Abstract
Background
Gastric carcinoma is characterised by numerous genetic and epigenetic alterations that influence cell cycle progression, apoptosis and DNA repair. These alterations include down‐regulation of the cyclin‐dependent kinase (CDK) inhibitors p21WAF1/CIP1 and p27Kip1, and mutations of the tumour suppressor protein p53 and the cell adhesion molecule E‐cadherin. Combined evaluation of the prognostic significance of these alterations has not been reported in Mexican Mestizo patients.
Aims
To evaluate p21WAF1/CIP1, p27Kip1, p53 and E‐cadherin protein expression, including mutant E‐cadherin variants with deletion of exon 8 (del 8) or 9 (del 9), in gastric cancer from Mexican patients.
Methods
Immunohistochemistry for the above‐mentioned markers, including mutation‐specific E‐cadherin antibodies, was carried out in 69 gastric carcinomas; expression levels were correlated with histotype, tumour stage and prognosis.
Results
Expression of p21WAF1/CIP1 alone or in combination with p27Kip1 or in the absence of p53 was associated with favourable prognosis. Staining of del 8 and del 9 E‐cadherin was found exclusively in patients negative for p53 and positive for p21WAF1/CIP1, suggesting that the p21WAF1/CIP1 regulatory function of p53 was intact.
Conclusion
Combined evaluation of the prognostic significance of cell cycle regulators and E‐cadherin should be performed. Even though patients negative for p53 and positive for p21WAF1/CIP1 have a favourable prognosis, it may have a negative influence on prognosis if they acquire in addition E‐cadherin mutations which have been shown previously to be associated with poor survival.
Keywords: p21WAF1/CIP1 , p27Kip1 , p53, E‐cadherin, gastric cancer
Gastric carcinoma is one of the most frequent malignancies worldwide and one of the leading causes of cancer mortality in Mexico, with a higher prevalence of diffuse versus intestinal type gastric carcinoma in low‐income Mestizo descendants.1 In contrast, subtype distribution is comparable to that in western countries in medium‐income patients.2 These differences might result from dietary or environmental influences. The question whether gene expression patterns of gastric carcinomas of Mexican origin is different from tumours of European or Asian countries remains open. Common genetic and epigenetic alterations in gastric cancer are mutations in the tumour suppressors E‐cadherin and p53.3 In addition, p21WAF1/CIP1 and p27Kip1, CDK inhibitors of the CIP/Kip family that control the G1‐S transition, are frequently down‐regulated. Although a multitude of studies has examined the prognostic significance of the markers mentioned above, results are controversial, and little is known about their prognostic relevance in high risk groups, such as Mexican Mestizo patients.
Decrease of p21WAF1/CIP14,5,6 and p27Kip1 expression has frequently been associated with poor prognosis in gastric cancer,7,8,9 while some authors failed to detect a prognostic significance of both markers.10,11 In gastric cancer, the prognostic value of p53 is under discussion, since most of the studies show an association of p53 with patient survival,12,13,14,15 while other investigations do not support these findings.4 E‐cadherin participates in cell cycle regulation by up‐regulating p27Kip1.16 Somatic E‐cadherin mutations occur predominantly in diffuse type gastric carcinomas.17,18,19,20,21,22 The most common mutations in gastric cancer are splice‐site mutations and in‐frame deletions located in exons 8 or 9.22,23 We have recently shown that abnormal E‐cadherin expression on the protein level in gastric adenocarcinomas from Mexican Mestizo patients has low impact on patient survival.24 However, patients carrying deletions of exons 8 or 9, observed in 5.3% of tumours in this series, had a worse prognosis than patients without these alterations.24
In the present study, we examined the expression and prognostic significance of p21WAF1/CIP1, p27Kip1, p53 and E‐cadherin in a Mexican series of gastric adenocarcinomas.
Materials and methods
Patient selection
Patients with a diagnosis of gastric adenocarcinoma who had undergone partial or total gastrectomy from 1982 to 2001 in the Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, with available clinical information and follow‐up were included in the study. A blinded review of the slides was made by two pathologists (AG‐D, I Becker) and a diagnosis according to Laurén's classification of gastric adenocarcinoma was established.24,25 Mexican Mestizo patients with available paraffin material and a morphological diagnosis of poorly differentiated intestinal, mixed or diffuse‐type adenocarcinoma in which UICC (International Union against Cancer) staging criteria could be applied, were included.26
Immunohistochemical analysis
Antibodies against p53 (clone DO‐7, M7001, dilution 1:20) and Ki‐67 (clone MIB‐1, M7240, dilution 1:50) were purchased from DakoDiagnostika (Hamburg, Germany) Staining was carried out on automated immunostainers (Nexes, Benchmark, Ventana, Tucson, Arizona, USA) with the recommended reagents. Antigen retrieval was performed in CC1 buffer from Ventana in a water bath (1 h, 100°C). A manual staining protocol was used for p21WAF1/CIP1 and p27Kip1 staining with the following antibodies and dilutions: p21WAF1/CIP1 antibody (DakoDiagnostika, clone SX118, M7202, dilution 1:20), p27Kip1 antibody (clone F‐8, sc‐1641, Santa Cruz Biotechnology, Heidelberg, Germany, dilution 1:10). Staining was carried out with LSAB‐DAB from Dako. Ten representative high power fields were counted for each antibody staining and the percentage of positive tumour cells was calculated. Immunohistochemical analysis of the E‐cadherin expression profile and of mutant E‐cadherin variants (del 8 and del 9) was carried out as previously described.24 In brief, antibody clone 36 against E‐cadherin (Transduction Laboratories, Lexington, Kentucky, USA, dilution 1:1000) and the mutation‐specific antibodies E‐cad delta 8‐1 and E‐cad delta 9‐1, recognising del 8 or del 9 E‐cadherin, have been used.27,28 For qualitative analysis of the E‐cadherin staining pattern, three different staining patterns were analysed: 1, normal; 2, abnormal (including atypical with partial membrane staining and cytoplasmic staining and heterogeneous staining with positive and negative tumour cells in the same slide); or 3, negative staining. Tumours were considered as del 8 or del 9 positive when membranous staining was detected in tumour cells, without quantifying the percentage of positive tumour cells.
Statistical analysis
Evaluation was performed by three pathologists (AG‐D, SS, FF) who were unaware of clinical features and survival. Statistical analyses were performed using Fisher's exact, Kruskal–Wallis or χ2 tests when appropriate. Kaplan–Meier survival time analysis was used to correlate the investigated markers with pT, pN, and pM status with clinical evolution. Cox regression analysis was performed correlating the investigated markers with prognosis. A two sided p value <0.05 was considered to be statistically significant.
Results
Table 1 shows the clinicopathological features of 69 Mexican Mestizo patients with gastric cancer.
Table 1 Clinicopathological features of 69 patients with gastric cancer.
| Age (y) | ||
| Mean | 59.2 | |
| Median | 62.0 | |
| SD | 15.4 | |
| Range | 14–86 | |
| n | % | |
| Gender | ||
| Female | 35 | 50.7 |
| Male | 34 | 49.3 |
| Tumour location | ||
| Antrum | 22 | 31.9 |
| Corpus/fundus | 25 | 36.2 |
| Gastro‐oesophagic junction | 11 | 15.9 |
| More than one zone | 11 | 15.9 |
| Histotype (Laurén) | ||
| Intestinal | 28 | 40.6 |
| Diffuse | 39 | 56.5 |
| Mixed | 2 | 2.9 |
| Stage (UICC) | ||
| IA | 2 | 2.9 |
| IB | 1 | 1.4 |
| II | 15 | 21.7 |
| IIIA | 17 | 24.6 |
| IIIB | 12 | 17.4 |
| IV | 22 | 31.9 |
| Residual disease | ||
| R0 | 54 | 78.3 |
| R1 | 15 | 21.7 |
UICC, International Union against Cancer.
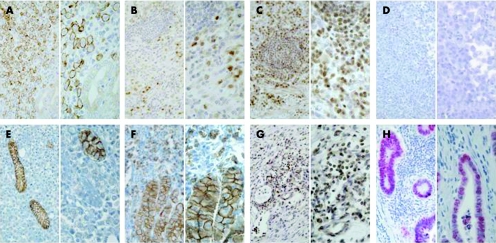
The 69 cases were investigated immunohistochemically for expression of p21WAF1/CIP1, p27Kip1, p53, Ki‐67 and E‐cadherin (including mutant E‐cadherin variants with deletions of exons 8 or 9). Figure 1 shows an example of a gastric cancer sample with tumour cell staining for del 9 E‐cadherin (A), p21WAF1/CIP1 (B) and p27Kip1 (C) and absence of p53 staining in neoplastic cells (D). In addition, examples of tumours lacking E‐cadherin expression (E) or with abnormal E‐cadherin expression (F) in neoplastic cells as well as a case without p27Kip1 expression in neoplastic cells (G) and a p53 positive tumour (H) are depicted.
Figure 1 Immunohistochemical staining for del 9 E‐cadherin, p53, p21WAF1/CIP1, and p27Kip1 in a diffuse type gastric cancer. (A) Staining with delta 9‐1 antibody recognising E‐cadherin lacking exon 9. Note negativity of residual normal glands. (B) Nuclear expression of p21WAF1/CIP1 was detected in 30% of gastric carcinoma cells and not in lymphocytes. (C) Nuclear p27Kip1 staining was present in 50% of tumour cells and in lymphocytes. (D) Complete absence of p53 staining. (E, F) Negative (E) or abnormal heterogeneous (F) E‐cadherin expression in diffuse type gastric cancer cases is shown. (G) Absence of p27Kip1 expression in neoplastic cells in an example of a diffuse type gastric cancer. Note reactivity of lymphocytes. (H) Positive p53 staining in 60% of tumour cells was detected in an intestinal type gastric carcinoma. Original magnification: left panel ×200, right panel ×400.
The percentage of positivity was correlated with the clinicopathological parameters histotype and tumour node metastasis (TNM) stage (table 2). p27Kip1 expression was significantly associated with histotype (p = 0.004), with a higher median expression level in diffuse (32.5%) compared to intestinal type gastric carcinoma (9.5%). Ki‐67 expression was also significantly correlated with histotype, with a median positivity of 40.0% in intestinal versus 20.0% in diffuse type gastric cancer (p = 0.001). These results indicate an inverse correlation between p27Kip1 and Ki‐67 expression. Ki‐67 staining was correlated with the perigastric lymph node status (p = 0.022), but not with pT stage, or distant metastasis formation. No association between p27Kip1 reactivity and TNM stage was identified. For p21WAF1/CIP1 and p53, no correlation was detectable with histotype or TNM status.
Table 2 Relationship between the percentage of p21WAF1/CIP1, p27Kip1, p53 or Ki‐67 positive tumour cells and clinicopathological parameters.
| n | Median (range) in % | ||||
|---|---|---|---|---|---|
| p21WAF1/CIP1 | p27Kip1 | p53 | Ki‐67 | ||
| Histotype (Laurén) | |||||
| Intestinal | 28 | 15.0 (0–50) | 9.5 (0–60) | 5.0 (0–80) | 40.0 (10–70) |
| Mixed | 2 | 19.5 (9–30) | 30.0 (0–60) | 45.0 (0–90) | 32.5 (15–50) |
| Diffuse | 39 | 20.0 (0–70) | 32.5 (0–65) | 17.5 (0–90) | 20.0 (0–55) |
| p value | 0.712 | 0.004 | 0.578 | 0.001 | |
| Tumour invasion | |||||
| pT 1–2 | 6 | 25.0 (13–70) | 20.0 (0–50) | 0.0 (0–40) | 20.0 (10–65) |
| pT 3–4 | 63 | 20.0 (0–70) | 20.0 (0–65) | 15.0 (0–90) | 25.0 (0–70) |
| p value | 0.195 | 0.874 | 0.218 | 0.844 | |
| Perigastric lymph node status | |||||
| pN0 | 18 | 30.0 (0–70) | 35.0 (0–65) | 20.0 (0–70) | 15.0 (5–55) |
| pN1–2 | 51 | 15.0 (0–70) | 20.0 (0–60) | 10.0 (0–90) | 25.0 (0–70) |
| p value | 0.086 | 0.621 | 0.482 | 0.022 | |
| Distant metastases | |||||
| pM0 | 56 | 20.0 (0–70) | 15.0 (0–65) | 15.0 (0–90) | 22.5 (0–70) |
| pM1 | 13 | 10.0 (0–60) | 20.0 (9–60) | 9.5 (0–80) | 25.0 (10–70) |
| p value | 0.225 | 0.091 | 0.940 | 0.829 | |
p values were obtained by Kruskal–Wallis test.
The optimal cut‐offs separating positive and negative cases were searched by correlating p21WAF1/CIP1, p27Kip1 or p53 expression with patient survival using the log rank test. Cut‐offs used in the literature did not discriminate well within our Mexican patient collective, explaining the requirement of a statistical search for the best cut‐offs. Tumours were considered as positive when they expressed >15% p21WAF1/CIP1, >35% p27Kip1 or >30% p53. Of the 69 carcinomas examined, 41 (59.4%) showed positive tumour cell staining for p21WAF1/CIP1, 23 (34.3%) for p27Kip1 and 24 (34.8%) for p53.
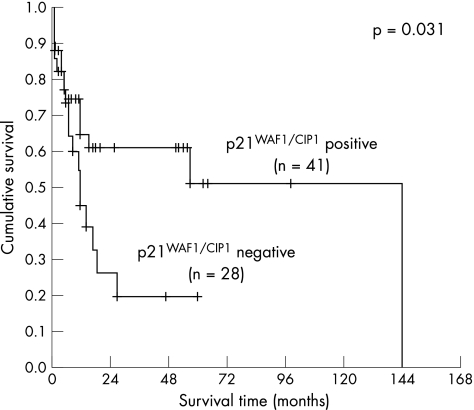
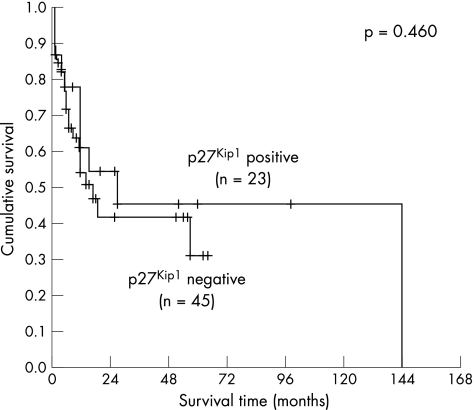
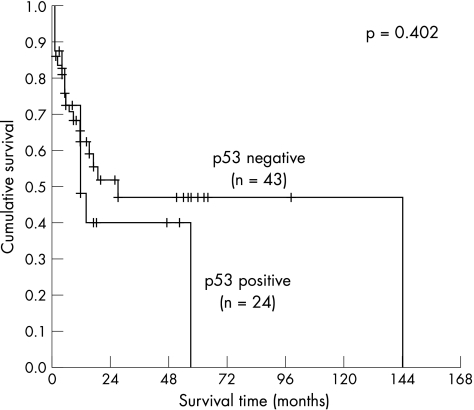
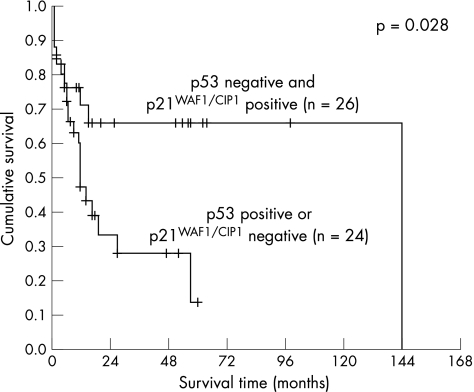
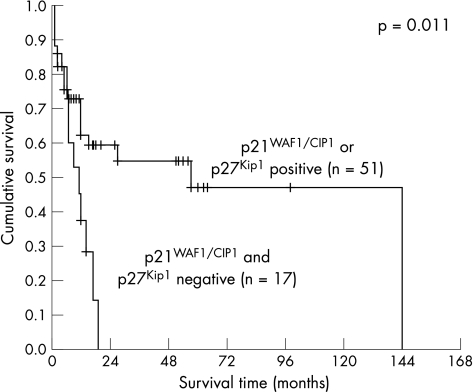
The Kaplan–Meier method was used to correlate p21WAF1/CIP1, p27Kip1 and p53 expression alone and in combination with patient survival. The mean and median of the overall patient follow‐up were 19.3 or 11.0 months, respectively (range 1–144 months, SD 25.5 months). A significant correlation with survival was detected for p21WAF1/CIP1 (fig 2, log‐rank 0.031), but not for p27Kip1 (fig 3) or p53 (fig 4). When a combination of p53 negative and p21WAF1/CIP1 positive cases was compared with p53 positive or p21WAF1/CIP1 negative cases, a significant association with survival was observed (fig 5, log‐rank 0.028). Patients expressing either p21WAF1/CIP1or p27Kip1 had a significantly better prognosis than patients negative for both CDK inhibitors (fig 6, log‐rank 0.011).
Figure 2 Survival impact of p21WAF1/CIP1 expression. Kaplan–Meier survival curve for the 69 patients with gastric carcinoma stratified according to p21WAF1/CIP1 expression status in gastric carcinoma cells. A cut‐off of 15% was used.
Figure 3 Survival impact of p27Kip1 expression. Kaplan–Meier survival curve for 68 patients with gastric carcinoma stratified by p27Kip1 expression in tumour cells. A cut‐off of 35% was used.
Figure 4 Survival impact of p53 expression. Kaplan–Meier survival curve for 67 patients with gastric carcinoma stratified by p53 expression in neoplastic cells. A cut‐off of 30% was used.
Figure 5 Survival impact of a combination of p53 and p21WAF1/CIP1 expression. Kaplan–Meier survival curve for 68 patients with gastric carcinoma divided into two groups by absence of p53 staining in combination with positivity for p21WAF1/CIP1 (group 1) and p53 positivity in combination with a lack of p21WAF1/CIP1 (group 2).
Figure 6 Survival impact of a combination of p21WAF1/CIP1 and p27Kip1 expression. Kaplan–Meier survival curve for 68 gastric carcinoma patients stratified into two groups by positivity for either p21WAF1/CIP1 or p27Kip1 staining (group 1) and p53 positivity in combination with absence of p21WAF1/CIP1 and p27Kip1 (group 2).
The prognostic value of p21WAF1/CIP1, p27Kip1, and p53 was also investigated in combination with E‐cadherin expression because E‐cadherin plays a role in growth regulation and E‐cadherin mutations are associated with poor prognosis.24 Four cases with del 8 or del 9 reactivity were included (5.8%), two cases with del 8, and two cases with del 9 E‐cadherin (three cases were of diffuse and one of mixed type). All cases with exon 8 or 9 deletion were p53 negative (maximal 5% p53 positive tumour cells) and p21WAF1/CIP1 positive (table 3, p = 0.009). No significant association between absence of p53 staining and presence of p21WAF1/CIP1 was detected with abnormal E‐cadherin expression (clone 36 staining), although a trend was also observed here (p = 0.075). Cases with normal E‐cadherin expression were either p53 positive or p21WAF1/CIP1 negative, but never p53 negative and p21WAF1/CIP1 positive.
Table 3 Correlation of E‐cadherin expression with p53 and p21WAF1/CIP1 expression.
| E‐cadherin (clone 36) | E‐cadherin del 8/9 reactivity | ||||
|---|---|---|---|---|---|
| Normal | Abnormal | − | + | − | |
| p53 – and p21WAF1/CIP1 + | 0 | 24 | 2 | 4 | 22 |
| p53 + or p21WAF1/CIP1 − | 6 | 30 | 6 | 0 | 42 |
| p = 0.075 | p = 0.009 | ||||
+, positive; −, negative; p values were obtained by χ2 test.
A multivariate analysis using Cox's proportional hazard model was used to correlate expression of p21WAF1/CIP1, p27Kip1, p53, Ki‐67, E‐cadherin (including del 8 and del 9 E‐cadherin), with prognosis. p21WAF1/CIP1 in combination with p27Kip1 (p21WAF1/CIP1 or p27Kip1 positive versus p21WAF1/CIP1 and p27Kip1 negative) was significantly correlated with survival and is an independent predictor of patient survival (p = 0.019). Residual disease status and stage were significantly correlated with each other (p = 0.001). Even if the residual disease status was included into the multivariate analysis, only the combination of p21WAF1/CIP1 and p27Kip1 expression resulted in statistical significance.
Discussion
In this study, we obtained evidence that combined evaluation of several markers important for cell proliferation in a Mexican series of gastric carcinomas is of predictive value for the estimation of patients' survival. Expression of p21WAF1/CIP1 alone or in combination with p27Kip1 or in the absence of p53 was associated with favourable prognosis. E‐cadherin was also investigated, because it acts as a growth suppressor16 and E‐cadherin mutations have been shown to be associated with poor survival.24 Staining of del 8 and del 9 E‐cadherin was found exclusively in patients negative for p53 and positive for p21WAF1/CIP1, suggesting that the p21WAF1/CIP1 regulatory function of p53 was intact. These data suggest a selection for mutations in either the E‐cadherin or the p53‐p21WAF1/CIP1 pathway. Of note, the investigated cohort was of relatively small size, limiting the conclusions that can be drawn, especially with regard to the cases with del 8 and del 9 reactivity.
Our finding of a prognostic value of p21WAF1/CIP1 in gastric carcinoma is in accordance with previous publications.4,15 p27Kip1 was described as an independent prognostic factor in gastric carcinomas of Asia and Europe,7,29,30,31 but there are also reports that loss of p27Kip1 does not predict patient survival.32 A combination of p21WAF1/CIP1 and p27Kip1 with expression of at least one of the CDK inhibitors was of prognostic value in a Japanese tumour collective.29 In our patient collective, expression of both cell cycle regulators p21WAF1/CIP1 and p27Kip1 was not correlated with each other, while such an association was found in this study of Japanese tumours.29
Take‐home messages
Gastric carcinoma is characterised by numerous alterations including down‐regulation of p21WAF1/CIP1 and p27Kip1 as well as genomic mutations of p53 and E‐cadherin.
Combined evaluation of the prognostic significance of p21WAF1/CIP1, p27Kip1, p53 and E‐cadherin protein expression, including mutant E‐cadherin variants with deletion of exon 8 (del 8) or 9 (del 9), was performed in 69 gastric carcinomas from Mexican patients.
Expression of p21WAF1/CIP1 alone or in combination with p27Kip1 or in the absence of p53 was associated with favourable prognosis.
Staining of del 8 and del 9 E‐cadherin was found exclusively in patients negative for p53 and positive for p21WAF1/CIP1.
Combined evaluation of the prognostic significance of cell cycle regulators and E‐cadherin should be performed.
Our result of no prognostic significance of p53 is independent of the chosen cut‐off, because statistical significance was not reached using cut‐offs of 10%, 20%, or 30%. Expression of p21WAF1/CIP1 in combination with a lack of p53 expression was significantly associated with prognosis which is in concordance with a previous report.15 Moreover, we observed that all tumours harbouring E‐cadherin mutations in exons 8 or 9 were negative for p53 and positive for p21WAF1/CIP1. Our present finding is in accordance with our previous investigation of p53 expression in gastric carcinoma from European patients where we show that p53 accumulation occurs more frequently in tumours without E‐cadherin mutations compared to patients harbouring E‐cadherin mutations determined by sequence analysis.33
Taken together, our data indicate that cell cycle regulators should be investigated in combination with the E‐cadherin mutation status. Even though Mexican Mestizo patients negative for p53 and positive for p21WAF1/CIP1 have good prognostic factors, it may have a negative influence on prognosis if they acquire in addition E‐cadherin mutations which have been shown to be associated with poor survival.24
Acknowledgements
The authors thank I Becker for reviewing slides for a diagnosis according to Laurén's classification and C Hartmann for excellent technical assistance.
Abbreviations
CDK - cyclin dependent kinase
del 8 E‐cadherin - E‐cadherin with deletion of exon 8
del 9 E‐cadherin - E‐cadherin with deletion of exon 9
TNM - tumour node metastasis
Footnotes
Funding: The study was supported by a grant to Drs B Luber and I Becker from the Wilhelm‐Sander‐Stiftung (Nr 1999.118.2).
Competing interests: None.
References
- 1.Rubio C A, Jessurum J, de Ruiz P A. Geographic variations in the histologic characteristics of the gastric mucosa. Am J Clin Pathol 199196330–333. [DOI] [PubMed] [Google Scholar]
- 2.Lopez‐Carrillo L, Vega‐Ramos B, Costa‐Dias R.et al Histological types of gastric cancer in Mexico. Int J Epidemiol 1997261166–1171. [DOI] [PubMed] [Google Scholar]
- 3.Tahara E. Molecular aspects of invasion and metastasis of stomach cancer. Verh Dtsch Ges Pathol 20008443–49. [PubMed] [Google Scholar]
- 4.Gomyo Y, Ikeda M, Osaki M.et al Expression of p21 (waf1/cip1/sdi1), but not p53 protein, is a factor in the survival of patients with advanced gastric carcinoma. Cancer 1997792067–2072. [DOI] [PubMed] [Google Scholar]
- 5.Ikeguchi M, Saito H, Katano K.et al Expression of p53 and p21 are independent prognostic factors in patients with serosal invasion by gastric carcinoma. Dig Dis Sci 199843964–970. [DOI] [PubMed] [Google Scholar]
- 6.Jang S J, Ahn M J, Paik S S.et al Expression of cyclin dependent kinase inhibitor p21WAF1 alone and in combination with p27KIP1 shows prognostic value in gastric carcinoma. J Korean Med Sci 199813369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori M, Mimori K, Shiraishi T.et al p27 expression and gastric carcinoma. Nat Med 19973593. [DOI] [PubMed] [Google Scholar]
- 8.Ohtani M, Isozaki H, Fujii K.et al Impact of the expression of cyclin‐dependent kinase inhibitor p27Kip1 and apoptosis in tumor cells on the overall survival of patients with non‐early stage gastric carcinoma. Cancer 1999851711–1718. [PubMed] [Google Scholar]
- 9.Takano Y, Kato Y, van Diest P J.et al Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases. Am J Pathol 2000156585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller W, Noguchi T, Wirtz H C.et al Expression of cell‐cycle regulatory proteins cyclin D1, cyclin E, and their inhibitor p21 WAF1/CIP1 in gastric cancer. J Pathol 1999189186–193. [DOI] [PubMed] [Google Scholar]
- 11.Muller W, Grabsch H, Takeno S.et al Prognostic value of the cyclin‐dependent kinase inhibitor p27Kip1 in gastric cancer. Anticancer Res 2000201787–1792. [PubMed] [Google Scholar]
- 12.Joypaul B V, Hopwood D, Newman E L.et al The prognostic significance of the accumulation of p53 tumour‐suppressor gene protein in gastric adenocarcinoma. Br J Cancer 199469943–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichiyoshi Y, Oiwa H, Tomisaki S.et al Overexpression of p53 is associated with growth pattern and prognosis in advanced gastric cancer. Hepatogastroenterology 199744546–553. [PubMed] [Google Scholar]
- 14.Sakaguchi T, Watanabe A, Sawada H.et al Prognostic value of cyclin E and p53 expression in gastric carcinoma. Cancer 1998821238–1243. [DOI] [PubMed] [Google Scholar]
- 15.Okuyama T, Maehara Y, Kabashima A.et al Combined evaluation of expressions of p53 and p21 proteins as prognostic factors for patients with gastric carcinoma. Oncology 200263353–361. [DOI] [PubMed] [Google Scholar]
- 16.St Croix B, Sheehan C, Rak J W.et al E‐Cadherin‐dependent growth suppression is mediated by the cyclin‐dependent kinase inhibitor p27(KIP1). J Cell Biol 1998142557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker K F, Atkinson M J, Reich U.et al E‐cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994543845–3852. [PubMed] [Google Scholar]
- 18.Muta H, Noguchi M, Kanai Y.et al E‐cadherin gene mutations in signet ring cell carcinoma of the stomach. Jpn J Cancer Res 199687843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura G, Sakata K, Nishizuka S.et al Inactivation of the E‐cadherin gene in primary gastric carcinomas and gastric carcinoma cell lines. Jpn J Cancer Res 1996871153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado J C, Soares P, Carneiro F.et al E‐cadherin gene mutations provide a genetic basis for the phenotypic divergence of mixed gastric carcinomas. Lab Invest 199979459–465. [PubMed] [Google Scholar]
- 21.Oda T, Kanai Y, Oyama T.et al E‐cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA 1994911858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker K F, Atkinson M J, Reich U.et al Exon skipping in the E‐cadherin gene transcript in metastatic human gastric carcinomas. Hum Mol Genet 19932803–804. [DOI] [PubMed] [Google Scholar]
- 23.Berx G, Becker K F, Hofler H.et al Mutations of the human E‐cadherin (CDH1) gene. Hum Mutat 199812226–237. [DOI] [PubMed] [Google Scholar]
- 24.Gamboa‐Dominguez A, Dominguez‐Fonseca C, Chavarri‐Guerra Y.et al E‐cadherin expression in sporadic gastric cancer from Mexico: exon 8 and 9 deletions are infrequent events associated with poor survival. Hum Pathol 20053629–35. [DOI] [PubMed] [Google Scholar]
- 25.Laurén P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type of carcinoma. Acta Pathol Microbiol Scand 19656431–49. [DOI] [PubMed] [Google Scholar]
- 26.Spiessl B, Beahrs O H, Hermanek P.et alUICC, International Union against Cancer: TNM Atlas. Berlin, Heidelberg: Springer‐Verlag, 1992
- 27.Becker K F, Kremmer E, Eulitz M.et al Analysis of E‐cadherin in diffuse‐type gastric cancer using a mutation‐specific monoclonal antibody. Am J Pathol 19991551803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker K F, Kremmer E, Eulitz M.et al Functional allelic loss detected at the protein level in archival human tumours using allele‐specific E‐cadherin monoclonal antibodies. J Pathol 2002197567–574. [DOI] [PubMed] [Google Scholar]
- 29.Liu X P, Kawauchi S, Oga A.et al Combined examination of p27(Kip1), p21(Waf1/Cip1) and p53 expression allows precise estimation of prognosis in patients with gastric carcinoma. Histopathology 200139603–610. [DOI] [PubMed] [Google Scholar]
- 30.Nitti D, Belluco C, Mammano E.et al Low level of p27(Kip1) protein expression in gastric adenocarcinoma is associated with disease progression and poor outcome. J Surg Oncol 200281167–176. [DOI] [PubMed] [Google Scholar]
- 31.Sgambato A, Migaldi M, Leocata P.et al Loss of p27Kip1 expression is a strong independent prognostic factor of reduced survival in N0 gastric carcinomas. Cancer 2000892247–2257. [DOI] [PubMed] [Google Scholar]
- 32.Feakins R M, Mulcahy H E, Quaglia A.et al p27(Kip1) loss does not predict survival in patients with advanced gastric carcinoma. Cancer 2000891684–1691. [DOI] [PubMed] [Google Scholar]
- 33.Fricke E, Keller G, Becker I.et al Relationship between E‐cadherin gene mutation and p53 gene mutation, p53 accumulation, Bcl‐2 expression and Ki‐67 staining in diffuse‐type gastric carcinoma. Int J Cancer 200310460–65. [DOI] [PubMed] [Google Scholar]