Abstract
Background
Loss of mismatch repair (MMR) gene expression has been associated with fewer metastases and improved prognosis in various tumour types.
Aims
To evaluate the predictive and prognostic significance of loss of MMR protein MSH2 in early stage cervical cancer.
Methods
Specimens from 218 consecutive patients with early stage, surgically treated cervical cancer were analysed. Median age was 42 years (interquartile range 35–53). International Federation of Gynecology and Obstetrics (FIGO) stages were IB1 (57%), IB2 (25%) and IIA (18%). Histology was 70% squamous cell, 6% adenosquamous and 24% adenocarcinoma. Pelvic lymph node metastasis was present in 66 (30%) patients. Median follow‐up was 5.2 years (interquartile range 2.5–7.9). Tissue microarrays (TMAs) were constructed containing three cores of paraffin‐embedded tumour per case. MSH2 expression was assessed by immunohistochemistry on TMAs and full sections.
Results
In TMAs MSH2 expression could be analysed in 184/218 (84%) tumours. Loss of MSH2 was observed in 58/184 (32%) tumours, with a moderately strong concordance between TMAs and full sections (κ = 0.47). In tumours with loss of MSH2, pelvic lymph node metastasis and cancer invasion beyond 10 mm were more frequent (48% vs 25%, and 59% vs 37%, respectively). However, loss of MSH2 expression was not related to recurrence or survival.
Conclusion
TMAs are powerful tools for high throughput screening of biological markers for prognostic value in cervical cancer. Absence of MSH2 expression is associated with a high‐risk profile in early stage cervical cancer, but does not predict lymph node status with sufficient accuracy to be used in the clinic.
Keywords: uterine cervical neoplasms, tissue microarray, prognostic marker, MSH2 protein, human
Cervical cancer, when diagnosed at an early stage, has a favourable prognosis, with a 5‐year survival rate of 80%.1 For a variety of reasons most gynaecological oncologists consider radical hysterectomy with pelvic lymph node dissection to be the therapy of choice in otherwise healthy, early stage cervical cancer patients. Pelvic lymph node metastasis is the most important independent prognostic factor in early stage cervical cancer.2 Definitive information on lymph node status only emerges on histological evaluation after surgery. Knowledge of lymph node status prior to surgical treatment could aid in identifying patients with a higher risk of recurrence, enabling a more optimal patient tailored choice of treatment. Currently, characteristics known prior to treatment are International Federation of Gynecology and Obstetrics (FIGO) stage and pathological characteristics of the tumour, as determined by analysis of biopsy material. Tumour characteristics such as histological subtype and grade of differentiation do not have significant prognostic value.3 Lymphatic vascular space involvement (LVSI), when present in biopsy material, indicates an unfavourable prognosis, but its absence is of no significance.4 Much effort has been spent in attempts to find biological markers that can predict pelvic lymph node metastasis or prognosis.5,6,7,8 To date, there is no biological marker available that accurately predicts the presence or absence of pelvic lymph node metastasis prior to surgery.
Loss of mismatch repair (MMR) protein expression has been observed in several tumour types.9,10,11,12,13,14,15 MSH2 is one of the most important members of the MMR gene family.16 DNA becomes microsatellite unstable when MMR genes are lost or dysfunctional. This type of instability is thought to predispose cells to the accumulation of other genetic alterations, accelerating the process of malignant transformation. Hereditary non‐polyposis colorectal carcinoma syndrome (HNPCC) was the first type of malignancy in which this pathophysiological mechanism for carcinogenesis was described.17 Microsatellite instability (MSI) is furthermore observed in 12–15% of sporadic colon cancers. In colon cancer, absence of one of the two most frequently involved MMR proteins, MLH1 and MSH2, is a good predictor of MSI, while colon cancers with MSI show fewer lymph node metastases and have a better prognosis.18,19 Few data are available on the role of MSI in cervical carcinogenesis,20,21,22,23,24,25 and even less on MMR protein expression in cervical cancer.20,26,27 MSI has been observed in 6–25% of invasive cervical cancers.20,21,22,23,24,25 Furthermore, Chung et al showed loss of MSH2 expression in 7 of 50 squamous cervical cancers and Giarnieri et al showed loss of MSH2 in 10 of 23 cervical cancers.20,26 Ciavattini et al recently found MSH2 and Mlh1 expression to be lower in 28 invasive squamous cell cervical cancers compared to cervical intraepithelial lesions.27 These studies were too small to correlate findings to clinicopathological characteristics.
Recently new technologies have become available that facilitate high throughput analysis of biological markers. Tissue microarray (TMA) technology is an example of such a technique, allowing the evaluation of paraffin‐embedded tissue from hundreds of tumours at once, generating vast amounts of data and saving time as well as money. New biological markers can thus be easily evaluated for their predictive and/or prognostic value. TMA technology was first described in 1998 by Kononen et al and has since been validated and used by others.28,29,30,31,32
The aim of our study was to evaluate MSH2 protein expression and its predictive and prognostic value in a large, clinically well‐defined population of cervical cancer patients. For this purpose a TMA containing FIGO stage IB and IIA cervical cancers was constructed. The observed heterogeneous staining pattern of MSH2 on cervical cancer tissue prompted us to validate our TMAs by analysing MSH2 expression in the original full sections and comparing the staining results with the TMAs. To further investigate the biological consequences of our immunohistochemical findings, we performed MSI analysis on a subset of MSH2 negative and positive tumours.
Patients and methods
Patients
Since 1987, clinical and histological data of patients referred to the Department of Gynecologic Oncology of the University Medical Center Groningen, the Netherlands, have been prospectively collected and stored in a computerised database. For the present study, all patients (n = 218) diagnosed with FIGO stage IB or IIA cervical cancer and treated primarily with surgery between January 1987 and December 1998 were identified.
Institutional Review Board approval
Both clinicopathological and follow‐up data as well as paraffin embedded tissue specimens were obtained during standard treatment and follow‐up. For the present study all relevant data were retrieved from our computerised database and entered into a separate, anonymous, password protected database. Protection of patient identity was guaranteed by assigning study‐specific, unique patient numbers. Codes were only known to two dedicated data managers, who also have daily responsibility for the larger database. In case of uncertainties with respect to clinicopathological and follow‐up data, the larger databases could only be consulted through the data managers, thereby ascertaining the protection of the patient's identity. Therefore, according to Dutch law no further Institutional Review Board approval was needed for this study.
Staging and treatment
In all patients, bimanual examination under general anaesthesia was performed for clinical staging, in accordance with the FIGO guidelines.33 Primary surgical treatment consisted of a type II radical hysterectomy and pelvic lymphadenectomy. Adjuvant external beam radiation therapy of the pelvis was applied when any of lymph node metastases, parametrial invasion or positive resection margins was present.
Designing and constructing the TMAs
TMAs were constructed using paraffin‐embedded tumour tissue of cervical cancer patients. Morphologically representative areas of tumour were marked on H&E‐stained sections. Three cores of 0.6 mm diameter were taken from the marked areas out of the corresponding tissue block and placed in a recipient blank paraffin block on predefined array locations, using a precision instrument (Beecher Instruments, Silver Spring, Maryland, USA). Three arrays were constructed, each containing three cores per tumour. Cores of histologically normal cervical tissue and cervical cancer tissue originating from the same donor blocks were incorporated in all three arrays to serve as internal controls for intra‐run variability. Sections (3 μm) were cut from the arrays using a sectioning tape system and transferred to adhesive coated slides. The slides were placed under an UV light for 35 seconds, after which the tape was removed using a solvent degreaser.
Immunohistochemistry
Conventional immunohistochemistry on whole sections was performed on sections (3 μm) cut from the same formalin‐fixed paraffin‐embedded tumours used to construct the TMAs. These sections were mounted on slides with 3‐aminopropyl triethoxysilane coating (Sigma–Aldrich, Diesenhofen, Germany). Immunohistochemical procedures were the same for the TMA and full tumour sections. Slides were cleared in xylene, rehydrated through a graded ethanol series to distilled water and subjected to antigen retrieval using an autoclave. The sections were heated for 5 minutes, three times at 115°C in a blocking reagent (2% block and 0.2% sodium dodecylsulphate in maleic acid, pH 6.0 (Boehringer–Mannheim, Mannheim, Germany)). Slides were incubated with a mouse monoclonal antibody against hMSH2 for 30 minutes (Ab‐2, Oncogene, Boston, Massachusetts, USA (1:100)). Staining was performed using the DAKO autostainer. Diaminobenzidine was used as the chromogen to visualise the antibody. The nuclei were counterstained with Mayer's haematoxylin and the slides were dehydrated in graded ethanols, dried and coverslipped. Protein expression in the basal layer of normal cervical epithelium served as an internal positive control.
Analysis of immunohistochemical staining on TMA
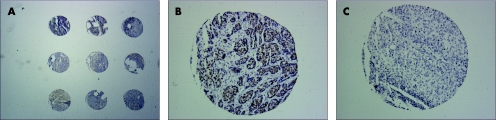
Staining intensity for MSH2 was scored as negative (−), faint (+), positive (++) or strong (+++) nuclear staining. Two independent observers scored the TMAs (KH and EN) and a concordance of >95% was found. The discordant cases were reviewed and scores were reassigned on consensus of opinion. Percentage of positively stained cells could not be assessed on 0.6 mm cores. Figure 1 shows three cores from our TMAs.
Figure 1 Overview of cores from the TMA and close up of a positive and negative core, stained with hMSH2 monoclonal antibody. (A) Overview of cores from the TMA. (B) Squamous cell carcinoma, scored as positive. (C) Squamous cell carcinoma, scored as negative.
Analysis of immunohistochemical staining on full tumour sections
Two investigators (KH and EN) scored MSH2 staining on full sections of paraffin‐embedded tissue separately from the TMAs. Staining was semi‐quantitatively scored based on staining intensity (−, +, ++ or +++) and percentage of positively stained (++ or +++) tumour cells. Percentage of tumour cells stained ++ or +++ was assessed and subgroups were identified, with <1%, 1% to <5%, 5% to <10%, 10% to <50% or ⩾50% of the tumour stained positive for MSH2.
Microsatellite instability analysis
Ten tumours that showed no expression of MSH2 and 10 tumours that were stained highly positive were analysed for microsatellite instability. Tumour DNA was extracted from paraffin‐embedded sections of 10 μm thickness. In addition, DNA was obtained from lymph nodes without metastases from the same patients. MSI primers were used as recommended by the National Cancer Institute (NCI).34 The analysed loci consisted of two mononucleotide repeats (BAT‐25 and BAT‐26) and three dinucleotide repeats (D2S123, D5S346 and D17S250). PCR products were analysed as described previously.35 A phenotype was considered to be MSI‐high if deletions were present in two or more of the five markers and MSI‐low if only one of the five markers showed deletions in the tumour DNA when compared with normal DNA.
Statistical analysis
All analyses were performed using SPSS V.11. Differences between groups were tested using the χ2 test. Multiple logistic regression analysis was performed with cancer invasion beyond 10 mm, presence of lymph node metastasis, 5‐year disease free survival (DFS) and 5‐year overall survival (OS) as dependent variables, and MSH2 staining on full sections, FIGO stage, grade and tumour histology as independent variables, entered simultaneously into the model. In order to determine the optimal cut‐off value for MSH2 staining on full sections, MSH2 cut‐off values <1%, <5%, <10% and <50% were entered separately into the model (table 2). The concordance between immunohistochemical analysis on full sections and TMA was determined using Cohen's κ. A κ value of 0.4–0.6 was considered to denote a moderately strong concordance and a κ value of >0.6 was considered to denote a strong concordance.36 p‐Values of <0.05 were considered statistically significant.
Table 2 Multiple logistic regression analysis: MSH2 and variables known prior to surgery.
| Variables | OR (95% CI) | p‐Value | OR (95% CI) | p‐Value |
|---|---|---|---|---|
| Lymph node metastasis | Cancer invasion >10 mm | |||
| MSH2 <1% | 0.4 (0.182 to 0.808) | 0.012 | 0.5 (0.221 to 0.984) | 0.045 |
| FIGO stage | 1.6 (1.149 to 2.210) | <0.005 | 1.8 (1.310 to 2.590) | <0.0005 |
| Grade | 1.2 (0.839 to 1.836) | 0.281 | 1.5 (0.987 to 2.148) | 0.059 |
| Histology | 1.1 (0.730 to 1.586) | 0.710 | 0.8 (0.513 to 1.112) | 0.156 |
| MSH2 <5% | 0.5 (0.237 to 0.967) | 0.040 | 0.4 (0.220 to 0.904) | 0.025 |
| FIGO stage | 1.6 (1.132 to 2.165) | 0.007 | 1.8 (1.297 to 2.562) | 0.001 |
| Grade | 1.2 (0.827 to 1.803) | 0.314 | 1.4 (0.975 to 2.124) | 0.067 |
| Histology | 1.1 (0.722 to 1.557) | 0.766 | 0.8 (0.515 to 1.116) | 0.161 |
| MSH2 <10% | 0.5 (0.264 to 1.013) | 0.055 | 0.5 (0.259 to 0.966) | 0.039 |
| FIGO stage | 1.6 (1.148 to 2.197) | 0.005 | 1.8 (1.313 to 2.594) | 0.000 |
| Grade | 1.2 (0.818 to 1.786) | 0.342 | 1.4 (0.960 to 2.094) | 0.079 |
| Histology | 1.1 (0.731 to 1.586) | 0.709 | 0.8 (0.523 to 1.136) | 0.188 |
| MSH2 <50% | 0.6 (0.295 to 1.131) | 0.109 | 0.5 (0.264 to 0.971) | 0.041 |
| FIGO stage | 1.5 (1.106 to 2.110) | 0.010 | 1.8 (1.261 to 2.495) | 0.001 |
| Grade | 1.2 (0.827 to 1.803) | 0.315 | 1.4 (0.968 to 2.106) | 0.072 |
| Histology | 1.1 (0.739 to 1.623) | 0.651 | 0.8 (0.538 to 1.182) | 0.259 |
| 5‐year disease‐free survival | 5‐year overall survival | |||
| MSH2 <1% | 0.5 (0.240 to 1.237) | 0.147 | 0.5 (0.290 to 1.450) | 0.107 |
| FIGO stage | 1.3 (0.885 to 1.818) | 0.196 | 1.4 (0.955 to 2.008) | 0.086 |
| Grade | 1.6 (1.005 to 2.399) | 0.048 | 1.6 (0.999 to 2.448) | 0.051 |
| Histology | 1.2 (0.804 to 1.872) | 0.342 | 1.5 (0.990 to 2.343) | 0.056 |
| MSH2 <5% | 0.5 (0.225 to 1.066) | 0.072 | 0.5 (0.222 to 1.151) | 0.104 |
| FIGO stage | 1.3 (0.875 to 1.805) | 0.215 | 1.4 (0.946 to 1.990) | 0.095 |
| Grade | 1.5 (0.996 to 2.377) | 0.052 | 1.5 (0.990 to 2.421) | 0.055 |
| Histology | 1.2 (0.813 to 1.899) | 0.315 | 1.5 (0.992 to 2.347) | 0.055 |
| MSH2 <10% | 0.7 (0.350 to 1.581) | 0.442 | 0.9 (0.386 to 1.927) | 0.719 |
| FIGO stage | 1.3 (0.886 to 1.814) | 0.194 | 1.4 (0.953 to 1.989) | 0.089 |
| Grade | 1.5 (0.995 to 2.371) | 0.053 | 1.6 (0.996 to 2.432) | 0.052 |
| Histology | 1.2 (0.795 to 1.849) | 0.372 | 1.5 (0.955 to 2.244) | 0.080 |
| MSH2 <50% | 0.5 (0.244 to 1.126) | 0.098 | 0.6 (0.290 to 1.450) | 0.292 |
| FIGO stage | 1.2 (0.859 to 1.765) | 0.258 | 1.4 (0.935 to 1.956) | 0.108 |
| Grade | 1.5 (0.996 to 2.391) | 0.052 | 1.6 (0.994 to 2.435) | 0.053 |
| Histology | 1.3 (0.842 to 2.018) | 0.235 | 1.5 (0.993 to 2.405) | 0.054 |
Dependent variables: lymph node metastasis, cancer invasion >10 mm, 5‐year overall survival and disease recurrence.
Independent variables: MSH2 at 1–50% cut‐off, FIGO stage, grade and tumour histology.
FIGO, International Federation of Gynecology and Obstetrics.
Table 4 Concordance between tissue microarray (TMA) and full sections (Cohen's κ).
| MSH2 on full section (1% cut‐off) | ||||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| MSH2 on TMA | Negative | 30 | 28 | 58 |
| Positive | 11 | 115 | 126 | |
| Total | 41 | 143 | 184 | |
κ = 0.47.
Results
Clinicopathological characteristics
Follow‐up was collected until 1 December 2004. Median age at time of diagnosis was 42 years (interquartile range 35–53). Median follow‐up time was 5.2 years (interquartile range 2.5–7.9). Cervical cancers were histologically typed as squamous cell carcinomas (70%), adenocarcinomas (24%) and adenosquamous carcinomas (6%). Patients were classified as FIGO stage IB1 (57%), IB2 (25%) and IIA (18%). Lymph node metastases were present in 66 (30%) patients and LVSI in 113 (52%) patients. Adjuvant radiation therapy was given to 84 (39%) patients; 47 patients (22%) developed recurrent disease and 43 (20%) died as a result of cervical cancer within our follow‐up, of which 39 (18%) died within 5 years after diagnosis. Table 1 summarises clinicopathological characteristics and their relationship to 5‐year recurrence free survival (RFS) and 5‐year OS. In our population, lymph node metastasis, cancer invasion beyond 10 mm and grade of differentiation was related to both 5‐year RFS and 5‐year OS in univariate analysis.
Table 1 Clinicopathological characteristics related to recurrence free and overall survival.
| Clinicopathological characteristics | n | % | 5‐year RFS | 5‐year OS | ||
|---|---|---|---|---|---|---|
| % | p‐Value | % | p‐Value | |||
| FIGO stage | 0.251 | 0.269 | ||||
| IB1 | 124 | 57 | 84 | 86 | ||
| IB2 | 55 | 25 | 71 | 76 | ||
| IIA | 39 | 18 | 77 | 77 | ||
| Histology | 0.631 | 0.309 | ||||
| Squamous cell | 135 | 70 | 80 | 84 | ||
| Adenosquamous | 13 | 6 | 85 | 85 | ||
| Adenocarcinoma | 52 | 24 | 75 | 75 | ||
| Grade (n = 212) | 0.019 | 0.027 | ||||
| 1 | 35 | 17 | 80 | 83 | ||
| 2 | 92 | 43 | 88 | 90 | ||
| 3 | 85 | 40 | 69 | 73 | ||
| Tumour volume | 0.053 | 0.036 | ||||
| 0–2 cm | 43 | 20 | 93 | 95 | ||
| 2–4 cm | 101 | 46 | 79 | 82 | ||
| 4–6 cm | 68 | 31 | 72 | 74 | ||
| >6 cm | 6 | 3 | 67 | 83 | ||
| LNM | <0.0005 | <0.0005 | ||||
| No | 152 | 70 | 88 | 91 | ||
| Yes | 66 | 30 | 59 | 62 | ||
| Cancer invasion | 0.009 | 0.004 | ||||
| ⩽10 mm | 129 | 59 | 85 | 88 | ||
| >10 mm | 89 | 41 | 71 | 73 | ||
| LVSI | 0.117 | 0.016 | ||||
| No | 105 | 48 | 84 | 89 | ||
| Yes | 113 | 52 | 75 | 76 | ||
FIGO, International Federation of Gynecology and Obstetrics; LNM, lymph node metastasis; LVSI, lymph vascular space involvement; OS, overall survival; RFS, recurrence‐free survival.
Immunohistochemistry of TMAs
Of the 218 tumours in the TMAs, 184 had cores of sufficient quality to allow analysis of MSH2 staining. In 104 of 184 tumours (55%) all three cores were available, in 50 tumours (27%) two cores were available and in 28 tumours (15%) one core was available for immunohistochemical evaluation. The core “loss” was randomly divided over cases. The majority of tumours showed the same staining intensity in all cores (108/154, 70%). Absence of MSH2 expression was observed in 58/184 (32%) tumours. Lymph node metastasis (48% vs 25%, p = 0.001), cancer invasion >10 mm (59% vs 37%, p = 0.007) and LVSI (60% vs 45%, p = 0.057) were more frequently observed in tumours that were MSH2 negative (table 3).
Table 3 Univariate analysis of MSH2 and lymph node metastasis, cancer invasion beyond 10 mm and lymph vascular space involvement (LVSI), for tissue microarrays (TMAs) as well as full sections.
| MSH2 on TMA | MSH2 on full sections | |||
|---|---|---|---|---|
| Negative (%) | Positive (%) | Negative (%) | Positive (%) | |
| Lymph node metastasis | ||||
| Present | 28 (48) | 31 (25) | 20 (49) | 39 (27) |
| Absent | 30 (52) | 95 (75) | 21 (51) | 104 (73) |
| p‐Value | 0.001 | 0.009 | ||
| Cancer invasion >10 mm | ||||
| Present | 34 (59) | 47 (37) | 24 (58) | 57 (40) |
| Absent | 24 (41) | 79 (63) | 17 (42) | 86 (60) |
| p‐Value | 0.007 | 0.034 | ||
| LVSI | ||||
| Present | 35 (60) | 57 (45) | 26 (63) | 66 (46) |
| Absent | 23 (40) | 69 (55) | 15 (37) | 77 (54) |
| p‐Value | 0.057 | 0.051 | ||
Immunohistochemistry of full sections
Full sections were cut from the 184 tumours that could be analysed on the TMAs and stained for MSH2. In order to determine the optimal cut‐off value for MSH2 staining on full sections with respect to its predictive value for lymph node metastasis and/or survival, MSH2 cut‐off values <1%, <5%, <10% and <50% were entered into a multiple logistic regression model (table 2). At the cut‐off value of 1%, MSH2 staining on full sections had the best odds ratio (OR) and p‐value, therefore it was considered the most accurate in predicting lymph node metastasis. Subsequently this 1% cut‐off value was chosen for all our further analyses for MSH2 on full sections. At the 1% cut‐off value, 41/184 (22%) patients were MSH2 negative on full sections. In a univariate analysis, lymph node metastasis (49% vs 27%, p = 0.009), cancer invasion >10 mm (58% vs 40%, p = 0.034) and LVSI (63% vs 46%, p = 0.051) were more frequently observed in MSH2 negative tumours (table 3). In multivariate analysis, absence of MSH2 expression at the 1% cut‐off value independently predicted the presence of lymph node metastasis (OR = 0.4, 95% CI 0.182 to 0.808, p = 0.012) and cancer invasion beyond 10 mm (OR = 0.5, 95% CI 0.221 to 0.984, p = 0.045). There was no relationship between absence of MSH2 and disease recurrence or survival on either TMA or full sections.
MSI analysis
For MSI analysis, we selected 10 extremes on each side of the spectrum. Of the MSH2 negative tumours, two showed loss of heterozygosity (LOH), both at the D17S250 locus, and two had an MSI high phenotype. None of the MSH2 positive tumours showed LOH or an MSI‐high phenotype. However, two MSH2 positive tumours showed an MSI‐low phenotype (table 5). In total, 20 tumours were analysed for MSI (10 MSH2 negative and 10 MSH2 positive tumours). Table 5 shows patient characteristics of the six cases with MSI.
Table 5 Patient characteristics of the six cases with microrosatellite instability (MSI).
| MSI‐high | MSI‐high | MSI‐low | MSI‐low | LOH | LOH | |
|---|---|---|---|---|---|---|
| Age (years) | 68 | 51 | 36 | 47 | 39 | 35 |
| Follow‐up (years) | 10.2 | 6.9 | 10.1 | 6.4 | 9.6 | 8.3 |
| FIGO stage | IIA | IB1 | IIA | IB1 | IB1 | IB2 |
| Histology | Squamous | Adeno | Squamous | Squamous | Squamous | Squamous |
| Grade | 2 | 3 | 3 | 2 | 2 | 3 |
| LNM | Present | Absent | Absent | Absent | Absent | Present |
| Cancer invasion | >10 mm | >10 mm | >10 mm | 5–10 mm | >10 mm | 5–10 mm |
| LVSI | Present | Present | Absent | Present | Present | Absent |
| Recurrence | Absent | Absent | Absent | Absent | Absent | Absent |
| MSH2 | Negative | Negative | Positive | Positive | Negative | Negative |
| MSI/LOH locus | D5S346 | BAT25 | D17S250 | D17S250 | D17S250 | D17S250 |
| D17S250 | BAT26 |
FIGO, International Federation of Gynecology and Obstetrics; LNM, lymph node metastasis; LOH, loss of heterozygosity; LVSI, lymph vascular space involvement.
Using MSH2 as a predictive marker
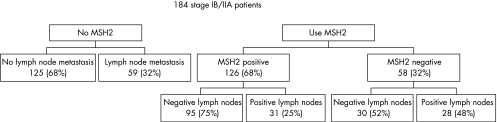
Pelvic lymph node metastasis was present in 59 of 184 patients (32%). Absence of MSH2 immunostaining on TMA had a sensitivity of 0.47 in predicting presence of lymph node metastasis, with a specificity of 0.76. At the 1% cut‐off value, MSH2 on full sections had a sensitivity of 0.34 with a specificity of 0.83. We created a flowchart of our patient population to visualise the possible clinical relevance of MSH2 (fig 2). Pelvic lymph node metastases were found in 48% of patients with MSH2 negative tumours, compared to only 25% of patients with MSH2 positive tumours.
Figure 2 The implications of using MSH2 in predicting presence of lymph node metastasis in early stage cervical cancer patients (tissue microarray data).
Discussion
In early stage cervical cancer, ideal predictive markers should identify patients with pelvic lymph node metastases, allowing better preoperative decision making and patient counselling. Many predictive markers have been evaluated for this purpose in previous studies in cervical cancer, but none are presently used in the clinic.
Our study on MSH2 has been performed in a large, well‐documented population of patients with early stage cervical cancer that appears to be representative of early stage cervical cancer, since the distribution of clinicopathological characteristics and outcome in our study population are comparable to those in previous studies.37 Lymph node status is the most important predictor of disease recurrence and survival, and this was again shown in our population.
Our study shows that loss of MSH2 is associated with lymph node metastasis and cancer invasion beyond 10 mm in early stage cervical cancer. In a variety of malignancies data on the impact of loss of MMR gene expression are contradictory. Loss of MMR genes has shown to be of favourable prognostic value in sporadic colon cancers. Gryfe et al showed reduced metastases and improved survival for patients diagnosed before 50 years of age with sporadic colon cancer of MSI‐high phenotype (cancers with loss of MMR gene expression, mainly MLH1 and MSH2).18 In prostate cancer, loss of MSH2 or MLH1 has also been reported to be associated with a better prognosis.15 Studies of soft tissue sarcoma and biliary tract carcinoma reported a poor prognosis in tumours with reduced expression of MSH2 and reduced expression of MMR genes combined with O6‐methylguanine‐DNA methyltransferase, respectively.12,14 In ovarian cancer, lower expression of MSH2 was found in tumours that did not respond to chemotherapy.10 Loss of MMR protein expression therefore appears to have different effects in different types of tumour.
In cervical cancer the prognostic value of loss of MMR protein expression has hitherto been unclear. Only two studies have previously assessed MSH2 and MLH1 status in cervical cancers of squamous origin, but no solid conclusions could be drawn as to its prognostic value, mainly because of an insufficient number of patients.20 In our study, loss of MSH2 expression was related to lymph node metastasis and cancer invasion beyond 10 mm, but not to disease recurrence or disease specific death. Presence of lymph node metastasis is the most important prognostic factor for survival, shown also in our population. Loss of MSH2 significantly predicts presence of lymph node metastases, but is not a strong enough marker to predict disease‐free and overall survival. MSH2 negative tumours do have a worse survival in a Kaplan–Meier curve; however, this was not significant.
In our study comparable results were found for MSH2 staining on TMAs and full sections with respect to relation to lymph node metastasis, cancer invasion beyond 10 mm and LVSI. Despite a heterogeneous staining pattern, the observed concordance between TMA and full section data shows that in cervical cancer TMAs are a good representation of the full sections and therefore useful as a research tool in the screening of markers for their predictive and prognostic potential.
Several studies have investigated the role of MSI in cervical cancer and have shown MSI to be an infrequent phenomenon, although different MSI markers were used in these studies.21,22,23,24,25 Our study did not show a relationship between loss of MSH2 and MSI, using the marker panel recommended by the NCI, since only two of 10 MSH2 negative tumours showed an MSI‐high phenotype. Several explanations for this discrepancy can be envisioned. First, we investigated early stage cervical cancers. It might be that loss of MSH2 only results in MSI over a longer period of time, and it might not be demonstrable in a population of early stage cervical cancer patients. Second, the MSI marker panel of the NCI may not be the ideal marker set for cervical cancer, since these markers were initially chosen to detect MSI in HNPCC‐related tumours.
Different mechanisms may lead to loss of MSH2 protein expression. One mechanism is through mutation in the MSH2 gene itself.38 Another explanation could be promoter hypermethylation. Hypermethylation of the promoter region of a gene can result in transcriptional silencing and is a phenomenon often seen in malignant transformation of different tumour types, including cervical cancer.39,40,41,42 Hypermethylation or mutation analysis of MSH2 was not performed in the present study.
Take‐home messages
MSH2 staining was absent in 32% of 184 early stage cervical cancers.
MSH2 expression on TMAs was representative of MSH2 expression on full sections; therefore TMAs are reliable tools for high throughput screening of biological markers for prognosis in cervical cancer.
A relationship between loss of MSH2 expression and microsatellite instability was not found in this study.
Loss of MSH2 expression was found to be associated with presence of pelvic lymph node metastasis and a deep stromal invasion, both in univariate as well as in multivariate analysis.
Loss of MSH2 expression alone is not a strong enough predictor of pelvic lymph node metastasis to be of possible use in the clinic.
Many studies in all kinds of malignancies have reported statistically significant relationships between a variety of cell biological markers and clinical parameters, for example pelvic lymph node metastases, as we now report for loss of MSH2 protein and cervical cancer in this study. However, these statistically significant relationships rarely result in clinically relevant prognostic markers. Figure 2 shows that almost half of MSH2 negative tumours had lymph node metastasis, compared to only 25% of MSH2 positive tumours. However, MSH2 can not be used in a clinical setting, since 30 patients (52%) with MSH2 negative tumours did not have lymph node metastasis. These patients would have been falsely identified preoperatively as being at high‐risk for lymph node metastasis. Despite the now reported significant relation between loss of MSH2 and pelvic lymph node metastasis, much stronger predictive factors are needed to be of possible use in the clinic.
In conclusion, TMA technology is useful as a screening tool for predictive markers in cervical cancer and even if expression of the marker is heterogeneous, three cores within a TMA are still representative of the full section. Absence of MSH2 appears to be a risk factor in early stage cervical cancer and may constitute a more malignant phenotype. Despite a relation between absence of MSH2 and presence of pelvic lymph node metastasis, its power as a predictive factor is insufficient to be used as a predictive marker in the clinic.
Acknowledgements
We thank Nicol Keith for the opportunity to visit the University of Glasgow. We wish to thank Robert Hofstra for performing the MSI analysis, and Inge Plateel and Tineke van der Sluis for cutting the full tumour sections.
Abbreviations
DFS - disease‐free survival
FIGO - International Federation of Gynecology and Obstetrics
HNPCC - hereditary non‐polyposis colorectal carcinoma
LNM - lymph node metastasis
LOH - loss of heterozygosity
LVSI - lymph vascular space involvement
MMR - mismatch repair
MSI - microrosatellite instability
NCI - National Cancer Institute
OR - odds ratio
OS - overall survival
RFS - recurrence‐free survival
RT - radiation therapy
TMA - tissue microarray
Footnotes
Competing interests: None.
References
- 1.Benedet J L, Odicino F, Maisonneuve P.et al Carcinoma of the cervix uteri. J Epidemiol Biostat 200167–43. [PubMed] [Google Scholar]
- 2.Creasman W T, Kohler M F. Is lymph vascular space involvement an independent prognostic factor in early cervical cancer? Gynecol Oncol 200492525–529. [DOI] [PubMed] [Google Scholar]
- 3.Ho C M, Chien T Y, Huang S H.et al Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol Oncol 200493458–464. [DOI] [PubMed] [Google Scholar]
- 4.Rutledge T L, Kamelle S A, Tillmanns T D.et al A comparison of stages IB1 and IB2 cervical cancers treated with radical hysterectomy. Is size the real difference? Gynecol Oncol 20049570–76. [DOI] [PubMed] [Google Scholar]
- 5.Birner P, Schindl M, Obermair A.et al Overexpression of hypoxia‐inducible factor 1‐alpha is a marker for an unfavorable prognosis in early‐stage invasive cervical cancer. Cancer Res 2000604693–4696. [PubMed] [Google Scholar]
- 6.Duk J M, Groenier K H, de Bruijn H W.et al Pretreatment serum squamous cell carcinoma antigen: a newly identified prognostic factor in early‐stage cervical carcinoma. J Clin Oncol 199614111–118. [DOI] [PubMed] [Google Scholar]
- 7.Ferrandina G, Lauriola L, Distefano M G.et al Increased cyclooxygenase‐2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol 200220973–981. [DOI] [PubMed] [Google Scholar]
- 8.van de P G, Holm R, Lie A K.et al Expression of p27, p21, and p16 protein in early squamous cervical cancer and its relation to prognosis. Gynecol Oncol 200389140–147. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Wang J, Fraig M M.et al Alterations in PMS2, MSH2 and MLH1 expression in human prostate cancer. Int J Oncol 2003221033–1043. [PubMed] [Google Scholar]
- 10.Geisler J P, Geisler H E, Miller G A.et al Immunohistochemical staining of the mismatch repair gene, hMSH2, and survival in patients with ovarian carcinoma. Eur J Gynaecol Oncol 200021237–240. [PubMed] [Google Scholar]
- 11.Hardisson D, Moreno‐Bueno G, Sanchez L.et al Tissue microarray immunohistochemical expression analysis of mismatch repair (hMLH1 and hMSH2 genes) in endometrial carcinoma and atypical endometrial hyperplasia: relationship with microsatellite instability. Mod Pathol 2003161148–1158. [DOI] [PubMed] [Google Scholar]
- 12.Kohya N, Miyazaki K, Matsukura S.et al Deficient expression of O(6)‐methylguanine‐DNA methyltransferase combined with mismatch‐repair proteins hMLH1 and hMSH2 is related to poor prognosis in human biliary tract carcinoma. Ann Surg Oncol 20029371–379. [DOI] [PubMed] [Google Scholar]
- 13.Leach F S, Polyak K, Burrell M.et al Expression of the human mismatch repair gene hMSH2 in normal and neoplastic tissues. Cancer Res 199656235–240. [PubMed] [Google Scholar]
- 14.Taubert H W, Bartel F, Kappler M.et al Reduced expression of hMSH2 protein is correlated to poor survival for soft tissue sarcoma patients. Cancer 2003972273–2278. [DOI] [PubMed] [Google Scholar]
- 15.Velasco A, Hewitt S M, Albert P S.et al Differential expression of the mismatch repair gene hMSH2 in malignant prostate tissue is associated with cancer recurrence. Cancer 200294690–699. [DOI] [PubMed] [Google Scholar]
- 16.Boland C R, Goel A. The silence of the genes: matching mismatch repair defects with tumors. Cancer 2003982091–2094. [DOI] [PubMed] [Google Scholar]
- 17.Peltomaki P, Lothe R A, Aaltonen L A.et al Microsatellite instability is associated with tumors that characterize the hereditary non‐polyposis colorectal carcinoma syndrome. Cancer Res 1993535853–5855. [PubMed] [Google Scholar]
- 18.Gryfe R, Kim H, Hsieh E T.et al Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 200034269–77. [DOI] [PubMed] [Google Scholar]
- 19.Parc Y, Gueroult S, Mourra N.et al Prognostic significance of microsatellite instability determined by immunohistochemical staining of MSH2 and MLH1 in sporadic T3N0M0 colon cancer. Gut 200453371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung T K, Cheung T H, Wang V W.et al Microsatellite instability, expression of hMSH2 and hMLH1 and HPV infection in cervical cancer and their clinico‐pathological association. Gynecol Obstet Invest 20015298–103. [DOI] [PubMed] [Google Scholar]
- 21.Chung T K, Ip T Y, Hampton G M.et al Microsatellite instability in cervical carcinoma. Eur J Obstet Gynecol Reprod Biol 200194121–124. [DOI] [PubMed] [Google Scholar]
- 22.Helland A, Borresen‐Dale A L, Peltomaki P.et al Microsatellite instability in cervical and endometrial carcinomas. Int J Cancer 199770499–501. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura M, Furumoto H, Kato T.et al Microsatellite instability is a late event in the carcinogenesis of uterine cervical cancer. Gynecol Oncol 200079201–206. [DOI] [PubMed] [Google Scholar]
- 24.Ou C Y, Chang J G, Tseng H H.et al Analysis of microsatellite instability in cervical cancer. Int J Gynecol Cancer 1999967–71. [DOI] [PubMed] [Google Scholar]
- 25.Wong Y F, Cheung T H, Poon K Y.et al The role of microsatellite instability in cervical intraepithelial neoplasia and squamous cell carcinoma of the cervix. Gynecol Oncol 200389434–439. [DOI] [PubMed] [Google Scholar]
- 26.Giarnieri E, Mancini R, Pisani T.et al Msh2, Mlh1, Fhit, p53, Bcl‐2, and Bax expression in invasive and in situ squamous cell carcinoma of the uterine cervix. Clin Cancer Res 200063600–3606. [PubMed] [Google Scholar]
- 27.Ciavattini A, Piccioni M, Tranquilli A L.et al Immunohistochemical expression of DNA mismatch repair (MMR) system proteins (hMLH1, hMSH2) in cervical preinvasive and invasive lesions. Pathol Res Pract 200520121–25. [DOI] [PubMed] [Google Scholar]
- 28.Camp R L, Charette L A, Rimm D L. Validation of tissue microarray technology in breast carcinoma. Lab Invest 2000801943–1949. [DOI] [PubMed] [Google Scholar]
- 29.Gulmann C, Butler D, Kay E.et al Biopsy of a biopsy: validation of immunoprofiling in gastric cancer biopsy tissue microarrays. Histopathology 20034270–76. [DOI] [PubMed] [Google Scholar]
- 30.Hendriks Y, Franken P, Dierssen J W.et al Conventional and tissue microarray immunohistochemical expression analysis of mismatch repair in hereditary colorectal tumors. Am J Pathol 2003162469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kallioniemi O P, Wagner U, Kononen J.et al Tissue microarray technology for high‐throughput molecular profiling of cancer. Hum Mol Genet 200110657–662. [DOI] [PubMed] [Google Scholar]
- 32.Kononen J, Bubendorf L, Kallioniemi A.et al Tissue microarrays for high‐throughput molecular profiling of tumor specimens. Nat Med 19984844–847. [DOI] [PubMed] [Google Scholar]
- 33.Finan M A, DeCesare S, Fiorica J V.et al Radical hysterectomy for stage IB1 vs IB2 carcinoma of the cervix: does the new staging system predict morbidity and survival? Gynecol Oncol 199662139–147. [DOI] [PubMed] [Google Scholar]
- 34.Boland C R, Thibodeau S N, Hamilton S R.et al A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998585248–5257. [PubMed] [Google Scholar]
- 35.Berends M J, Hollema H, Wu Y.et al MLH1 and MSH2 protein expression as a pre‐screening marker in hereditary and non‐hereditary endometrial hyperplasia and cancer. Int J Cancer 200192398–403. [DOI] [PubMed] [Google Scholar]
- 36.Landis J R, Koch G G. The measurement of observer agreement for categorical data. Biometrics 197733159–174. [PubMed] [Google Scholar]
- 37.Ho C M, Chien T Y, Huang S H.et al Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol Oncol 200493458–464. [DOI] [PubMed] [Google Scholar]
- 38.Jascur T, Boland C R. Structure and function of the components of the human DNA mismatch repair system. Int J Cancer 20061192030–2035. [DOI] [PubMed] [Google Scholar]
- 39.Herman J G, Baylin S B. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 20033492042–2054. [DOI] [PubMed] [Google Scholar]
- 40.Wisman G B, Nijhuis E R, Hoque M O.et al Assessment of gene promoter hypermethylation for detection of cervical neoplasia. Int J Cancer 20061191908–1914. [DOI] [PubMed] [Google Scholar]
- 41.Feng Q, Balasubramanian A, Hawes S E.et al Detection of hypermethylated genes in women with and without cervical neoplasia. J Natl Cancer Inst 200597273–282. [DOI] [PubMed] [Google Scholar]
- 42.Virmani A K, Muller C, Rathi A.et al Aberrant methylation during cervical carcinogenesis. Clin Cancer Res 20017584–589. [PubMed] [Google Scholar]