Abstract
Background
Congenital vascular malformations (VMs) are mass‐forming lesions that usually progress slowly, but may become symptomatic because of episodes of sudden growth and pain, particularly those with a substantial component of arteriovenous shunting.
Aim
To systematically investigate the features of microvascular proliferation in a large series of surgically treated VMs.
Methods
107 resection specimens of clinically and histologically well‐documented VMs were screened for the presence and extent of microvascular proliferation, based on morphological parameters, microvessel density (MVD), mast cell density (MCD) and proliferative activity (Ki‐67 labelling index) of endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). The extent of microvascular proliferation was correlated with the histological type of VM and clinical characteristics of patients.
Results
Microvascular proliferation was observed in 32 (30%) of all VMs, of which 30 cases seemed to be arteriovenous malformations. MVD in areas of microvascular proliferation was 282 (186)/mm2 vs 13 (9)/mm2 in areas with mature vessels. Both ECs and VSMCs in these areas showed high Ki‐67 labelling indexes (mean (SD) 15 (18) and 17 (24)/mm2, respectively). In all lesions, a positive correlation was found between MCD and MVD. Age, sex and location of VM had no predictive value for the occurrence of microvascular proliferation. However, if present, the involved tissue areas were larger and the proliferative activity of EC was higher in male patients than in female patients.
Conclusions
Recognition of microvascular proliferation as a not uncommon feature, congenital arteriovenous malformations provide new insight into the growth behaviour and vascular composition of these lesions.
Vascular malformations (VMs) are congenital anomalies that result from localised errors of angiogenic development during embryonic life.1 VMs may occur at any topographic site, but have a predilection for skin and soft tissues of the head and extremities.2 Familial occurrence has been reported and although most VM are solitary lesions, they may also occur in various types of dysmorphic syndromes.3,4 VMs tend to progress slowly, but in the long term (usually after many years) serious complications may occur, which then require extensive surgical excision or even amputation. Moreover, because of ill‐defined borders, the rate of recurrence after excision is high.2
In children, VMs should be distinguished from infantile haemangiomas, which are the most common vascular tumours of infancy.5 Mulliken and Glowacki6 categorised vascular anomalies in either haemangiomas or malformations on the basis of difference in growth behaviour, endothelial cell (EC) turnover and mast cell density (MCD). Although not being absolute, this classic dichotomy is still used in the present classification accepted by the International Society for Study of Vascular Anomalies because of its simplicity and clinical relevance.5,7,8 According to this classification, haemangiomas are lesions with microvascular proliferation in the initial phases of growth, whereas VMs are stable lesions with mature vessels, which grow commensurately with the child. Despite this, few cases of florid proliferation of capillaries have been reported in the course of VM, and in our department we identified similar cases.
In the present study, we systematically investigated the presence and extent of microvascular proliferation in a large consecutive series of resection and amputation specimens of VM. Furthermore, features of microvascular proliferation were correlated with the clinical characteristics of patients and the histological type of VM.
Methods
Study patients
In a retrospective cohort study, we reviewed the resection or amputation records of a consecutive series of patients with VM of soft tissue and skin who were treated between between 1984 and 2003 (n = 179) in the Academisch Medisch Centrum, University of Amsterdam, Amsterdam, The Netherlands, which is a referral centre for the management of vascular anomalies. Indication for surgical treatment consisted of pain or ulceration, and/or functional impairment and or rapid growth (in a period of weeks to months) complicating the vascular anomaly. Only resection or amputation specimens >3 cm and of which one or more tissue blocks per centimetre of malformation were available were included for this study. On this basis, 109 cases were enrolled. Of all patients included, age, sex and topographic location of VM were recorded. The study was performed in accordance with the Declaration of Helsinki and the institutional medical ethics committee.
Histopathology
By using H&E and Elastica von Gieson‐stained tissue sections, VMs of all patients were classified as (1) venous vascular malformation (VVM): lesions composed of veins of variable sizes, often with thick vascular walls; (2) lymphatic malformation (LM): substantial component of dilated thin‐walled lymphatic vessels of different sizes; (3) deep arteriovenous malformation (AVM): large numbers of tortuous arteries and/or reactive intimal changes in arteries and veins with fibrointimal thickening due to haemodynamic stress; and (4) superficial arteriovenous malformation/acral arteriovenous tumour (aAVT): localised nodular tumour of thick‐walled arteries and veins of skin and subcutis.
Tissue blocks of all VMs were screened for the presence of microvascular proliferation, defined as “solid” areas of densely packed capillary vessels lined with plump endothelium. For evaluation of the extent of microvascular proliferation, a semiquantitative score was applied as follows: 0, absent; 1, single cluster of immature capillaries (<50 vessels); 2, multiple clusters; 3, extensive diffuse and solid proliferation, splitting up pre‐existent tissue. Adjacent serial sections were mounted for additional immunostains.
Glucose transporter type 1 reactivity of endothelium
Glucose transporter type 1 (GLUT‐1) is a protein specifically expressed by ECs of juvenile angioma, both in the proliferative and in the mature phase of evolution of lesions. By contrast, the endothelium of vessels of VM always stains negative with this antibody.9 To exclude the possible cases of juvenile angioma in our series, we immunostained representative sections of each specimen with anti‐GLUT‐1 antibody.
Microvessel density
For determination of microvessel density (MVD), sections immunostained with antivascular smooth muscle cell (VSMC) (smooth muscle actin‐1SMA‐1) antibody were scanned at a low‐power magnification (×40) to identify the areas of highest MVD (hot spots).10 In these areas, the number of vessels was counted at ×200 magnification in 10 non‐overlapping fields with the manual count option, Image Pro Plus. Similarly, MVD was calculated in 10 random areas of the same VM with only large mature vessels.
Mast cell density
The same areas, traced in adjacent sections immunostained with antitryptase antibody, were screened for the presence of mast cells. Numbers of mast cells were counted by an automated macro with the use of image analysis software, and expressed as cells/mm2.
Proliferative activity of vascular wall cells
Proliferative activity of both ECs and VSMCs was assessed with anti‐Ki‐67 antibody, recognising a nuclear protein with a fundamental function in cell proliferation.11 For this purpose, sections were double immunostained with Ki‐67/CD31 and Ki‐67/SMA‐1, respectively. Proliferating Ki‐67‐positive cells (ECs and VSMCs) were counted in 10 fields at ×400 magnification both in hot spots and in non‐proliferating areas.
To gain insight into the diameter of vessels with a high proliferative activity, we calculated the number of Ki‐67 and ECs per mm EC lining (lumen circumference) and number of Ki‐67 and SMCs per mm2 media in relation to the vessel diameter of 150 vessels from 4 patients.
Immunohistochemical methods
Sections mounted for immunohistochemistry were deparaffinised and subjected to antigen retrieval in a microwave oven before staining. We used citrate buffer for GLUT‐1 and tryptase immunostaining and EDTA for the Ki‐67 double immunostainings. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in methanol for 20 min. For immunostaining with anti‐mouse tryptase (dilution 1:1000, Chemicon, Temecula, California, USA), direct alkaline phosphatase visualisation with Vector Red (Vector, Burlingame, California, USA) was applied. For the immunostaining with anti‐GLUT‐1 (dilution 1:100; Dako, Glostrup, Denmark), a streptavidin–biotin complex/horsesradish peroxidase method was used and antibody reactivity was visualised with diaminobenzidine tetrachloride.
Ki‐67/CD31 and Ki‐67/SMA‐1 double immunostaining was performed in a multistep technique using Ki‐67 antibody (Neomarkers, clone SP6, undiluted (Labvison/Neomarkers, Fremont, California, USA)), visualised by diaminobenzidine tetrachloride in a brown nuclear precipitate, followed by application of the second primary antibody (either anti‐CD31, clone JC70, dilution 1:20, Dako; or anti‐α1 actin, SMA‐1, dilution 1:200, Dako) visualised by permanent red in a red cytoplasmic precipitate.
Positive controls (sections of skin and juvenile capillary angioma) and non‐immune negative control sections were evaluated for each stain.
Image analysis
Digital images of immunostained sections were captured using a Roper Coolsnap CF digital camera and an RGB liquid crystal display filter (Cambridge Research Instrumentation, Woburn, Massachusetts, USA) mounted on an Olympus BX60 microscope. Image analysis was performed using Image Pro Plus 4.5 (Media Cybernetics, Silver Spring, Maryland, USA).
Statistical analysis
Binary logistic regression models were used to assess the relationship between clinical characteristics (sex, age, topographic location and histological type) and the occurrence of microvascular proliferation.
Normality was analysed using the Kolmorov–Smirnov test. A paired t test was used for data with normal distribution (MVD, mass cell density (MCD) and proliferative activity of EC), and Mann–Whitney U test when data were not normally distributed (proliferative activity of VSMC). For comparison of more than two variables (proliferative activity of EC/endothelial lining and proliferative activity of VSMC/median area), the Kruskall–Wallace one‐way analysis of variance was used, followed by post hoc Mann–Whitney U test. The paired t test was used for differences in proliferative activity of ECs and SMCs between men and women. Fischer's exact test was used for differences in the extent of vascular proliferation between men and women. p Values <0.05 were considered significant. In all instances, statistical analysis was performed using SPSS V.11.5.
Results
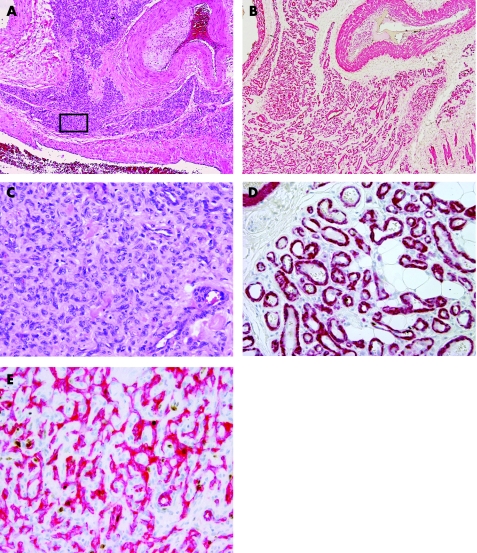
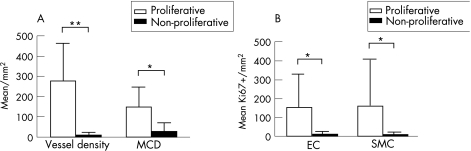
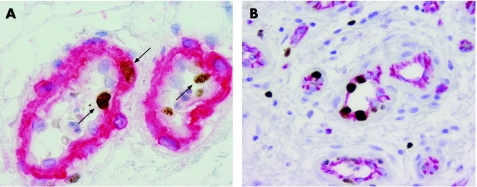
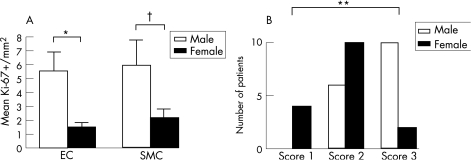
Table 1 shows clinical characteristics and histopathological diagnosis of all cases. Of 109 vascular specimens, two showed strong GLUT‐1 immunoreactivity of endothelial lining of small mature vessels, which is highly specific for the mature phase of juvenile capillary angioma. Accordingly, these lesions were excluded from the series of VM. The remaining 107 cases were classified as AVM (n = 71), VVM (n = 21), LM (n = 8) or aAVT (n = 7). The areas of microvascular proliferation were found amid the mature vessels of the malformation in 32 out of 107 (30%) cases. Most cases showed multiple small clusters (50%) or even diffuse infiltration of pre‐existent tissues by immature vessels (38%; fig 1), whereas only 12% of cases had a single cluster of microvascular proliferation in the resected material. Microvascular proliferation was observed in almost 42% of AVMs encountered in this series, and also almost exclusively in this type of malformation (30 of 32 positive cases were AVM). The other two positive cases were VVM, which showed only a single small cluster of microvessels. In LM and aAVT, vascular proliferations were absent. There was no association between age, sex or localisation and the presence of microvascular proliferation (table 1). MVD in areas of microvascular proliferation was significantly higher (282 (186)/mm2) than in adjacent areas of the malformation (13 (9)/mm2; paired t test p<0.05). Similarly, MCD was significantly higher in areas of microvascular proliferation (154 (97)/mm2 in proliferating areas vs 35 (35)/mm2 in areas with mature vessels; paired t test p<0.001; fig 2A). Both ECs and VSMCs showed high Ki‐67 labelling indexes in proliferating areas (EC: 15 (18) vs 1 (2)/mm2 and VSMC/pericytes: 17 (24) vs 1 (1)/mm2; fig 2B). For this purpose, double immunostaining allowed to discriminate clearly between Ki‐67 immunoreactivity of ECs and VSMCs in vessels (fig 3). The correlation between Ki‐67 indexes of both cell types with vessel diameter showed proliferative activity predominantly in vessels <20 μm (representing the size of capillaries, arterioles and venules). Although sex was not predictive for the occurrence of vascular proliferation, we found that the Ki‐67 labelling indexes in both ECs and SMCs were higher in proliferative areas of male patients than in female patients (fig 4A). Moreover, the extent of microvascular proliferation throughout the lesion appeared also more prominent in male patients than in female patients (fig 4B).
Table 1 Clinical and histological characteristics of all patients with vascular malformation (VM) and the subgroup of patients with capillary angiogenesis in VM.
| All patients with VM | Patients with VM with vascular proliferation | p Values | |
|---|---|---|---|
| n = 107 | n = 32 | ||
| Sex (M:F) | 58:49 (1.2:1) | 16:16 (1:1) | NS |
| Age (years) | 23 (0–67) | 25 (5–64) | NS |
| Localisation of VM, n (%) | |||
| Head and neck | 49 (46) | 13 (41) | NS |
| Trunk | 17 (16) | 3 (9) | NS |
| Upper extremities | 17 (16) | 6 (19) | NS |
| Lower extremities | 24 (22) | 10 (31) | NS |
| Histological type of VM, n (%) | |||
| AVM | 71 (66) | 30 (94) | p<0.001 |
| VVM | 21 (20) | 2 (6) | NS |
| LVM | 8 (7) | 0 | NS |
| aAVT | 7 (7) | 0 | NS |
AVM, arteriovenous malformation; aAVT, acral arteriovenous tumour; F, female; LVM, left ventriuclar mass; M, male; NS, statistically not significant; VM, vascular malformations; VVM, venous vascular malformation.
Figure 1 (A) Extensive (grade 3) solid microvascular proliferation amid the large vessels of an arteriovenous malformation (H&E). (B) Same area as in fig 3A showing distinct closely packed vascular structures in anti‐1a actin immunostain (SMA‐1). (C) Details taken from boxed area in fig 3A showing solid‐appearing growth of swollen vascular cells with inconspicuous lumina (H&E). (D) Details taken from boxed area showing well‐outlined vascular walls of small vessels in SMA‐1 immunostain. (E) Details taken from boxed area showing the luminal endothelial component of the vascular proliferation in anti‐CD31 immunostain.
Figure 2 (A) Microvessel density and mast cell density (MCD) in proliferative (open bars) and mature (black bars) areas of vascular malformations of 32 patients with capillary angiogenesis. (B) Ki‐67 labelling indexes of endothelial cells and vascular smooth muscle cells in proliferative (open bars) and mature (black bars) areas of the same patient group *p<0.001, **p<0.05. EC, endothelial cell; SMC, smooth muscle cell.
Figure 3 Details of double immunostaining to visualise Ki‐67 labelling in vascular smooth muscle cells and endothelial cells (ECs) respectively. (A) Double immunostain with Ki‐67 (brown) and smooth muscle actin‐1 (SMA‐1; red), showing Ki‐67 positive nuclei of smooth muscle cells (SMCs) within the immunostained area (long arrow) and Ki‐67‐positive nuclei of ECs on the outside of the immunostained area (short arrows; Ki‐67/SMA‐1). (B) Double immunostain with Ki‐67 (brown) and CD31 (red), showing several Ki‐67 positive nuclei of ECs within the immunostained vessel area (Ki‐67/CD31).
Figure 4 (A) Labelling indexes of endothelial cells (ECs) and vascular smooth muscle cells in proliferative areas of vascular malformation of male (open bars) and female (black bars) patients. EC proliferation appears significantly higher in male patients than in female patients. (B) Also, the extent of vascular proliferation, using a semiquantitative score (see text), is significantly higher in male patients. *p<0.05, **p<0.005, †NS. SMC, smooth muscle cell.
Discussion
According to current insights into the biology of benign vascular lesions, congenital VMs represent slowly progressive growing vascular masses composed of dysplastic but mature vessels. Despite this view, we identified areas of microvascular proliferation, interpreted as angiogenesis, in 30% of cases of a large series of 107 surgically removed VMs. These areas of microvascular proliferation contained closely packed small vessels (<20 μm) with a high Ki‐67 labelling index of both ECs and SMCs. In most cases, those areas were multicentric (50%) or displayed a solid growth pattern, splitting up pre‐existent adipose tissue and skeletal muscle (38%). Pain, swelling, rapid growth (in a period of weeks to months) and/or functional impairment were symptoms most frequently encountered in this population of patients, and these symptoms served as indicators for surgical intervention. It could be therefore that microvascular proliferation is involved in causing symptoms due to the mass‐forming effect (fig 1A, B). However, our data files did not allow to correlate reliably specific symptoms of each patient with corresponding histological findings in all cases; moreover, other (well‐known) tissue complications such as thrombosis or haemorrhage may also be encountered. Therefore, a potential role of microvascular proliferation in the onset of symptoms of patients remains purely speculative.
In our patient population, sex distribution was equal, and neither age or sex nor topographic location of malformations had a predictive value for the occurrence of foci of microvascular proliferations. Only the histological type of VM correlated significantly with the occurrence of microvascular proliferation, since of all positive cases >90% were AVM. This observation suggests that a high flow through arteriovenous communications, which is a hallmark of AVM, could play a part in microvascular proliferation. The exact mechanism, however, remains to be elucidated. Despite equal sex distribution among patients with microvascular proliferation, we found that AVMs of men showed a higher proliferation rate in both ECs and VSMCs than those occurring in women (fig 4A). Moreover, the semiquantitative score used to evaluate the extent of microvascular proliferation in VM was significantly higher in male patients (fig 4B). This implies that, once it occurs, microvascular proliferation appears more prominent in male than in female patients with AVM. Gender differences in the growth of VSMCs have been described only in animal models and have, to our knowledge, not been reported in human pathology. These differences in proliferation rate probably relate to a shorter cell cycle in male VSMCs than in those from female VSMCs. This could be because of effects of sex hormones, although, for example, oestrogens seem to accelerate rather than inhibit angiogenesis.12,13,14
Angiosarcoma has been reported as a rare complication of AVM,15 and in cases with extensive solid proliferations a suspicion of malignancy (angiosarcoma) was raised. Despite this, malignancy was excluded based on detailed histological features, indicating benign capillary vessels, and clinical follow‐up of patients.
The question whether microvascular proliferation in AVM should be considered as a reactive phenomenon or alternatively as part of the congenital malformation is more realistic. Morphologically, microvascular proliferation in our study group was indiscernible from that occurring in proliferating lesions of juvenile angioma, pyogenic granuloma and reactive angioendotheliomatosis.16
Takahashi et al17 have reported a high proliferative activity, defined by a high expression of proliferating cell nuclear antigens, in the growth phase of juvenile angiomas, and almost no staining in the involuting stage of the same type of tumours. In this study proliferative activity was barely detectable in five VMs included, which contradicts to our findings. This difference probably relates to sampling because of the small sample size and the fact that only one AVM was investigated.
Take‐home messages
Florid microvascular proliferations are found in up to 30% of congenital vascular malformations (VMs), especially in those with a significant arteriovenous component.
Traditionally, VMs are slowly growing lesions composed of mature vessels. By contrast, vasoproliferative growth is considered unique for infantile haemangiomas and reactive vascular proliferations. The outcome of this study is therefore important for differential diagnostic aspects of benign vascular tumours, and may provide new insights into the biology of VMs.
Sex, age and topographic location of VM are not predictive for the onset of microvascular proliferation. However, once it occurs in a patient, the vasoproliferative growth seems to be significantly more extensive in men than in women.
Mast cells, which are a rich source of growth factors that induce or modulate angiogenesis,18,19 were found in significantly increased numbers in areas of microvascular proliferation of AVM. Beyond those areas, the mast cell counts were low in all VMs. A similar correlation between areas of high MVD, morphological immaturity and high MCD has previously been reported also for juvenile angiomas and reactive proliferations such as pyogenic granuloma.20 In the regressive stages of these lesions, when vessels mature or disappear, the amount of mast cells indeed decreases.17,21,22
The endothelial lining of capillaries in juvenile angioma is characterised by strong immunoreactivity with GLUT‐1 antibody,9 whereas in AVM all vessels, including the immature capillary vessels we describe in our study, are GLUT‐1 negative. Pyogenic granuloma (which is GLUT‐1 negative), diffuse dermal angiomatosis (GLUT‐1 reactivity not reported) and acro‐angiodermatitis (GLUT‐1 negative) are all considered reactive capillary lesions, and although their presence is limited to the dermal skin, such morphologic and immunophenotypic similarities could favour a reactive nature of microvascular proliferation in VM.
Indeed, Bavikatty et al23 recently described florid vascular proliferations of the colon wall in five patients, of whom two had a possible AVM of the intestinal wall. These authors also believed this process to be of a reactive rather than a neoplastic nature, probably resulting from local tissue hypoxia, because of concomitant intussusception.
One could speculate that capillary structures in VM undergo maturation by the time, as is also a well‐known feature of the immature capillary vessels in juvenile angioma and pyogenic granuloma, and finally develop vessel areas indistinguishable from the areas of thin‐walled small mature vessels that are present in most, if not all, AVMs of soft tissue.2
In summary, the present report provides new insights into the growth behaviour and vascular composition of VMs, particularly in those with an arteriovenous component.
Acknowledgements
We thank Ms JC Korevaar of the Department of Clinical Epidemiology & Biostatistics for her assistance in statistical analysis.
Abbreviations
aAVT - acral arteriovenous tumour
AVM - arteriovenous malformation
EC - endothelial cell
GLUT - glucose transporter type
LM - lymphatic malformation
MCD - mast cell density
MVD - microvessel density
SMA‐1 - smooth muscle actin 1
VM - vascular malformation
VSMC - vascular smooth muscle cell
VVM - venous vascular malformation
Footnotes
Competing interests: None declared.
References
- 1.Vikkula M, Boon L M, Mulliken J B. Molecular genetics of vascular malformations. Matrix Biol 200120327–335. [DOI] [PubMed] [Google Scholar]
- 2.Calonje E. Haemangiomas. In: Fletcher CDM, K Unni, Mertens F, eds. World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press, 2002
- 3.Esterly N B. Cutaneous hemangiomas, vascular stains and malformations, and associated syndromes. Curr Probl Pediatr 1996263–39. [DOI] [PubMed] [Google Scholar]
- 4.Brouillard P, Vikkula M. Vascular malformations: localized defects in vascular morphogenesis. Clin Genet 200363340–351. [DOI] [PubMed] [Google Scholar]
- 5.Bruckner A L, Frieden I J. Hemangiomas of infancy. J Am Acad Dermatol 200348477–93 quiz 4946. [DOI] [PubMed] [Google Scholar]
- 6.Mulliken J B, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 198269412–422. [DOI] [PubMed] [Google Scholar]
- 7.Enjolras O, Mulliken J B. Vascular tumors and vascular malformations (new issues). Adv Dermatol 199713375–423. [PubMed] [Google Scholar]
- 8.Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol 199724701–710. [DOI] [PubMed] [Google Scholar]
- 9.North P E, Waner M, Mizeracki A.et al GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol 20003111–22. [DOI] [PubMed] [Google Scholar]
- 10.Elpek G O, Gelen T, Aksoy N H.et al The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol 200154940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D C, Gatter K C. Ki67 protein: the immaculate deception? Histopathology 2002402–11. [DOI] [PubMed] [Google Scholar]
- 12.Bacakova L, Kunes J. Gender differences in growth of vascular smooth muscle cells isolated from hypertensive and normotensive rats. Clin Exp Hypertens 20002233–44. [DOI] [PubMed] [Google Scholar]
- 13.Loukotova J, Bacakova L, Zicha J.et al The influence of angiotensin II on sex‐dependent proliferation of aortic VSMC isolated from SHR. Physiol Res 199847501–505. [PubMed] [Google Scholar]
- 14.Rubanyi G M, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol 20023889–98. [DOI] [PubMed] [Google Scholar]
- 15.Rossi S, Fletcher C D. Angiosarcoma arising in hemangioma/vascular malformation: report of four cases and review of the literature. Am J Surg Pathol 2002261319–1329. [DOI] [PubMed] [Google Scholar]
- 16.McMenamin M E, Fletcher C D. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol 200226685–697. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Mulliken J B, Kozakewich H P.et al Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest 1994932357–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribatti D, Vacca A, Nico B.et al The role of mast cells in tumour angiogenesis. Br J Haematol 2001115514–521. [DOI] [PubMed] [Google Scholar]
- 19.Crivellato E, Beltrami C A, Mallardi F.et al The mast cell: an active participant or an innocent bystander? Histol Histopathol 200419259–270. [DOI] [PubMed] [Google Scholar]
- 20.Hagiwara K, Khaskhely N M, Uezato H.et al Mast cell “densities” in vascular proliferations: a preliminary study of pyogenic granuloma, portwine stain, cavernous hemangioma, cherry angioma, Kaposi's sarcoma, and malignant hemangioendothelioma. J Dermatol 199926577–586. [DOI] [PubMed] [Google Scholar]
- 21.Glowacki J, Mulliken J B. Mast cells in hemangiomas and vascular malformations. Pediatrics 19827048–51. [PubMed] [Google Scholar]
- 22.Pasyk K A, Cherry G W, Grabb W C.et al Quantitative evaluation of mast cells in cellularly dynamic and adynamic vascular malformations. Plast Reconstr Surg 19847369–77. [DOI] [PubMed] [Google Scholar]
- 23.Bavikatty N R, Goldblum J R, Abdul‐Karim F W.et al Florid vascular proliferation of the colon related to intussusception and mucosal prolapse: potential diagnostic confusion with angiosarcoma. Mod Pathol 2001141114–1118. [DOI] [PubMed] [Google Scholar]