Abstract
The role of substance P (SP) in physiological haematopoiesis is well established. However, it also seems to be important in the neoplastic transformation of bone marrow, leading to the development of acute leukaemia in children, and also metastases to bone marrow of solid tumours (particularly neuroblastoma and breast cancer) in early stages of these diseases. This review summarises the available data on SP involvement in both processes. In the future, SP antagonists may be used as anti‐neoplastic drugs, for example by direct or indirect blocking of tumour cell proliferation through inhibition of growth factor production and interleukin‐1b synthesis.
Keywords: substance P, tachykinins, hematopoiesis, malignancy
Studies on bone marrow (BM) innervation have a long history,1 and even though the presence of nerves in BM has been sufficiently well documented, the significance of substances released by the nerves during haematopoiesis remains unclear. In general, nerve fibres are provided to bones from an appropriate branch of the spinal nerve supplying a given region of the body. They include both extraganglionic sympathetic fibres and afferent sensory fibres originating from spinal ganglia. The classical and still referred to studies of de Castro2 showed that the nerves penetrate the BM cavity in common with the nutrient artery, split into branches and follow bone marrow arterioles, entwining them in the form of a dense network. A more complete illustration of BM innervation and relations between nerve endings and microelements of the BM environment was presented by Yamazaki and Allen.3 As many as 61.5% of myelinated fibres were found to terminate between cells of arterial adventitia, 37.8% between haematopoietic cells and only 0.7% on cells forming BM sinuses. The significantly more numerous non‐myelinated fibres most frequently terminate between adventitial cells (66.9%), on smooth muscle cells forming walls of BM blood vessels (4.9%), on cells forming BM sinuses (9.7%), and directly between BM cells (18.5%). This indicates that substances released from nerve endings may participate in formation of the haematopoietic microenvironment.
The most richly represented nerve endings in BM include those which release substance P (SP) and calcitonin‐gene related peptide.4 Others, which are less frequent in BM nerve endings, release neuropeptide Y and vasoactive intestinal peptide.5
Haematopoiesis
There are at least two stem cells in adult BM: the haematopoietic stem cell (HSC) and the mesenchymal stem cell (MSC).6,7 The major function of HSC, which is typical also for most of the studied stem cells, is continuous replacement of all cells which constitute the immune and blood systems. The process known as haematopoiesis involves multidirectional regulations among haematopoietic cells, BM stromal cells and nerve endings.7 HSCs are found in the low‐oxygen areas of BM, close to the endosteum.8 MSCs are located in the inner region of blood vessels.9 Despite the anatomical distance between HSCs and MSCs, these two stem cells are functionally interconnected since MSCs are the source of the supporting stromal cells.10,11
Haematopoiesis within the BM microenvironment depends on multidirectional stimuli.12 Cellular responses cause production of soluble factors such as cytokines, chemokines, neurotrophic factors and neuropeptides.13 Innervation of the BM with peptidergic, including SP, and sympathetic fibres14,15,16,17 suggests that the functions, and possibly the properties of HSCs could be influenced by the neural system. However, homoeostasis in the haematopoietic system is attained by multiple intracellular pathways triggered by receptors and their respective ligands. One group is composed of neurokinin receptors (NK‐Rs) and neurotransmitters belonging to the tachykinin family of peptides.18,19
Tachykinins
Neuropeptides belonging to the tachykinin family are encoded by three genes, namely TAC1, TAC3 and TAC4 (known previously as PPT‐A, PPT‐B and PPT‐C belonging to the preprotachykinin (PPT) gene family).20 The tachykinins are mostly 10–12‐amino acid peptides that present a common carboxyl terminus, Phe–X–Gly–Leu–Met–NH2, where X is either an aromatic or branched aliphatic residue.21TAC1 encodes two of the most studied tachykinins, SP and neurokinin (NK)‐A.21 SP and NK‐A are 11‐ and 10‐amino acid peptides, respectively. PPT‐A comprises seven exons, which can be alternately spliced and modified to form four transcripts: α, β, γ, and δ. SP is encoded by exon 3, present in each transcript, and NK‐A is encoded by exon 6, present only in transcripts β and γ.21TAC1 gene is expressed in neural (peripheral and central) and non‐neural tissues, BM, and immune cells.22,23TAC3 has seven exons, with exon 5 encoding nerokinin B (NK‐B)20; it is expressed in the brain and in peripheral tissues.21TAC4 gene produces haemokinin 1 (HK‐1), expressed in haematopoietic cells.17,24,25 It appears that HK‐1 is distinctively expressed outside the neural system and has a prominent role in the regulation of lymphopoiesis.25 It is encoded by exon 2, found in each transcript.21 Finally, C14TLK‐1 gene gives origin to endokinins A and B, expressed in heart, liver and placenta.26
Traditionally, neurons have been identified as the major source of SP, NK‐A and NK‐B, but now it is well established that tachykinins and their receptors are expressed in the cardiovascular system, salivary gland, skin, muscles, respiratory system, digestive tract, genitourinary tracts, thyroid gland and immune system.25,26,27,28 Because of such diffuse expression and regulation of disparate physiological functions, tachykinins can also be implicated in the pathogenesis of many diseases, including neoplasms.
Tachykinin receptor subtypes
The tachykinins interact with three natural tachykinin (neurokinin) receptors: NK‐1R, NK‐2R, and NK‐3R.13 They belong to the family of 7‐transmembrane, G‐protein coupled receptors.20 SP and HK‐1 exhibit binding preference for NK‐1R, whereas NK‐A show binding preferences for NK‐2R.21,29 The tachykinins can, however, interact with weak binding affinity to other NK‐Rs.9 NK‐Rs are widely expressed in neural and non‐neural systems. BM stroma, immune, and haematopoietic cells also express NK‐Rs.13,20
Substance P and its significance in physiological haematopoiesis
Substance P (H–Arg–Pro–Lys–Pro–Gln–Gln–Phe–Phe–Gly–Leu–Met–NH2) shows wide distribution in the nervous system, in which it plays the role of a neuromediator.30,31 Outside the central nervous system SP is antidromally released from sensory nerve endings of C type nerve fibres32,33 in response to mechanical, chemical, and thermal insults as well as in response to factors released at sites of tissue injury.33,34,35,36,37
According to several authors, the presence of peptidergic nerve endings in the closest vicinity of immunocompetent cells in organs most exposed to contact with foreign antigens represents an anatomical exponent of functional links between the mentioned fibres and cells and, more generally, between nervous system and immune system.38,39,40
In humans, receptors for SP can be demonstrated on around 40% of peripheral blood lymphocytes.36 Compared to mature lymphocytes, lymphoblasts carry around 3–4‐fold higher amounts of receptors for SP.41 Apart from lymphocytes, receptors for SP are also found on monocytes,42 endothelial cells,43 fibroblasts44 and haematopoietic cells.45 Substance P augments proliferative activity of human and mouse T lymphocytes,46,47 human smooth muscle cells,48 mouse fibroblasts,49 fibroblasts of human skin,44 smooth muscle fibres of arterial walls,50 human synovial cells51 and human cells forming colonies of granulocytes and monocytes or of erythrocytes.52
SP stimulates production of cytokines such as interleukin‐1 (IL),53,54 IL‐2,36,55 IL‐3, IL‐6, tumour necrosis factor‐α,51 interferon‐γ,56 granulocyte monocyte colony stimulating factor (GM‐CSF) and stem cell factor (SCF).55 It may intensify expression of adhesion molecules, i.e. intercellular adhesion molecule 1, which promote implantation of grafted haematopoietic cells.57 Due to the presence in BM peptidergic nerve endings, SP has an easy access both to haematopoietic cells and to cells forming sublayers of BM.58 Earlier studies showed that SP is also released in BM from macrophages,59 eosinophils60,61 and cells of vascular endothelium.62 Most of cells present in BM, i.e. haematopoietic cells52 and cells forming BM stroma,44,63 as well as lymphocytes present there, particularly T lymphocytes,64 are equipped with the SP‐specific receptor, NK‐1R. Tested in short term cultures of human BM in methylcellulose, SP alone was shown to support haematopoiesis in vitro.52 The authors showed that SP, at a concentration of 10−11–10−8 mol/l could substitute for IL‐3, granulocyte colony stimulating factor (G‐CSF) and GM‐CSF, the presence of which was indispensable for growth of colonies. On the other hand, substance P could not substitute for erythropoietin although, when added together, it augmented activity of the latter. Specificity of this stimulatory action of SP was confirmed by administering it together with blockers of the known subtypes of SP receptor. Such a parallel administration of SP and a blocker for a subtype of NK‐1R receptor yielded results at the control level. However, blocking of the NK‐2R receptor had no effect on SP activity.45 SP was also found to affect haematopoietic cells in an indirect manner, i.e. through the stromal cells, stimulating their production of cytokines. Supplementation of SP‐stimulated cultures with antibodies specific for IL‐1, IL‐3, IL‐6 and GM‐CSF resulted in partial inhibition of cell growth, proving that SP can act through the induction of the cytokine synthesis. SP also induces synthesis of IL‐1 and SCF in BM stromal cells.65
Cytokines linked to the haematopoietic functions of SP include IL‐1, IL‐3, GM‐CSF and SCF.23 SP induces production of these cytokines, which exhibit stimulatory effects on haematopoiesis. Alternatively, the cytokines induced by SP could activate BM cells through an autocrine and/or paracrine mechanism to produce other cytokines with haematopoiesis‐stimulatory effects.66 For example, SP induces the production of IL‐1, which stimulates the induction of haematopoietic factors with direct and indirect effects on HSCs.66 In contrast to SP, the haematopoietic effects of NK‐A could be stimulatory or inhibitory, depending on the particular haematopoietic lineage.52,67 NK‐A inhibits the proliferation of granulocyte‐monocyte progenitors, but stimulates erythrocyte progenitors.58 The negative functions of NK‐A can be explained by the production of haematopoietic suppressors, macrophage inflammatory protein 1α and transforming growth factor β.23
SP has been shown to be involved in haemorrhagic shock, a time at which the replacement of blood and immune cells68,69 is urgently needed. During haemorrhagic shock, SP exhibits functional pleiotropism so as to maintain a balance in haematopoiesis. Hypoxia, which is linked to haemorrhagic shock, activates the transcription of hypoxia‐inducible factor 1α, which interacts with the PPT‐1 promoter to induce its expression.68
The induction of SP stimulates haematopoiesis so as to replace immune and blood cells.68,69 During the period of haemorrhagic shock, SP also acts as an anti‐apoptotic factor so as to protect BM cells from the insults caused by acute lowering of oxygen in the BM.68
SP is inactivated by the neutral endopeptidase of renal brush border.70 Activity of the enzyme was also shown on the surface of neutrophiles,71 lymphocytes,72 enterocytes,73 fibroblasts74 and endothelial cells.75
Moreover, SP is believed to have an essential role as a modulator of synaptic transmission in sympathetic nerve fibres.76 However, the sympathetic nervous system regulates the egress of stem and progenitor cells from their niche in BM.77 This was shown in a model study using UDP‐galactose ceramide galoctosyltransferase‐deficient mice, which exhibited aberrant nerve conduction and displayed no stem and progenitor cells escape from BM following G‐CSF administration. These results also raise the interesting possibility that SP‐derived alterations in sympathetic tone may explain the variability in mobilisation efficiencies among normal donors78; modulation of sympathetic outflow to the stem cell niche represents a novel strategy to increase the efficiency of haematopoietic stem and progenitor cell harvests for stem cell‐based therapeutics.77 Figure 1 summarises the data presented above.
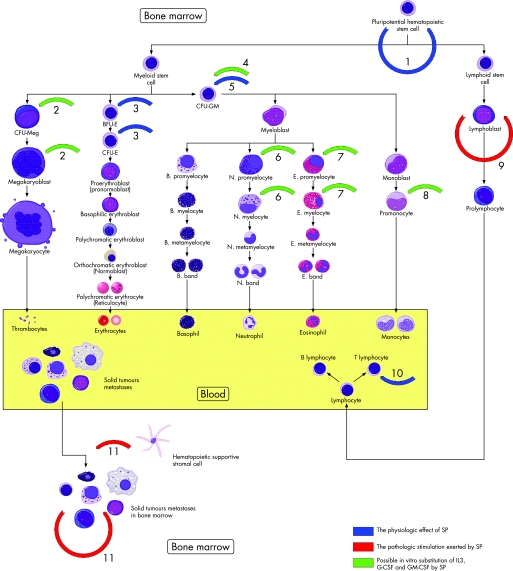
Figure 1 Diagrammatic representation of substance P (SP) effects on haematopoiesis and solid tumour metastases in bone marrow (BM). 1, Stimulation of BM pluripotent haematopoietic cells for self‐renewal by release of stem cell factor.55 2, 4, 6–8, The potent role of SP in substitution of IL‐3, G‐CSF and GM‐CSF in experimental studies.52 3, 5, Stimulatory effect of SP on proliferation of BFU‐E, CFU‐E and CFU‐GM.52,96 9, Autostimulatory effect of SP on proliferation of neoplastically transformed lymphoblasts in acute leukaemia.41,79,80 10, SP augments proliferation of T lymphocytes.46,47 11, SP induces formation and homing of neuroblastoma and breast cancer metastases in bone marrow by autostimulation or direct influence of stromal cells.97,98,99,100,101,102,103,104,105,106,107,108 CFU‐GM, colony forming unit, granulocyte monocyte; CFU‐Meg, colony forming unit megakaryocyte; CFU‐E, colony forming unit erythroblast; BFU‐E, burst forming unit erythroblast; IL, interleukin; G‐CSF, granulocyte colony stimulating factor; GM‐CSF, granulocyte monocyte colony stimulating factor.
Relation of SP to NK‐A and haemokinin‐1 in haematopoiesis
In normal haematopoiesis, SP and NK‐A, through the production of distinct cytokines, exert opposite effects with respect to proliferation of haematopoietic progenitors. The negative effects of NK‐A on proliferation of BM progenitors suggest that NK‐A might be protective to HSCs.65 A protective role for NK‐A is construed based on the predominant types of TAC1 transcripts found in normal BM cells and in leukaemia cells. Normal BM stromal cells express transcript β, while leukaemic cells express only transcript α. While the former is capable of producing both NK‐A and SP, the latter can only produce SP. This suggests that SP and NK‐A might be able to regulate the proliferation of HSC through autocrine and/or paracrine mechanisms. Such a regulatory mechanism might not be possible in leukaemic cells, which produce only SP.79,80,81 This argument is supported by reports showing an autocrine role for SP in proliferation of basophilic leukaemia cells.82
HK‐1 has been also implicated in haematopoiesis.25 A link between HK‐1 and tachykinins derived from the TAC1 gene is currently unclear. HK‐1 regulates B and T lymphopoiesis25,83 and directly affects the transition from pro‐B to pre‐B cells.25 HK‐1 promotes the survival and expansion of B cell lineage84,85,86 and similarly facilitates T cell development at specific stages.16 Regulation of the PPT‐1 gene and/or functions of TAC1 peptides could be altered, leading to BM disruption.
Substance P and malignant haematopoiesis
Physiological haematopoiesis could be displaced by malignant tumours developing in BM. Neoplastic cells present there can originate directly from BM progenitors (leukaemia) or might be derived from metastatic cells of different solid tumours. Substance P plays an important role in pathogenesis of both groups of diseases.
Leukaemia
The leukaemic process involves abnormal differentiation and proliferation of neoplastically transformed stem cells. The cells infiltrate BM, leading to inhibited growth and differentiation of the remaining normal stem cells. The clinical signs reflect anaemia, leucopenia, low blood platelet level as well as involvement of tissues other than BM by the neoplastic process. Leukaemia can affect almost every organ, but lymph nodes, spleen, liver, central nervous system and skin in particular. Traditional classification of acute leukaemia was mainly based on morphological traits of cells examined by light microscopy. Subsequently, cytochemical and cytogenetic techniques were introduced; recently the armamentarium of techniques supporting morphological classification has been enriched by immunology and molecular biology. Since the control processes of normal haematopoiesis involve several pathways and several aspects, it is not easy to select a single in vivo factor, in this case SP, and to directly prove its significance for pathogenesis of acute leukaemia. However, the agent may play role in the pathogenesis of leukaemia since it is involved in the physiological control of haematopoiesis. The suggestion has been confirmed by results of the above mentioned studies on expression of TAC1 transcripts.
An attempt was first made to clarify such a potential by monitoring the fate of patients in whom acute lymphoblastic leukaemia was diagnosed; SP expression was determined in blast cells, at the level of respective mRNA (classical in situ hybridisation) and the protein (immunocytochemistry). It was shown that in children diagnosed with acute lymphoblastic leukaemia of the B cell line phenotype, who were in the low risk group, expression of SP in blast cells before the start of treatment (and before introducing steroids in particular) represented an unfavourable prognostic index.79 These children had a higher number of relapses, compared to children in the low risk group who showed no SP expression.
In the high risk group, the original expression of SP on BM blast cells had no prognostic significance. Interestingly, the expression of SP, in both high and low risk groups, showed no relationship to the risk of death resulting from progression of the disease. However, this might be explained by parallel involvement of several other variables, which together could result in death of the patients. Therefore, it proved impossible to select SP as the dominating factor.79
Similar results were obtained by De Giorgio et al.87 Using flow cytometry, they estimated a strong SP immunoreactivity in lymphocyte‐like cells derived from patients with acute non‐lymphoblastic leukaemia, chronic myeloid leukaemia and B‐chronic lymphoblastic leukaemia. In comparison to these neoplastically transformed cells, normal lymphocytes were, in general, negative or weakly positive for SP, suggesting that SP expression in neoplastic cells may be indicative of their activation state. Moreover, expression of SP in these types of leukaemia also indicated an unfavourable prognosis.
In our preliminary, not yet published studies, experimental 2‐hour incubation of blast cells with an SP agonist was found to result in a significant increase of IL‐1b concentration in BM sampled from children with acute lymphocytic leukaemia (ALL) who showed no SP expression in leukaemic cells. Incubation of the cells with an SP antagonist (spantide) resulted in control levels of IL‐1b. However, in children with ALL and original expression of SP in blast cells, incubation of leukaemic cells with an SP agonist decreased by half the original IL‐1b concentration. A similar concentration was obtained following incubation of the cells with spantide. The IL‐1b concentration in the supernatant of the incubated cells from SP‐positive children was twofold higher than that from SP‐negative children. This might suggest that SP indirectly stimulated proliferation of leukaemic cells by enhancement of IL‐1b synthesis in the cells. It should also be stressed that the phenomenon was observed within a relatively narrow range of SP concentrations of 10−10 to 10−8 mol/l.
Thus, the correlation between the original expression of SP in BM blast cells on the one hand and relapse of the disease in children in the low risk group on the other might suggest that SP is involved in the pathogenesis of acute lymphoblastic leukaemia in children. We have thus decided to also define SP expression in cases of bone marrow hypoplasia.
Bone marrow hypoplasia
BM hypoplasia appears as a morphological equivalent of clinically diagnosed haematopoietic insufficiency.88 It is defined by the following laboratory values: haemoglobin <8.5 g/dl, mean corpuscular volume <88 fl, white blood cell count <2.0 g/l with neutrophil count <1.0 g/l, platelet count <50 g/l and reticulocytes <0.1%.88,89 BM aspirate reveals hypocellularity. Transient BM hypoplasia is usually caused by infections (both viral and bacterial) or by different chemical (including iatrogenic) and/or physical factors.90,91 Inherited aplastic anaemia, or BM hypoplasia prior to the presence of non‐haematopoietic tissue in BM (i.e., neuroblastoma metastases in BM), is a much more rare phenomenon. A considerable number of cases of BM hypoplasia may be subject to spontaneous remission and may not even be recognised. The first hospitalisation of a child with BM hypoplasia usually takes place after two or more ineffective courses of antibiotic therapy (for some undiagnosed chronic infection), followed by symptoms of anaemia and thrombocytopenia. In such cases BM hypoplasia can evolve into myelodysplastic syndrome, severe aplastic anaemia or neoplasia. Establishing the correct diagnosis usually requires several weeks during which the affected children are not treated but are monitored (laboratory evaluation of peripheral blood, BM, chest radiographs, etc) at least once a month.
BM hypoplasia is not a malignant disease, but because of its close relation to the development of different types of acute leukaemia, could be regarded as a pre‐neoplastic disorder. Acute leukaemia of either a lymphoblastic or non‐lymphoblastic type that subsequently evolves into BM hypoplasia used to have a poor prognosis.88 This may, at least in part, result from late introduction of a chemotherapy regimen. It is obvious that, in cases of neoplastic transformation, the type of chemotherapy used will depend on precise recognition of the tumour in question. Such treatment, however, could probably be introduced much earlier if expression of certain markers of early neoplastic transformation could be detected. The current obstacle is that the full list of possible markers is not universally accepted. However, some authors emphasise the fact that several of the cytokines that regulate physiological haematopoiesis may themselves represent potential risk factors of neoplastic transformation in BM hypoplasia.31,32 The search for causes and aids to the diagnosis of BM hypoplasia involves taking a thorough history, a detailed physical examination and a number of laboratory tests, including microscopic analysis of BM, sampled by needle biopsy or during surgery, tests for infectious diseases and, occasionally, genetic studies on the patient and his/her family. Application of immunocytochemical techniques in the diagnosis of BM hypoplasia has already been tested.92 However, attempts to use this technique have been restricted to differentiation of myelodysplastic syndromes from various grades of BM aplasia.92,93,94 Among others, Ki67 antigen and proliferating cell nuclear antigen have been shown to be useful for the purpose.94 In patients with BM aplasia, the markers could not be shown in nucleated cells, while in myelodysplastic syndromes the percentage of immunopositive cells has ranged from 20% to 60%.94 These studies, however, have not attempted to determine the future trend of such BM hypoplastic lesions. Our selection of SP as an indicator of the potential trend of BM hypoplasia evolution was prompted by elucidation of its role, and the role of other neuropeptides, in the physiological control of haematopoiesis.
Our studies performed on BM of healthy individuals (who formed the control group) showed that the immunocytochemical expression of peripherally located SP (most probably coupled to NK‐1R) on nucleated cells of the BM involved a 5% or lower fraction of the cells.79,80 The results prompted us to perform analogous phenotyping of the material sampled from patients with clinical symptoms of BM hypoplasia.95 In some, the proportion of SP‐positive cells among all nucleated cells amounted to 67.6–95.8% (mean 81.5% cells for immunocytochemistry and 84.3% with in situ hybridisation) in the absence of neoplastic cells. Subsequent observation of these patients showed that they developed a proliferative disease of the BM. Throughout the time which preceded the neoplastic transformation, an increased number of SP‐positive cells was noted, although the proportion of Ki67‐positive cells was similar to control values. The neoplastic transformation was manifested by the appearance in BM of poorly differentiated cells with a positive reaction for Ki67 (39.6–49.8%). In line with the above, we suggest that presence of SP in B lymphocytes of normal appearance in hypoplastic BM becomes the additional (to nerve endings) source of this peptide, which may stimulate other neoplastically transformed cells to uncontrolled proliferation. Thus, SP might accelerate the already initiated development of leukaemia.95 The origin of leukaemia, however, seems to be independent of SP expression.96 The expression of SP in apparently normal BM lymphocytes before their neoplastic transformation could not predict death of the patient; this was related to the insufficient number of patients with neoplastic transformation and to different chemotherapy protocols used in the treatment of ALL and acute non‐lymphocytic leukaemia. BM hypoplasia, followed by neoplastic transformation, frequently remains asymptomatic. Few patients receive specialist care before the symptoms of BM proliferative disease become evident. In these few patients the diagnosis of BM hypoplasia leads to consecutive check‐ups, in the course of which immunocytochemical analysis of cellular inducers of differentiation on the surface of BM cells may provide a simple screening test, pointing to the potential trend of evolution of the lesion.
Solid tumours: neuroblastoma, breast cancer
Neuroblastoma is the third most common paediatric malignancy developing from neoplastically transformed ganglionic cells in the peripheral nervous system.97 SP, together with other neuroendocrine markers such as neuropeptide Y, vasoactive intestinal peptide, somatostatin, and corticotrophin‐releasing hormone are commonly expressed in ganglionic cells as well as in ganglioneuroblastoma samples98 or metastatic cells in bone marrow.99,100 Human neuroblastoma cell lines (SY5Y and CHP212) were found to express TAC1 and the genes for NK‐1R and NK‐2R, at the levels of both mRNA and protein.101TAC1 and NK receptors genes were also important for neuroblastoma cell proliferation and ability to establish metastatic foci in bone marrow.101 The NK‐1R deficient neuroblastoma cells did not proliferate when they were co‐cultured with bone marrow stroma, which suggests that NK‐1R signalling is important for the survival of neuroblastoma cells in the bone marrow.101 It must be emphasised that expression of TAC1 and the genes for NK‐Rs is also a common event in other neuroblastoma endocrine‐associated neoplasms such as breast cancer.102,103
Breast cancer is the most common malignant disease and the second leading cause of cancer mortality in women.104 Compared to normal mammary epithelial cells and benign breast biopsy specimens, several breast cancer cell lines have shown increased expression of TAC1 and NK‐Rs.101 Considering that TAC1 peptides are haematopoietic modulators, the autocrine expression in breast cancer cells might explain their early integration in the bone marrow which is a preferred site of metastasis.105 Moreover, bone marrow metastases of breast cancer correlate with poor prognosis and it is highly probable that metastatic cells settle in the bone marrow long before clinical detection of the tumour.105 Studies by Rao et al showed that normal non‐tumourigenic breast cells are not able to survive when co‐cultured with bone marrow stroma.106 This situation could be however be reversed when the above mentioned breast cells were genetically engineered to express TAC1. However, suppression of TAC1 in breast cancer cells limited their malignancy and affected the process of bone marrow colonisation in knock‐out mice.106 Moreover, SP has an established stimulatory effect on the migration and metastatogenic phenotype in collagen matrix of MDA‐MB‐468, the human oestrogen receptor‐negative breast carcinoma cell line; these effects can be prevented by certain NK‐1R antagonists.107 In this mechanism, SP is believed to up‐regulate expression of α2 integrin (an essential adhesion receptor for collagen in migration), and down‐regulate gelsolin (the tumour suppressor agent).107 It is also possible that its total effect could be augmented by the potent role of SP in the initiation of angiogenesis.108
Take‐home messages
Substance P (SP) has a role in both normal and pathological haematopoiesis. In the latter it interferes with the process of blast proliferation, acting as a tumour growth factor, or disturbs the physiological course of bone marrow cell maturation by promoting metastases of different solid tumours to bone marrow.
The tachykinin system represents a potent therapeutic target, especially in tumours unresponsive to standard chemotherapy regimens. SP antagonists might be considered for use as anti‐neoplastic drugs, for example by direct or indirect blocking of tumour cell proliferation through inhibition of growth factor production, including IL‐1b synthesis.
In line with the above, SP must be regarded not only as a growth factor in different tumour cells, but also as an regulating agent, promoting formation of metastases in bone marrow and impeding the physiological haematopoiesis. It must be emphasised that a similar mechanism involving SP could be also utilised in another cancers with a preference for bone marrow metastases: lung, prostate, and to a lesser extent, colon.109
Acknowledgement
The authors wish to thank Professor Geoffrey Shaw for his help in English text editing.
Abbreviations
ALL - acute lymphocytic leukaemia
BM - bone marrow
G‐CSF - granulocyte colony stimulating factor
GM‐CSF - granulocyte monocyte colony stimulating factor
HK - haemokinin
HSC - haematopoietic stem cell
IL - interleukin
MSC - mesenchymal stem cell
NK - neurokinin
NK‐R - neurokinin receptor
PPT - preprotachykinin
SCF - stem cell factor
SP - substance P
Footnotes
Competing interests: None.
References
- 1.Dąbrowski Z, Tabarowski Z. History of discoveries of bone marrow and bone vascularization and innervation. In: Schoutens A, et al eds. Bone circulation and vascularization in normal and pathological conditions. New York: Plenum Press, 199349–54.
- 2.de Castro F. Quelques observations sur l'intervention du systeme nerveux autonome dans l'ossification. Innervation du tissu osseux et de la moelle osseuse. Laboratoire de Recherches Biologiques de l'Universite de Madrid 1930215–244.
- 3.Yamazaki K, Allen T D. Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: ‘the neuro‐reticular complex'. Am J Anatomy 1990187261–276. [DOI] [PubMed] [Google Scholar]
- 4.Imai S, Tokunaga Y, Maeda T.et al Calcitonine‐gene related peptide, substance P and tyrosine hydroxylase‐immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanism. J Orthop Research 199715133–140. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed M, Bjurholm A, Kreibergs A.et al Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide‐immunoreactive nerve fibers in the vertebral bosies, discs, dura mater and spinal ligaments of the rat lumbar spine. Spine 199318268–273. [DOI] [PubMed] [Google Scholar]
- 6.Bianco P, Riminucci M, Gronthos S.et al Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 200119180–192. [DOI] [PubMed] [Google Scholar]
- 7.Zon L I. Developmental biology of hematopoiesis. Blood 1995862876–2891. [PubMed] [Google Scholar]
- 8.Kondo M, Wagers A J, Manz M G.et al Biology of hematopoietic stem cells and progenitors: implications for clinical application. Ann Rev Immunol 200321759–806. [DOI] [PubMed] [Google Scholar]
- 9.Deans R J, Moseley A B. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 200028875–884. [DOI] [PubMed] [Google Scholar]
- 10.Arai F, Ohneda O, Miyamoto T.et al Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med 20021951549–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caplan A I, Bruder S P. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 20017259–264. [DOI] [PubMed] [Google Scholar]
- 12.Kaushansky K, Karplus A P. Hematopoietic growth factors: understanding functional diversity in structural terms. Blood 1993823229–3236. [PubMed] [Google Scholar]
- 13.Greco S J, Corcoran K E, Cho K J.et al Tachykinins in the emerging immune system: relevance to bone marrow homeostasis and maintenance of hematopoietic stem cells. Front Biosci 200491782–1793. [DOI] [PubMed] [Google Scholar]
- 14.Fras C, Kravetz P, Mody D R.et al Substance P‐containing nerves within the human vertebral body: an immunohistochemical study of the basiverterbral nerve. Spine J 2003363–67. [DOI] [PubMed] [Google Scholar]
- 15.Tabarowski Z, Gibson‐Berry K, Felten S Y. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem 199698453–457. [DOI] [PubMed] [Google Scholar]
- 16.van Hagen P M, Krenning E P, Kwekkeboom D J.et al Somatostatin and the immune and haematopoietic system: a review. Eur J Clin Invest 19942491–99. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Paige C J. T‐cell developmental blockage by tachykinins antagonists and the role of hemokinin 1 in T lymphopoiesis. Blood 20031022165–2172. [DOI] [PubMed] [Google Scholar]
- 18.Patacchini R, Lecci A, Holzer P.et al Newly discovered tachykinins raise new questions about their peripheral roles and the tachykinin nomenclature. Trends Pharmacol Sci 2004251–3. [DOI] [PubMed] [Google Scholar]
- 19.Quartara L, Maggi C A. The tachykinin NK1 receptor: part II. Distribution and pathophysiological roles. Neuropeptides 1998321–49. [DOI] [PubMed] [Google Scholar]
- 20.Beaujouan J C, Torrens Y, Saffroy M.et al A 25 year adventure in the field of tachykinins. Peptides 200425339–357. [DOI] [PubMed] [Google Scholar]
- 21.Pennefather J N, Lecci A, Candenas M L.et al Tachykinins and tachykinin receptors: a growing family. Life Sci 2004741445–1463. [DOI] [PubMed] [Google Scholar]
- 22.Rameshwar P, Gascón P. Hematopoietic modulation by the tachykinins. Acta Haematol 19979859–64. [DOI] [PubMed] [Google Scholar]
- 23.Rameshwar P, Poddar A, Gascón P. Hematopoietic regulation mediated by interactions among the neurokinins and cytokines. Leuk Lymphoma 1997281–10. [DOI] [PubMed] [Google Scholar]
- 24.Morteau O, Lu B, Gerard C.et al Hemokinin I is a full agonist at the substance P receptor. Nat Immunol 200121088–1092. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Lu L, Furlonger C.et al Hemokinin is a hematopoietic specific tachykinin that regulates B lymphopoiesis. Nat Immunol 20001392–397. [DOI] [PubMed] [Google Scholar]
- 26.Page N M, Bell N J, Gardiner S M.et al Characterization of the endokinins: human tachykinins with cardiovascular activity. Proc Natl Acad Sci USA 20031006245–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milne C D, Fleming H E, Zhang Y.et al Mechanism of selection mediated by interleukin‐7, the preBCR, and hemokinin‐1 during B‐cell development. Immunol Rev 200419775–88. [DOI] [PubMed] [Google Scholar]
- 28.Pinto F M, Almeida T A, Hernandez M.et al mRNA expression of tachykinins and tachykinin receptors in different human tissues. Eur J Pharmacol 2004494233–239. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly D, Maudsley S, Gent J P.et al Conserved polar residues in the transmembrane domain of the human tachykinin NK2 receptor: functional roles and structural implications. Biochem J 199933955–61. [PMC free article] [PubMed] [Google Scholar]
- 30.Aronin N. Substance P and the tachykinins. Endocrinology and metabolism. In: Becker KL, ed. Principles and practice of endocrinology and metabolism. Philadelphia: JB Lippincott Company, 19901309–1322.
- 31.Maggio J. Tachykinins. Ann Rev Neurosci 19901113–26. [DOI] [PubMed] [Google Scholar]
- 32.Pernow B. Substance P. Pharmacol Rev 19833585–98. [PubMed] [Google Scholar]
- 33.Holzer P. Local effector functions of capsaicin‐sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene‐related peptide and other neuropeptides. Neuroscience 19882439–44. [DOI] [PubMed] [Google Scholar]
- 34.Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilatation and neurogenic plasma extravasation. Naunyn‐Schmiedeberg's Arch Pharmacol 1979310175–180. [DOI] [PubMed] [Google Scholar]
- 35.Rang H, Bevan S, Dray A. Chemical activation of nociceptive peripheral neurones. Br Med Bull 199147534–543. [DOI] [PubMed] [Google Scholar]
- 36.Calvo C, Chavanel G, Senik A. Substance P enhances IL‐2 expression in activated human T‐cells. J Immunol 19921483498–3513. [PubMed] [Google Scholar]
- 37.Ansel J, Brown J, Payan D.et al Substance P selectively activates TNF‐α gene expression in murine mast cell. J Immunol 19931504478–4483. [PubMed] [Google Scholar]
- 38.Stead R, Bienenstock J, Stanisz A. Neuropeptide regulation of mucosal immunity. Immunol Rev 198710033–43. [DOI] [PubMed] [Google Scholar]
- 39.Shanahan F, Anton P. Neuroendocrine modulation of the immune system. Dig Dis Sci 198833123–144. [DOI] [PubMed] [Google Scholar]
- 40.Savino W, Dardenne M. Immuno‐neuroendocrine interactions. Immunol Today 199516318–329. [DOI] [PubMed] [Google Scholar]
- 41.Sirinek L, O'Dorisio M. Modulation of immune function by intestinal neuropeptides. Acta Oncol 199130509–518. [DOI] [PubMed] [Google Scholar]
- 42.Bost K, Breeding S, Pascual D. Modulation of the mRNAs encoding substance P and its receptor in rat macrophages by LPS. Reg Immunol 19924105–112. [PubMed] [Google Scholar]
- 43.Linnik D, Moskowitz A. Identification of immunoreactive substance P in human and other mammalian endothelial cells. Peptides 198910957–963. [DOI] [PubMed] [Google Scholar]
- 44.Ziche M, Morbidelli L, Pacini M.et al NK‐1 receptors mediate the proliferative response of human fibroblasts to tachykinins. Br J Pharmacol 199010011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rameshwar P, Gascon P. Substance P (SP) mediates production of stem cell factor and interleukin‐1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction Blood199586482–490. [PubMed] [Google Scholar]
- 46.Payan D, Brewster D, Goetzl E. Specific stimulation of human T lymphocytes by substance P. J Immunol 19831311613–1622. [PubMed] [Google Scholar]
- 47.Scicchitano R, Biennestock J, Stanisz A. In vivo immunomodulation by the neuropeptide substance P. Immunology 198863733–742. [PMC free article] [PubMed] [Google Scholar]
- 48.Payan D. Receptor‐mediated mitogenic effect of substance P on cultured smooth muscle cells. Biochem Biophys Res Commun 1985130104–113. [DOI] [PubMed] [Google Scholar]
- 49.Woll P, Rosengurt E. Neuropeptides as growth regulators. Br Med Bull 198945492–499. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson G, Pernow B, Fisher G.et al Presence of substance P‐ like immunoreactivity in plasma from man and dog. Acta Physiol Scand 197594542–551. [DOI] [PubMed] [Google Scholar]
- 51.Lotz M, Carson D, Vaughan J. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science 1987235893–905. [DOI] [PubMed] [Google Scholar]
- 52.Rameshwar P, Ganea D, Gascón P. In vitro stimulatory effect of substance P on hematopoiesis. Blood 199381391–398. [PubMed] [Google Scholar]
- 53.Cozens P, Rowe F. Substance P is a potent inducer of TNF and IL‐1 secretion by macrophages. Immunology 19871757–16. [Google Scholar]
- 54.Laurenzi M, Persson M, Dalsgaard C.et al The neuroendopeptide substance P stimulates production of interleukin 1 in human blood monocytes: activated cells are preferentially influenced by the neuropeptide. Scand J Immunol 199031529–538. [DOI] [PubMed] [Google Scholar]
- 55.Rameshwar P, Gascón P, Ganea D. Immunoregulatory effects of neuropeptides. Stimulation of interleukin‐ 2 production by substance P. J Neuroimmunol 19923765–74. [DOI] [PubMed] [Google Scholar]
- 56.Wagner F, Fink R, Hart R.et al Substance P enhances interferon‐ gamma production by human peripheral blood mononuclear cells. Regul Pept 198719355–361. [DOI] [PubMed] [Google Scholar]
- 57.Buzby J, Knoppel E, Cairo M. Coordinate regulation of Steel factor, its receptor (kit) and cytoadhesion molecule (ICAM‐1 and ECAM‐1) mRNA expression in human vascular endothelial cellsof differing origins. Exp Hematol 199422122–131. [PubMed] [Google Scholar]
- 58.Felten S, Felten D, Bellinger D.et al Noradrenergic and peptidergic innervation of lymphoid organs. Neuroimmunoendocrinology 19922567–77. [Google Scholar]
- 59.Pascual D, Bost K. Substance P production by P388D1 macrophages: a possible autocrine function for this neuropeptide. Immunology 19907152–77. [PMC free article] [PubMed] [Google Scholar]
- 60.Weinstock J, Blum A, Walder J.et al Eosinophils from granulomas in murine Schistosomatosis mansoni produce substance P. J Immunol 1988141961–966. [PubMed] [Google Scholar]
- 61.Weinstock J, Blum A. Tachykinin production by granuloma eosinophils in murine Schistosomatosis mansoni. J Immunol 19891423256–3261. [PubMed] [Google Scholar]
- 62.Walsh D, Wharton J, Blake D.et al Neural and endothelial regulatory peptides, their possible involvement in inflammation. Int J Tissue React 199214101–112. [PubMed] [Google Scholar]
- 63.Greeno E, Mantych P, Vercellotti G.et al Functional neurokinin 1 receptors for substance P are expressed by human vascular endothelium. J Exp Med 19931771269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Payan D, Brewster D, Missirian‐Bastian A.et al Substance P recognition by a subset of human T‐lymphocytes. J Clin Invest 1984741532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rameshwar P, Gascón P. Induction of negative hematopoietic regulators by neurokinin‐A in bone marrow stroma. Blood 19968898–106. [PubMed] [Google Scholar]
- 66.Rameshwar P. Substance P: a regulatory neuropeptide for hematopoiesis and immune functions. Clin Immunol Immunopathol 199785129–133. [DOI] [PubMed] [Google Scholar]
- 67.Rameshwar P, Gascón P. Neural regulation of hematopoiesis by the tachykinins. Mol Biol Hemat 19965463 [Google Scholar]
- 68.Qian J, Ramroop K, McLeod A.et al Induction of hypoxia‐inducible factor‐1α and caspase‐3 in hypoxic bone marrow stroma is negatively regulated by the delayed production of substance P. J Immunol 20011674600–4608. [DOI] [PubMed] [Google Scholar]
- 69.Quinlan D, Jr, Rameshwar P, Qian J.et al Effect of hypoxia on the hematopoietic and immune modulator preprotachykinin‐1. Arch Surg 19981331328–1334. [DOI] [PubMed] [Google Scholar]
- 70.Kerr M, Kenny A. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J 1974137477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skidgel R, Jackman H, Erdos E. Metabolism of substance P and bradykinin by human neutrophils. Biochem Pharmacol 1991411335–1341. [DOI] [PubMed] [Google Scholar]
- 72.Tran‐Paterson R, Willard H, Leterte M. The common acute lymphoblastic leukemia antigen (neutral endopeptidase) gene is located on human chromosome 3. Cancer Genet Cytogenet 198942129–141. [DOI] [PubMed] [Google Scholar]
- 73.Iwamoto I, Ueki I, Nadel J. Effect of neutral endopeptidase inhibitors on 3H‐substance P binding in rat ileum. Neuropeptides 198811185–196. [DOI] [PubMed] [Google Scholar]
- 74.Johnson A, Ashton J, Schultz W.et al Neutral metalloendopeptidase from rabbit kidney brush border. Am Rev Respir Dis 1985132564–573. [DOI] [PubMed] [Google Scholar]
- 75.Erdos E, Skidgel R. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J 19893145–156. [PubMed] [Google Scholar]
- 76.Jobling P, Messenger J P, Gibbins I L. Differential expression of functionally identified and immunohistochemically identified NK(1) receptors on sympathetic neurons. J Neurophysiol 2001851888–1898. [DOI] [PubMed] [Google Scholar]
- 77.Katayama Y, Battista M, Kao W M.et al Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006124407–421. [DOI] [PubMed] [Google Scholar]
- 78.Korbling M, Huh Y O, Durett A.et al Allogeneic blood stem cell transplantation: peripheralization and yield of donor‐derived primitive hematopoietic progenitor cells (CD34+ Thy‐1dim) and lymphoid subsets, and possible predictors of engraftment and graft‐versus‐host disease. Blood 1995862842–2848. [PubMed] [Google Scholar]
- 79.Nowicki M, Miskowiak B. Substance P—a potent risk factor in childhood lymphoblastic leukaemia. Leukemia 200361096–1099. [DOI] [PubMed] [Google Scholar]
- 80.Nowicki M, Miskowiak B, Ostalska‐Nowicka D. Detection of substance P and its mRNA in human blast cells in childhood lymphoblastic leukaemia using immunocytochemistry and in situ hybridisation. Folia Histochem Cytobiol 20034133–36. [PubMed] [Google Scholar]
- 81.Rameshwar P, Oh H S, Yook C.et al Substance P‐fibronectin cytokine interactions in myeloproliferative disorder with bone marrow fibrosis. Acta Haematol 20031091–10. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki R, Furuno T, McKay D M.et al Direct neurite‐mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol 19991632410–2415. [PubMed] [Google Scholar]
- 83.Zhou D, Kusnecov A W, Shurin M R.et al Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic‐pituitary‐adrenal axis. Endocrinology 19931332523–2530. [DOI] [PubMed] [Google Scholar]
- 84.Coa Y A, Wagers A J, Beilhack A.et al Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA 2004101221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maestroni G J M. Adrenergic regulation of haematopoiesis. Pharmacol Res 199532249–253. [DOI] [PubMed] [Google Scholar]
- 86.Morrison S J, Shah N M, Anderson D J. Regulatory mechanisms in stem cell biology. Cell 199788287–298. [DOI] [PubMed] [Google Scholar]
- 87.De Giorgio R, Tazzari P L, Barbara G.et al Detection of substance P immunoreactivity in human peripheral leukocytes. J Neuroimmunol 199882175–181. [DOI] [PubMed] [Google Scholar]
- 88.Stockman J A. Aplastic anemia (in Polish). In: Behrman RE, Kliegman R, Jenson AB, eds. Nelson textbook of pediatrics. 14th edn. Warsaw: PWN, 19961426–1428.
- 89.Alter B P, Potter N U. Classification and aetiology of the aplastic anaemias. Clin Haematol 19787431–439. [PubMed] [Google Scholar]
- 90.Williams D M, Lynch R E, Cartwright G E. Drug‐induced aplastic anemia. Semin Hematol 197310195–201. [PubMed] [Google Scholar]
- 91.Baranski B, Armstrong G, Truman J T.et al Epstein‐Barr virus in the bone marrow of patients with aplastic anemia. Ann Intern Med 1988109695–704. [DOI] [PubMed] [Google Scholar]
- 92.Elias J, Gown A, Nakamura R. Special report: Quality control in immunohistochemistry. Am J Clin Pathol 198992836–843. [DOI] [PubMed] [Google Scholar]
- 93.Sadahira Y, Wada H, Manabe T.et al Immunohistochemical assessment of human bone marrow macrophages in hematologic disorders. Pathol Int 199949626–632. [DOI] [PubMed] [Google Scholar]
- 94.Kitagawa M, Kamiyama R, Kasuga T. Expression of the proliferating cell nuclear antigen in bone marrow cells from patients with myelodysplastic syndromes and aplastic anemia. Hum Pathol 199324359–363. [DOI] [PubMed] [Google Scholar]
- 95.Nowicki M, Ostalska‐Nowicka D, Konwerska A.et al The predicting role of substance P in the neoplastic transformation of the hypoplastic bone marrow. J Clin Pathol 200659935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang H S, Trzaska K A, Corcoran K.et al Neurokinin receptors: relevance to the emerging immune system. Arch Immunol Ther Exp 200452328–347. [PubMed] [Google Scholar]
- 97.Weinstein J L, Katzenstein H M, Cohn S L. Advances in the diagnosis and treatment of neuroblastoma. Oncologist 20038278–292. [DOI] [PubMed] [Google Scholar]
- 98.Kimura N, Yamamoto H, Okamoto H.et al Multiple‐hormone gene expression in ganglioneuroblastoma with water diarrhea, hypokalemia, and achlorhydria syndrome. Cancer 1993712841–2846. [DOI] [PubMed] [Google Scholar]
- 99.Nowicki M, Miskowiak B. Comparison of the cell immunophenotype of metastatic and primary foci in stage IV‐S neuroblastoma. Folia Histochem Cytobiol 200240297–303. [PubMed] [Google Scholar]
- 100.Nowicki M, Ostalska‐Nowicka D, Miskowiak B. Prognostic value of stage IV neuroblastoma metastatic immunophenotype in the bone marrow: preliminary report. J Clin Pathol 200659150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mukerji L, Ramkssoon S H, Reddy K K R.et al Autocrine proliferation of neuroblastoma cells is partly mediated through neurokinin receptors: relevance to bone marrow metastasis. J Neurooncol 20057191–98. [DOI] [PubMed] [Google Scholar]
- 102.Singh D, Joshi D D, Hameed M.et al Increased expression of preprotachykinin‐I and neurokinin receptors in human breast cancer cells: implications for bone marrow metastasis. Proc Natl Acad Sci USA 200097388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oh H S, Moharita A, Potian J G.et al Bone marrow stroma influences transforming growth factor‐beta production in breast cancer cells to regulate c‐myc activation of the preprotachykinin‐I gene in breast cancer cells. Cancer Res 2004646327–6336. [DOI] [PubMed] [Google Scholar]
- 104.Landis S H, Murray T, Bolden S.et al Cancer statistics, 1999. CA Cancer J Clin 1999498–31. [DOI] [PubMed] [Google Scholar]
- 105.Gluck S. Autologous transplantation for patients with advanced breast cancer with emphasis on bony metastasis. Can J Oncol 19955(Suppl 1)58–62. [PubMed] [Google Scholar]
- 106.Rao G, Patel P S, Idler S P.et al Facilitating role of preprotachykinin‐I gene in the integration of breast cancer cells within the stromal compartment of the bone marrow: a model of early cancer progression. Cancer Res 2004152874–2881. [DOI] [PubMed] [Google Scholar]
- 107.Lang K, Drell T L, 4th, Lindecke A.et al Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer 2004112231–238. [DOI] [PubMed] [Google Scholar]
- 108.Kato H, Shichiri M, Marumo F.et al Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology 19971382615–2620. [DOI] [PubMed] [Google Scholar]
- 109.Palma C. Tachykinins and their receptors in human malignancies. Curr Drug Targets 200681043–1052. [DOI] [PubMed] [Google Scholar]