycle delay in G2, it was possible that pressure stress activated Wee1 kinase, thereby maintaining Cdc2 kinase in an inactive form. The mutant cdc2-1w is refractile to Wee1 activity and is characterized by a wee phenotype (Russell and Nurse, 1987). Therefore, to test the above-mentioned possibility, cell cycle progression in cdc2-1w cells (TH319) was monitored in both treated (70.8 MPa) and untreated samples as described above. Delays in both nuclear division and septation (data not shown) were observed in pressure-treated cdc2-1w cells compared with that of unstressed controls (Figure 3B). These findings suggested that Wee1 was either not involved in the pressure-induced cell cycle delay or that Wee1 did not act independently to cause this delay.
To test whether Cdc25 and Wee1 could function redundantly to delay entry into mitosis in response to pressure stress, a potential dual role for Cdc25 and Wee1 in coordinating a pressure-induced cell cycle delay was investigated using cdc25-22 wee1-50 cells encoding temperature-sensitive mutations in both Cdc25 and Wee1 (TH370). When shifted to the restrictive temperature of 35.5°C, the function of these alleles is abrogated (Fantes, 1979). Thus, TH370 was elutriated at 35.5°C to obtain G2-synchronized cells, and cell cycle progression was monitored in both pressure-treated (70.8-MPa) and untreated samples maintained at 35.5°C. Surprisingly, the delay in nuclear division and septation was abrogated in the double mutant cdc25-22 wee1-50 at 35.5°C (Figure 3, C and D). In contrast, the pressure stress-induced delay in nuclear division and septation was still observed in wild-type cells at 35.5°C, indicating that the abrogation of cell cycle delay observed in pressure-treated cdc25-22 wee1-50 cells was not a result of performing the experiment at this elevated temperature (Figure 3, E and F). These data identified a requirement for both Cdc25 and Wee1 for cell cycle delay induced by hydrostatic pressure. Furthermore, these results are consistent with a model in which Cdc2-Y15 is the target of a pressure-induced cell cycle delay. Cell viability was also examined at the restrictive temperature after exposure to pressure stress (70.8 MPa), and it was reduced in cdc25-22 wee1-50 cells compared with wild type, but not significantly (Figure 3G).
These findings raised the possibility that pressure stress might lead to a reduction in Cdc25 or an increase in Wee1 protein levels, which might subsequently lead to a cell cycle delay. However, neither Cdc25 nor Wee1 protein levels were found to be significantly affected after exposure to 70.8-MPa pressure stress (Supplemental Figure 2).
The Spc1 MAPK Pathway Is Activated in Response to Pressure Stress
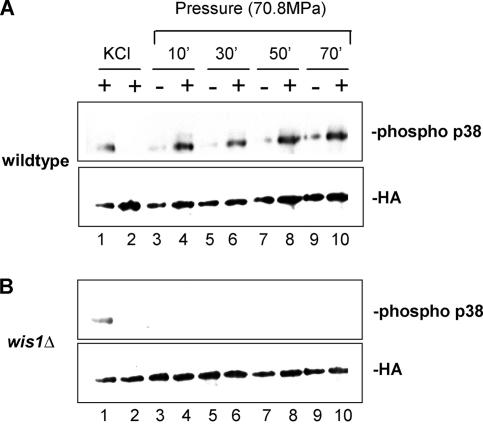
Spc1 MAPK is activated through Wis1-dependent phosphorylation at residues Thr171 and Tyr173 in response to a variety of stresses in S. pombe (for review, see Toone and Jones, 1998). To determine whether Spc1 was activated in response to pressure stress, phosphorylation of HA-Spc1 was examined by Western blot by using an anti-phospho-p38 antibody at different times after pressure stress. Wild-type cells (TH172) exhibited an increase in HA-Spc1 phosphorylation soon after exposure to pressure stress (70.8 MPa; Figure 4A). Some HA-Spc1 phosphorylation was also observed in untreated samples at later time points (60 min) due to stress from experimental conditions (Figure 4A, lane 9). A role for Wis1 in phosphorylating Spc1 after pressure stress was also examined, where in contrast to wild-type (TH172), Spc1 phosphorylation was not detected in a wis1Δ background (TH1728), with or without prior exposure to pressure stress. Thus, Wis1 was required for activation of Spc1 in response to pressure stress (Figure 4B).
Figure 4.
Hydrostatic pressure activates the Spc1 MAPK pathway. Western blot analysis of HA-Spc1 phosphorylation in wild-type cells (TH172) (A) and wis1Δ cells (TH1728) (B) after 10-min exposure to 70.8-MPa HP or untreated, after times indicated (lanes 3–10). Western blots were probed with anti-phospho-p38 antibody (top) and anti-HA antibody (bottom). KCl treatment (0.6 M for 20 min) of TH172 (A and B, lane 1) and TH1728 (A and B, lane 2) were used as positive and negative controls, respectively. Data are representative of at least three separate experiments.
Spc1 Is Required to Facilitate Cell Cycle Recovery after Pressure Stress
A role for the Spc1 MAPK pathway in cell cycle control has been identified in fission yeast (for review, see Karlsson-Rosenthal and Millar, 2006). To examine a possible role for Spc1 in regulation of the pressure-induced cell cycle delay, G2-synchronized spc1-m13 (TH123), a null allele of Spc1, was subjected to 47.2-MPa HP (maximal pressure for cell survival for spc1-m13; Figure 1A) for 10 min, and cell cycle progression was examined. Exposure of spc1-m13 cells to this pressure resulted in a delay in nuclear division and septation of ∼60 min, consistent with a cell cycle delay in G2 or early mitosis (Figure 5, A and B). Importantly, because no cell cycle delay was observed in wild-type cells under these conditions (Figure 2, A and B), these data indicated a role for Spc1 in facilitating cell cycle progression after pressure stress.
Figure 5.
Role of Spc1 and Cdc25 in maintaining cell cycle kinetics after pressure stress. The percentage of binucleate or septated cells was determined (as indicated) at times shown after exposure of G2-synchronized cells to either 47.2- or 70.8-MPa HP (as indicated) for 10 min by using spc1-m13 (TH123) (A and B), spc1Δ plo1.S402A (IH3286) (C), spc1Δ plo1.S402E (IH3287) (D), plo1.S402A (IH3124) (E), plo1.S402E (IH3125) (F), and cdc25-9A (S1299) (G and H). Untreated (solid squares) or pressure treated (open circles) cells are shown. Data are representative of at least three separate experiments.
Spc1-mediated phosphorylation of polo kinase Plo1 on Ser402 is observed in response to heat or centrifugal stress, and it was found to promote cell cycle recovery after heat stress (Petersen and Hagan, 2005). To determine whether Spc1-dependent Plo1 Ser402 phosphorylation was required to promote cell cycle recovery in response to hydrostatic pressure, cell cycle progression was examined in spc1Δ cells containing either plo1.S402A (IH3286), which abrogates phosphorylation, or plo1.S402E (IH3287), which mimics phosphorylation and compensates for the spc1Δ mitotic commitment defect (Petersen and Hagan, 2005). After exposure of G2-synchronized strains to 47.2-MPa HP for 10 min, spc1Δ plo1.S402A exhibited a cell cycle delay equivalent to that observed in spc1-m13 (compare Figure 5, A and C). In contrast, spc1Δ plo1.S402E exhibited a reduced cell cycle delay compared with that of spc1Δ plo1.S402A or spc1-m13 (compare Figure 5, C and D). These findings are consistent with a role for Spc1-mediated phosphorylation of Plo1 Ser402 in promoting cell cycle progression after 47.2-MPa HP hydrostatic pressure. Moreover, plo1.S402E cells (IH3125) were found to exhibit a reduced cell cycle delay compared with plo1.S402A cells (IH3124) after exposure to 70.8-MPa HP (compare Figure 5, E and F). These findings strongly support a role for Spc1-dependent phosphorylation of Plo1 Ser402 in cell cycle recovery from pressure stress.
To test whether Cdc25 phosphorylation is required for cell cycle progression in response to hydrostatic pressure, cdc25-9A cells (S1299) were exposed to 47.2 MPa of pressure stress for 10 min. In contrast to wild-type cells (Figure 2, A and B), a modest delay in both nuclear division and septation was observed in cdc25-9A cells after exposure to 47.2-MPa pressure stress (Figure 5, G and H), with cells recovering in the next cell cycle. This suggests that Cdc25 phosphorylation at one or more of the sites mutated in cdc25-9A plays a role in maintaining cell cycle progression in response to pressure stress in wild-type cells.
The Spc1 MAPK Pathway Is Required for Cell Viability under Pressure Stress
A possible role for the Spc1 MAPK pathway was assessed in maintaining viability under conditions of pressure stress. Exposure of spc1-m13 to a range of hydrostatic pressures resulted in reduced viability compared with wild-type cells (Figure 1A). From these experiments, the maximum pressure dose for which there was no loss in viability in spc1-m13 cells was considerably lower than that observed for wild-type cells, and it was determined to be 47.2 MPa, whereas complete loss of viability was observed after exposure to 118-MPa HP (Figure 1A). These results indicated a role for Spc1 in maintaining cell viability under pressure stress. In contrast, only modest loss of viability was observed under these conditions in a strain in which the Spm1/Pmk1 MAPK was deleted, which functions in the cell integrity pathway (Zaitsevskaya-Carter and Cooper, 1997) (Supplemental Figure 3A). The roles for MAPKK kinases Win1 and Wak1, the MAPK kinase Wis1, and the MAPK Spc1 in maintaining cell viability under pressure stress were examined further. In contrast to wild-type cells, the mutants win1Δ wak1Δ, wis1Δ and spc1-m13 each showed a significant reduction in viability after exposure to pressure stress, with the greatest loss of viability observed in wis1Δ and spc1-m13 cells (70.8 MPa; Figure 6A).
Figure 6.
Spc1 MAPK pathway is required for cell viability after hydrostatic pressure. Cell viability of stress-kinase mutants win1-1 Δwak1 (TH972), Δwis1 (TH815), and spc1-m13 (TH123) (A); upstream phospho-relay mutants mak1Δ mak2Δ mak3Δ (TH973), mcs4Δ (TH971), and prr1Δ (TH970) (B); and downstream effector mutants atf1Δ (TH968), pap1Δ (TH454), and atf1Δ pap1Δ (TH474) (C) was compared with wild-type (black column) in response to 70.8-MPa HP for 10 min. Data are representative of at least three experiments. Significant values (Student's t test) relative to wild-type viability (100%) are indicated (*p < 0.05 and **p < 0.005).
The role of the upstream sensor histidine kinases and response regulators of the Spc1 MAPK pathway were also investigated, where it was found that mcs4Δ was significantly less viable than mak1Δmak2Δmak3Δ and prr1Δ cells under pressure stress (Figure 6B). This indicated a role for the response regulator Mcs4 in the pressure-stress response and further suggested that this stress was unlikely to be sensed by the histidine kinases Mak1, Mak2, and Mak3.
The role of the downstream effector Atf1 and Pap1 in maintaining cell viability under pressure stress was also assessed. Cell viability was significantly reduced compared with wild-type cells in an atf1Δ background after exposure to pressure stress (Figure 6C). The dead atf1Δ cells did not exhibit an elongated phenotype, suggesting that loss of viability was not a result of failed cell cycle recovery (Supplemental Figure 4). Moreover, spc1Δ plo1.S402E did not exhibit significantly increased viability despite exhibiting increased cell cycle recovery (Supplemental Figure 3B). In contrast, little loss of viability was observed in a pap1Δ background in response to pressure stress (Figure 6C). Because there was no further loss of viability in an atf1Δpap1Δ double compared with atf1Δ alone, this indicated that Atf1 but not Pap1 played a key role in maintaining cell viability under conditions of pressure stress. Loss of viability in atf1Δ or atf1Δpap1Δ was greater than that of spc1-m13, suggesting a possible role for Atf1 in maintaining viability after exposure to hydrostatic pressure independently of Spc1.
Examining Stress Tolerance to Hydrostatic Pressure in S. pombe
Exposure to mild stress can lead to cells acquiring resistance to various stresses that would normally be lethal. Such stress tolerance suggests a general stress response, and it has been observed in S. cerevisiae in response to a variety of stress agents (Palhano et al., 2004). To examine the specificity of the pressure-stress response, barotolerance was investigated after prestressing of wild-type TH9 cells with various agents, including pressure (23.6 and 70.8 MPa for 10 min), oxidative stress (0.5 mM H2O2 for 30 min), osmotic stress (1 M sorbitol for 30 min), and heat (39°C for 30 min). These conditions were based on studies showing both a maximal gene induction in S. pombe and also their contribution to barotolerance in S. cerevisiae (Chen et al., 2003; Abe, 2004). Prestressed cells were left at room temperature at normal atmospheric conditions for 10 min before exposure to hydrostatic pressure dose of 94.4 MPa for 10 min, which results in ∼50% loss in viability in wild type (Figure 1A). Analysis of cell viability after prestress with pressure, osmotic or oxidative stresses indicated that tolerance to hydrostatic pressure was not enhanced compared with nonprestressed controls (Figure 7). However, prior exposure of cells to heat (39°C) was found to significantly increase barotolerance (p < 0.05) (Figure 7).
Figure 7.
Study of stress-induced adaptation to hydrostatic pressure in S. pombe. Asynchronous log-phase cultures of wild-type S. pombe (TH9) were prestressed with either pressure (23.6 or 70.8 MPa), oxidative (0.5 mM H2O2), osmotic (1 M sorbitol), or heat (39°C) stress before subjecting cells to a pressure dose of 94.4 MPa for 10 min, and then cell viability was determined. Data are representative of three separate experiments. Significant value (*p < 0.05) is indicated relative to viability of cells at 94.4-MPa HP.
DISCUSSION
In this study, the cellular responses to hydrostatic pressure in S. pombe were examined. Exposure to sublethal levels of hydrostatic pressure (70.8 MPa) was found to trigger a cell cycle delay. Further analysis of the underlying mechanism of this response indicated that the pressure-induced G2 cell cycle delay resulted from Cdc2 inactivation through tyrosine (Y15) phosphorylation. Consistent with this was the finding that pressure-induced cell cycle delay correlated with increased Cdc2-Y15 phosphorylation levels. Moreover, pressure-induced cell cycle delay was abrogated in a cdc25-22 wee1-50 double mutant, in which the key regulators of Cdc2-Y15 phosphorylation were simultaneously inactivated. Mutants that bypassed either Cdc25 inactivation (cdc2-3w) or Wee1 activation (cdc2-1w) still underwent a pressure-induced cell cycle delay, which suggests that pressure-induced cell cycle delay results from simultaneous inactivation of Cdc25 phosphatase and activation of Wee1 kinase activities (Figure 8).
Figure 8.
Model for the regulation of the cell cycle and stress responses to hydrostatic pressure in S. pombe. See text for details.
Abrogation of a cell cycle delay through genetic mutation suggested the activation of a cell cycle checkpoint in response to pressure stress. Further genetic analysis ruled out the possibility that the pressure-induced cell cycle delay resulted through activation of the DNA damage checkpoint as the pressure induced cell cycle delay was still observed in rad3Δ, chk1Δ, rad24Δ, cdc25-9A, and cdc2-3w strains, which abrogate the DNA integrity checkpoint pathways (Carr, 2002). The pressure-induced cell cycle delay was also observed in a clp1Δ strain, indicating that the cytokinesis checkpoint was also not responsible for the pressure-induced delay (Mishra et al., 2004). A role for the spindle orientation checkpoint, which causes an intramitotic delay in response to actin depolymerization, was also unlikely to be responsible for the pressure-induced delay, because although mutations in Spc1 or Atf1 would normally abrogate this checkpoint (Gachet et al., 2001), spc1-m13 exacerbated the pressure-induced cell cycle delay. Furthermore, the cell size checkpoint, which can work through Cdc25 or Wee1 under different conditions (Nurse, 1975; Fantes and Nurse, 1978; Rupes et al., 2001), was also unlikely to have been responsible for the delay, because oversized cells resulting from Cdc25 inactivation still underwent a pressure-induced cell cycle delay.
Therefore, it is possible that pressure stress may activate a novel checkpoint pathway that arrests the cell cycle in response to pressure-induced damage through Cdc25 inactivation and Wee1 activation. Such a checkpoint-dependent cell cycle delay would presumably allow time for pressure-induced damage to be repaired. Alternatively, pressure stress may produce a wide spectrum of damage, leading to the simultaneous activation of more than one checkpoint controlling entry into mitosis, and hence mutations that abrogate the DNA integrity, cytokinesis, or cell size checkpoints may not individually abrogate the pressure-induced cell cycle delay.
It is also possible that the pressure-induced cell cycle delay could result from a checkpoint-independent mechanism, resulting instead from reduced levels and/or availability of proteins required for cell cycle progression. Possible mechanisms by which pressure stress could modify cell cycle regulators include protein destabilization or protein synthesis inhibition. Indeed, global analysis of gene expression in S. cerevisiae after pressure stress identified genes involved in cell cycle progression and protein synthesis to be the most repressed (Fernandes et al., 2004). Moreover, protein synthesis is one of the most barosensitive cellular functions, and it is completely blocked at 67-MPa HP in Escherichia coli and several mammalian cell lines (Gross and Jaenicke, 1994; Mentre and Hoa, 2001). Importantly, inhibiting protein synthesis can lead to cell cycle delay through both activating Wee1 (Suda et al., 2000) and by inhibiting Cdc25 and Cdc13 (Daga and Jimenez, 1999; Grallert et al., 2000). Furthermore, Wee1 is positively regulated at the transcriptional and posttranscriptional levels in response to protein synthesis inhibition, and Spc1 MAPK is required for its transcriptional regulation (Suda et al., 2000). High pressure is also known to cause protein denaturation and dissociation, both of which are reversible after pressures up to 100–300 MPa (Mentre et al., 1999). Stress tolerance to pressure obtained after mild heat-shock in the study presented here suggested that one main effect of baroinjury could be protein unfolding. Indeed, heat-shock proteins and their cochaperones have been shown to be involved in cell cycle regulation. The heat-shock protein (Hsp)90/Swo1 chaperone complexes with S. pombe Wee1 or Mik1 and it is required for stability of the complex and maintenance of function (Goes and Martin, 2001). Wos2 (homologous to human p23), a cochaperone of Hsp90, has been suggested to be involved in the regulation of Wee1 and Cdc2 at normal growth temperatures in fission yeast (Munoz et al., 1999). Although we observed no significant change in either Cdc25 or Wee1 protein levels after exposure to hydrostatic pressure, it is possible that pressure-induced cell cycle delay results from down-regulation or denaturation of a distinct mitotic activator, the absence of which can be suppressed by inactivation of both Cdc25 and Wee1.
A role for the Spc1 MAPK was identified in cell cycle recovery in response to hydrostatic pressure. Further studies suggest Spc1 facilitates cell cycle recovery from pressure stress, in part, through mediating Plo1 phosphorylation on Ser402. These findings are consistent with a recently identified role for Spc1-dependent Plo1 phosphorylation in mitotic commitment and cell cycle recovery from heat stress (Petersen and Hagan, 2005). In this respect, Plo1 phosphorylation may contribute to the activation of Cdc25 and inactivation of Wee1, thereby facilitating cell cycle recovery from pressure stress (Figure 8). Spc1-dependent Plo1 phosphorylation ensures the return of actin to cell tips and efficient reinitiation of cell tip growth after either heat or centrifugal stress (Petersen and Hagan, 2005). We have observed the kinetics of actin remodeling to be delayed in wild-type cells after exposure to hydrostatic pressure (our unpublished data), suggesting that Spc1-dependent Plo1 phosphorylation may function similarly to ensure efficient reinitiation of tip growth during recovery from hydrostatic pressure. The finding that the double mutant cdc25-22 wee1.50 strain does not seem to lose viability as a result of failure to undergo a cell cycle delay in response to elevated pressure suggests that it is the ability to recover from the arrest and not the arrest per se that is important in this response.
An important role for the Spc1 MAPK pathway was also identified in maintaining cell viability under pressure stress. A comprehensive analysis of mutants in this pathway revealed that the upstream stress response regulator (Mcs4), the MAPK cassette (comprising the MAPKKKs Win1 and Wak1), the MAPKK (Wis1), the MAPK (Spc1), and, more crucially, the downstream bZip transcription factor Atf1 were required to maintain viability under pressure stress. The histidine kinases (Mak1, Mak2, and Mak3) did not contribute significantly to stress-responsive cell survival, suggesting the existence of an alternative sensor in the pressure-stress response. Importantly, Atf1 functions to maintain cell viability in response to a number of environmental stresses, independently of a role in cell cycle control (Takeda et al., 1995; Shiozaki and Russell, 1996; Wilkinson et al., 1996). Furthermore, Atf1 is not required for heat-stress induced phosphorylation of Plo1, which facilitates cell cycle recovery (Petersen and Hagan, 2005). Consistent with this, no cell cycle arrest phenotype was associated with cell death in atf1Δ cells after exposure to pressure stress treatment, indicating that the roles of the Spc1 MAPK pathway in facilitating cell cycle progression and in maintaining cell viability under pressure stress conditions are distinct (Figure 8). Interestingly, tolerance to pressure stress was obtained only in cells prestressed with heat, suggesting a likely role for heat-induced genes downstream of Atf1 in maintaining viability in response to pressure stress. Such genes may include Hsps and genes involved in trehalose metabolism, which are known to induce barotolerance in S. cerevisiae (Abe, 2004). However, no increase in barotolerance was observed after prestressing cells with hydrostatic pressure, suggesting that there was little or no overlap in gene expression induced by pressure and heat stress.
Understanding the molecular responses to hydrostatic pressure is of medical as well as biological interest. Cardiac hypertrophy is an adaptive physiological growth response of the constituent ventricular myocytes of the heart myocardium to increased demand for blood flow in the body (for review, see Bicknell et al., 2003). Although this is a normal developmental process, hypertrophy also can be pathophysiological, when it occurs in response to stresses such as chronic hypertension and/or myocardial infarction. Furthermore, chronic or extreme acute stress can lead to heart failure and even death. In accordance with the results of our present study in yeast, it has been demonstrated previously that pressure overload-induced left ventricular hypertrophy, after aortic constriction in adult rats, leads to a significant increase in the number of myocyte nuclei arresting in the G2 phase of the cell cycle in hypertrophied hearts compared with untreated controls (Li and Brooks, 1999). Although several cell cycle regulators responsible for the hypertrophic transition from G0/G1 to G2 have been studied in cardiac myocytes (Bicknell et al., 2004), an explanation for the G2 arrest is lacking. It is possible that the mechanisms of pressure-induced G2 arrest identified in our study may contribute to our understanding of diseases such as detrimental cardiac hypertrophy and heart failure. The stress-activated protein kinase pathway has been found to be activated in cardiac myocytes by a variety of different stresses, including ischemia (for review, see Tibbles and Woodgett, 1999). More recently, p38α was found to play a critical role in cardiac myocyte survival in response to pressure overload (Nishida et al., 2004). Thus, our findings suggest that the role for p38 MAPK in the cellular response to pressure stress may be generally conserved. Moreover, it might be predicted that homologues of the bZIP transcription factor Atf1 might also function in response to pressure overload in cardiac myocytes. It is hoped that this study will contribute to the understanding of the mechanisms by which homeostasis is maintained in response to hydrostatic pressure in eukaryotes.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Hill and D. Stevens for help in initial development of the pressure stress system; R. Aligue, K. Gould, I. Hagan, J. Hayles, J. Millar, C. Norbury, and M. O'Connell for reagents; S. Kearsey for technical assistance; and J. Thacker and B. Johnson for helpful discussions. V.G. was supported by the Medical Research Council and a Universities UK Overseas Research Scholarship at The University of Reading.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1141) on August 15, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Abe F. Piezophysiology of yeast: occurrence and significance. Cell Mol. Biol. 2004;50:437–445. [PubMed] [Google Scholar]
- al-Khodairy F., Carr A. M. DNA-repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K., Mitsubayashi Y., Aiba H., Mizuno T. Spy1, a histidine-containing phosphotransfer signaling protein, regulates the fission yeast cell cycle through the Mcs4 response regulator. J. Bacteriol. 2000;182:4868–4874. doi: 10.1128/jb.182.17.4868-4874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell K. A., Coxon C. H., Brooks G. Forced expression of the cyclin B1-CDC2 complex induces proliferation in adult rat cardiomyocytes. Biochem. J. 2004;382:411–416. doi: 10.1042/BJ20031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell K. A., Surry E. L., Brooks G. Targeting the cell cycle machinery for the treatment of cardiovascular disease. J. Pharm. Pharmacol. 2003;55:571–591. doi: 10.1211/002235703765344487. [DOI] [PubMed] [Google Scholar]
- Buck V., Quinn J., Pino T. S., Martin H., Saldanha J., Makino K., Morgan B. A., Millar J.B.A. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mo. Biol. Cell. 2001;12:407–419. doi: 10.1091/mbc.12.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A. M. DNA structure dependent checkpoints as regulators of DNA repair. DNA Repair. 2002;1:983–994. doi: 10.1016/s1568-7864(02)00165-9. [DOI] [PubMed] [Google Scholar]
- Chen D. R., Toone W. M., Mata J., Lyne R., Burns G., Kivinen K., Brazma A., Jones N., Bahler J. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell. 2003;14:214–229. doi: 10.1091/mbc.E02-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga R. R., Jimenez J. Translational control of the Cdc25 cell cycle phosphatase: a molecular mechanism coupling mitosis to cell growth. J. Cell Sci. 1999;112:3137–3146. doi: 10.1242/jcs.112.18.3137. [DOI] [PubMed] [Google Scholar]
- Enoch T., Carr A. M., Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA-replication. Genes Dev. 1992;6:2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- Fantes P. Epistatic gene interactions in the control of division in fission yeast. Nature. 1979;279:428–430. doi: 10.1038/279428a0. [DOI] [PubMed] [Google Scholar]
- Fantes P., Nurse P. Control of cell size at division in fission yeast by a growth-modulated size control over nuclear division. Exp. Cell Res. 1977;107:377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- Fantes P. A., Nurse P. Control of timing of cell-division in fission yeast–cell-size mutants reveal a 2nd control pathway. Exp. Cell Res. 1978;115:317–329. doi: 10.1016/0014-4827(78)90286-0. [DOI] [PubMed] [Google Scholar]
- Fernandes P.M.B., Domitrovic T., Kao C. M., Kurtenbach E. Genomic expression pattern in Saccharomyces cerevisiae cells in response to high hydrostatic pressure. FEBS Lett. 2004;556:153–160. doi: 10.1016/s0014-5793(03)01396-6. [DOI] [PubMed] [Google Scholar]
- Gachet Y., Tournier S., Millar J.B.A., Hyams J. S. A MAP kinase-dependent actin checkpoint ensures proper spindle orientation in fission yeast. Nature. 2001;412:352–355. doi: 10.1038/35085604. [DOI] [PubMed] [Google Scholar]
- Gaits F., Degols G., Shiozaki K., Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goes F. S., Martin J. Hsp90 chaperone complexes are required for the activity and stability of yeast protein kinases Mik1, Wee1 and Swe1. Eur. J. Biochem. 2001;268:2281–2289. doi: 10.1046/j.1432-1327.2001.02105.x. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast Cdc2+ protein-kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Grallert B., Kearsey S. E., Lenhard M., Carlson C. R., Nurse P., Boye E., Labib K. A fission yeast general translation factor reveals links between protein synthesis and cell cycle controls. J. Cell Sci. 2000;113:1447–1458. doi: 10.1242/jcs.113.8.1447. [DOI] [PubMed] [Google Scholar]
- Greenall A., Hadcroft A. P., Malakasi P., Jones N., Morgan B. A., Hoffman C. S., Whitehall S. K. Role of fission yeast Tup1-like repressors and Prr1 transcription factor in response to salt stress. Mol. Biol. Cell. 2002;13:2977–2989. doi: 10.1091/mbc.01-12-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Jaenicke R. Proteins under Pressure–the influence of High hydrostatic-pressure on structure, function and assembly of proteins and protein complexes. Eur. J. Biochem. 1994;221:617–630. doi: 10.1111/j.1432-1033.1994.tb18774.x. [DOI] [PubMed] [Google Scholar]
- Hamada K., Nakatomi Y., Osumi M., Shimada S. Direct induction of homozygous diploidization in the fission yeast Schizosaccharomyces pombe by pressure stress. FEMS Microsc. Lett. 1996;136:257–262. [Google Scholar]
- Hamada K., Nakatomi Y., Shimada S. Direct induction of tetraploids or homozygous diploids in the industrial yeast Saccharomyces cerevisiae by hydrostatic pressure. Curr. Genet. 1992;22:371–376. doi: 10.1007/BF00352438. [DOI] [PubMed] [Google Scholar]
- Karlsson-Rosenthal C., Millar J. B. Cdc 25, mechanisms of checkpoint inhibition and recovery. Trends Cell Biol. 2006;16:285–292. doi: 10.1016/j.tcb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kawarai T., Arai S., Furukawa S., Ogihara H., Yamasaki M. High-hydrostatic-pressure treatment impairs actin cables and budding in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2006;101:515–518. doi: 10.1263/jbb.101.515. [DOI] [PubMed] [Google Scholar]
- Li J. M., Brooks G. Cell cycle regulatory molecules (cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors) and the cardiovascular system–potential targets for therapy? Eur. Heart J. 1999;20:406–420. doi: 10.1053/euhj.1998.1308. [DOI] [PubMed] [Google Scholar]
- Lopez-Aviles S., Grande M., Gonzalez M., Helgesen A. L., Alemany V., Sanchez-Piris M., Bachs O., Millar J.B.A., Aligue R. Inactivation of the Cdc25 phosphatase by the stress-activated Srk1 kinase in fission yeast. Mol. Cell. 2005;17:49–59. doi: 10.1016/j.molcel.2004.11.043. [DOI] [PubMed] [Google Scholar]
- MacNeill S. A., Nurse P. Cell cycle control in fission yeast. In: Pringle J. R., Broach J., Jones E. W., editors. Yeast III. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 697–763. [Google Scholar]
- Mentre P., Hamraoui L., Hoa G.H.B., Debey P. Pressure-sensitivity of endoplasmic reticulum membrane and nucleolus as revealed by electron microscopy. Cell Mol. Biol. 1999;45:353–362. [PubMed] [Google Scholar]
- Mentre P., Hoa G.H.B. Effects of high hydrostatic pressures on living cells: a consequence of the properties of macromolecules and macromolecule-associated water. Int. Rev. Cytol. 2001;201:1–84. doi: 10.1016/s0074-7696(01)01001-4. [DOI] [PubMed] [Google Scholar]
- Millar J.B.A., Buck V., Wilkinson M. G. Pyp1 and Pyp2 Ptpases dephosphorylate an osmosensing Map kinase controlling cell-size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Mishra M., Karagiannis J., Sevugan M., Singh P., Balasubramanian M. K. The 14-3-3 protein rad24p modulates function of the cdc14p family phosphatase clp1p/flp1p in fission yeast. Curr. Biol. 2005;15:1376–1383. doi: 10.1016/j.cub.2005.06.070. [DOI] [PubMed] [Google Scholar]
- Mishra M., Karagiannis J., Trautmann S., Wang H., McCollum D., Balasubramanian M. K. The Clp1p/Flp1p phosphatase ensures completion of cytokinesis in response to minor perturbation of the cell division machinery in Schizosaccharomyces pombe. J. Cell Sci. 2004;117:3897–3910. doi: 10.1242/jcs.01244. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Munoz M. J., Bejarano E. R., Daga R. R., Jimenez J. The identification of Wos2, a p23 homologue that interacts with Wee1 and Cdc2 in the mitotic control of fission yeasts. Genetics. 1999;153:1561–1572. doi: 10.1093/genetics/153.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A. N., Lee A., Place W., Shiozaki K. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mo. Biol. Cell. 2000;11:1169–1181. doi: 10.1091/mbc.11.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K., et al. p38 alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol. Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Genetic-control of cell-size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Palhano F. L., Orlando M.T.D., Fernandes P.M.B. Induction of baroresistance by hydrogen peroxide, ethanol and cold-shock in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2004;233:139–145. doi: 10.1016/j.femsle.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Paredes V., Franco A., Soto T., Vicente-Soler J., Gacto M., Cansado J. Different roles for the stress-activated protein kinase pathway in the regulation of trehalose metabolism in Schizosaccharomyces pombe. Microbiology. 2003;149:1745–1752. doi: 10.1099/mic.0.26279-0. [DOI] [PubMed] [Google Scholar]
- Petersen J., Hagan I. M. Polo kinase links the stress pathway to cell cycle control and tip growth in fission yeast. Nature. 2005;435:507–512. doi: 10.1038/nature03590. [DOI] [PubMed] [Google Scholar]
- Rao P. N. Mitotic synchrony in mammalian cells treated with nitrous oxide at high pressure. Science. 1968;160:774–776. doi: 10.1126/science.160.3829.774. [DOI] [PubMed] [Google Scholar]
- Rupes I., Webb B. A., Mak A., Young P. G. G2/M arrest caused by actin disruption is a manifestation of the cell size checkpoint in fission yeast. Mol. Biol. Cell. 2001;12:3892–3903. doi: 10.1091/mbc.12.12.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein-kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Samejima I., Mackie S., Fantes P. A. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Kobori H., Ishijima S. A., Feng Z. H., Hamada K., Shimada S., Osumi M. Schizosaccharomyces pombe is more sensitive to pressure stress than Saccharomyces cerevisiae. Cell Struct. Funct. 1996;21:167–174. doi: 10.1247/csf.21.167. [DOI] [PubMed] [Google Scholar]
- Sato M., Kobori H., Shimada S., Osumi M. Pressure-stress effects on the ultrastructure of cells of the dimorphic yeast Candida tropicalis. FEMS Microbiol. Lett. 1995;131:11–15. [Google Scholar]
- Shieh J. C., Wilkinson M. G., Buck V., Morgan B. A., Makino K., Millar J.B.A. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shiozaki K., Russell P. Cell-cycle control linked to extracellular environment by Map kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K., Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- Shiozaki K., Shiozaki M., Russell P. Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism. Mol. Biol. Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda M., Yamada S., Toda T., Miyakawa T., Hirata D. Regulation of Wee1 kinase in response to protein synthesis inhibition. FEBS Lett. 2000;486:305–309. doi: 10.1016/s0014-5793(00)02299-7. [DOI] [PubMed] [Google Scholar]
- Takeda T., Toda T., Kominami K. I., Kohnosu A., Yanagida M., Jones N. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbles L. A., Woodgett J. R. The stress-activated protein kinase pathways. Cell Mol. Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toone W. M., Jones N. Stress-activated signalling pathways in yeast. Genes Cells. 1998;3:485–498. doi: 10.1046/j.1365-2443.1998.00211.x. [DOI] [PubMed] [Google Scholar]
- Toone W. M., Kuge S., Samuels M., Morgan B. A., Toda T., Jones N. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 1998;12:1453–1463. doi: 10.1101/gad.12.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann S., Wolfe B. A., Jorgensen P., Tyers M., Gould K. L., McCollum D. Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr. Biol. 2001;11:931–940. doi: 10.1016/s0960-9822(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Walker G. M. Synchronization of yeast cell populations. Methods Cell Sci. 1999;21:87–93. doi: 10.1023/a:1009824520278. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. G., Samuels M., Takeda T., Toone W. M., Shieh J. C., Toda T., Millar J.B.A., Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Wolfe B. A., Gould K. L. Fission yeast Clp1p phosphatase affects G(2)/M transition and mitotic exit through Cdc25p inactivation. EMBO J. 2004;23:919–929. doi: 10.1038/sj.emboj.7600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsevskaya-Carter T., Cooper J. A. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S. pombe. EMBO J. 1997;16:1318–1331. doi: 10.1093/emboj/16.6.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Piwnica-Worms H. DNA damage and replication checkpoints in fission yeast require nuclear exclusion of the Cdc25 phosphatase via 14-3-3 binding. Mol. Cell Biol. 1999;19:7410–7419. doi: 10.1128/mcb.19.11.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.