Abstract
Premature activation of digestive enzymes within the pancreas which leads to autodigestion of the gland is an early step in the pathogenesis of pancreatitis. Pancreatic injury is followed by other manifestations of inflammation including plasma extravasation, edema, and neutrophil infiltration which constitute the features of pancreatitis. Recent studies indicate that neural innervation of the pancreas may play an important role in the initiation and maintenance of the inflammatory response to injury. The pancreas is innervated by vagal, sympathetic and parasympathetic neurons, as well as sensory neurons. Activation of pancreatic primary sensory neurons causes the release of inflammatory neuropeptides both in the spinal cord to signal pain and in the pancreas itself where they produce plasma extravasation and neutrophil infiltration. Recent studies indicate that primary sensory neurons of the pancreas express transient receptor potential V1 (TRPV1) channels whose activation induces pancreatic inflammation. Moreover, blockade of these TRP channels significantly ameliorates experimental pancreatitis. This review describes our current understanding of the role of TRPV1 channels in pancreatitis and illustrates how this mechanism might be used to direct future treatments of pancreatic diseases.
Keywords: acute pancreatitis, chronic pancreatitis, experimental pancreatitis, neurogenic inflammation, substance P, vanilloid receptor, VR1, capsaicin receptor
Introduction
The exocrine pancreas synthesizes, stores, and secretes digestive enzymes essential for normal digestion. Pancreatic injury often initiates conversion of the proenzyme trypsinogen to its active form trypsin. When typsin exceeds the capacity of endogenous trypsin inhibitors, activation of other zymogens occurs within the pancreas producing a proteolytic cascade that ultimately causes autodigestion of the gland [1]. Pancreatitis develops when this destructive process is accompanied by inflammatory features including cytokine release, vasodilation, edema, and neutrophil infiltration [2].
The pancreas is innervated by the vagus nerve containing cholinergic neurons that regulate exocrine section as well as parasympathetic and sympathetic neurons that modulate both endocrine and exocrine function [3]. The vagus nerve and splanchnic nervous system contain both stimulatory and inhibitory neurotransmitters that precisely regulate the secretion of pancreatic enzymes, fluid, and bicarbonate necessary for digestion of a meal. In addition, the pancreas is supplied with sensory neurons that contain vasoactive and inflammatory neuropeptides such as bradykinin, calcitonin gene-related peptide (CGRP) and substance P [4–6]. These nerves are responsible for signaling painful stimuli originating in the pancreas. It has long been recognized that certain neural influences may induce inflammation [7]. This process known as neurogenic inflammation is characterized by vasodilation, edema, and neutrophil infiltration and can be reproduced by specific neuropeptides such as bradykinin, CGRP and substance P [8].
These actions raise the possibility that sensory neurotransmitters may contribute to the inflammatory response following pancreatic injury. Only recently has the role of sensory nerves in the pancreas been extensively examined.
Features of Acute Pancreatitis
Acute pancreatitis is associated with intra-acinar cell activation of digestive enzymes [9]. Under normal conditions, small amounts of trypsinogen may become spontaneously activated, but further proteolytic activation of other enzymes does not occur because trypsin is inactivated by either endogenous trypsin inhibitors or enzymatic autodigestion of trypsin itself. However, when the amount of activated trypsin is high, as occurs with certain forms of pancreatic injury, and exceeds the trypsin inhibitory capacity of the pancreas, uncontrolled proteolytic activation of pancreatic proenzymes transpires leading to acinar cell autolysis and necrosis [10, 11]. Secretion of digestive enzymes is reduced and fusion of zymogen granules with lysosomes can be observed [12]. Zymogen-lysosomal granule fusion may contribute further to enzyme activation [13]. As is true in many examples of tissue injury, pancreatic damage and initiation of the autodigestive process is followed by release of cytokines and other inflammatory mediators that ultimately cause acute inflammation [14]. Pancreatic inflammation may cause more extensive pancreatic injury before resolution occurs. Severe pancreatitis is associated with extensive tissue necrosis.
Experimentally, acute pancreatitis can be reproducibly induced by administering supraphysiological amounts of the secretagogue, caerulein; feeding a diet deficient in choline and supplemented with ethionine; ligating the bile-pancreatic duct; or injecting high amounts of arginine [1]. Although the severity of pancreatitis and tissue necrosis varies among these different models, the fundamental features of zymogen activation, reduction in enzyme secretion, and plasma extravasation, edema, and neutrophil infiltration within the pancreas are common to all of these models.
Neurogenic inflammation
Neurogenic inflammation occurs following activation of sensory neurons and release of bioactive substances that produce vasodilation, edema, and other manifestations of local inflammation. Primary sensory neurons innervate the entire body (except the brain) and are responsible for the detection of pain and transmission of painful stimuli to the central nervous system. The cell bodies of primary sensory neurons reside in dorsal root ganglia and send axonal projections centrally to the spinal cord and peripherally to innervate specific organs including the pancreas. Neurotransmitters are released from sensory neurons following activation by dorsal root reflexes, axonal reflexes, or local depolarization [8]. The role of sensory neurons is to relay information from the periphery to the central nervous system. When a sensory nerve is stimulated an orthodromic signal is generated and neurotransmitters are released in the spinal cord which, when relayed to the brain, signal pain. However, when neuronal depolarization occurs, an antidromic signal is also generated and neurotransmitters are simultaneously released at the peripheral end of the neuron. Therefore, stimulation of primary sensory neurons has the ability to activate neurons in the spinal cord to signal pain as well as release neurotransmitters in the periphery that exert other bioactive effects.
Primary sensory neurons contain a number of bioactive transmitters some of which cause local inflammation. Two subpopulations of unmyelinated neurons, known as C and Aδ fibers, are particularly relevant since these contain the tachykinins neurokinin A, neurokinin B, and substance P as well as calcitonin gene-related peptide (CGRP), glutamate, and adenosine, all of which have proinflammatory actions. Local substance P release has been measured using a novel immunological technique to detect substance P-stimulated internalization of the substance P receptor [known as neurokinin 1 (NK1) receptor] [15]. These studies have demonstrated that substance P is released in the spinal cord and in the periphery following sensory nerve stimulation [16, 17].
Substance P and CGRP appear to be responsible for many of the features of neurogenic inflammation. Not only are both neuropeptides released by C and Aδ fibers but they have the abilities to interact with endothelial cells, arterioles, mast cells and other immune cells to induce vasodilation, edema and inflammatory cell infiltration. The manifestations of neurogenic inflammation can be mimicked by local administration of substance P and CGRP and these effects can be blocked by administration of immunoneutralizing peptide antibodies or substance P or CGRP receptor antagonists. Moreover, inflammatory responses to injury or substance P are substantially reduced in mice with genetic deletion of substance P receptors [18].
The effects of substance P and CGRP are transient; however, following severe injury, the manifestations of neurogenic inflammation are more profound. Under pathological conditions, neutrophil infiltration and mast cell degranulation amplify the inflammatory response by releasing proinflammatory molecules that cause neutrophil adhesion, migration, and release of reactive oxygen species [19–21].
Sensory neurons respond to physical and chemical stimuli. C and Aδ fibers are particularly noteworthy because they are sensitive to capsaicin, the active ingredient in hot peppers. Capsaicin, acting through C and Aδ neurons, induces feelings of warmth and in high concentrations causes inflammation. The actions of capsaicin on the bronchial tree, skin, joints, and gastrointestinal tract have been well documented [22]. Through its effects on sensory neurons, injection of capsaicin causes hypersensitivity, redness due to vasodilation, and edema due to plasma extravasation. However, these phenomena can be prevented by repeated administration of capsaicin which depletes neurotransmitters from nerve terminals. Interestingly, administration of very high amounts of capsaicin, particularly to neonatal animals, which are exquisitely sensitive to the compound, destroys C and Aδ neurons and renders animals insensitive to capsaicin-induced inflammation [23].
A major advance was the discovery and molecular cloning of the capsaicin receptor [24] which is expressed on C and Aδ fibers. Based on the structure of its ligand, the capsaicin receptor was originally named the vanilloid receptor 1 (VR1) but it was soon recognized that it was a cation channel and has since been renamed transient receptor potential vanilloid type 1 (TRPV1). TRPV1 is a non-selective cation channel and when activated, permits the flow of cations such as sodium and calcium from the outside to the inside of the cell. Cation influx, of which sodium is likely to be the most important, causes the depolarization of TRPV1-bearing neurons and leads to release of neurotransmitters, such as substance P, from nerve terminals. Capsaicin, by activating TRPV1, is highly specific for C and Aδ fibers and causes neuronal release of substance P and CGRP.
TRPV1 is not only activated by capsaicin, but also by heat and protons [25]. Increasing the temperature of tissues reduces the threshold for activation of TRPV1 and leads to substance P and CGRP release in both the spinal cord and periphery. Mice in which the TRPV1 gene was deleted are less sensitive to the application of painful heat [26]. Thus, TRPV1 is one of the mechanisms that sense local increase in temperature. In addition, acidification of the extracellular environment also activates TRPV1. The convergence of heat, acid and capsaicin on TRPV1 explains, in part, why pepper (i.e., capsaicin) feels hot. In low concentrations, capsaicin induces feelings of warmth, but in high concentrations, through the release of proinflammatory neuropeptides such as substance P, is highly inflammatory and causes plasma extravasation. Moderate acidity (pH≤6.4) lowers the temperature threshold for activation of TRPV1 such that activation can occur at 37°C. Thus, the burning sensations associated with application of acid or local acidification of tissue appears to occur through activation of TRPV1 neurons [27]. Importantly, it appears that one of the major effects of protons on sensory nerves is to potentiate the responses of other activators of TRPV1 [28].
Under physiological conditions, capsaicin-sensitive sensory nerves are probably responsible for signaling local increases in temperature which are interpreted as painful heat. Release of inflammatory neuropeptides may produce local edema and other sequelae that lead to the process of normal repair following tissue injury. Under pathophysiological conditions, more extensive involvement of sensory neurons produces excessive neuropeptide release that may exacerbate inflammation. This may explain why substance P pathways have been implicated in diseases such as arthritis, asthma, and inflammatory bowel disease [28].
Capsaicin is a plant molecule that is not produced by mammalian species. Therefore, following the discovery of TRPV1, considerable investigation has been devoted to identifying endogenous activators of the “capsaicin receptor” TRPV1 and the mechanisms that signal heat hyperalgesia [29]. It has since been recognized that the temperature threshold of TRPV1 is lowered by proinflammatory mediators such as bradykinin, ATP and other signaling molecules that activate protein kinase C (PKC) [30–32]. TRPV1 is also sensitized by phosphatidylinositol-4,5-bisphosphate (PIP2) hydrolysis after phospholipase C activation [33]. Interestingly, protease-activated receptor 2 (PAR-2), which may have a role in pancreatitis, has been shown to potentiate TRPV1 activation through a PKC-dependent mechanism [34]. By lowering the temperature threshold, TRPV1 can be activated at temperatures lower than 42°C, thus increasing the sensitivity of the ion channel to other endogenous mediators. Other proinflammatory mediators such as prostaglandins which activate protein kinase A (PKA) phosphorylate TRPV1 and cause a reduction in desensitization of the channel [35]. Nerve growth factor (NGF) has been shown to regulate TRPV1 expression through two mechanisms. First, NGF sensitizes TRPV1-bearing neurons by increasing the number of ion channels in the cell membrane [36]. NGF activates PI3 kinase which, through Src phosphorylation, induces the rapid movement of TRPV1 channels to the plasma membrane. A second, long-term effect is NGF’s ability to upregulate de novo expression of TRPV1 through activation of the Ras-MAPK pathway [37, 38].
Capsaicin is similar in structure to some endogenous lipid molecules including members of the arachidonic acid family. This similarity raised the possibility that related molecules may be able to interact with TRPV1. Subsequently, the lipid mediator anandamide and the proinflammatory leukotriene B4 (LTB4) have recently been shown to directly activate TRPV1 and play essential roles in the inflammatory response following an injurious insult [39, 40]. These findings indicate that endogenous TRPV1 signaling molecules exist in several forms. Since molecules like anandamide and LTB4 are generated during tissue injury, they are primed to activate primary sensory neurons through their actions on TRPV1 which leads to the release of other inflammatory mediators such as substance P.
Trypsin has been shown to activate primary sensory neurons [41]. This observation is particularly pertinent in pancreatitis, since activation of trypsinogen to trypsin is a key step in the initiation of the disease. Primary sensory neurons possess proteinase-activated receptors (PARs) which are a family of G protein-coupled receptors that are activated by proteases such as trypsin or thrombin [42]. The unique feature of these receptors is that they are activated when proteases cleave the amino terminus of the extracellular region, exposing a new amino sequence that functions as a tethered ligand. This new amino terminus binds to and activates the PAR. Four PARs have been identified (PARs 1-4). PARs 1, 3, and 4 are activated by thrombin but PAR-2 is unique in that it is activated by trypsin and tryptase. PAR-2 is expressed on neutrophils, endothelial, epithelial, and mast cells and dorsal root ganglion neurons [43]. Activation of PAR-2 on vascular endothelial cells causes vasodilation and in neurons PAR-2s are linked to sensory neurotransmission. It was recently demonstrated in sensory neurons that PAR-2 sensitizes TRPV1 by activating PKCε and PKA to cause thermal hyperalgesia [44]. This mechanism may also contribute to inflammatory pain, where multiple proteases are generated that could activate PAR-2.
It is now apparent that proinflammatory mediators may regulate TRPV1 directly or indirectly through other receptors on sensory nerves. For example, capsaicin, protons, and heat directly activate TRPV1. Lipid mediators such as anandamide, which is structurally similar to capsaicin, also have direct effects on TRPV1. In contrast, bradykinin binds to the B2 receptor, which resides on primary sensory neurons and, through PKC, stimulates primary sensory neurons and modulates TRPV1 activity [45]. The PAR-2 receptor is activated by extracellular proteases and stimulates sensory neurons independently of TRPV1. Leukotriene B4 is interesting because it can directly activate TRPV1 or, by binding to its own LTB4 receptor, activate intraneuronal signaling pathways that could indirectly modulate TRPV1.
There are a number of compelling findings to indicate that neural influences contribute to the pathogenesis of pancreatitis [46]. First, in other systems, release of neuropeptides such as substance P and CGRP induce pain and cause inflammation. Second, antagonism of sensory neuropeptides (e.g., substance P and CGRP) ameliorates inflammation. Third, agents that activate TRPV1-bearing sensory neurons stimulate neuropeptide release and induce inflammation. Fourth, sensory neurons containing potentially inflammatory mediators such as substance P are present in the pancreas.
Neuropeptides & Substance P in pancreatitis
Several studies indicate that neurogenic inflammation may be important in the development of acute pancreatitis. Substance P is released from sensory nerves and binds to the neurokinin 1 (NK1) receptor on endothelial and epithelial cells. In the pancreas, acinar cells express NK1 receptors and substance P-containing neurons are abundant. Substance P-induced plasma extravasation in the pancreas is blocked by NK1 receptor antagonists [47]. It was recently demonstrated that pancreatic levels of substance P are elevated and NK1 receptor expression is increased in a model of caerulein-induced acute pancreatitis [48]. Importantly, hyperamylasemia, hyperlipasemia, neutrophil sequestration in the pancreas, and pancreatic acinar cell necrosis as well as pancreatitis-associated lung injury were significantly reduced in NK1R knockout mice when compared with wild-type animals [48]. These findings indicate that substance P acting through NK1 receptors is proinflammatory and is responsible, at least in part, for the severity of acute pancreatitis. Similar protective effects were observed with hemorrhagic pancreatitis induced by feeding mice a diet deficient in choline, supplemented with ethionine (CDE diet) in NK1 receptor knockout mice [49]. The precise roles of substance P and its receptor were further investigated using selective NK1 receptor agonists and antagonists [50]. In rats, both substance P and NK1 receptor agonists and caerulein administration stimulated pancreatic plasma extravasation and this effect was blocked by an NK1 receptor antagonist. In contrast to wild type mice, administration of exogenous substance P did not induce pancreatic edema in NK1 receptor knockout mice [50]. These findings indicate that substance P and its receptor are essential for development of inflammatory edema in acute pancreatitis.
The actions of substance P are terminated at the cell surface of the effector cell by the cell surface enzyme neutral endopeptidase, which degrades substance P. In a lethal model of hemorrhagic pancreatitis induced by the CDE diet, genetic deletion of the NK1 receptor prevented diet-induced mortality from pancreatitis and reduced acinar cell necrosis [51]. Conversely, genetic deletion of neutral endopeptidase significantly worsened survival and exacerbated the severity of diet-induced pancreatitis and associated lung injury. These findings further support the important role of substance P in necrotizing pancreatitis. It is interesting to speculate that defects in neutral endopeptidase may play a role in clinical pancreatitis.
TRPV1 in pancreatitis
Experimental models have been extremely useful for defining the organ-specific and cellular pathological processes that occur during the development and evolution of pancreatitis. Considerable data indicate that intra-pancreatic activation of digestive enzymes are a necessary and early event and may result from direct pancreatic injury [9, 52]. The severity of pancreatitis that follows is related to a number of interacting factors that either exacerbate injury (e.g., generation of proinflammatory mediators, ischemia, oxygen-derived free radical generation, etc.), or protect the pancreas (e.g., endogenous trypsin inhibitors, mesotrypsin, etc.) that impact the mode of cell death which itself may be deleterious. Among the proinflammatory mediators that may contribute to pancreatitis severity, substance P has received particular attention. In the gastrointestinal tract and pancreas, substance P has been shown to stimulate plasma extravasation from postcapillary venules [47]. Moreover, blocking substance P’s actions with specific NK1 receptor antagonists, or genetic deletion of the NK1 receptor, reduced pancreatic edema and neutrophil infiltration [47, 48]. Innervation of the pancreas with sensory neurons that contain proinflammatory transmitters such as substance P and CGRP and localization of TRPV1 on these neurons raise the possibility that sensory nerve activation in the pancreas is instrumental in a neurogenic process that contributes to the pathogenesis of pancreatitis.
If neurogenic components contributed significantly to the severity of pancreatitis, it seemed reasonable that blocking sensory nerves should reduce pancreatic inflammation. Capsazepine is a synthetic competitive antagonist of capsaicin and serves as a TRPV1 inhibitor. It has been studied in vitro and blocks TRPV1 activation by capsaicin, heat and protons [25, 27]. It was recently demonstrated in vivo that capsazepine significantly reduced inflammation and pancreatic injury in a model of caerulein-induced acute pancreatitis [53].
One of the ways in which substance P activity is terminated is through enzymatic degradation by the cell-surface metalloprotease, neutral endopeptidase. This is particularly relevant in pancreatitis since it has been shown that genetic deletion of neutral endopeptidase exacerbates experimental pancreatitis and its associated lung injury [54]. Although neutral endopeptidase clearly plays an important role in terminating substance P-induced plasma extravasation, its effects on pulmonary inflammation appear to be mediated through effects on elastase which is injurious to the lung [55].
It is now well established that substance P is produced by a subset of primary sensory neurons and is released following nerve stimulation and depolarization. Using retrograde tracing and immunohistochemistry it was shown that the pancreas is innervated by substance P-containing nerve fibers that originate from dorsal root ganglion cells [56] indicating that substance P neurons are of extrinsic origin consistent with primary sensory neurons. In addition, TRPV1 has been detected in nerve fibers close in proximity to pancreatic acini [57]. The functional role of these neurons was investigated in rats in which primary sensory neurons expressing the capsaicin receptor (TRPV1) were ablated during neonatal life by administration of neurotoxic doses of capsaicin [58]. Systemic sensory denervation was documented by loss of eye-wipe responses to local application of capsaicin. Importantly, when subjected to either of two types of experimental pancreatitis (caerulein-induced pancreatitis or pancreatitis produced by ligation of the common bile-pancreatic duct), capsaicinized animals incurred much less pancreatitis compared to non-capsaicinized animals. These findings demonstrated that primary sensory neurons play a significant role in the inflammatory cascade leading to pancreatitis. Moreover, since only capsaicin-sensitive neurons (which are those expressing TRPV1) are destroyed by capsaicin administration, this study demonstrated that it is TRPV1-bearing neurons that mediate neurogenic inflammation in the pancreas.
There is now substantial experimental and even clinical evidence that substance P release with subsequent activation of the NK1 receptor plays a key role in the initiation of neurogenic inflammation and parenchymal injury. Like other G protein-coupled receptors, the NK1 receptor that normally resides on the plasma membrane is internalized into the cell following ligand binding. Both in vitro and in vivo studies have shown that after substance P binding, the NK1 receptor undergoes rapid internalization and is then recycled to the plasma membrane after degradation of bound substance P. This process of receptor endocytosis has been used as a measure of ligand activity at the cell surface. Using an antiserum specific for the carboxyl terminus of the NK1 receptor, it has been possible to measure local substance P activity by quantifying NK1 receptor immunoreactive endosomes [15, 16, 59]. This technique has the advantage of indicating substance P at the cellular level since it is a measure of substance P-stimulated receptor activation, not just substance P content in tissue. It was shown that experimental pancreatitis produced by repeated caerulein injection in mice caused substance P release within the pancreas and induced biochemical and histological evidence of acute pancreatitis [53, 60]. Moreover, pharmacological blockade of TRPV1 with capsazepine reduced NK1 receptor endocytosis (indicating reduced substance P release) and reduced the severity of caerulein-induced pancreatitis. These findings suggest that TRPV1 plays a central role in the evolution from pancreatic injury to the full manifestations of pancreatitis. Thus, it appears that supraphysiological doses of caerulein generate a signal that activates TRPV1 on primary sensory neurons resulting in substance P release. Substance P in turn propagates the inflammatory cascade that, in the pancreas, is manifest as acute pancreatitis. Inhibition of TRPV1 diminishes substance P release and reduces the severity of pancreatitis.
Serum amylase is commonly used as a marker of pancreatitis. In several studies in which pancreatitis was reduced through TRPV1 blockade, serum amylase levels were elevated following the induction of pancreatic injury. Interestingly, however, even though pancreatitis severity was reduced by TRPV1 blockade or elimination of primary sensory neurons, serum amylase levels were not significantly lower [53, 58]. These findings suggest that amylase release is an early event in the course of pancreatitis and is independent of primary sensory neurons. Thus, serum amylase is a marker of pancreatic injury but is not synonymous with pancreatitis.
The mechanisms underlying TRPV1 activation in pancreatitis are unknown. It has been well established that TRPV1 on primary sensory neurons is directly activated by heat and protons. Acidic conditions, in which the pH is ≤6.4, are capable of lowering the temperature threshold for TRPV1 at 37°C. It is interesting to note that pancreatic vacuole and lysosomal compartments have high proton contents that are increased under conditions of experimental pancreatitis [61, 62]. It is conceivable that these acidic compartments when damaged following pancreatic injury help lower the tissue pH sufficiently to activate TRPV1 at physiologic temperatures. In addition to the possible proton contributions from lysosomes and pancreatic vacuoles, it is known that high proton concentrations occur locally in several injurious processes such as tissue ischemia, infection, and inflammation [63–65]. Thus, the inflammation that accompanies pancreatitis may be a source of protons that can lower the temperature threshold for TRPV1 activation. It is also possible that in addition to increasing proton concentrations, pancreatic injury generates a molecule that activates TRPV1. One possibility is the endocannabinoid anandamide which is thought to play a role in initiating neurogenic inflammation [66]. It has been shown that anandamide, which was previously known to activate the cannabinoid CB1 receptor, regulates inflammatory neuropeptide release from capsaicin-sensitive primary sensory neurons through TRPV1 [67]. In addition, endogenous leukotriene B4 can also activate primary sensory nerves either through the LTB4 receptor or by direct interaction with TRPV1 [28, 39]. Many of the substances that activate TRPV1 or sensitize the ion channel to other agents are generated early in the development of pancreatitis; therefore, there are multiple complementary pathways in which TRPV1 can be activated during the course of the disease.
It is well established that substance P targets NK1 receptors on postcapillary venules and causes intercellular gaps to form and it is this cellular site of action that is responsible for the plasma extravasation and edema that occurs following substance P administration [50]. However, the contributions of the NK1 receptor on acinar cells to pancreatic inflammation are less well understood. Although NK1 receptor endocytosis in acinar cells provides a very useful model for quantifying substance P action within the pancreas, the physiological significance of substance P stimulation of pancreatic acinar cells in pancreatitis is unclear. In vitro studies have shown that substance P exposure stimulates amylase release from pancreatic acinar cells. Whether the effects of substance P on acinar cells during the evolution of pancreatitis are important for the progression of pancreatitis has yet to be determined.
TRPV1 and pancreatic pain
It is well documented that TRPV1 is important in a variety of pain models. Thus, in addition to its contributions to pancreatic inflammation, it is likely that TRPV1 plays a role in the pain response in pancreatitis [25, 68]. Activation of TRPV1 on neurons causes the release of both substance P and CGRP in the dorsal horn of the spinal cord which is critical for transmitting pain signals from the periphery to the central nervous system. A number of subsequent studies have illustrated the role of TRPV1 in mediating the effects of peripheral signals into pain perceptions. Shortly after the cloning of TRPV1 it was shown that genetic deletion of TRPV1 in mice impaired thermal hypersensitivity associated with tissue inflammation [28]. Intrathecal injection of antagonists to the NK1 or CGRP receptor (calcitonin-like receptor, CLR) has been shown to inhibit the frequency of abdominal contractions in response to colorectal distention which is a measure of allodynia [69]. Injection of capsaicin into the pancreatic duct of rats has been shown to induce expression of the protooncogene c-fos in the dorsal horn of spinal neurons which is an indication of nociceptive sensory neuron activation. The specificity of this finding for capsaicin indicates the involvement of TRPV1 [70].
In a model of experimental pancreatitis induced by injection of L-arginine it was shown that mechanical hyperalgesia was associated with increased expression of substance P and CGRP mRNA in the dorsal root ganglia indicating that pancreatitis produced sensory signals mediated by inflammatory transmitters of primary sensory neurons [56]. In a comprehensive study in rats it was shown that TRPV1, substance P, and CGRP are expressed in the same nerve fibers of the pancreas and injection of capsaicin resulted in NK1 receptor endocytosis in spinal dorsal horn neurons indicative of substance P release [57]. Moreover, in a separate study of L-arginine-induced necrotizing pancreatitis, an increase in c-fos expression in dorsal horn neurons receiving innervation from the pancreas was observed providing additional evidence for activation of nociceptive pathways in pancreatitis. This coincided with an increase in spontaneous abdominal contractions, a sign of referred pancreatic pain. Both spinal c-fos expression and abdominal contractions were inhibited by the TRPV1 antagonist capsazepine. Additionally, c-fos expression was reduced by intrathecal but not systemic administration of substance P and CGRP antagonists. These findings indicate that acute necrotizing pancreatitis activates TRPV1 on primary sensory neurons causing the release of substance P and CGRP in the dorsal horn to produce a nociceptive response. In contrast to other studies which induced mild to moderately severe pancreatitis with caerulein or pancreatico-bile duct obstruction [53, 58], this study used L-arginine to produce severe necrotizing pancreatitis [57]. L-arginine is directly toxic to acinar cells and is known to cause oxygen free radical formation. These differences in pancreatic injury may explain why this study did not demonstrate a protective effect of capsazepine on pancreatitis severity.
PAR-2 is expressed on primary sensory neurons and is activated by trypsin to expose its tethered ligand. Using a synthetic peptide with the same amino acid sequence as the PAR-2 ligand, it was shown that injection of the ligand into the pancreas activated nociceptive neurons and sensitized them to capsaicin, indicating sensitization of TRPV1 [70]. It seems logical to propose that activated trypsin, which generates a natural PAR-2 agonist, may also be capable of producing a nociceptive signal during the course of pancreatitis [71]. PAR-2 is expressed by virtually all nociceptive neurons in thoracic dorsal root ganglia that provide nerves to the pancreas and is co-expressed with TRPV1 [71]. Moreover, intraductal trypsin increased c-fos expression, indicating activation of spinal dorsal horn neurons, and induced a behavioral pain response. These effects could be ameliorated by pre-infusion of the pancreatic duct with PAR-2 activating peptide that desensitized the response to trypsin. These findings indicate that PAR-2 activation may also stimulate primary sensory neurons and induce pancreatic pain.
Most studies investigating the role of neurogenic inflammation in pancreatitis have used systemic pharmacological interventions or gene deletion methods that affect primary afferent neurons throughout the body. One recent approach examined the effect of interrupting pancreatic sensory nerves at a local level to determine if a more confined strategy may be reasonable for assessing possible therapeutic interventions [72]. Primary sensory nerves of the pancreas course through the celiac ganglion. It was hypothesized that disruption of the celiac ganglion by surgical excision or inhibition of C and Aδ fibers through blockade of TRPV1 would reduce the severity of pancreatitis. In a rat model of caerulein-induced pancreatitis, the celiac ganglion was either surgically removed or the TRPV1 agonist resiniferatoxin was applied to the surface of the ganglion. In high concentrations, resiniferatoxin selectively destroys C and Aδ fibers. Animals treated with resiniferatoxin did not demonstrate evidence of systemic sensory denervation, thus confirming that the effects were local to the region on the celiac ganglion. It was found that local application of resiniferatoxin to the celiac ganglion or surgical excision of the ganglion both reduced NK1 receptor endocytosis in pancreatic acinar cells (indicating reduced substance P release) and ameliorated pancreatitis. These findings confirm that the neurogenic component of pancreatic inflammation is mediated by TRPV1-bearing sensory nerves of which most, if not all, course through the celiac ganglion. It is interesting to speculate but remains to be determined if the celiac ganglion will provide a potential site for selectively targeting sensory nerves for treatment of pancreatic pain.
It is difficult to evaluate the contributions of neurogenic inflammation to the pathogenesis of human pancreatitis although several histopathological studies provide interesting insights. In human chronic pancreatitis, increases in neural tissue, enlargement of intrapancreatic nerves, and perineural inflammation have been found [73]. Consistent with this increase in neural tissue, substance P content was found to be elevated in pancreatic nerves from chronic pancreatitis patients [74]. Some of these changes may result from trophic effects of local nerve growth factor, particularly since NGF receptors are known to exist on primary sensory nerves [75]. Precisely how these changes in sensory nerves may be related to pancreatitis-associated pain remains to be determined.
TRPV1 has been detected in non-neuronal tissues usually under certain pathological conditions. TRPV1 immunostaining has been detected in human pancreatic cancer (acinar, ductal and nerve cells) but staining intensity was greatest in nerves of inflamed tissue surrounding the cancer [76]. TRPV1 was also upregulated at the mRNA and protein levels in human pancreatic cancer and in human chronic pancreatitis when compared to normal control pancreas. Interestingly, elevated TRPV1 expression was associated with pancreatic pain in pancreatic cancer patients. Treatment of pancreatic cancer cells in vitro with resiniferatoxin inhibited cell growth, produced oxidative stress, and induced apoptosis possibly through inhibition of mitochondrial respiration. These findings suggest that TRPV1 expression may be upregulated in pancreatic cancer and possibly chronic pancreatitis and may be involved in pancreatic pain. It remains to be determined if cytotoxic therapies directed at TRPV1 are reasonable for treatment of pancreatic cancer.
Other TRP channels
Although TRPV1 has been studied most extensively, other TRP channels have been located in the pancreas. TRPV5 and TRPV6 have been studied extensively in other epithelial tissues where they have distinctive roles in regulating calcium homeostasis and may have similar roles in pancreas [77, 78]. TRP-melastatin 5 (TRPM5), although less well characterized, is also present in pancreas [79]. A detailed study localized the mRNAs of three TRPC channels in the pancreas [80]. TRPC3, TRPC5 and TRPC6 were all found in acinar cells of the pancreas and only TRPC3 was found in pancreatic ducts. None of the TRPC channels were found in pancreatic endothelium. Localization of TRP channels in the pancreas is an important step in defining the function of these channels in pancreatic physiology; however, any potential role in pancreatitis is as yet unclear. It is possible that TRP channels located on pancreatic acinar cells could participate in pancreatic injury or secretion but this action would be distinct from the better defined role of TRPV1 in neurogenic inflammation. In contrast, however, TRPV4 resides on primary afferent neurons and senses even mild changes in osmolarity [81, 82]. It was recently shown that PAR-2 sensitizes TRPV4 to cause mechanical hyperalgesia through phospholipase Cβ and protein kinase A, C, and D pathways [83]. It is unknown whether TRPV4 contributes to the neurogenic component of pancreatitis but it is possible that it plays a role in pancreatic pain.
Conclusions
A number of other experimental approaches have helped elucidate the role of sensory neurons in the pancreas. Identification of sensory neurons through the use of novel immunological stains, sensitive histological approaches, and electrophysiological techniques have illustrated the anatomical and physiological properties of primary sensory neural pathways. The ability to identify the specific neuropeptides within primary sensory neurons through co-localization studies, understanding the regulation of their release, and defining their proinflammatory properties have been instrumental in defining the role of neurogenic inflammation.
Characterization of TRPV1 channels on primary sensory neurons has been a major advance in the study of neurogenic inflammation and has greatly expanded our understanding of the activation of sensory pathways. The consequences of sensory nerve stimulation has been brought to light through the use of TRPV1 agonists and antagonists and new mouse models with genetic modifications of TRPV1, neuropeptides and their receptors including NK1 receptors. Additional techniques such as quantification of NK1 receptor endocytosis have opened new doors for understanding not only receptor biology but basic mechanisms of neurogenic inflammation. As these findings have been applied to the pancreas we have gained new insights into the pathogenesis of pancreatitis. There is a growing body of evidence that activation of primary sensory nerves is an early event in the development of acute pancreatitis and causes the release of proinflammatory neuropeptides such as substance P and CGRP both in the spinal cord to signal pain and in the periphery to induce local inflammation. Stimulation of primary sensory neurons occurs in large part through activation of TRPV1 although other receptors are also involved. The specific endogenous agonists that are released during the course of pancreatic injury have not yet been definitively identified, although a number of reasonable candidates exist.
Although tremendous progress has been made in understanding the role of TRP channels in pancreatitis, a number of other important questions remain. Specifically, what are the endogenous activators of TRPV1, what is their source, and how are they generated? What is the relationship between TRP channel activation and pancreatic enzyme activation in the pancreas? Is neurogenic inflammation or TRPV1 activation a necessary requirement for the generation of pancreatitis? Does neurogenic inflammation play a role in chronic pancreatitis? The answer to each of these questions will be important in order to fully understand the role of TRP channels in pancreatitis. Ultimately, we hope this information will lead us to new strategies for preventing or treating human pancreatitis.
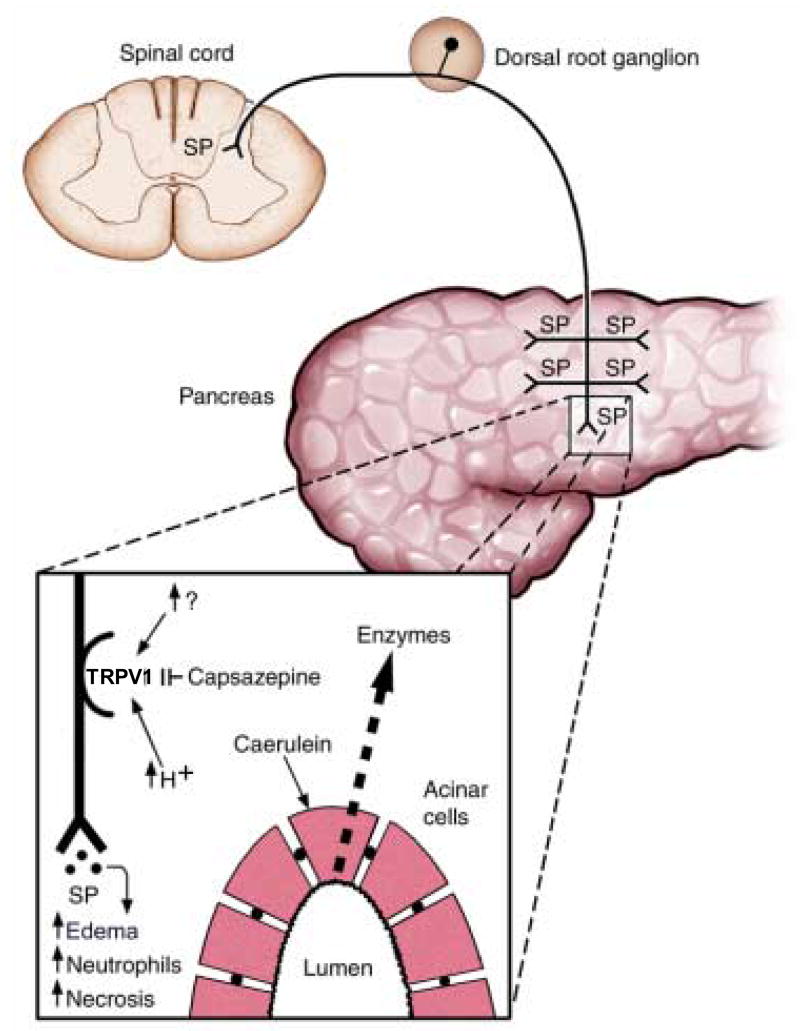
Figure 1. A schematic diagram of the proposed role of TRPV1 in a model of caerulein-induced pancreatitis.
Hyperstimulation of the pancreas with caerulein causes intra-pancreatic activation and release of digestive enzymes. Subsequent tissue acidification (↑H+) or release of an endogenous TRPV1 agonist (↑?) stimulates TRPV1 on primary sensory neurons. TRPV1 activation causes neuronal depolarization and the release of substance P in both the spinal cord and pancreas. Substance P in the pancreas causes tissue edema, neutrophil infiltration and necrosis. Antagonism of TRPV1 with capsazepine does not prevent pancreatic enzyme release but blocks TRPV1 and limits pancreatic inflammation. (Modified from [53], permission pending).
Table 1.
Putative mechanisms regulating TRPV1 [84]. It should be noted that the temperature threshold for activation is lowered in the presence of other channel activators.
| Physical Activators | Chemical Activators | Temperature Range | Sensory Nerve |
|---|---|---|---|
| Thermal | Protons | >42°C | C fibers |
| Osmotic | Capsaicin | Aδ fibers | |
| Endocannabinoids | |||
| Diphenyl compounds | |||
| Leukotrienes |
Footnotes
Guest Editor: Professor Bernd Nilius
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steer ML, Saluja AK. Experimental acute pancreatitis: studies of the early events that lead to cell injury. In: Go VLW, DiMagno JD, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas: Biology, Pathophysiology and Disease. 2. Raven Press; New York: 1993. pp. 489–500. [Google Scholar]
- 2.Steer ML, Meldolesi J. Pathogenesis of acute pancreatitis. Annu Rev Med. 1988;39:95–105. doi: 10.1146/annurev.me.39.020188.000523. [DOI] [PubMed] [Google Scholar]
- 3.Niebergall-Roth E, Singer MV. Central and peripheral neural control of pancreatic exocrine secretion. J Physiol Pharmacol. 2001;52:523–538. [PubMed] [Google Scholar]
- 4.Kirchgessner AL, Liu MT. Neurohormonal regulation of the pancreas. In: Singer MV, Krammer H-J, editors. Neurogastroenterology: From the Basics to the Clinics. Kluwer; Dordrecht: 2000. pp. 267–287. [Google Scholar]
- 5.Larsson LI. Innervation of the pancreas by substance P, enkephalin, vasoactive intestinal polypeptide and gastrin/CCK immunoractive nerves. J Histochem Cytochem. 1979;27:1283–1284. doi: 10.1177/27.9.479572. [DOI] [PubMed] [Google Scholar]
- 6.Seifert H, Sawchenko P, Chesnut J, Rivier J, Vale W, Pandol SJ. Receptor for calcitonin gene-related peptide: binding to exocrine pancreas mediates biological actions. Am J Physiol. 1985;249:G147–151. doi: 10.1152/ajpgi.1985.249.1.G147. [DOI] [PubMed] [Google Scholar]
- 7.Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb and on the nature of these fibres. J Physiol. 1901;26:173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 9.Steer ML. Frank Brooks memorial Lecture: The early intraacinar cell events which occur during acute pancreatitis. Pancreas. 1998;17:31–37. doi: 10.1159/000026152. [DOI] [PubMed] [Google Scholar]
- 10.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 11.Nathan JD, Romac J, Peng RY, Peyton M, Macdonald RJ, Liddle RA. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–727. doi: 10.1053/j.gastro.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 12.Gorelick FS, Adler G, Kern H. Cerulein-Induced Pancreatitis. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele G, editors. The Pancreas: Biology, Pathobiology, and Disease. 2. Raven Press; New York: 1993. pp. 501–526. [Google Scholar]
- 13.Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh A, Gukovskaya AS, Edderkaoui M, Daghighian MS, Reeve JR, Jr, Shimosegawa T, Pandol SJ. Tumor necrosis factor-alpha mediates pancreatitis responses in acinar cells via protein kinase C and proline-rich tyrosine kinase 2. Gastroenterology. 2005;129:639–651. doi: 10.1016/j.gastro.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A. 1995;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PW Mantyh, E DeMaster, A Malhotra, Ghilardi JR, SD Rogers, CR Mantyh, H Liu, AI Basbaum, Vigna SR, JE Maggio, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- 18.Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 19.Baluk P, Bertrand C, Geppetti P, McDonald DM, Nadel JA. NK1 receptors mediate leukocyte adhesion in neurogenic inflammation in the rat trachea. Am J Physiol. 1995;268:L263–269. doi: 10.1152/ajplung.1995.268.2.L263. [DOI] [PubMed] [Google Scholar]
- 20.Saban MR, Saban R, Bjorling D, Haak-Frendscho M. Involvement of leukotrienes, TNF-alpha, and the LFA-1/ICAM-1 interaction in substance P-induced granulocyte infiltration. J Leukoc Biol. 1997;61:445–451. doi: 10.1002/jlb.61.4.445. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe T, Otani H, Bao L, Mikami Y, Yasukura T, Ninomiya T, Ogawa R, Inagaki C. Intracellular signaling pathway of substance P-induced superoxide production in human neutrophils. Eur J Pharmacol. 1996;299:187–195. doi: 10.1016/0014-2999(95)00816-0. [DOI] [PubMed] [Google Scholar]
- 22.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 23.Holzer P. Capsaicin as a tool for studying sensory neuron functions. Adv Exp Med Biol. 1991;298:3–16. doi: 10.1007/978-1-4899-0744-8_1. [DOI] [PubMed] [Google Scholar]
- 24.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 25.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 26.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 27.Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, McNaughton PA. Why pain gets worse: the mechanism of heat hyperalgesia. J Gen Physiol. 2006;128:491–493. doi: 10.1085/jgp.200609676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga A, Bolcskei K, Szoke E, Almasi R, Czeh G, Szolcsanyi J, Petho G. Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo. Neuroscience. 2006;140:645–657. doi: 10.1016/j.neuroscience.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- 34.Gatti R, Andre E, Amadesi S, Dinh TQ, Fischer A, Bunnett NW, Harrison S, Geppetti P, Trevisani M. Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol. 2006;101:506–511. doi: 10.1152/japplphysiol.01558.2005. [DOI] [PubMed] [Google Scholar]
- 35.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 38.Bron R, Klesse LJ, Shah K, Parada LF, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol Cell Neurosci. 2003;22:118–132. doi: 10.1016/s1044-7431(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 39.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McVey DC, Vigna SR. The role of leukotriene B4 in Clostridium difficile toxin A-induced ileitis in rats. Gastroenterology. 2005;128:1306–1316. doi: 10.1053/j.gastro.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 42.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 43.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 44.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geppetti P, Bertrand C, Ricciardolo FL, Nadel JA. New aspects on the role of kinins in neurogenic inflammation. Can J Physiol Pharmacol. 1995;73:843–847. doi: 10.1139/y95-115. [DOI] [PubMed] [Google Scholar]
- 46.Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551–559. doi: 10.1159/000082180. discussion 559–560. [DOI] [PubMed] [Google Scholar]
- 47.Figini M, Emanueli C, Grady EF, Kirkwood K, Payan DG, Ansel J, Gerard C, Geppetti P, Bunnett N. Substance P and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am J Physiol. 1997;272:G785–793. doi: 10.1152/ajpgi.1997.272.4.G785. [DOI] [PubMed] [Google Scholar]
- 48.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maa J, Grady EF, Yoshimi SK, Kim EH, Hutter MM, Bunnett NW, Kirkwood KS. Genetic deletion of the neurokinin-1 receptor improves survival in diet-induced hemorrhagic pancreatitis in mice. Surg Forum. 1999;50:34–35. [Google Scholar]
- 50.Grady EF, Yoshimi SK, Maa J, Valeroso D, Vartanian RK, Rahim S, Kim EH, Gerard C, Gerard N, Bunnett NW, Kirkwood KS. Substance P mediates inflammatory oedema in acute pancreatitis via activation of the neurokinin-1 receptor in rats and mice. Br J Pharmacol. 2000;130:505–512. doi: 10.1038/sj.bjp.0703343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maa J, Grady EF, Yoshimi SK, Drasin TE, Kim EH, Hutter MM, Bunnett NW, Kirkwood KS. Substance P is a determinant of lethality in diet-induced hemorrhagic pancreatitis in mice. Surgery. 2000;128:232–239. doi: 10.1067/msy.2000.107378. [DOI] [PubMed] [Google Scholar]
- 52.Luthen R, Owen RL, Sarbia M, Grendell JH, Niederau C. Premature trypsinogen activation during cerulein pancreatitis in rats occurs inside pancreatic acinar cells. Pancreas. 1998;17:38–43. doi: 10.1097/00006676-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Nathan JD, Patel AA, McVey DC, Thomas JE, Prpic V, Vigna SR, Liddle RA. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1322–1328. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- 54.Maa J, Grady EF, Kim EH, Yoshimi SK, Hutter MM, Bunnett NW, Kirkwood KS. NK-1 receptor desensitization and neutral endopeptidase terminate SP-induced pancreatic plasma extravasation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G726–732. doi: 10.1152/ajpgi.2000.279.4.G726. [DOI] [PubMed] [Google Scholar]
- 55.Day AL, Wick E, Jordan TH, Jaffray CE, Bunnett NW, Grady EF, Kirkwood KS. Neutral endopeptidase determines the severity of pancreatitis-associated lung injury. J Surg Res. 2005;128:21–27. doi: 10.1016/j.jss.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Won MH, Park HS, Jeong YG, Park HJ. Afferent innervation of the rat pancreas: retrograde tracing and immunohistochemistry in the dorsal root ganglia. Pancreas. 1998;16:80–87. doi: 10.1097/00006676-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Wick EC, Hoge SG, Grahn SW, Kim E, Divino LA, Grady EF, Bunnett NW, Kirkwood KS. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G959–969. doi: 10.1152/ajpgi.00154.2005. [DOI] [PubMed] [Google Scholar]
- 58.Nathan JD, Peng RY, Wang Y, McVey DC, Vigna SR, Liddle RA. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G938–946. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 59.Mantyh CR, Pappas TN, Lapp JA, Washington MK, Neville LM, Ghilardi JR, Rogers SD, Mantyh PW, Vigna SR. Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology. 1996;111:1272–1280. doi: 10.1053/gast.1996.v111.pm8898641. [DOI] [PubMed] [Google Scholar]
- 60.Hutter MM, Wick EC, Day AL, Maa J, Zerega EC, Richmond AC, Jordan TH, Grady EF, Mulvihill SJ, Bunnett NW, Kirkwood KS. Transient receptor potential vanilloid (TRPV-1) promotes neurogenic inflammation in the pancreas via activation of the neurokinin-1 receptor (NK-1R) Pancreas. 2005;30:260–265. doi: 10.1097/01.mpa.0000153616.63384.24. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe O, Baccino FM, Steer ML, Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984;246:G457–467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- 62.Niederau C, Grendell JH. Intracellular vacuoles in experimental acute pancreatitis in rats and mice are an acidified compartment. J Clin Invest. 1988;81:229–236. doi: 10.1172/JCI113300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 64.Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993;154:113–116. doi: 10.1016/0304-3940(93)90184-m. [DOI] [PubMed] [Google Scholar]
- 65.Stevens CR, Williams RB, Farrell AJ, Blake DR. Hypoxia and inflammatory synovitis: observations and speculation. Ann Rheum Dis. 1991;50:124–132. doi: 10.1136/ard.50.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 67.Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci. 2003;17:2611–2618. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- 68.Kwak JY, Jung JY, Hwang SW, Lee WT, Oh U. A capsaicin-receptor antagonist, capsazepine, reduces inflammation-induced hyperalgesic responses in the rat: evidence for an endogenous capsaicin-like substance. Neuroscience. 1998;86:619–626. doi: 10.1016/s0306-4522(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 69.Plourde V, St-Pierre S, Quirion R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am J Physiol. 1997;273:G191–196. doi: 10.1152/ajpgi.1997.273.1.G191. [DOI] [PubMed] [Google Scholar]
- 70.Hoogerwerf WA, Zou L, Shenoy M, Sun D, Micci MA, Lee-Hellmich H, Xiao SY, Winston JH, Pasricha PJ. The proteinase-activated receptor 2 is involved in nociception. J Neurosci. 2001;21:9036–9042. doi: 10.1523/JNEUROSCI.21-22-09036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoogerwerf WA, Shenoy M, Winston JH, Xiao SY, He Z, Pasricha PJ. Trypsin mediates nociception via the proteinase-activated receptor 2: a potentially novel role in pancreatic pain. Gastroenterology. 2004;127:883–891. doi: 10.1053/j.gastro.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Noble MD, Romac J, Wang Y, Hsu J, Humphrey JE, Liddle RA. Local disruption of the celiac ganglion inhibits substance P release and ameliorates caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G128–134. doi: 10.1152/ajpgi.00442.2005. [DOI] [PubMed] [Google Scholar]
- 73.Friess H, Shrikhande S, Shrikhande M, Martignoni M, Kulli C, Zimmermann A, Kappeler A, Ramesh H, Buchler M. Neural alterations in surgical stage chronic pancreatitis are independent of the underlying aetiology. Gut. 2002;50:682–686. doi: 10.1136/gut.50.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Sebastiano P, di Mola FF, Di Febbo C, Baccante G, Porreca E, Innocenti P, Friess H, Buchler MW. Expression of interleukin 8 (IL-8) and substance P in human chronic pancreatitis. Gut. 2000;47:423–428. doi: 10.1136/gut.47.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friess H, Zhu ZW, di Mola FF, Kulli C, Graber HU, Andren-Sandberg A, Zimmermann A, Korc M, Reinshagen M, Buchler MW. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg. 1999;230:615–624. doi: 10.1097/00000658-199911000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartel M, di Mola FF, Selvaggi F, Mascetta G, Wente MN, Felix K, Giese NA, Hinz U, Di Sebastiano P, Buchler MW, Friess H. Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut. 2006;55:519–528. doi: 10.1136/gut.2005.073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoenderop JG, Nilius B, Bindels RJ. Epithelial calcium channels: from identification to function and regulation. Pflugers Arch. 2003;446:304–308. doi: 10.1007/s00424-003-1045-8. [DOI] [PubMed] [Google Scholar]
- 78.den Dekker E, Hoenderop JG, Nilius B, Bindels RJ. The epithelial calcium channels, TRPV5 & TRPV6: from identification towards regulation. Cell Calcium. 2003;33:497–507. doi: 10.1016/s0143-4160(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 79.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 80.Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 82.Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]