Abstract
Nonylphenol (NP) and its parent compounds, the nonylphenol ethoxylates are some of the most prevalent chemicals found in U.S. waterways. NP is also resistant to biodegradation and is a known environmental estrogen, which makes NP a chemical of concern. Our data show that NP also activates the constitutive androstane receptor (CAR), an orphan nuclear receptor important in the induction of detoxification enzymes, including the P450s. Transactivation assays demonstrate that NP increases murine CAR (mCAR) transcriptional activity, and NP treatment can overcome the inhibitory effects of the inverse agonist, androstanol, on mCAR activation. Treatment of wild-type (CAR +/+) mice with NP at 50 or 75 mg/kg/day increases Cyp2b protein expression in a dose-dependent manner as demonstrated by Western blotting, and was confirmed by quantitative reverse transcription–PCR of Cyp2b10 transcript levels. CAR-null (CAR −/−) mice show no increased expression of Cyp2b following NP treatment, indicating that CAR is required for NP-mediated Cyp2b induction. In addition, NP increases the translocation of CAR into the nucleus, which is the key step in the commencement of CAR's transcriptional activity. NP also induced CYP2B6 in primary human hepatocytes, and increased Cyp2b10 messenger RNA and protein expression in humanized CAR mice, indicating that NP is an activator of human CAR as well. In conclusion, NP is a CAR activator, and this was demonstrated in vitro with transactivation assays and in vivo with transgenic CAR mouse models.
Keywords: Nonylphenol, CAR, PXR, Cyp2b10, P450
Nonylphenols (NP) are toxic degradation products of the alkylphenol ethoxylates (APEs). Research has identified NP as the most critical metabolite of APEs because of its resistance to biodegradation, its ability to bioaccumulate, and its toxicity (Ahel et al., 1994; Tyler et al., 1998). Therefore, both the European Union and Canada have placed limits or banned specific uses of nonylphenol ethoxylates (Renner, 1997). Research indicates that NP is one of the most prevalent toxicants in the United States (Kolpin et al., 2002), and Europe (Ahel et al., 1991). The U.S. Geological Survey performed an extensive survey of 139 lakes and rivers of the Unites States in 30 different states for 95 organic water contaminants. This survey showed that NP is found in more than 50% of our surface waters and is the seventh most common chemical. However, when present, NP is the third most prevalent chemical in the United States, and NP's parent compound nonylphenol monoethoxylate is the most prevalent chemical (Kolpin et al., 2002).
NP is also found in some food products, cosmetics, and drinking water. NP has been detected in drinking water (Petrovic et al., 2003), and has been reported to leach from tubing for milk processing and from plastics used in food packaging (Fernandes et al., 2003; Gilbert et al., 1992; Loyo-Rosales et al., 2004). Recent studies have demonstrated that para-NP is found on most of our fruits and vegetables, probably because of the widespread use of NP as a carrier in pesticide formulations (Yang and Ding, 2005). Furthermore, 100% of edible marine species contain NP. For example, in the Adriatic Sea, NP concentrations in marine organisms have increased over the past 5 years and vary from 0.4 to 1.5 ppm in edible crustaceans and fish (Ferrara et al., 2001, 2005).
NP is a mixture of para-, ortho-, and meta-isomers with the estrogenic, para-NP (4-NP) comprising approximately 85% of the isomers. 4-NP is estrogenic at supraphysiological concentrations and is widely studied primarily because of its widespread use and endocrine disrupting effects in vitro (Soto et al., 1991; White et al., 1994) and in vivo (Acevedo et al., 2005; Baldwin et al., 1997; Laws et al., 2000). Some studies have demonstrated increased P450 expression in association with reduced fecundity in daphnids (Baldwin et al., 1997), or increased mammary cancer incidence in mice (Acevedo et al., 2005). NP has been shown to increase Cyp3a in fish and rodents (Arukwe et al., 1997; Baldwin et al., 2005; Lee et al., 1996), presumably due to its interactions with the pregnane X-receptor (PXR) (Masuyama et al., 2000). Recent research indicates that Cyp2b induction is greater than Cyp3a induction in mice (Acevedo et al., 2005; Hernandez et al., 2006), and this has been confirmed in rats (Fu et al., 2006). This is consistent with activation of the constitutive androstane receptor (CAR) by NP; however, NP's ability to activate CAR has not been directly investigated.
CAR (NR1I3) and PXR (NR1I2) are orphan nuclear receptors that act as master regulators of the phase I, phase II, and phase III enzymes and transporters critical for detoxification of steroids, bile acids, and xenobiotics (Chang, 2006; Qatanani and Moore, 2005; Wei et al., 2000; Zollner et al., 2006). PXR and potentially CAR are activated by a wide range of endocrine disrupting chemicals and therefore may be important in protecting the integrity of the endocrine system from natural and man-made chemicals (Kretschmer and Baldwin, 2005). The early research suggested that PXR primarily induced the transcription of CYP3A family members, and CAR primarily induced the transcription of CYP2B family members when activated. More recent research indicates that these receptors are involved in “cross-talk” in which they are activated by similar chemicals (Moore et al., 2000, 2002), and regulate the expression of analogous genes by stimulating similar response elements (Wei et al., 2002; Xie and Evans, 2001) with PXR showing broader effects on CYP2B and CYP3A than CAR (Faucette et al., 2007). Thus, PXR and CAR regulate overlapping, but distinct sets of genes involved in protecting the cell from toxicants (Maglich et al., 2002).
CAR's ligand-binding pocket is smaller and less flexible than PXR's (Suino et al., 2004; Watkins et al., 2001). The smaller ligand-binding domain is thought to be the reason CAR is less promiscuous than PXR (Suino et al., 2004). However, ligand binding is not a prerequisite for activation of CAR as demonstrated by phenobarbital (PB) (Kawamoto et al., 1999), which activates CAR through an adenosine monophosphate kinase phosphorylation cascade (Rencurel et al., 2005; Shindo et al., 2007). It has been hypothesized that the majority of CAR activators work through an indirect pathway (Shindo et al., 2007).
Activation of CAR is implicated in several drug–drug interactions. For example, CAR activation and the subsequent induction of CYP3A4 have been associated with increased vitamin D metabolism. This provides a potential association between CAR-activating antiepileptic drugs, such as PB and phenytoin, and a decline in bone mineral density (Xu et al., 2006). The production of the toxic metabolite of acetaminophen, 4-hydroxyacetanilide, N-acetyl-paminophenol, is significantly enhanced by CAR activation, and mice lacking CAR are protected from acetaminophen toxicity (Zhang et al., 2002). Furthermore, hydroxylation of methoxychlor, polychlorinated biphenyls, and possibly polybrominated diphenyl ethers by P450s is necessary to produce their endocrine disrupting metabolites (Connor et al., 1995, 1997; Dehal and Kupfer, 1994; Meerts et al., 2001), and accordingly induction of P450s by CAR activation may enhance toxicity of these environmental pollutants.
Therefore, exposure to environmental and occupational chemicals, such as the ubiquitous contaminant NP, may induce P450s and lead to altered toxicity and clearance of endogenous wastes, pharmaceuticals, pesticides, and other xenobiotics due to CAR activation. Consequently, we set out to determine if NP activates CAR using transient transactivation assays, CAR-null mice, and humanized (hCAR) transgenic mice to determine if NP activates CAR in vitro and in vivo.
MATERIALS AND METHODS
Chemicals
Technical grade 4-NP (∼85% para-isomers) was obtained from Fluka Chemical Co. (Seelze, Germany). Absolute ethanol, zoxazolamine (2-amino-5-chlorobenzoxazole 97% purity) (ZOX), 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), ketamine HCl/xylazine HCl (800 mg/120 mg) PB, androstanol, pregnenolone 16α-carbonitrile (97%) (PCN), and rifampicin (95%) were obtained from Sigma-Aldrich (St Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from Fisher Scientific (Houston, TX). 6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (CITCO) was purchased from BIOMOL Research Labs, Inc. (Plymouth Meeting, PA).
Transactivation assays
The rat PXR (rPXR) and human PXR (hPXR) were generous gifts of Dr Bingfan Yan and Dr Ronald Evans, respectively (Blumberg et al., 1998; Zhang et al., 1999). The mouse CAR (mCAR) expression plasmid was described previously (Forman et al., 1998). The CAR reporter, a generous gift from Dr Masahiko Negishi, is the −1.8 kb PBREM from CYP2B6 (Wang et al., 2003). The PXR reporter (PXREluc) was a generous gift from Dr Russell Prough and contains two copies of the PXRE from the CYP3A23 promoter (Ripp et al., 2002).
Transactivation assays were performed in HepG2 human hepatoma cells (ATCC, Rockville MD) cultured in phenol red free Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 1% Penicillin/Streptomycin (Invitrogen) under 5% CO2 at 37°C. Transient transfections were performed as previously described (Song et al., 2005; Wyde et al., 2003). For mCAR transfections, HepG2 cells were plated in 12-well plates at 100,000 cells per well, and transfected 24 h later with 20 ng of the mCAR expression vector, and 100 ng of the pGL3 reporter plasmid using Effectene reagent according to the manufacturer's instructions (Qiagen, Valencia, CA). Transfected cells were treated the next day with NP, 10μM androstanol, or 10μM androstanol as an inverse agonist in combination with 250 nm TCPOBOP or increasing NP concentrations. The use of an inverse agonist (androstanol) is necessary for repressing CAR's constitutive activity and increasing the sensitivity of the transactivation assay (Forman et al., 1998). All chemicals were dissolved in DMSO and therefore all samples, treated and untreated, received 0.2% DMSO. Firefly luciferase activity was measured 24 h after the chemical treatment with the Steady-Glo luciferase reporter assay system (Promega, Madison, WI) according to manufacturer's protocol as described previously (Wyde et al., 2005). The luminescence signal was normalized to transfected, untreated cells, or transfected, androstanol-treated cells by determining the ratio of the normalized signal from chemically treated cells over that of the CAR-transfected, or CAR-transfected, androstanol-treated cells. This is reported as fold activation. Empty vector, reporter plasmid only, and expression plasmid only transfections were performed (data not shown). Data are presented as the mean of triplicate assays ± standard deviation from a minimum of three separate assays. Statistical differences (p ≤ 0.05) were determined by analysis of variance (ANOVA) followed by Fisher's protected least significant difference (PLSD) as post hoc test using StatView software.
For hPXR and rPXR transfected HepG2 cells, the assays were performed similarly with a couple of exceptions. Cells were transfected with 100 ng PXR expression plasmid, 100 ng PXR luciferase reporter plasmid, and 10 ng of pRLTK Renilla luciferase reporter plasmid as an internal control for transfection efficiency. The next day cells were treated with rifampicin, PCN, or NP. Twenty-four hours later, the luciferase assays were performed with the Dual-Glo Luciferase Assay System (Promega). Firefly luciferase reporter activity was normalized to Renilla luciferase activity to control for transfection efficiency. The data were then normalized to untreated (DMSO) samples by comparing the ratio of firefly luciferase activity in treated samples to untreated samples, and reported as fold activation. Data are presented as the mean of triplicate assays ± standard deviation from a minimum of three separate assays. Statistical differences (p ≤ 0.05) were determined by ANOVA followed by Fisher's PLSD as the post hoc test using StatView software.
Mice
All studies were carried out according to National Institutes of Health (NIH) guidelines for humane use of research animals and were preapproved by the Baylor College of Medicine or University of Texas at El Paso Animal Care and Use Committee. B6129PF1/J mice were obtained from Jackson's Laboratory (Bar Harbor, ME). CAR-null mice, on a B6129 background, were previously described (Wei et al., 2000), and are housed at both the Baylor College of Medicine and the University of Texas at El Paso. Humanized CAR (hCAR) mice were produced by adding hCAR on the albumin promoter to CAR-null mice and have been described previously (Zhang et al., 2002). hCAR mice were housed and treated at the Baylor College of Medicine. Mice were provided water, and fed ad libitum prior to treatments.
NP treatment of mice
Eight to 10-week-old wild-type and CAR-null female mice were randomly split into five treatment groups each. A group of mice were fed 0, 50, or 75 mg/kg/day NP mixed in 100 μl of honey for 7 days. A second group was injected with 100 μl corn oil or 3 mg/kg/day TCPOBOP mixed in corn oil one day prior to necropsy. hCAR mice were treated at The Baylor Medical School and livers were shipped on dry ice to The University of Texas at El Paso. Mice were provided corn oil injections or 30 mg/kg/day CITCO in corn oil for 3 consecutive days as a positive control, in addition to the hCAR mice treated as described above for 7 days with NP (0, 50, 75 mg/kg/day in honey). All mice were anesthetized by ketamine injection and then sacrificed by CO2 asphyxiation. Livers were excised, diced into several pieces; half of the liver was used for microsome preparation, and the other half was placed in TRIReagent (Sigma-Aldrich) for RNA extraction. In addition, 0.1 g of liver was removed from the protein fraction and used to prepare nuclear fractions. All samples were stored at −80°C.
ZOX-induced paralysis in NP-treated mice
Seventeen wild-type female and 14 CAR-null mice were randomly split into three treatment groups. Wild-type mice were treated with honey for 7 days followed by a corn oil injection on day 7, 50 mg/kg/day NP mixed in honey followed by a corn oil injection on day 7, or treated with honey for 7 days followed by an injection of 3 mg/kg TCPOBOP dissolved in corn oil. CAR-null mice were also split into untreated, 50 mg/kg/day NP, and 3 mg/kg/day TCPOBOP-treated groups as described above. On day 8, mice were injected ip with 300 mg/kg ZOX in corn oil. After injection, initial paralysis was noted, and paralysis time was measured by placing the mice on their backs and measuring the time until they were able to consistently right themselves. All statistical tests were performed with StatView software (SAS Institute Inc., Cary, NC). Data were analyzed by ANOVA followed by Fisher's PLSD as the post hoc test. A p-value ≤ 0.05 was regarded as statistically significant.
Primary human hepatocytes
Human hepatocytes plated in collagen I coated 12-well plates were obtained from CellzDirect (Pittsboro, NC), and provided hepatocyte culture medium (MEM Alpha Medium, Invitrogen Corporation, Carlsbad, CA) supplemented with 0.5% Penicillin/Streptomycin, 1% Insulin–Transferrin–Selenium (Invitrogen), and 100nM dexamethasone immediately after arrival and every 24 h thereafter. Human liver donor information is provided in Table 1. Cells were treated with DMSO as a vehicle, NP, or PB for 24 h. Cells were harvested with a cell scraper after a 10-min treatment with 1% trypsin, RNA was extracted, and quantitative reverse transcriptase–PCR (Q-PCR) performed as described below with primers to CYP2B6, CYP3A4, and 18S ribosomal RNA (rRNA).
TABLE 1.
General Information about Each Human Hepatocyte Donor
| Hepatocyte # | Age | Weight (lbs) | Height (in.) | Viability (%) | Sex | Race |
|---|---|---|---|---|---|---|
| Hu420 | 52 | 172 | 63 | 86 | F | C |
| Hu435 | 29 | 131 | 63 | 86 | F | C |
| Hu504 | 77 | 125 | 64 | 88 | F | C |
Note. F = female; C = Caucasian.
Sample preparations
RNA was extracted from approximately half of the liver with TRI-reagent according to the manufacturer's instructions followed by DNAse (Promega Corporation, Madison, WI) digestion to remove residual genomic DNA. RNA concentrations were determined spectrophotometrically at 260/280 nm (Molecular Devices, Ramsey, MN). Reverse transcription was performed to make complementary DNA (cDNA) using 200 units Moloney Murine Leukemia Virus–reverse transcriptase (Promega Corporation, Madison, WI), a 10mM deoxy-nucleotidyl tri-phosphate mixture, and 0.05 mg random hexamers.
For microsome preparation, approximately half of the liver was individually homogenized with a Dounce Homogenizer and microsomes were prepared as described previously (Van der Hoeven and Coon, 1974). Nuclear fractions were prepared by homogenizing individual liver tissue samples with a Dounce homogenizer using the Panomics (Redwood City, CA) nuclear extraction protocol. Protein concentrations were determined with the Bio-Rad protein assay (Bio-Rad, Hercules, CA) according to manufacturer's instructions.
Quantitative RT-PCR
Q-PCR was performed on mouse liver and human hepatocytes cDNA using the following primers: mouse Cyp2b10 (forward 5′-CTGAATCCGCTCCTCCACACTC-3′, reverse 5′-TGAGCCAACCTTCAA GGAATAT-3′); human CYP2B6 (forward 5′- AAGCGGATTTGTCTTGGTGAA-3′, reverse 5′-TGGAGGATGGTGGTGAAGAAG-3′); 18S rRNA was used as the housekeeping gene to normalize gene expression. This gene is highly conserved, so primers were designed that recognize both mouse and human (forward 5′-ATGGCCGTTCTTAGTTGGTG-3′; reverse 5′-ATGCCAGAGTCTCGTTCGTT-3′). Briefly, 320 ng of cDNA was incubated with Cyp2b10 primer, a 10mM dNTP mixture, 0.25×SybrGreen, and Taq polymerase (Qiagen, Valencia, CA). During PCR, samples were denatured at 95°C for 30 s, then lowered to the proper annealing temperature for 30 s, and extended at 72°C for 30 s. Annealing temperatures were 63.5°C (Cyp2b10), 63.5°C (CYP2B6), and 61.7°C (18S). Q-PCR was performed using a MyiQ Single Color Reverse Transcription PCR detection system (Bio-Rad Laboratories). Relative quantities of Cyp2b10 and CYP2B6 were determined as described previously using the equation (NE = {Eref}Ct-ref/{Etarget}Ct-target), where NE is the normalized gene expression, E is the efficiency of amplification for a particular gene, Ct is the threshold cycle, ref is the housekeeping gene (18S), and target is the gene of interest (Hernandez et al., 2006; Muller et al., 2002). ANOVA was performed followed by Fisher's PLSD as the post hoc statistical test. A p-value of ≤ 0.05 was regarded as significantly different from control values and is shown in figures with an asterisk.
Western blots
Western blots were performed on 50 μg of microsomal protein to measure P450 levels. Proteins were separated by polyacrylamide gel electrophoresis, in a 10% gel (Laemmli, 1970), and then transferred to 0.45-μm nitrocellulose (Bio-Rad Laboratories). Nitrocellulose was blocked, using 5% skim milk in 0.3% Tween 20 dissolved in phosphate buffered saline, for 15 min for Cyp2b10 and hCAR, and 45 min for β-actin. The blots were incubated with a rat anti-mouse Cyp2b10 antibody (generous gift from Dr Randy Rose), rabbit anti-mouse CAR antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or mouse anti-human β-actin (Sigma-Aldrich) at a 1:500 dilution. β-Actin was used as a housekeeper to ensure equal loading of samples. Goat anti-mouse (β-actin), goat anti-rat (Cyp2b10), and goat anti-rabbit (hCAR) alkaline phosphatase coupled secondary IgG (Bio-Rad) were used to recognize the primary antibodies. Bands were visualized colorimetrically with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as substrates (Bio-Rad Laboratories), and scanned on a GS-710 densitometer (Bio-Rad). Cyp2b10 and β-actin were quantified with LabWorks image analysis software (UVP laboratory products, Upland, CA), and statistical analysis was performed by ANOVA followed by Fisher's PLSD. A p-value of ≤ 0.05 was regarded as significantly different from control and is shown in figures with an asterisk.
RESULTS
Luciferase transactivation assays utilizing rPXR and hPXR reiterate previous results demonstrating NP activates rodent PXR (Masuyama et al., 2000; Mikamo et al., 2003). NP activated rPXR in a dose-dependent fashion with a maximal response of fourfold induction over untreated cells. PCN a known rPXR agonist was used as a positive control, and increased reporter expression by nearly 100-fold over controls (Fig. 1A). Transactivation of hPXR by NP was 5.5-fold greater than the controls, similar to the positive control, rifampicin (Fig. 1B). Therefore, these experiments confirm that NP activates rodent PXR and demonstrates that NP activates hPXR.
FIG. 1.
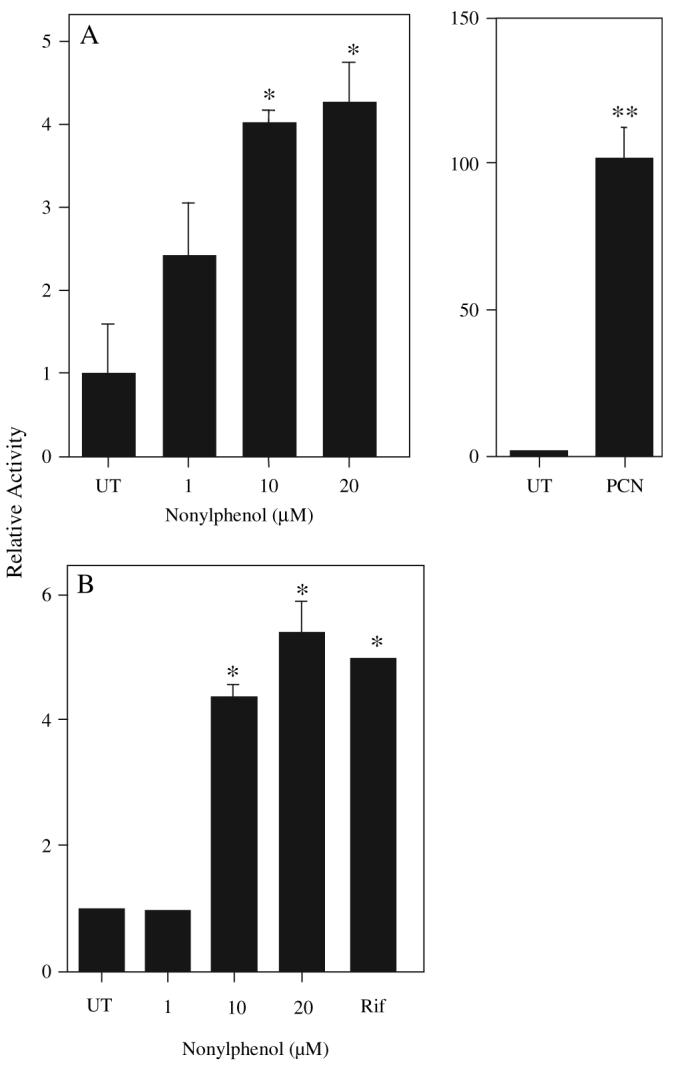
NP activates both the rPXR and hPXR. HepG2 cells were transfected with either the rPXR (A) or hPXR (B), and activation of PXR by NP determined using a luciferase-coupled reporter containing CYP3A23 PXRE. PCN (10μlM) and rifampicin (10μM) were used as positive controls for activation of rPXR and hPXR, respectively. The results are shown as fold induction compared to the solvent control, mean ± SD (n = 3 rPXR, n = 4 hPXR). Asterisk indicate a significant difference from the DMSO control (UT) cells by ANOVA followed by Fisher's PLSD as the post hoc test (*p < 0.05, **p < 0.01).
Previous results in our lab that demonstrated NP increased cyp2b RNA and protein expression, and testosterone 16β-hydroxylase activity, a biomarker of Cyp2b activity, suggested activation of CAR (Acevedo et al., 2005; Hernandez et al., 2006). Therefore, we performed mCAR transactivation assays in HepG2 cells to determine if mCAR was activated by NP in vitro. NP increased mCAR activity in a dose-dependent manner without androstanol present (Fig. 2A). Assays were also performed in the presence of the inverse agonist androstanol. Inhibition of CAR's constitutive activity by androstanol is necessary to increase the sensitivity and reliability of CAR transactivation assays. NP was able to reverse the androstanol-induced repression in a dose-dependent manner at concentrations as low as 1μM NP (Fig. 2B) with a maximum activation of 2.6-fold over the control at 20μM. In comparison, the potent CAR activator, TCPOBOP induced mCAR activity 3.6-fold (Fig. 2B). These studies indicate that NP is a relatively potent mCAR activator.
FIG. 2.
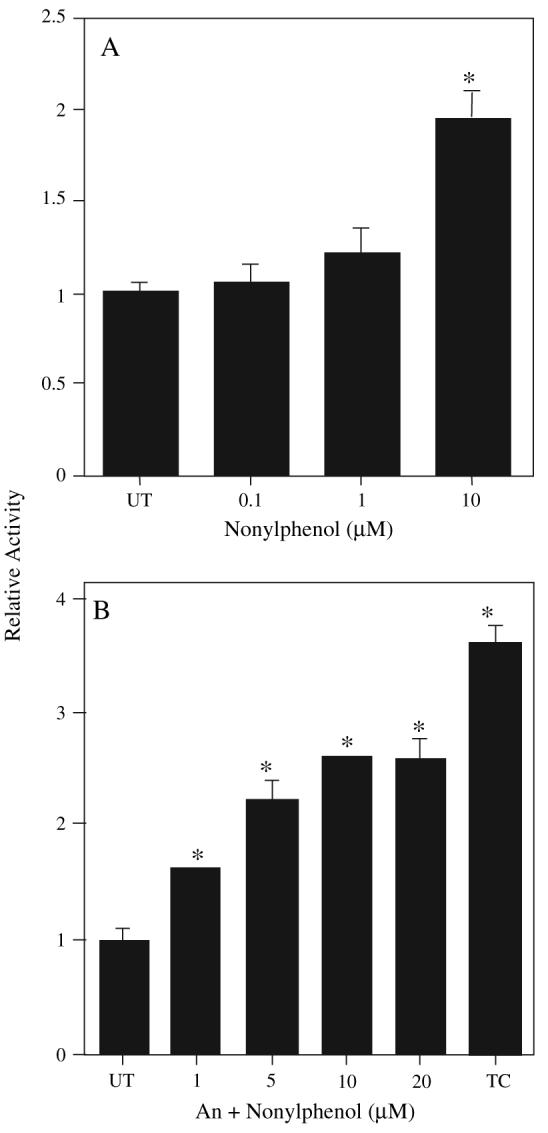
NP activates mCAR. HepG2 cells were cotransfected with an expression plasmid for mCAR and the luciferase reporter plasmid, containing CYP2B6 PBREM, were then treated with an increasing amount of NP (A). In graph (B) all mCAR-transfected cells were cotreated with the inverse agonist androstanol (An) to reduce mCAR's constitutive activity and increase the sensitivity of the assay. The potent mCAR agonist, TCPOBOP (TC) at 250nM, was used as a positive control. Data are presented as mean ± SD. Statistical analysis was performed by ANOVA followed by Fisher's PLSD as the post hoc test (n = 3). An asterisk indicates statistical significance from the androstanol control (p < 0.01).
Experiments were performed with wild-type and CAR-null mice to determine if NP could also activate CAR in vivo. Following treatment, mice were euthanized, livers excised, and translocation of CAR from the cytosol to the nucleus investigated because it is a key step in CAR transcriptional activity (Kawamoto et al., 1999). Western blots were performed on the nuclear extracts to detect the amount of CAR in the nucleus. CAR was not detectable in the nucleus in untreated wild-type mice. CAR was not detectable in TCPOBOP-treated CAR-null mice as expected (Fig. 3A), indicating the lack of CAR in this model. However the potent CAR agonist TCPOBOP markedly increased CAR protein levels in the nucleus of wild-type mice (Fig. 3B). Lastly, wild-type mice treated with 75 mg/kg/day NP also showed an increase in CAR protein levels in the nucleus (Fig. 3C), indicating that NP increased the nuclear translocation of CAR, which may lead to transcription of Cyp2b10 and other target genes.
FIG. 3.
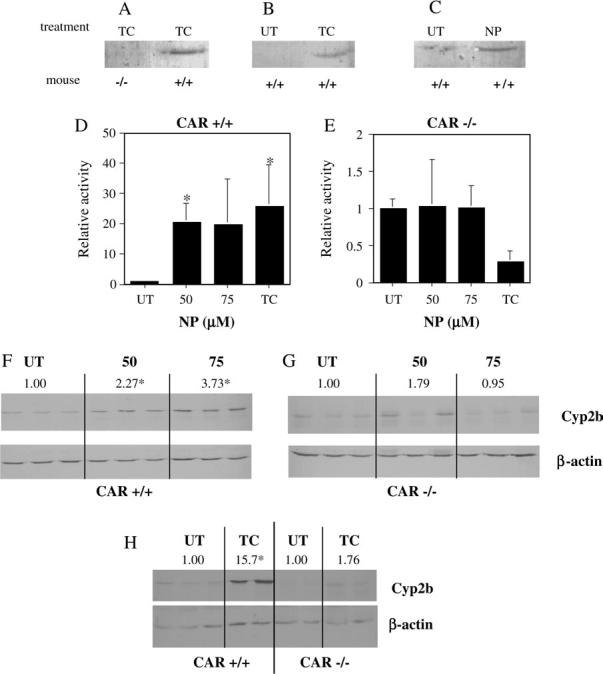
NP activation of CAR. NP increased the translocation of CAR into the nucleus as determined by Western blotting of nuclear fractions from wild-type (+/+) and CAR-null (−/−) mice. (A) CAR is detected in the nucleus of TCPOBOP (TC)–treated wild-type, but not TC-treated CAR-null mice. (B) Increased translocation of CAR into the nucleus following treatment with TC at 3 mg/kg/day. (C) Increased translocation of CAR into the nucleus following treatment with NP at 75 mg/kg/day (UT = untreated). Q-PCR of hepatic Cyp2b10 after treatment with NP or TC in (D) wild-type or (E) CAR-null mice demonstrates CAR-dependent induction of Cyp2b10. Data are presented as mean ± SD. Western blots were used to confirm changes in Cyp2b protein concentrations in wild-type (F) and CAR-null (G) mice treated with NP. A Western blot of Cyp2b levels in TC-treated wild-type and CAR-null mice are shown in (H). Below each Cyp2b Western blot picture is the housekeeping gene β-actin. Relative protein induction is denoted by the values above the Western blots. An asterisk indicates a statistical difference (p < 0.05) determined by an ANOVA followed by Fisher's PLSD (n = 3; Western blots, n = 3-4 Q-PCR).
We examined Cyp2b10 induction, a biomarker commonly used to measure CAR activation. Livers from wild-type (CAR +/+) mice treated with 50 or 75 mg/kg/day NP, showed a 20- or 19-fold induction of Cyp2b10, respectively, compared to untreated mice as measured by Q-PCR (Fig. 3D). In contrast, CAR-null (CAR −/−) mice showed no change in Cyp2b10 transcript levels following NP treatment (Fig. 3E). Both results were in concert with the well-characterized positive control, TCPOBOP (Figs. 3D and 3E). Interestingly, TCPOBOP caused a substantial but not statistically significant decrease in Cyp2b10 transcript levels (Fig. 3E). Transcript induction was confirmed by Western blots of Cyp2b protein. Cyp2b protein was induced in NP-treated wild-type mouse livers nearly fourfold over wild-type controls (Fig. 3F); while CAR-null mice treated with NP showed no change in Cyp2b protein levels (Fig. 3G). In comparison, TCPOBOP-treated wild-type mice showed a 15-fold induction of Cyp2b protein levels, and CAR-null mice showed no significant change in Cyp2b protein levels (Fig. 3H). Our data demonstrate that Cyp2b induction by TCPOBOP and NP is CAR dependent.
ZOX challenges were performed to determine the potential pharmacological impact of P450 induction. Previously we showed that NP decreases ZOX paralysis time similarly to the CAR activator TCPOBOP (Hernandez et al., 2006). These ZOX assays have the added benefit of determining whether an NP-mediated decrease in ZOX paralysis is CAR dependent. Untreated wild-type mice exhibited an average paralysis time of 55 min, which was markedly decreased by NP and TCPOBOP as both NP and TCPOBOP treatment decreased paralysis time to almost 0 min (Fig. 4). In contrast, all CAR-null mice, treated or untreated, had a longer paralysis time than the untreated wild-type mice indicating the importance of CAR in the maintenance and induction of P450s. For example, untreated CAR-null mice had an average paralysis time two times longer than untreated wild-type mice. TCPOBOP-treated CAR-null mice did not show a decrease in paralysis time compared to untreated CAR-null mice. However, NP-treated CAR-null mice showed a statistically significantly shorter paralysis time than untreated CAR-null mice suggesting a role for PXR in the induction of P450s by NP.
FIG. 4.
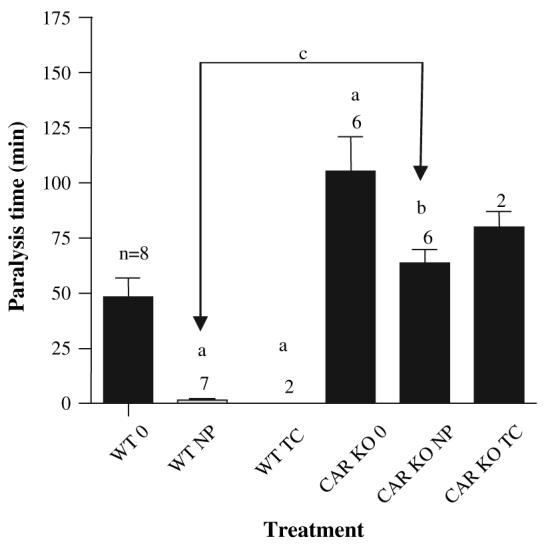
ZOX-induced paralysis in wild-type (WT) and CAR-null (CAR KO) mice treated with 50 mg/kg/day NP or 3 mg/kg/day TCPOBOP (TC). The number of mice treated in each group is provided above the error bars. An (a) indicates a statistical difference from the untreated wild-type mice (WT 0). A (b) indicates a statistical difference from the untreated CAR-null mice (CAR KO 0), and (c) indicates a significant difference from the wild-type mice treated with NP (WT NP) as determined by ANOVA followed with Fisher's PLSD as the post hoc test (p < 0.05). Data are presented as mean ± SEM.
CAR ligand activation profiles vary among species because CAR, like PXR, has undergone significant positive selection (Iyer et al., 2006; Krasowski et al., 2005). This can make extrapolating from mouse to human difficult. Therefore, we performed studies with hCAR mice. The humanized mouse model is especially useful since hCAR transactivation assays are unreliable because of its extensive constitutive activity and the lack of a potent inverse agonist. NP treatment at 50 or 75 mg/kg/ day, showed an induction of Cyp2b10 transcript levels of 7- and 2.5-fold, respectively, compared to untreated mice (Fig. 5A). Our lack of a dose–response in Cyp2b10 transcript levels in the hCAR mice is not understood. However, the Cyp2b protein levels in the NP-treated mice were induced in a nearly identical fashion (3.8-and 3.1-fold, respectively, at 50 and 75 mg/kg/day) at both doses provided (Fig. 5B). In comparison, the known hCAR agonist, CITCO that activates only hCAR and not mCAR (Maglich et al., 2003), induced Cyp2b10 messenger RNA (mRNA) 32-fold, but protein was induced only twofold (Figs. 5A and 5B). Overall, this work indicates that NP activates hCAR in addition to mCAR.
FIG. 5.
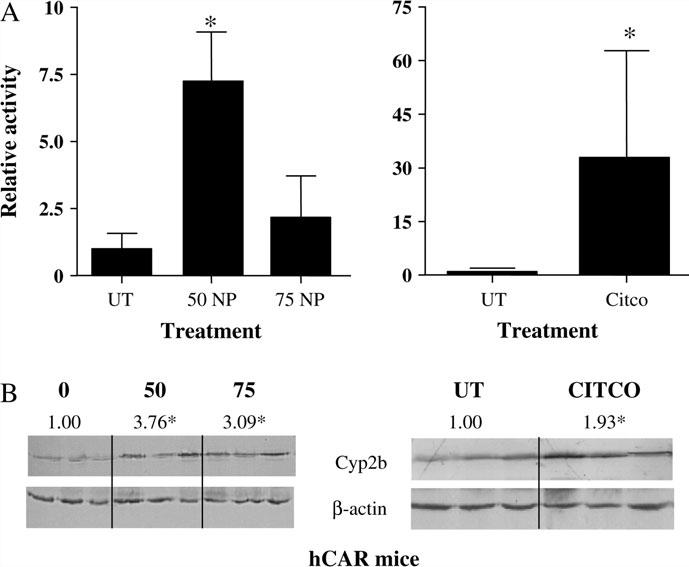
NP induces Cyp2b in humanized mice. CAR-null mice containing a transgene for human CAR (hCAR) on the albumin promoter were treated with 0, 50, or 75 mg/kg/day NP or 30 mg/kg/day CITCO. Q-PCR (A) and Western blots (B) of hepatic Cyp2b10 in hCAR mice indicate that NP activates hCAR in addition to mCAR. Data are presented as mean ± SD. An asterisk indicates a statistical difference (p < 0.05) determined by an ANOVA followed by Fisher's PLSD (n = 3; Western blots, n = 3–4 Q-PCR).
To confirm the hCAR results and measure the potential for NP-mediated P450 induction in humans, primary hepatocytes from three donors were treated with NP or PB. Primary hepatocytes are used extensively to determine potential P450 induction by pharmaceuticals (Faucette et al., 2004). NP induced CYP2B6, the human homolog to mouse Cyp2b10, as measured by Q-PCR in all three donors (Fig. 6). However, induction was highly variable and markedly less in NP-treated hepatocytes than PB-treated hepatocytes. NP induced CYP2B6 3.7 to 8.6-fold; PB induced CYP2B6 9- to 34-fold. CYP3A4 was also examined and produced similar but muted induction compared to CYP2B6 (data not shown). Taken together, these data indicates that NP is a moderate hCAR agonist and P450 inducer in humans.
FIG. 6.
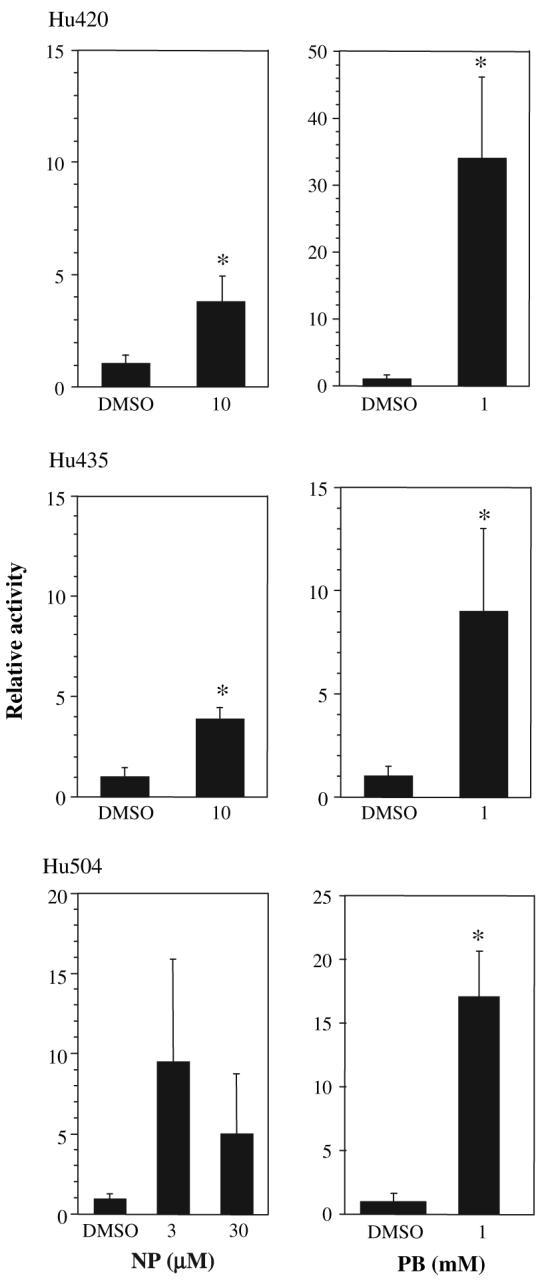
Induction of CYP2B6 in human hepatocytes by NP. Primary hepatocytes from three donors obtained from CellzDirect were treated with NP or PB for 24 h and CYP2B6 induction measured by Q-PCR. Data are presented as mean ± SD. An asterisk indicates a statistical difference (p < 0.05) as determined by a Student's t-test (n = 4 wells, Hu420, Hu435; n = 3 wells, Hu504).
DISCUSSION
NP, one of the most prevalent anthropogenic chemicals found in the United States, is a known P450 inducer (Acevedo et al., 2005; Hernandez et al., 2006; Lee et al., 1996; Masuyama et al., 2000; Mikamo et al., 2003). Research has demonstrated that NP activates PXR and in turn induces Cyp3a (Masuyama et al., 2000; Mikamo et al., 2003); however, recent research shows greater Cyp2b induction than Cyp3a indicating activation of CAR (Acevedo et al., 2005; Hernandez et al., 2006). Our data demonstrate that NP induces Cyp2b10 by activating CAR. Transactivation assays demonstrate CAR activation by NP, and in vivo results using CAR-null and wild-type mice confirmed these results and demonstrated that CAR is necessary for Cyp2b10 induction. Studies using ZOX showed pharmacologically relevant perturbations at high doses of NP. Lastly, studies with primary human hepatocytes and hCAR mice demonstrate the potential for hCAR-mediated P450 induction in humans.
Because previous research demonstrated that NP could activate murine PXR (Masuyama et al., 2000; Mikamo et al., 2003), we repeated these studies using rPXR. Our data confirmed that NP can activate rPXR, but is a weak partial agonist when compared to PCN. In contrast, hPXR is activated equally by both rifampicin and NP, which may be important in NP's ability to induce P450s in humans. To our knowledge, activation of hPXR by NP has not been previously reported.
Previous studies implicate CAR in NP-mediated Cyp2b10 induction (Hernandez et al., 2006); therefore, we tested whether NP activates CAR. Transactivation assays demonstrated that NP activates mCAR and can out-compete androstanol inhibition and induce luciferase activity. Androstanol also increased the sensitivity of the CAR transactivation assay, thus demonstrating significant activation of CAR at concentrations of NP as low as 1μM. The development of CAR-null mice (Wei et al., 2000) allowed us to determine that NP also activates CAR and induces Cyp2b10 in a CAR-dependent fashion in vivo. Western blots were used to confirm the Q-PCR results and demonstrated a 3.8-fold induction of Cyp2b that is much less than the 20-fold induction measured by Q-PCR. Antibodies for the Cyp2b's most likely recognize multiple Cyp2b isoforms in addition to Cyp2b10, and therefore we refer to the protein as only Cyp2b and not Cyp2b10. The recognition of other Cyp2b subfamily members may also have caused the observed differences between Q-PCR and Western blot results as some Cyp2b's may not be induced by NP (Hernandez et al., 2006).
ZOX paralysis has widely been used to determine the pharmacological relevance of P450 induction in vivo and determine possible drug–drug or drug–toxicant interactions. Increased paralysis indicates P450 inhibition and decreased paralysis indicates P450 induction. ZOX-induced paralysis time was decreased greater than 96% in NP-treated wild-type mice, nearly the same as ZOX-induced paralysis was decreased by the potent agonist, TCPOBOP. This demonstrates that NP activation induces several important drug metabolizing enzymes involved in the metabolism of ZOX, including Cyp2b10. Interestingly, ZOX paralysis was significantly greater in CAR-null mice than wild-type mice, further demonstrating that CAR is necessary for the maintenance of P450s in addition to P450 induction (Wei et al., 2000). Though a direct comparison of basal P450 or Cyp2b levels in wild-type and CAR-null mice was not compared in this paper, currently our preliminary unpublished results suggest a fourfold decrease in basal Cyp2b10 mRNA levels in CAR-null mice compared to the wild-type mice. Therefore, an increase in paralysis time in CAR-null mice may be in part due to a decrease in Cyp2b10 expression.
ZOX-induced paralysis was also decreased 39% in the NP-treated CAR-null mice, suggesting that PXR is also involved in the NP-mediated induction of P450s. However, the overall decrease in ZOX metabolism was much less in the CAR-null mice, suggesting that CAR's role in P450 induction is more prominent than PXRs. Alternatively, ZOX may be preferentially metabolized by Cyp2b's and therefore this assay is more sensitive in CAR-null mice. To our knowledge, the P450s responsible for ZOX metabolism have not been published, but increases in Cyp1a, Cyp2b, and Cyp3a activity have been correlated with decreased ZOX-induced paralysis (Hernandez et al., 2006; Kapitulnik et al., 1976; Staudinger et al., 2001; Wei et al., 2000). Furthermore, CAR regulates several phase II and III enzymes and transporters involved in xenobiotic clearance (Qatanani and Moore, 2005; Wei et al., 2000). Therefore, we can not omit the possibility that induction of these detoxification proteins may also be involved in increased ZOX clearance.
Currently, hCAR transactivation assays are nearly impossible and at best very unreliable because of hCAR's high constitutive activity and resistance to marked inhibition by inverse agonists (Zhang et al., 2002); thus, the recent development of hCAR mice provides an important mechanistic model for extrapolating CAR activation to humans. Treatment of hCAR mice with NP showed Cyp2b induction as measured by Western blotting and Q-PCR. The Q-PCR data were not dose dependent as Cyp2b10 increased sevenfold at 50 mg/kg/day, but only 2.5-fold at 75 mg/kg/day. Wild-type mice did not show a similar decrease in Cyp2b10 induction at 75 mg/kg/day (Fig. 3D). The reason for a lack of a dose–response in hCAR mice is unknown, but may be related to temporal effects caused by euthanasia 24 h following the last treatment. This may have caused mRNA levels to peak prior to euthanasia, but protein levels were still left at relatively high levels. The effect may also be related to preliminary unpublished results suggesting that hCAR levels in these mice are lower than the respective mCAR levels in wild-type mice. We highly doubt the decrease in Cyp2b10 transcript levels is caused by toxicity to the hCAR mice since neither wild-type nor CAR-null mice showed toxicity. Interestingly, protein levels as measured by western blots did not show a decrease in expression at 75 mg/kg/day relative to 50 mg/kg/day. However, both CITCO and NP showed less induction by Western blotting than Q-PCR similar to TCPOBOP and NP following treatment in wild-type mice. As mentioned previously, this may be due to the sensitivity of the assay or the antibody recognizing other Cyp2b isoforms that are not induced by CAR activation.
Furthermore, we measured Cyp2b induction in primary human hepatocytes. Though P450 induction in humans can show great variability, three different donors showed CYP2B6 induction. Patient Hu504 did not show statistically significant induction following NP treatment; however, only three replicates were performed for this donor and therefore the statistical power was reduced. Three replicates were performed because we were interested in investigating a potential dose–response and the addition of a second NP concentration reduced the number of wells available at each concentration. Interestingly, the lower dose showed the greatest increase in CYP2B6 levels measured in the donors, but this observation was not statistically significant. The lack of a dose–response to NP at the higher doses was also observed in hCAR mice, and in ZOX challenged FVB/NJ mice in a previous study (Hernandez et al., 2006). Therefore, this effect does not appear to be a species-specific effect mediated through hCAR, but this cannot be completely ruled out. Previously, we attributed the lack of a dose–response in the FVB/NJ mice to P450 inhibition by NP, but this does not explain the reduction in transcript relative to lower doses. NP-mediated toxicity seems unlikely as concentrations as high as 100μM caused no discernable toxicity as measured by an MTS assays (data not shown). Overall, the induction of CYP2B6 in NP-treated primary human hepatocytes also indicates potential drug–toxicant interactions in highly exposed and susceptible individuals.
The list of hCAR activators is short compared with the extensive list of chemicals known to activate mCAR. Very few environmentally relevant chemicals have been shown to activate hCAR and in turn induce P450s; therefore these data demonstrating the activation of hCAR by NP is unique. Several structurally diverse environmental estrogens have been shown to induce P450 expression by activating mCAR or PXR, including estradiol, NP, dichloro-diphenyl-trichloroethane, diethylhexylphthlate, and bisphenol A (Kretschmer and Baldwin, 2005). Future research in our laboratory and others should test whether hCAR also interacts with a diverse set of plasticizers, detergents, and environmental estrogens. Lastly, it is interesting to speculate as to whether other effects of NP such as its ability to increase adipocyte formation and proliferation (Masuno et al., 2003) are at least in part mediated by CAR, because CAR is also critical in regulating genes involved in energy and lipid metabolism (Shiraki et al., 2003; Ueda et al., 2002).
NP is one of the most ubiquitous chemicals in the environment and specific uses are being banned in several countries because of its estrogenicity, propensity to bioaccumulate, and toxicity. We have demonstrated in this manuscript that NP can induce Cyp2b10 through mCAR and NP is a CYP2B6 inducer in humans, probably through hCAR. Other chemicals (plasticizers, pesticides, detergents, dietary supplements, other occupational, and environmental chemicals) add to the load of CAR activators (Kretschmer and Baldwin, 2005) that we are exposed and may act in concert in a predictable chemical-addition manner (Rider and LeBlanc, 2005) to increase P450 induction and drug–toxicant interactions. At this time a chemical-addition model for CAR has not been developed, but the potential for drug–toxicant interactions mediated by CAR activation may grow as the list of environmental chemicals that activate CAR increases. Thus, identifying the chemicals that activate CAR may help us predict adverse drug reactions that cannot be predicted based on pharmaceutical exposure alone.
ACKNOWLEDGMENTS
The authors would like to thank Dr Li You, Dr Binfang Yan, and Dr Fan Zhang for their help with the transactivation assays, Dr Randy Rose for donating his Cyp2b10 antibody, and Dr Julia Bader for help with the statistical analysis. Research support for this study was provided by NIH grants GM008012-35 and G12RR08124. Juan P. Hernandez is supported in part by a National Science Foundation-Alliance for Graduate Education and the Professoriate award and an NIH Kirschstein fellowship, 1F31ES014113-01A1.
REFERENCES
- Acevedo R, Villanueva H, Parnell PG, Chapman LM, Gimenez T, Gray SL, Baldwin WS. The contribution of hepatic steroid metabolism to serum estradiol and estriol concentrations in nonylphenol treated MMTVneu mice and its potential effects on breast cancer incidence and latency. J. Appl. Toxicol. 2005;25:339–353. doi: 10.1002/jat.1078. [DOI] [PubMed] [Google Scholar]
- Ahel M, Giger W, Koch M. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment I. Occurrence and transformation in sewage treatment. Water Res. 1994;23:1131–1142. [Google Scholar]
- Ahel M, Giger W, Schaffner C. Environmental occurrence and behaviour of alkylphenol polyethoxylates and their degradation products in rivers and groundwaters. Swedish EPA Seminar on Nonylphenol Ethoxylate/Nonylphenol held in Saltsjobaden; Sweden. February 6-8.1991. pp. 105–151. [Google Scholar]
- Arukwe A, Forlin L, Goksoyr A. Xenobiotic and steroid biotransformation enzymes in Atlantic Salmon (Salmo salar) liver treated with an estrogenic compound, 4-nonylphenol. Environ. Toxicol. Chem. 1997;16:2576–2583. [Google Scholar]
- Baldwin WS, Graham SE, Shea D, LeBlanc GA. Metabolic androgenization of female Daphnia magna by the xenoestrogen 4-nonylphenol. Environ. Toxicol. Chem. 1997;16:1905–1911. [Google Scholar]
- Baldwin WS, Roling JA, Peterson S, Chapman LM. Effects of nonylphenol on hepatic testosterone metabolism and the expression of acute phase proteins in winter flounder (Pleuronectes americanus): Comparison to the effects of Saint John's Wort. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2005;140:87–96. doi: 10.1016/j.cca.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh WJ, Juguilon H, Bolado JJ, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TKH. Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR) Drug Metab. Rev. 2006;38:51–73. doi: 10.1080/03602530600569828. [DOI] [PubMed] [Google Scholar]
- Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: Structure-activity relationships. Toxicol. Appl. Pharmacol. 1997;145:111–123. doi: 10.1006/taap.1997.8169. [DOI] [PubMed] [Google Scholar]
- Connor K, Safe S, Jefcoate CR, Larsen M. Structure-dependent induction of CYP2B by polychlorinated biphenyl congeners in female Sprague-Dawley rats. Biochem. Pharmacol. 1995;50:1913–1920. doi: 10.1016/0006-2952(95)02087-x. [DOI] [PubMed] [Google Scholar]
- Dehal SS, Kupfer D. Metabolism of the proestrogenic pesticide methoxychlor by hepatic P450 monooxygenases in rats and humans. Dual pathways involving novel orthoring-hydroxylation by CYP2B. Drug Metab. Dispos. 1994;22:937–946. [PubMed] [Google Scholar]
- Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J. Pharmacol. Exp. Ther. 2007;320:72–80. doi: 10.1124/jpet.105.098160. [DOI] [PubMed] [Google Scholar]
- Faucette SR, Wang H, Hamilton GA, Jolley SL, Gilbert D, Lindley C, Yan B, Negishi M, LeCluyse EL. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab. Dispos. 2004;32:348–358. doi: 10.1124/dmd.32.3.348. [DOI] [PubMed] [Google Scholar]
- Fernandes AR, Costley CT, Rose M. Determination of 4-octylphenol and 4-nonylphenol congeners in composite foods. Food Addit. Contam. 2003;20:846–852. doi: 10.1080/0265203031000138303. [DOI] [PubMed] [Google Scholar]
- Ferrara F, Fabietti F, Delise M, Bocca AP, Funari E. Alkylphenolic compounds in edible molluscs of the Adriatic Sea (Italy) Environ. Sci. Technol. 2001;35:3109–3112. doi: 10.1021/es010508h. [DOI] [PubMed] [Google Scholar]
- Ferrara F, Fabietti F, Delise M, Funari E. Alkylphenols and alkylphenol ethoxylates contamination of crustaceans and fishes from the Adriatic Sea (Italy) Chemosphere. 2005;59:1145–1150. doi: 10.1016/j.chemosphere.2004.11.085. [DOI] [PubMed] [Google Scholar]
- Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- Fu X, Cooper SM, Delclos KB. Induction of hepatic enzyme expression by p-nonylphenol (NP) is modulated by diet in male Sprague-Dawley rats (#1829) The Toxicologist CD—An official Journal of the Society of Toxicology. 2006;90:S-1. [Google Scholar]
- Gilbert MA, Shepherd MK, Startin JR, Wallwork MA. Identification by gas chromatography-mass spectrometry of vinylchloride oligomers and other low molecular weight components of poly (vinylchloride) resins for food package applications. J. Chromatogr. 1992;237:249–261. [Google Scholar]
- Hernandez JP, Chapman LM, Kretschmer XC, Baldwin WS. Gender specific induction of cytochrome P450s in nonylphenol-treated FVB/NJ mice. Toxicol. Appl. Pharmacol. 2006;216:186–196. doi: 10.1016/j.taap.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer M, Reschly EJ, Krasowski MD. Functional evolution of the pregnane X receptor. Expert Opin. Drug Metab. Toxicol. 2006;2:381–397. doi: 10.1517/17425255.2.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitulnik J, Levin W, Poppers PJ, Tomaszewski JE, Jerina DM, Conney AH. Comparison of the hydroxylation of zoxazolamine and benzo[a]pyrene in human placenta: Effect of cigarette smoking. Clin. Pharmacol. Ther. 1976;20:557–564. doi: 10.1002/cpt1976205557. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999-2000: A National Reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Yasuda K, Hagey LR, Schuetz EG. Evolutionary selection across the nuclear hormone receptor superfamily with a focus on the NR1I subfamily (vitamin D, pregnane X, and constitutive androstane receptors) Nucl. Recept. 2005;3:2. doi: 10.1186/1478-1336-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. CAR and PXR: Xenosensors of endocrine disrupters? Chem. Biol. Interact. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol. Sci. 2000;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- Lee PC, Patra SC, Struve M. Modulation of rat hepatic CYP3A by nonylphenol. Xenobiotica. 1996;26:831–838. doi: 10.3109/00498259609046753. [DOI] [PubMed] [Google Scholar]
- Loyo-Rosales JE, Rosales-Rivera GC, Lynch AM, Rice CP, Torrents A. Migration of nonylphenol from plastic containers to water and a milk surrogate. J. Agric. Food Chem. 2004;52:2016–2020. doi: 10.1021/jf0345696. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Masuno H, Okamoto S, J I, Honda K, Shiosaka T, Kidani T, Sakayama K, Yamamoto H. Effect of 4-nonylphenol on cell proliferation and adipocyte formation in cultures of fully differentiated 3T3-L1 cells. Toxicol. Sci. 2003;75:314–320. doi: 10.1093/toxsci/kfg203. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y, Kunitomi M, Takafumi K, MacDonald PN. Endocrine disrupting chemicals, phthalic acid and nonylphenol, activate Pregnane X receptor-mediated transcription. Mol. Endocrinol. 2000;14:421–428. doi: 10.1210/mend.14.3.0424. [DOI] [PubMed] [Google Scholar]
- Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, Van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikamo E, Harada S, Nishikawa J, Nishihara T. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol. Appl. Pharmacol. 2003;193:66–72. doi: 10.1016/j.taap.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, Lambert MH, Moore JT. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol. Endocrinol. 2002;16:977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J. Biol. Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- Petrovic M, Diaz A, Ventura F, Barcelo D. Occurrence and removal of estrogenic short-chain ethoxy nonylphenolic compounds and their halogenated derivatives during drinking water production. Environ. Sci Technol. 2003;37:4442–4448. doi: 10.1021/es034139w. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Moore DD. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr. Drug Metab. 2005;6:329–339. doi: 10.2174/1389200054633899. [DOI] [PubMed] [Google Scholar]
- Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Roland C. AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J. Biol. Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- Renner R. European bans on surfactant trigger transatlantic debate. Environ. Sci. Technol. 1997;31:316A–319A. doi: 10.1021/es972366q. [DOI] [PubMed] [Google Scholar]
- Rider CV, LeBlanc GA. An integrated addition and interaction model for assessing toxicity of chemical mixtures. Toxicol. Sci. 2005;87:520–528. doi: 10.1093/toxsci/kfi247. [DOI] [PubMed] [Google Scholar]
- Ripp SL, Fitzpatrick JL, Peters JM, Prough RA. Induction of CYP3A expression by dehydroepiandrosterone: Involvement of the pregnane X receptor. Drug Metab. Dispos. 2002;30:570–575. doi: 10.1124/dmd.30.5.570. [DOI] [PubMed] [Google Scholar]
- Shindo S, Numazawa S, Yoshida T. A physiological role of AMP-activated protein kinase in phenobarbital-mediated constitutive androstane receptor activation and CYP2B induction. Biochem. J. 2007;401:735–741. doi: 10.1042/BJ20061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki T, Sakai N, Kanaya E, Jingami H. Activation of orphan nuclear constitutive androstane receptor requires subnuclear targeting by peroxisome proliferator-activated receptor gamma coactivator-1 alpha. A possible link between xenobiotic response and nutritional state. J. Biol. Chem. 2003;278:11344–11350. doi: 10.1074/jbc.M212859200. [DOI] [PubMed] [Google Scholar]
- Song X, Li Y, Liu J, Mukundan M, Yan B. Simultaneous substitution of phenylalanine-305 and aspartate-318 of rat pregnane X receptor with the corresponding human residues abolishes the ability to transactivate the CYP3A23 promoter. J. Pharmacol. Exp. Ther. 2005;312:571–582. doi: 10.1124/jpet.104.074971. [DOI] [PubMed] [Google Scholar]
- Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonylphenol: An estrogenic xenobiotic released from “modified” polystyrene. Environ. Health Perspect. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab. Dispos. 2001;29:1467–1472. [PubMed] [Google Scholar]
- Suino K, Peng L, Reynolds R, Li Y, Cha JY, Repa JJ, Kliewer SA, Xu HE. The nuclear xenobiotic receptor CAR: Structural determinants of constitutive activation and heterodimerization. Mol. Cell. 2004;16:893–905. doi: 10.1016/j.molcel.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: A critical review of the evidence. Crit. Rev. Toxicol. 1998;28:319–361. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Van der Hoeven TA, Coon MJ. Preparation and properties of partially purified cytochrome P450 and NADPH-cytochrome P450 reductase from rabbit liver microsomes. J. Biol. Chem. 1974;249:6302–6310. [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J. Biol. Chem. 2003;278:14146–14152. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. The human nuclear xenobiotic receptor PXR: Structural determinants of directed promiscuity. Science. 2001;292:2329–2333. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacog. J. 2002;2:117–126. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135:175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Wyde ME, Bartolucci E, Ueda A, Zhang H, Yan B, Negishi M, You L. The environmental pollutant 1,1-dichloro-2,2-bis (p-chlorophenyl)ethylene induces rat hepatic cytochrome P450 2B and 3A expression through the constitutive androstane receptor and pregnane X receptor. Mol. Pharmacol. 2003;64:474–481. doi: 10.1124/mol.64.2.474. [DOI] [PubMed] [Google Scholar]
- Wyde ME, Kirwan SE, Zhang F, Laughter A, Hoffman HB, Barolucci-Page E, Gaido KW, Yan B, You L. Di-n-Butyl phthalate activates constitutive androstane receptor and pregnane X receptor and enhances the expression of steroid-metabolizing enzymes in the liver of rat fetuses. Toxicol. Sci. 2005;86:281–290. doi: 10.1093/toxsci/kfi204. [DOI] [PubMed] [Google Scholar]
- Xie W, Evans RM. Orphan nuclear receptors: The exotics of xenobiotics. J. Biol. Chem. 2001;276:37739–37742. doi: 10.1074/jbc.R100033200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hashizume T, Shuhart MC, Davis CL, Nelson WL, Sakaki T, Kalhorn TF, Watkins PB, Schuetz EG, Thummel KE. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1 alpha, 25-dihydroxyvitamin D(3): Implications for drug-induced osteomalacia. Mol. Pharmacol. 2006;69:56–65. doi: 10.1124/mol.105.017392. [DOI] [PubMed] [Google Scholar]
- Yang DK, Ding WH. Determination of alkylphenolic residues in fresh fruits and vegetables by extractive steam distillation and gas chromatography-mass spectrometry. J. Chromatogr. A. 2005;1088:200–204. doi: 10.1016/j.chroma.2004.11.063. [DOI] [PubMed] [Google Scholar]
- Zhang H, LeCulyse E, Liu L, Hu M, Matoney L, Zhu W, Yan B. Rat pregnane X receptor: Molecular cloning, tissue distribution, and xenobiotic regulation. Arch. Biochem. Biophys. 1999;368:14–22. doi: 10.1006/abbi.1999.1307. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: Pathogenetic and therapeutic considerations. Mol. Pharmacol. 2006;3:231–251. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]


