Abstract
Microbial adhesion to the host tissue represents an early, critical step in the pathogenesis of most infectious diseases. Borrelia burgdorferi, the causative agent of Lyme disease (LD), expresses two surface-exposed decorin-binding adhesins, DbpA and DbpB. A decorin-deficient (Dcn–/–) mouse was recently developed and found to have a relatively mild phenotype. We have now examined the process of experimental LD in Dcn–/– mice using both needle inoculation and tick transmission of spirochetes. When exposed to low doses of the infective agent, Dcn–/– mice had fewer Borrelia-positive cultures from most tissues analyzed than did Dcn+/+ or Dcn+/– mice. When the infection dose was increased, similar differences were not observed in most tissues but were seen in bacterial colonization of joints and the extent of Borrelia-induced arthritis. Quantitative PCR demonstrated that joints harvested from Dcn–/– mice had diminished Borrelia numbers compared with issues harvested from Dcn+/+ controls. Histological examination also revealed a low incidence and severity of arthritis in Dcn–/– mice. Conversely, no differences in the numbers of Borrelia-positive skin cultures were observed among the different genotypes regardless of the infection dose. These differences, which were observed regardless of genetic background of the mice (BALB/c or C3H/HeN) or method of infection, demonstrate the importance of decorin in the pathogenesis of LD.
Introduction
Borrelia burgdorferi sensu lato is the causative agent of Lyme disease (LD). It is transmitted by infected ticks that deposit a small number of organisms in the dermis of a host animal during feeding, leading to a localized infection (1). The initial skin infection is usually accompanied by a local rash (erythema migrans) and can include other manifestations such as fever, muscle pain, and headaches (2–4). Bacterial dissemination and colonization of many different organs may follow. Late stages of LD can include neurological, ocular, cutaneous, and cardiac disease in addition to arthritis (1–3). Early antibiotic therapy will usually resolve B. burgdorferi infection. However, symptoms sometimes may reappear, and up to 10% of patients with Lyme arthritis are classified as treatment resistant (1, 5, 6).
Microbial adhesion to and colonization of host tissues is an early, critical event in most infection processes (7). Pathogenic bacteria often express several adhesins that can participate in parallel and independent attachment mechanisms that result in tissue colonization. In LD, bacterial adhesion to host tissue may be important in determining both the fate of the initial challenge organisms in the dermis and their ability to disseminate. Spirochetes deposited in the dermis are found associated with collagen fibers in the extracellular matrix (ECM). However, B. burgdorferi do not attach directly to collagen but to decorin, a small leucine-rich proteoglycan (SLRP) that is associated with and “decorates” collagen fibers (8, 9). Decorin is distributed throughout the mammalian body and could be a target for B. burgdorferi adherence during dissemination, as these spirochetes express two decorin-binding proteins, DbpA and DbpA. However, alternate adhesion mechanisms also may be used by the spirochetes depending on the tissue target and stage of dissemination (10, 11). In addition to the two decorin-binding microbial surface component–recognizing adhesive matrix molecules (MSCRAMMs), Borrelia also express a fibronectin-binding MSCRAMM, BBK32, and a putative glycosaminoglycan-binding MSCRAMM (12–14). B. burgdorferi have been shown to attach to a variety of mammalian cells in vitro, and the organisms can bind to the integrins αMβ2, αIIbβ3, α5β1, and αvβ3 (8, 9, 15–19).
Recently, a decorin-deficient (Dcn–/–) mouse was developed and characterized (20). The phenotype of this mouse was relatively mild and appeared to be restricted to skin laxity and fragility, a consequence of irregular collagen fibers in the dermis (20). This result was somewhat surprising given that decorin can have profound effects in vitro. The proteoglycan can regulate the proliferation of cells, the activity of TGF-β and the complement system (C1q), as well as the adherence of mammalian cells to fibronectin (21–27). The availability of a Dcn–/– mouse provided an opportunity to assess the importance of this proteoglycan in B. burgdorferi dissemination to various organs and tissue colonization in vivo. It has been previously shown that the method of B. burgdorferi transmission may affect the progression of LD and that different strains of mice can vary in the development and presentation of LD (28, 29). Therefore, we examined the role of decorin in the development of LD by infecting Dcn–/– mice that have been backcrossed onto genetic backgrounds that exhibit either severe (C3H/Hen) or relatively mild arthritis (BALB/c). Although the ID50 for both strains of mice is similar, spirochete dissemination and arthritis develop at different rates and intensities (28–30). Additionally, we examined the effect of decorin during different infection processes by comparing disease development in mice that had been infected using either cultured organisms administered by needle or arthropod-adapted spirochetes transmitted by ticks (31).
Methods
Mice.
Pathogen-free (MTV–) Dcn–/– mice (BL/Swiss × 129Sv) (20) were backcrossed for five and ten generations into BALB/c or C3H/HeN backgrounds (Harlan Sprague Dawley, Indianapolis, Indiana, USA). Mice were genotyped for the decorin allele by PCR analysis of mouse tail DNA as described previously (20). BALB/c and C3H/Hen mice used in all experiments were backcrossed to the fifth generation unless otherwise specified. The animals were maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care in accordance with current regulations and standards of the United States Department of Agriculture, Department of Health and Human Services, and NIH. All animal procedures were approved by the Institutional Animal Care and Use Committee of Texas A&M University Health Science Center Institute of Biosciences and Technology. Female Dcn–/–, Dcn+/–, and Dcn+/+ mice ranging in age from 8 to 10 weeks were used at the start of the experiments.
Borrelia infections.
Borrelia burgdorferi sensu stricto, strain B31, was obtained at in vitro passage 5 from S.J. Norris (The University of Texas Medical School at Houston, Houston, Texas, USA) (32) and inoculated into BSK-II medium supplemented with antibiotics (50 μg/ml rifampicin and 100 μg/ml phosphomycin) at 34°C as described previously (33). Borrelia used for infections in all experiments described were cultured from aliquots of the same passage 5 frozen stock. Bacterial cultures contained in screw-cap tubes were incubated in a CO2-enriched atmosphere using a GasPak chamber (Becton Dickinson Microbiology Systems, Sparks, Maryland, USA) containing BBL GasPak Plus envelopes and a GasPak anaerobic indicator (Becton Dickinson, Cockeysville, Maryland, USA) until the cells reached a density between 5 × 107 and 1 × 108/ml. Borrelia were counted using dark-field microscopy and a Petroff-Hausser chamber. Tick- and needle-inoculation procedures were used during the course of these experiments. Borrelia (101–104) were needle-inoculated intradermally into shaved dorsal skin at the base of the tail (100 μl/mouse). For tick transmission, B. burgdorferi strain B31-infected Ixodes scapularis nymphs were allowed to feed to repletion. Mice were lightly anesthetized with Metofane (methoxyflurane; Pitman-Moore, Mundelein, Illinois, USA) during the tick attachment period. Immediately after tick placement, mice were individually housed in raised wire-bottom cages (Lab Products, Maywood, New Jersey, USA) containing approximately 20 ml of water at their base for the duration of the infection period (4–5 days). All cages were monitored three times daily during the first 3 days and four times daily during days 4 and 5, when replete ticks dropped from the mice (34).
Detection of Borrelia in replete ticks.
Replete ticks from each mouse were collected and stored in a humid chamber for 12 days. At the end of this incubation period, each tick was sterilized by incubating in 3% hydrogen peroxide (3 minutes) followed by a 10- to 15-minute wash in 70% ethanol. Ticks were crushed individually using flat-end tweezers in BSK II-filled tubes (6 ml). Tick-inoculated media was cultured for 2 weeks as already described here and were examined for the presence of Borrelia.
Culturing of blood and tissues.
At various time points after infection (days 3, 7, and 14) blood samples were collected from the mice and cultured for the presence of Borrelia. Tick-inoculated mice were maintained for 28 days after infection, and blood was cultured for the presence of Borrelia up to postinfection day 21. Their tails were sterilized with a propidium-iodine swab (Professional Disposables Inc., Orangeburg, New York, USA) and bled under a laminar flow biosafety cabinet. Blood dilutions ranging from 1:100 to 1:10,000 (final volume of 5 ml) were made with BSK II and incubated at 34°C. The cultures were checked 2 and 3 weeks later for the presence of viable Borrelia, using dark-field microscopy. Similarly, after sacrifice, joints, heart, bladder, and ear samples were aseptically removed and cultured for the presence of Borrelia (days 14 and 28 for needle- and tick-inoculated mice, respectively). Ear biopsy samples were collected by using a 3-mm Baker biopsy punch (Baker Norton Pharmaceuticals, Miami, Florida, USA). Care was taken to remove all skin from isolated joints. All instruments were sterilized between dissections (Dry Sterilizer IS-400; Inotech Biosystems International, Lansing, Michigan, USA).
Arthritis assessment.
Evidence of arthritis was determined by histopathological examination of formalin-fixed hind tibiotarsal joint samples. Tissues for histological examination were embedded in paraffin and stained with hematoxylin and eosin. All sections were examined without knowledge of the infection or genotype status of the mice. A positive/negative assessment of arthritis was made initially. To determine the severity of arthritis, joints were scored according to the levels of neutrophil infiltrate as follows: 0, no arthritis; 1, minimal or rare (≤ 10% tissue involvement); 2, mild (10–20%); frequent (20–50%); and 4, severe (>50%) (35).
Serum analysis (ELISA).
Assays were performed on Corning Easy Wash modified flat-bottom 96-well plates (Corning-Costar Corp., Cambridge, Massachusetts, USA) as described previously (35). Briefly, the titer of the serum samples was determined and tested for reactivity against Borrelia using antibodies directed against each murine antibody class/subclass (IgG1, IgG2a, IgG2b, IgG3, IgE, IgA, and IgM) as described previously (35).
DNA preparation for PCR quantitation.
A rear ankle joint (devoid of skin) and one ear were harvested from Dcn–/– and Dcn+/+ C3H/HeN mice 4 weeks after infection. Individual tissues were incubated in 0.1% collagenase A at 37°C overnight before addition of an equal volume of 0.2 mg/ml proteinase K (Sigma Chemical Co., St. Louis, Missouri, USA) as described previously (36). After an overnight incubation at 55°C, DNA was recovered by phenol-chloroform extraction and ethanol precipitation (36). After digestion with DNase-free RNase (Sigma Chemical Co.) at 1 mg/ml, samples were again extracted and DNA was recovered by precipitation. This precipitate was resuspended in 1.5 ml of water, and the DNA content was determined by measuring the absorbance at 260 nm (36).
Quantitation of Borrelia.
PCR analyses were performed in a fluorescence temperature cycler (LightCycler LC24; Idaho Technology, Idaho Falls, Idaho, USA). Amplification was performed on 200 ng of sample DNA in a 10 μl final volume containing 50 mM Tris (pH 8.3), 3 mM MgCl2, and 4.5 μg of BSA, 200 μM deoxynucleoside triphosphates, a 1:30,000 dilution of SYBR Green I (Molecular Probes Inc., Eugene, Oregon, USA), 5 μM of each primer, 0.5 U of Taq polymerase (Life Technologies Inc., Gaithersburg, Maryland, USA), and 110 ng of TaqStart antibody (CLONTECH Laboratories Inc., Palo Alto, California, USA). Amplification was performed for 40 cycles, with each cycle consisting of heating at 20°C per second to 95°C with a 1-second hold, cooling at 20°C per second to 60°C with a 1-second hold, and heating at 1°C per second to 84°C. This procedure continuously monitored the cycle-by-cycle accumulation of fluorescently labeled product. The cycle at which the product was first detected was used as an indicator of the relative starting copy number present in each sample. Copy numbers for the mouse nidogen gene and B. burgdorferi recA were calculated by using the LightCycle software, and recA values were corrected by normalization based on the nidogen gene copy number. The oligonucleotide primers used to detect murine nidogen were nidoF (5′-CCA GCC ACA GAA TCA CAT CC-3′) and nidoR (5′-GGA CAT ACT CTG CTG CCA TC-3′). The oligonucleotide primers used to detect B. burgdorferi recA were nTM17.F (5′-GTG GAT CTA TTG TAT TAG ATG AGG CTC TCG-3′) and nTM17.R (5′-GCC AAA GTT CTG CAA CAT TAA CAC CTA AAG-3′) (36, 37).
Results
Experimental Borrelia infection.
The involvement of decorin in the establishment and dissemination of B. burgdorferi in mice was examined using Dcn–/–, Dcn+/–, and Dcn+/+ mice. Because the genetic background of an infected mouse influences the severity of arthritis, the Dcn–/– mice were backcrossed to BALB/c and C3H/HeN animals. C3H/HeN mice are reported to develop severe arthritis when infected with B. burgdorferi, whereas BALB/c mice develop a relatively mild arthritis that is influenced by the dose of B. burgdorferi administered (28, 29, 38).
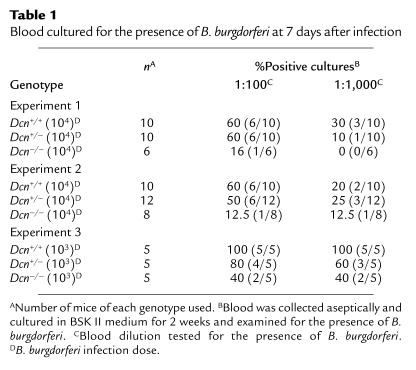
In initial experiments, BALB/c mice of each genotype were needle inoculated with 103 or 104 B. burgdorferi B31. Blood was collected at days 3, 7, and 14 days after infection and cultured to detect the presence of Borrelia (35). No spirochetes were detected in the blood 3 days after infection. Dcn–/– mice infected with either 103 or 104 B. burgdorferi consistently had a lower percentage of Borrelia-positive blood cultures 7 days after infection compared with Dcn+/+ and Dcn+/– mice at both 1:100 and 1:1,000 blood dilutions in three separate experiments (Table 1). These differences were not statistically significant in individual experiments. However, if the data for experiments 1 and 2 were combined (both used an infectious dose of 104 spirochetes), the difference in Borrelia-positive blood cultures between Dcn–/– (2/14) and Dcn+/+ (12/20) was significant for the 1:100 dilution (P < 0.05; Fisher’s exact test). Spirochetes were not detected in blood dilutions of ≥ 1:10,000. Blood samples collected 14 days after infection had a low incidence of Borrelia-positive cultures (<20%), and no significant difference was observed between the different Dcn genotypes (data not shown).
Table 1.
Blood cultured for the presence of B. burgdorferi at 7 days after infection
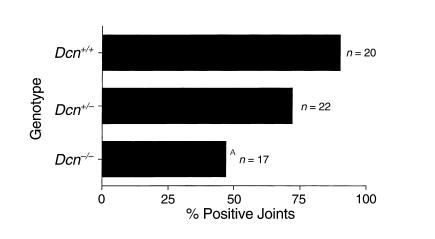
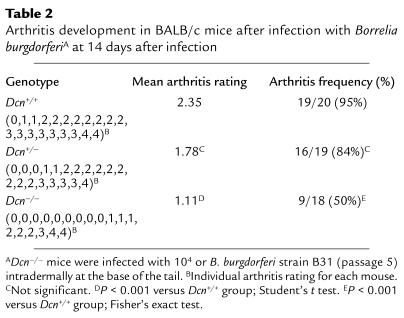
To examine whether any differences in the dissemination of Borrelia could be observed in these mice, the animals were sacrificed 14 days after infection, and ear biopsy samples, heart, bladder, and one hind tibiotarsal joint were harvested and cultured for the presence of spirochetes (35). Borrelia recovery from joints was significantly reduced in Dcn–/– mice (Figure 1), whereas essentially all cultures of other tissues examined were Borrelia-positive (data not shown). Only 47% of joints obtained from Dcn–/– mice were Borrelia-positive compared with 90% of joints obtained from Dcn+/+ mice (P < 0.01; Fisher’s exact test). Dcn+/– mice had an intermediate number of Borrelia-positive joints (72%). Histological examination of hematoxylin and eosin–stained, blind-coded sections of the contralateral hind tibiotarsal joint also revealed reduction in both arthritis incidence and severity in animals lacking decorin (Table 2). The average arthritis rating was only 1.11 in Dcn–/– mice but increased to 1.78 and 2.35 in Dcn+/– and Dcn+/+ mice, respectively. Severely arthritic joints (scored as 4) were found in all three genotypes. These data, and the reduced number of positive blood cultures in Dcn–/– mice, suggested that fewer Borrelia survived the initial infection and/or fewer Borrelia were able to colonize the joints after dissemination from the skin.
Figure 1.
Percent Borrelia-positive joint cultures. BALB/c Dcn–/– (n = 17), Dcn+/– (n = 22), and Dcn+/+ mice (n = 20) were infected with 104 B. burgdorferi. Joints were harvested 2 weeks post infection and inoculated into BSK II medium and examined for the presence of spirochetes 14 days later. AP < 0.01; Fisher’s exact test compared with wild-type controls.
Table 2.
Arthritis development in BALB/c mice after infection with Borrelia burgdorferiA at 14 days after infection
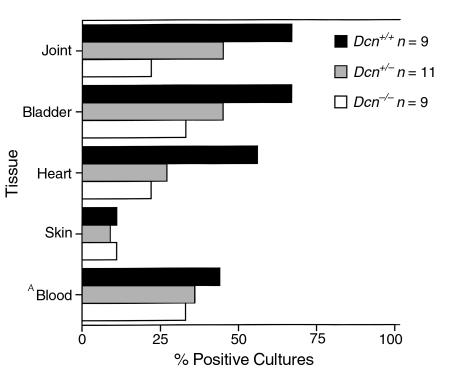
One possible explanation for these results is that the high inoculum dose used (≥ 103 Borrelia per mouse) might mask any discernible differences in colonization patterns for tissues other than the joints from the different genotypes. Because spirochetal persistence in tissues has been related to inoculum dose in BALB/c mice (31), we infected the three genotypes of mice with low doses of B. burgdorferi (101 or 102 Borrelia) and examined mice for spirochete dissemination 14 days after infection. All tissues examined from mice infected with 101 spirochetes were Borrelia negative by culture (data not shown). However, an infectious dose of 102 Borrelia revealed differences in colonization in most tissues examined. The most notable differences between genotypes were observed in joint and heart cultures; only 22% of joint and heart cultures harvested from Dcn–/– mice were Borrelia-positive (Figure 2), a marked but not statistically significant reduction at the sample size examined. Tissues from Dcn+/– mice had an intermediate level of Borrelia-positive cultures. Bladders were also colonized in a decorin-dependent manner with 33%, 45%, and 67% of the cultures scoring positive for Borrelia from the Dcn–/–, Dcn+/–, and Dcn+/+ mice, respectively. However, ear biopsy specimens cultured from infected mice of the various genotypes revealed no differences (Figure 2). The arthritic response by decorin genotype was also examined in mice that received 102 Borrelia. Histological analysis revealed that Dcn–/– mice had an arthritis incidence of 55%, whereas Dcn+/+ and Dcn+/– mice had an arthritis incidence of 100% and 44%, respectively (data not shown). The mean arthritis rating among the Dcn–/– and Dcn+/– mice (1.3 and 1.22, respectively) was also less than that observed for Dcn+/+ mice (1.87) (data not shown). These results demonstrated a reduced colonization of joint, bladder, and heart, but not blood or skin in Dcn–/– mice compared with Dcn+/+ controls when the infectious dose was low.
Figure 2.
Percent Borrelia-positive cultures. BALB/c Dcn–/– (n = 9), Dcn+/– (n = 11), and Dcn+/+ (n = 9) mice were infected with 102 B. burgdorferi. Tissues were aseptically removed 2 weeks after infection, inoculated into BSK II medium, and examined for the presence of spirochetes 14 days later. ABlood was cultured at postinfection day 7. Differences between genotypes were not statistically significant.
Quantification of Borrelia in tissues.
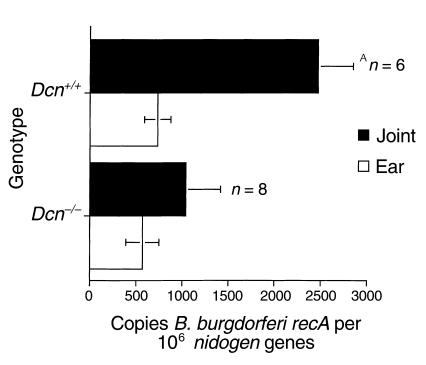
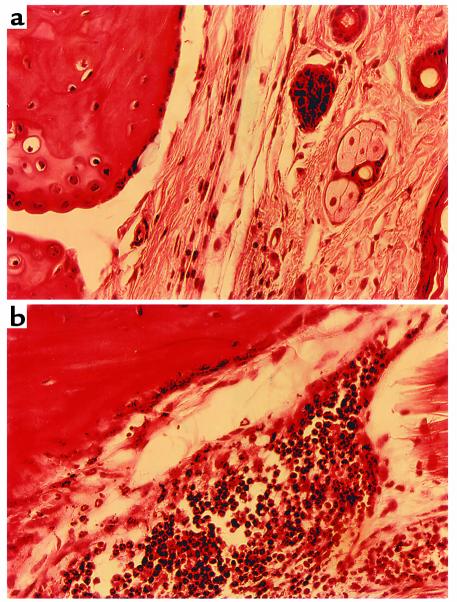
To examine whether the resistance to experimental Lyme arthritis observed in Dcn–/– BALB/c mice was also manifested in the C3H/HeN background, we infected Dcn–/– and Dcn+/+ C3H/HeN mice with 200 Borrelia intradermally and quantified the numbers of Borrelia in different tissue samples. DNA was purified from ears and joints at 4 weeks after infection, and the numbers of B. burgdorferi organisms present were determined by quantitative PCR (36). All samples were blind-coded before analysis. The results of a representative experiment are shown in Figure 3. Although Borrelia numbers were similar in ear samples, Dcn–/– C3H/HeN mice had significantly less Borrelia in joints than did Dcn+/+ controls. Differences were also apparent in the arthritis incidence and severity. Dcn–/– mice had 55% arthritis incidence and a mean arthritis score of 1.08 compared with 100% incidence and a 2.3 mean arthritis score in Dcn+/+ mice (P < 0.05; Fisher’s exact test and Student’s t test for percent incidence and severity, respectively). Figure 4 illustrates differences in hematoxylin and eosin–stained representative joint samples from Dcn–/– and Dcn+/+ mice (Figure 4, a and b, respectively). Joints harvested from Dcn+/+ mice had noticeably greater numbers of infiltrating cells, particularly neutrophils, compared with joints obtained from Dcn–/– mice.
Figure 3.
Detection of B. burgdorferi DNA. Borrelia was quantitated from ear and joint samples collected from Dcn–/– (n = 8) and Dcn+/+ (n = 6) C3H/HeN mice. Mice were infected with 200 B. burgdorferi. The indicated tissues were harvested 4 weeks after infection and assessed for B. burgdorferi-specific DNA by quantitative PCR of the B. burgdorferi recA gene. Results were normalized to those of the host nidogen gene. The data are expressed as mean ± SEM. AP < 0.05; Student’s t test.
Figure 4.
Hematoxylin and eosin–stained sections of hind tibiotarsal joints. C3H/HeN Dcn–/– (a) and Dcn+/+ (b) mice were infected with 200 borreliae. Mice were sacrificed and the joints harvested for histological analysis at 28 days after infection.
Tick inoculations.
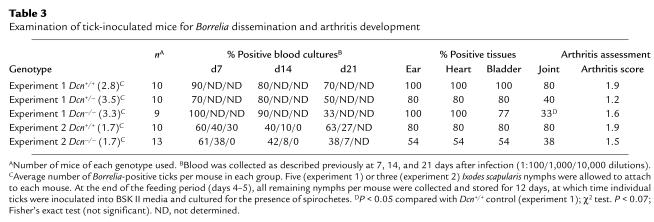
Lyme disease progression and development in mice, as well as the nature of the immune response generated against B. burgdorferi, differs between culture grown B. burgdorferi cells injected by needle- and tick-transmitted organisms (39, 40). We therefore also examined spirochete dissemination throughout the host and arthritis formation in Dcn–/– BALB/c mice after infection of animals by the natural vector. Borrelia-infected Ixodes scapularis nymphs (three or five nymphs per mouse) were allowed to feed to repletion on Dcn–/–, Dcn+/–, and Dcn+/+ mice, and blood samples were collected at postinfection days 7, 14, and 21 (34, 39). The animals were sacrificed 28 days after infection, and samples from ear, heart, bladder and joints were examined for the presence of B. burgdorferi (Table 3). To determine the number of Borrelia-positive ticks feeding on individual mice, ticks were cultured for the presence of Borrelia after the feeding period. Tick attrition during the course of the experiment, combined with Borrelia-negative ticks, reduced the mean number of infected ticks per mouse in both experiments to 2.8–3.5 and 1.7 (Table 3, experiments 1 and 2, respectively).
Table 3.
Examination of tick-inoculated mice for Borrelia dissemination and arthritis development
The number of Borrelia-positive ticks allowed to feed on individual mice of the different genotypes affected dissemination patterns of the bacteria in a manner similar to that observed in the high- and low-dose needle inoculation experiments. Similar to low-dose needle inoculations, Dcn–/– mice infested with less than two B. burgdorferi–infected ticks had fewer Borrelia-positive heart, bladder (54% positive), and joint cultures (38% positive) compared with Dcn+/+ controls (80% Borrelia-positive cultures for all tissues examined) (Table 3, experiment 2). Reduced numbers of Borrelia-positive cultures from ear biopsies collected from Dcn–/– mice compared with Dcn+/+ mice were observed when mice were tick inoculated but not needle inoculated. The reason for this difference is currently unknown. When the infectious dose was increased by allowing a larger number of infected ticks to feed on the mice, this difference was not generally observed (Table 3, experiment 1). However, the joints again represent an exception. Regardless of the number of infected ticks allowed to feed, Dcn–/– mice always had fewer Borrelia-positive joint cultures than did Dcn+/+ controls (Table 3). However, in contrast to needle-inoculated mice, the differences in arthritis scores were not significant. We observed differences in Borrelia-positive blood cultures for both high- and low-dose tick inoculations 21 days after infection (Table 3). When 2.8–3.5 infected ticks were allowed to feed on the mice, 33% of the blood cultures from Dcn–/– mice were Borrelia-positive at 21 days after infection compared with 70% and 50% positive in blood cultured from Dcn+/+ and Dcn+/– mice, respectively. When fewer ticks were allowed to feed to repletion, 38% compared with 63% of blood cultures (1:100 dilution) were Borrelia-positive from Dcn–/– and Dcn+/+ mice, respectively. Blood collected from the different genotypes earlier during the infection process did not show any considerable difference with respect to Borrelia-positive cultures (Table 3, experiment 2). Unless indicated, differences in Borrelia-positive cultures were not statistically significant (Table 3).
Immune responses in decorin-deficient mice.
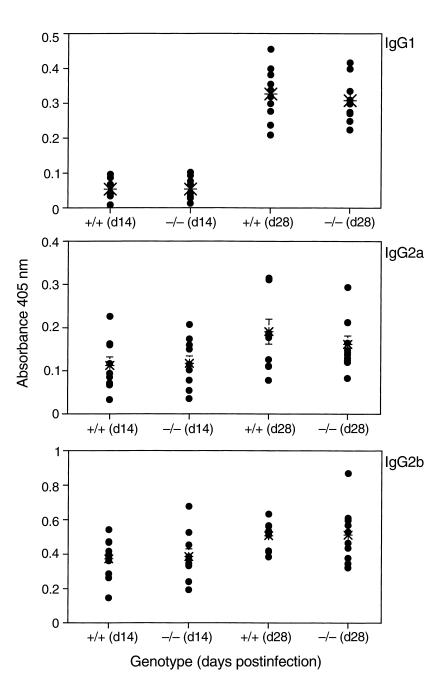
Decorin has been previously shown to affect a number of different mediators of immune responses, including TGF-β, in addition to affecting the complement system. What role decorin plays in regulating immune responses is not known. This raised the possibility that the observed increased resistance to Borrelia infection in Dcn–/– mice might be linked to decorin-related changes in immunity (21, 24–27, 41). To address this, both cellular and humoral responses in Dcn–/– and Dcn+/+ mice were examined. The serum from tick-inoculated mice (described in Table 3, experiment 2) was screened by ELISA for antibodies reactive to whole B. burgdorferi (Figure 5). Only IgG1, IgG2a, and IgG2b Borrelia-reactive antibodies were detected, and no differences between Dcn–/– and Dcn+/+ mice were observed. In addition, serum from needle-inoculated mice screened for DbpA reactivity revealed no differences among genotypes tested (Dcn–/–, Dcn+/–, or Dcn+/+) (data not shown). Cellular responses in Dcn–/– were also unaffected. Immunization with DbpA elicited a similar delayed-type hypersensitivity response in Dcn–/– and Dcn+/+ after challenge with DbpA (data not shown). Therefore, we concluded that the Dcn–/– background did not noticeably affect the development of either humoral or cellular immune responses.
Figure 5.
Antibody response to B. burgdorferi from tick-inoculated mice. Serum from infected mice described in Table 3 was tested for reactivity against B. burgdorferi 14 and 28 days after infection. Each data point represents the mean absorbance from triplicate wells from individual mice at 405 nm minus the substrate control. The mean absorbance value for each group is represented by a large asterisk. There was no statistical differences between the Dcn–/– and the Dcn+/+ mice.
Discussion
Bacterial adhesion to the host tissue is considered an early, critical step in the development of infection. Many pathogenic organisms have acquired several parallel and apparently independent mechanisms of adherence, probably reflecting the importance of this step in the disease process. Elimination of individual adhesins through genetic manipulations, nevertheless, can result in bacterial mutants with reduced virulence (42, 43). Heterologous expression of adhesins from pathogenic bacteria in less-virulent strains has also been used as a strategy to demonstrate a role of individual adhesins as virulence factors (42). Here we have taken a reverse approach by examining the infectious disease process in mice that are deficient in an adhesion ligand.
The adherence potential of B. burgdorferi is incompletely understood. So far, two decorin-binding (8) and one fibronectin-binding (13) MSCRAMM have been identified, as well as candidate adhesins for the observed adhesion of B. burgdorferi to β3 integrins and to glycosaminoglycans (12, 14, 16, 44–46). The Dcn–/– mice used in this study have a surprisingly mild phenotype considering the potent effects of decorin in vitro. Decorin is a member of the SLRP family of structurally and apparently functionally related molecules (41, 47). Some members of this family may have similar roles in vivo, resulting in a certain degree of redundancy. Furthermore, the inactivation of one SLRP gene in mice can result in upregulation of the expression of other SLRP genes and/or enhanced tissue retention of these molecules (48). The SLRP-binding spectra of the B. burgdorferi MSCRAMMs DbpA and DbpB have not been examined beyond work demonstrating that DbpA, in addition to binding decorin, also bound to the related proteoglycan, biglycan (E. Brown et al., unpublished observations). In view of the multiple adhesion mechanisms apparently available to the spirochete, it is remarkable that we have demonstrated differences in Borrelia infection in mice in which only one adhesion ligand was removed. Decorin binding, therefore, is a major mechanism by which B. burgdorferi disseminates. To our knowledge, this is the first report demonstrating in vivo the importance of an MSCRAMM ligand in an infectious disease process.
Most tissues examined from the Dcn–/– mice had fewer Borrelia-positive cultures compared with Dcn+/+ mice when the infection doses were low. In most cases, the differences were eliminated when the infection dose was increased. The joints were an exception in that this tissue from Dcn–/– mice had fewer Borrelia-positive cultures and reduced Borrelia numbers regardless of the infection dose. Furthermore, the joints were consistently less arthritic in Dcn–/– mice compared with the Dcn+/+ controls regardless of the genetic background of the mice. In general, the number of Borrelia-positive cultures were intermediate in Dcn+/– mice. This could reflect presumed intermediate levels of decorin in heterozygous mice based on decorin mRNA analysis (20). This difference suggested that decorin is a crucial substrate for the colonization of the joints by B. burgdorferi. One explanation for the apparent decorin-dependent colonization of joints observed in these studies is the very high content of the proteoglycan in cartilage. Fibronectin, another potential substrate for B. burgdorferi adhesins, is normally low in this tissue. Conversely, no differences in Borrelia-positive cultures were generally observed from skin biopsies regardless of genotype or infection dose, although Dcn–/– mice had fewer Borrelia-positive skin biopsies compared with Dcn+/+ mice when low-dose tick transmission was used. However, these results cannot be taken to indicate that Dbp-dependent adhesion mechanisms did not contribute to the ability of the spirochetes to colonize skin, as ligands for the Dbp’s other than decorin could be of importance. In fact, fluorescence microscopy of embryonic skin fibroblasts derived from Dcn–/– mice and probed with DbpA demonstrated the presence of additional ligands for the MSCRAMM (E. Brown and M. Höök, unpublished observations).
Substantial differences have been observed in the presentation of LD in mice depending on whether the spirochetes were culture grown and injected by needle or were tick transmitted (39, 40). However, Dcn–/– mice were resistant to LD regardless of the inoculation strategy used, and similar effects between the infection dose and tissue colonization by Borrelia were observed in mice infected by either inoculation procedure.
We have so far assumed that the observed resistance to LD in Dcn–/– mice was due to the proteoglycan’s role as a ligand to the Dbps and as a substrate to tissue adherence of B. burgdorferi. There are several observations that are consistent with this assumption: first, there is a tissue-specific effect that to some extent reflects the abundance of available decorin; second, heterozygous mice often showed intermediate colonization numbers, suggesting that the amounts of decorin available for Borrelia adherence could be limited; and third, with a high-dose inoculum the differences in positive cultures disappeared for most tissues, presumably because the spirochetes used alternative adhesion substrates. However, at this point we cannot exclude alternative explanations to the observed resistance to experimental LD in Dcn–/– mice. We considered the possibility that Dcn–/– mice might have an enhanced host defense against bacterial infections. The results presented here demonstrated that Dcn–/– mice could mount both humoral and cellular immune responses indistinguishable from Dcn+/+ controls. Furthermore, Dcn–/– BALB/c mice were found in preliminary experiments to be at least as susceptible as Dcn+/+ mice to Staphylococcus aureus–induced septic arthritis (E. Brown and M. Höök, unpublished observations). Thus, Dcn–/– mice did not appear to be dramatically different from Dcn+/+ mice in their general host defense potential.
In summary, we have demonstrated that Dcn–/– mice were more resistant to LD, which suggested that decorin could be a limiting factor as a substrate for the adherence of B. burgdorferi. However, this resistance could be overcome for most tissues examined by increasing the infection dose, suggesting that additional adhesion ligands could participate in the disease process in vivo.
Acknowledgments
This work was supported by a Department of Health and Human Services cooperative agreement from the Center for Disease Control (CCU614695 to E. Brown and M. Höök), a grant from the Neva Wesley West Foundation (to M. Höök), an NIH grant AR43521 (to J. Weis), an Arthritis Foundation Postdoctoral Fellowship (to R.M. Wooten), and grant 5P03-CA-42104 to the University of Utah. Special thanks are extended to Howell Hankamer for excellent technical assistance in the Program for Animal Resources.
Footnotes
Betty P. Guo’s present address is: Harvard Medical School, Department of Microbiology and Molecular Genetics, Boston, Massachusetts, USA.
References
- 1.Steere AC, et al. Successful parenteral penicillin therapy of established Lyme arthritis. N Engl J Med. 1985;312:869–874. doi: 10.1056/NEJM198504043121401. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 4.Szczepanski A, Benach JL. Lyme borreliosis: host responses to Borrelia burgdorferi. Microbiol Rev. 1991;55:21–34. doi: 10.1128/mr.55.1.21-34.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steere AC, et al. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37:878–888. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- 6.Dattwyler RJ, Halperin JJ. Failure of tetracycline therapy in early Lyme disease. Arthritis Rheum. 1987;30:448–450. doi: 10.1002/art.1780300414. [DOI] [PubMed] [Google Scholar]
- 7.Patti JM, Allen BL, McGavin MJ, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 8.Guo BP, Brown EL, Dorward DW, Rosenberg LC, Höök M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 9.Guo BP, Norris SJ, Rosenberg LC, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duray, P.H. 1992. Target organs of Borrelia burgdorferi infections: functional responses and histology. In Lyme disease: molecular and immunologic approaches. S.E. Schutzer, editor. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, New York, USA. 11–30.
- 11.Van Mierlo P, Jacob W, Dockx P. Erythema chronicum migrans: an electron-microscopic study. Dermatology. 1993;186:306–310. doi: 10.1159/000247384. [DOI] [PubMed] [Google Scholar]
- 12.Magoun L, et al. Variable small protein (Vsp)-dependent and Vsp-independent pathways for glycosaminoglycan recognition by relapsing fever spirochaetes. Mol Microbiol. 2000;36:886–897. doi: 10.1046/j.1365-2958.2000.01906.x. [DOI] [PubMed] [Google Scholar]
- 13.Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 14.Parveen N, Leong JM. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:1220–1234. doi: 10.1046/j.1365-2958.2000.01792.x. [DOI] [PubMed] [Google Scholar]
- 15.Cinco M, Murgia R, Presani G, Perticarari S. Integrin CR3 mediates the binding of nonspecifically opsonized Borrelia burgdorferi to human phagocytes and mammalian cells. Infect Immun. 1997;65:4784–4789. doi: 10.1128/iai.65.11.4784-4789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. Characterization of a candidate Borrelia burgdorferi beta3-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs RD. Borrelia burgdorferi bind to epithelial cell proteoglycans. J Clin Invest. 1994;93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klempner MS, Noring R, Rogers RA. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1993;167:1074–1081. doi: 10.1093/infdis/167.5.1074. [DOI] [PubMed] [Google Scholar]
- 19.Szczepanski A, Furie MB, Benach JL, Lane BP, Fleit HB. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990;85:1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danielson KG, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV. Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 22.Border WA, Ruoslahti E. Transforming growth factor-beta 1 induces extracellular matrix formation in glomerulonephritis. Cell Differ Dev. 1990;32:425–431. doi: 10.1016/0922-3371(90)90059-6. [DOI] [PubMed] [Google Scholar]
- 23.Breuer B, Schmidt G, Kresse H. Non-uniform influence of transforming growth factor-beta on the biosynthesis of different forms of small chondroitin sulphate/dermatan sulphate proteoglycan. Biochem J. 1990;269:551–554. doi: 10.1042/bj2690551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krumdieck R, Höök M, Rosenberg LC, Volanakis JE. The proteoglycan decorin binds C1q and inhibits the activity of the C1 complex. J Immunol. 1992;149:3695–3701. [PubMed] [Google Scholar]
- 25.Takagi M, Yamada T, Kamiya N, Kumagai T, Yamaguchi A. Effects of bone morphogenetic protein-2 and transforming growth factor-beta1 on gene expression of decorin and biglycan by cultured osteoblastic cells. Histochem J. 1999;31:403–409. doi: 10.1023/a:1003704425809. [DOI] [PubMed] [Google Scholar]
- 26.Winnemoller M, Schmidt G, Kresse H. Influence of decorin on fibroblast adhesion to fibronectin. Eur J Cell Biol. 1991;54:10–17. [PubMed] [Google Scholar]
- 27.Winnemoller M, Schon P, Vischer P, Kresse H. Interactions between thrombospondin and the small proteoglycan decorin: interference with cell attachment. Eur J Cell Biol. 1992;59:47–55. [PubMed] [Google Scholar]
- 28.Barthold SW, Sidman CL, Smith AL. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 29.Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, et al. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. J Immunol. 1998;161:1516–1524. [PubMed] [Google Scholar]
- 31.Brown CR, Reiner SL. Genetic control of experimental lyme arthritis in the absence of specific immunity. Infect Immun. 1999;67:1967–1973. doi: 10.1128/iai.67.4.1967-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 34.Piesman J. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 1993;30:199–203. doi: 10.1093/jmedent/30.1.199. [DOI] [PubMed] [Google Scholar]
- 35.Pride MW, et al. Specific Th1 cell lines that confer protective immunity against experimental Borrelia burgdorferi infection in mice. J Leukoc Biol. 1998;63:542–549. doi: 10.1002/jlb.63.5.542. [DOI] [PubMed] [Google Scholar]
- 36.Brown JP, Zachary JF, Teuscher C, Weis JJ, Wooten RM. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison TB, Ma Y, Weis JH, Weis JJ. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–992. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y, et al. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeidner N, et al. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–3106. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeidner N, Dreitz M, Belasco D, Fish D. Suppression of acute Ixodes scapularis-induced Borrelia burgdorferi infection using tumor necrosis factor-alpha, interleukin-2, and interferon-gamma. J Infect Dis. 1996;173:187–195. doi: 10.1093/infdis/173.1.187. [DOI] [PubMed] [Google Scholar]
- 41.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 42.Patti JM, et al. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun. 1994;62:152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaudaux PE, et al. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect Immun. 1995;63:585–590. doi: 10.1128/iai.63.2.585-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coburn J, Leong JM, Erban JK. Integrin alpha IIb beta 3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc Natl Acad Sci USA. 1993;90:7059–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coburn J, Barthold SW, Leong JM. Diverse Lyme disease spirochetes bind integrin alpha IIb beta 3 on human platelets. Infect Immun. 1994;62:5559–5567. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coburn J, Magoun L, Bodary SC, Leong JM. Integrins alpha(v)beta3 and alpha5beta1 mediate attachment of Lyme disease spirochetes to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iozzo RV. The family of small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 48.Svensson L, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]