Abstract
Aim
To study baseline and stimulated tissue factor (TF) production from a normal, albeit immortalised, human kidney proximal tubular cell line (HKC‐5), in order to establish a model for investigating the role of inflammatory mediators in the increased urinary TF (uTF) seen in inflammatory and neoplastic disease.
Methods
TF procoagulant activity, expression and secretion in HKC‐5 cells were investigated using TF activity and antigen assays, fluorescence confocal microscopy and immunocytochemistry. TF expression in the HKC‐5 cells was also studied using reverse transcription (RT)‐PCR and its synthesis was suppressed using antisense oligodeoxynucleotide (ODN), directed against human TF mRNA. Cells were stimulated, after serum deprivation, with bacterial lipopolysaccharide (LPS), an agonist known to enhance TF expression in monocytes. They were also subject to serum starvation.
Results
Analysis by RT‐PCR showed TF production by stimulated and actively metabolising HKC‐5 cells. Antisense ODN treatment resulted in approximately 50% suppression of TF synthesis compared to a mismatch ODN. The amount of TF produced by the HKC‐5 cells was time dependent and coincides with a decrease in the intracellular TF levels. LPS up‐regulated TF production in HKC‐5 cells. Reducing fetal calf serum concentrations in the culture medium decreased TF production and secretion.
Conclusion
Stimulated TF synthesis and secretion in vitro by HKC‐5 cells is consistent with the hypothesis that uTF is produced by tubular cells influenced by mediators of disease states and provides a model for further mechanistic investigations.
Keywords: tissue factor, human kidney, proximal tubules, HKC‐5 cell lines, immunostaining, antisense oligodeoxynucleotides, RT‐PCR
Urine possesses a powerful procoagulant activity1 due to tissue factor (TF), the primary physiological initiator of blood coagulation.2 Since urine is easily accessible, numerous studies have examined urinary TF (uTF) as a clinical marker. Abnormal uTF occurs in human inflammatory and malignant conditions.2,3,4,5,6,7 Beyond clinical correlations, mechanistic studies are relatively sparse in the literature and the cellular origin of uTF production remain poorly understood.
Tissue factor has been localised to the glomerulus,8,9,10,11,12 or renal tubules.13,14,15,16 Association with the renal tubules is supported by clinical and experimental evidence.13,15,17,18 Here we investigated TF expression and secretion in cells from a human kidney proximal tubular cell line (HKC‐5) under various experimental conditions using ELISA, fluorescence confocal microscopy, immunocytochemistry and reverse transcription (RT)‐PCR. Its synthesis was suppressed using antisense oligodeoxynucleotide (ODN), directed against human TF mRNA.
Materials and methods
HKC‐5 cell culture conditions
The HKC‐5 cell line was derived by Professor Racusen from virally transformed human proximal kidney tubule cells. They are non‐producers of the immortalising virus and non‐tumourigenic. The originators consider them to be a useful model for the study of human renal proximal tubular injury or disease.19,20,21,22
Here HKC‐5 cells were maintained in supplemented Hams17,18,19,20,21,22,23 at 37°C and 5% CO2. Variations for experimental purposes are as indicated. All experiments were performed initially on cells rendered quiescent with basal (serum free) medium overnight.
Fluorescence confocal microscopy
Cells were seeded into 8 chamber well Microtek slides (Nunc) and grown to 80% confluence. The medium was removed and the cells washed three times with 500 μl phosphate‐buffered saline (PBS) and fixed in 500 μl 4% (w/v) paraformaldehyde, in PBS, for 30 min at room temperature, before washing and permeabilising with 500 μl ice‐cold methanol (100%) for 5 min at −20°C. The cells were washed and then incubated in 250 μl PBS with 1% (w/v) bovine serum albumin (BSA), for 1 h at room temperature. An indirect immunostain was used, the primary antibody against recombinant human TF (American Diagnostica, Stamford, Connecticut, USA) and a secondary goat anti‐mouse F(ab′)2 (Alexa Fluor, Molecular Probes, Invitrogen Ltd, Paisley, UK). Preparations were washed with 500 μl PBS + 0.02% (v/v) Tween 20, and mounted in Vectashield (Vector Labs, Peterborough, UK). Samples were viewed using a Zeiss LSM 510 confocal microscope.
Immunoperoxidase staining
Cells were grown on chamber‐slides and fixed as detailed above. The primary antibody was as referred to above. A biotin–streptavidin peroxidase immunocytochemical detection system (Biogenex Super Sensitive Detection Kit) was used as described by the manufacturer.
RNA preparation and RT‐PCR
Total RNA was isolated from HKC‐5 cells using the commercially available RNeasy Mini kit according to the manufacturer's instructions (Qiagen). Human TF specific primers were: sense primer, 5′‐CAA CAG ACA CAG AGT GTG ACC TCA CCG ACG‐3′; anti‐sense primer, 5′‐TCT GAA TTC CCC TTT CTC CTG GCC C‐3′. Single tube RT‐PCR, using 1 μg total RNA, was carried out under the following conditions: reverse transcription, 48°C for 45 min, 94°C 2 min, followed by PCR, 30 cycles of 94°C 10 s, 57°C 10 s, 72°C 40 s followed by extension, 72°C for 2 min. A single RT‐PCR product (500 bp) was purified and verified by sequencing. Reactions in the absence of reverse transcriptase served as a control. Avian myeloblastosis virus reverse transcriptase was from Promega (Southampton, UK), DNA Taq polymerase was from AB Gene.
Suppression of TF synthesis in HKC‐5 cells using short interfering oligonucleotides
An antisense (OND) specific for human TF and a mismatched OND were obtained from MWG–Biotech Ltd (London, UK). The sequences were modified from Stephens and Rivers24 as follows. To facilitate uptake by the cells, 2′‐O‐methyl groups were incorporated onto the ends of the ODN. The sequences were: antisense, 5′‐GAAGATTTCCAATAA‐3′; mismatch, 5′‐AAAGGTCTCTAATAA‐3′. The cells were seeded into an 8‐well chamber slide (Chamber Slide System, Lab‐Tek), then incubated in media containing either antisense or mismatch ODN (8 μg/ml respectively) for 48 h. The cells were then washed in PBS, fixed in acetone:ethanol (1:1 v/v) at −20°C for 10 min and stained for TF antigen using the immunoperoxidase technique described above.
Measurements of TF procoagulant activity in HKC‐5 cells and FCS
The procoagulant activity of HKC‐5 cells was assessed using the ACTICHROME TF activity assay according to the manufacturer's instructions (American Diagnostica). TF activity was measured in neat FCS (cf the 10% v/v in standard medium) and, from the same cultures, conditioned medium, cell lysates and intact adherent cells. All cells were either rendered quiescent by serum starvation or, with intact cells, reversibly arrested in the cell cycle with hydroxyurea 2 mM.
Measurements of TF antigen in HKC‐5 cells
HKC‐5 cells were seeded into 24‐well plates (5×104 cells per well), grown to confluence and rendered quiescent by overnight incubation in serum‐free basal F12:DMEM culture medium, before refreshing with 250 μl fresh basal culture medium. After incubation the medium was collected, and the cells lysed in 100 μl buffer containing 10 mM Tris/HCl pH 7.4, 1% (v/v) Triton X‐100. Samples were cleared of large cellular material by centrifuging at 2000 × g for 10 min and then stored at −80°C. Detection of human TF antigen was made by the TF‐ELISA Kit (American Diagnostic).
Time course of TF expression and secretion by HKC‐5 cells
HKC‐5 cells were cultured over a 24 h time period and the culture medium and cell lysates harvested at 0, 2, 4, 8, and 24 h, respectively and assayed for TF antigen, as indicated above.
Effect of FCS concentration on TF expression by HKC‐5 cells
Cells were incubated in 25 cm2 flasks (Nunc) at 0%, 2%, 5% or 10% FCS, for 48 h at 37°C and 5% CO2. Conditioned media and cell lysates were obtained and assayed for TF antigen as described above.
Effect of LPS stimulation on TF expression by HKC‐5 cells
HKC‐5 cells were grown in 24‐well plates exposed to media containing 0, 25, 50, 100 and 200 μg/ml Escherichia coli lipopolysaccharide (LPS) for 16 h at 37°C and 5% CO2. The amount of TF in the cell media and cell lysates was measured as described above.
Quantification of TF on cultured HKC‐5 cells
Cells were grown, fixed and stained in chamber slides as described above and photographed. Images were digitised using a Umax, Astra 2400 s flat‐bed scanner. The images were analysed using a Zeiss, KS400, image analysis system. The mean field density and area of labelling was automatically measured for each image. The density measurements were expressed as grey level units and the area as pixels. The number of cells present in each image were then counted using a semi‐manual method and the mean area and density of labelling per cell was then calculated for 100 cells in each image.
Measurements of TFPI antigen in HKC‐5 cells
Total tissue factor pathway inhibitor (TFPI) antigen levels of the HKC‐5 cells were measured on conditioned media and cell lysates using commercially available kits (Imubind Total TFPI ELISA assay) according to the manufacturer's instructions (American Diagnostica).
Results
Immunofluorescence localisation of TF in HKC‐5 cells
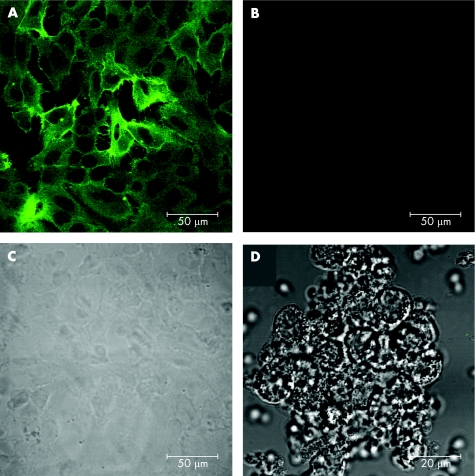
The localisation of TF antigen on fixed HKC‐5 cells was confirmed by fluorescence confocal microscopy. TF staining was observed throughout the HKC‐5 cells, both within the cytosol and on the plasma membrane (fig 1A). Control, no primary antibody, showed no fluorescent staining of the cells (fig 1B). A differential interference contrast (DIC) image (fig 1C) of the same field of cells shown in fig 1B. Specificity was controlled using leucocytes prepared with minimal processing that might cause activation and the same staining regimen as in fig 1A. Dark DIC is overlaid on a fluorescence image shown using a green look‐up table (fig 1D). The preparation is entirely negative.
Figure 1 Immunofluorescence localisation of tissue factor (TF) expressed by HKC‐5 cells using confocal microscopy. (A) Anti‐TF monoclonal antibody visualised by fluorescein isothiocyanate conjugated secondary antibody. (B) Control, no primary antibody. (C) Differential interference contrast (DIC) image of cells shown in (B). (D) Specificity control using (virtually non‐TF expressing) leucocyte preparation: DIC overlaid on fluorescence.
Detection of TF mRNA in HKC‐5 cells by RT‐PCR
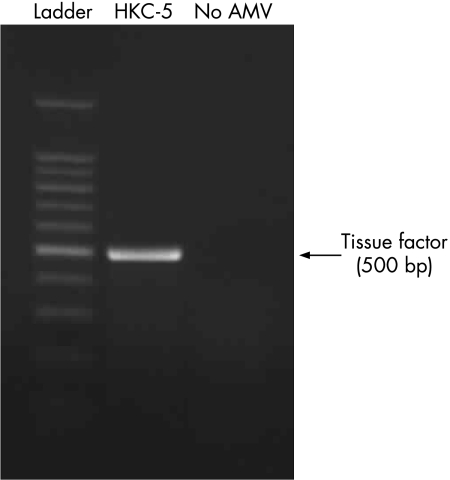
Total RNA was isolated from HKC‐5 cells and RT‐PCR carried out using specific human TF primers. Samples were analysed on an agarose gel and a single RT‐PCR product was observed at 500 bp (fig 2). The product was isolated, sequenced and confirmed to be identical with human TF mRNA. In the absence of avian myeloblastosis virus reverse transcriptase there were no non‐specific amplification products.
Figure 2 Expression of human tissue factor (TF) mRNA in HKC‐5 cells using reverse transcription‐PCR. A single amplification product specific to TF was observed at 500 bp. Control reactions were carried out in the absence of avian myeloblastosis virus reverse transcriptase. Data are representative of at least two experiments.
Effect of short interfering antisense ODN on TF synthesis by HKC‐5 cells
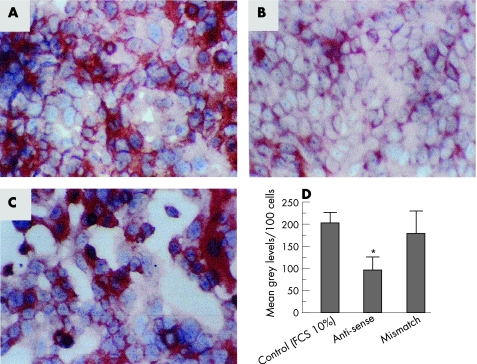
Treatment of HKC‐5 cells with an antisense ODN specific for TF mRNA (8 μg/ml for 48 h) resulted in a significant 2‐fold decrease in the amount of TF expressed in the cells compared to those in the absence of ODN (fig 3A,B). Similar treatment of the cells with a mismatch OND (8 µg/ml for 48 h) did not cause a decrease in the amount of expressed TF (fig 3C) and levels were similar to those in the control. The relative abundance of TF from these cells has been quantified and is shown in fig 3D.
Figure 3 Effect of sequence specific antisense oligodeoxynucleotide (ODN) on tissue factor (TF) synthesis in HKC‐5 cells. (A) TF expression in the absence of ODN; (B) TF expression in the presence of a human TF antisense ODN (8 μg/ml for 48 h); (C) TF expression in the presence of a mismatch TF ODN (8 μg/ml for 48 h). Images shown are representative of three preparations. (D) Relative abundance of TF expression in the cells following ODN treatment. Values represent mean (SEM); *p<0.05. FCS, fetal calf serum.
TF procoagulant activity of the HKC‐5 cells and FCS
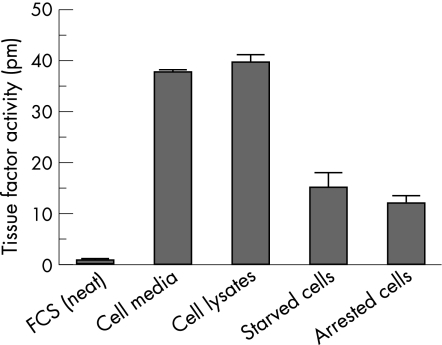
Even undiluted (neat) FCS contained negligible TF activity (fig 4), while conditioned media, cell lysates, and starved or arrested HKC‐5 cells showed high concentrations of TF procoagulant activity (fig 4). None of these contained serum at the time of assay except the arrested cells, which were in 10% v/v FCS.
Figure 4 Tissue factor procoagulant activity in fetal calf serum (FCS), cell media, cell lysates, starved and arrested cells. Results are shown as mean (SEM).
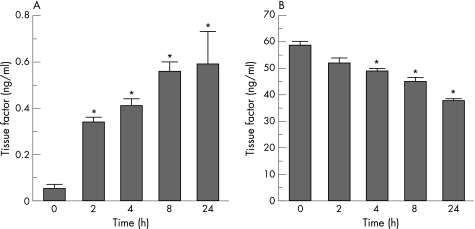
Time course of TF expression and secretion by HKC‐5 cells
When full growth media was restored there was a significant increase in TF secretion into the cell media in a time‐dependent manner, with 6.8‐fold, 8.2‐fold, 11.2‐fold and 11.8‐fold increases above baseline (0 h) for 2, 4, 8 and 24 h respectively (fig 5A). Concomitant with an increase in TF secretion, the relative amount of TF remaining in the cell lysates decreased in a time dependent manner, with 11.2%, 16.5%, 23.2% and 35.2% decreases from baseline for 2, 4, 8 and 24 h, respectively (fig 5B).
Figure 5 Time course of tissue factor expression in HKC‐5 cells. (A) Cell media. (B) Cell lysates. Values represent mean (SEM); *p<0.05 (n = 3).
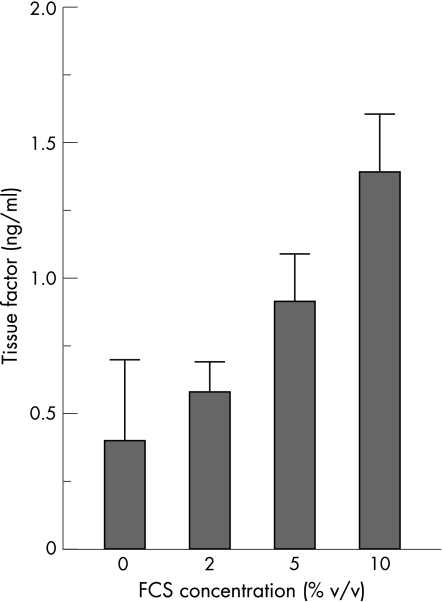
Effect of FCS concentration on TF expression by HKC‐5 cells
HKC‐5 cells were cultured in the presence of different FCS concentrations (0, 2, 5 and 10% v/v) for 48 h at 37°C, 5% CO2. Cells grown without FCS showed baseline levels of TF production. Increasing the FCS concentration to 2%, 5% and 10%, significantly increased the level of TF 1.4‐fold, 2.3‐fold and 3.5‐fold, respectively (fig 6).
Figure 6 Effect of increasing fetal calf serum (FCS) concentration on tissue factor expression in cell lysates. Values represent mean (SEM); *p<0.05 (n = 6).
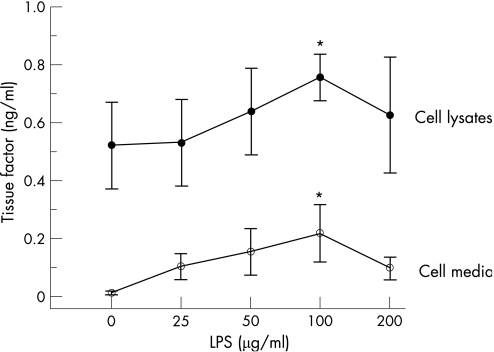
Effect of LPS stimulation on TF expression by HKC‐5 cells
HKC‐5 cells exposed to increasing concentrations of LPS (0–200 μg/ml) for 16 h at 37°C, 5% CO2 showed a dose‐dependent increase in TF secretion, with a statistically significant increase in TF being observed with 100 μg/ml LPS (fig 7). At higher concentrations of LPS (200 μg/ml), TF secretion from HKC‐5 cells began to decrease, possibly as a result of cytotoxic effects of the compound. In addition, we observed that increasing LPS concentrations caused an increase in TF within cell lysates. This also showed statistical significance with 100 μg/ml LPS (fig 7).
Figure 7 Effect of lipopolysaccharide (LPS) stimulation on tissue factor expression in HKC‐5 cells. Values represent mean (SEM); *p<0.05 (n = 4).
TFPI expression by HKC‐5 cells
TFPI levels in lysates of cells grown in 10% serum contained 10.8 ng/ml, reducing step‐wise on reduction of the serum concentration (table 1). TFPI liberated into conditioned medium was 8× higher when cells were grown in 10% serum than either 5% or 2% (table 1).
Table 1 Effect of increasing fetal calf serum (FCS) concentration on tissue factor pathway inhibitor (TFPI) levels in HKC‐5 cells.
| FCS | Total TFPI (ng/ml) | |
|---|---|---|
| Cell media | Cell lysates | |
| 2% | 0.5 | 1.3 |
| 5% | 0.5 | 3.1 |
| 10% | 4.1 | 10.8 |
Discussion
HKC‐5 cells, derived from normal kidney have been considered comparable to established human primary cultures.19 Here we show that these normal immortalised human kidney proximal tubule cells (HKC‐5) constitutively express TF message and secrete product. Production is enhanced by stimulation with LPS and compromised using a specific anti‐sense oligonucleotide. The subcellular distribution of TF was both cytosolic and on the plasma membrane. The use of leucocytes as a specificity control relies on them being non‐expressors of TF. These observations indicate that human proximal kidney cells are a source of inducible TF synthesis and secretion.
Epithelial cells are conventionally used in log‐phase growth conditions. Epithelial cells in vivo, however are predominantly in G0 in all but the transit‐amplifying compartment. This might change during repair or regeneration. In this study serum starvation slowed proliferation and reduced TF expression while the cells remained healthy and capable of resuming log‐phase growth, suggesting that proliferation and TF production correlate. Serum itself contributed negligible amounts of TF. Large amounts of TF appeared in conditioned medium and in quiescent cell lysates. Intact cells, either after serum deprivation or arrested with hydroxyurea (both models taking the cells out of the cell cycle) showed comparable TF procoagulant activity.
In the present study, we also found measurable levels of TF antigen in the HKC‐5 cell conditioned medium as well as in cell lysates. The TF‐antigen secretion was time‐dependent and up‐regulated by FCS and LPS. The release of TF is paralleled with a decrease in the intracellular TF levels. Increased apoptosis or cell detachment was not observed during these experiments and is not therefore thought to be responsible for TF release. In other systems, TF in conditioned media is bound to secreted microparticles.25 The hypothesis is that in inflammatory and malignant diseases, bioactive stimulants released into the vascular compartment are excreted via the kidneys, concentrating in urine to stimulate tubular cells in transit. In addition, LPS could cause a proinflammatory cytokine response in normal human proximal tubular cells.26 Increased TF expression by the kidney tubular cells could be of clinical significance in the peritubular capillary—and tubular basement membrane—fibrin deposition27,28 commonly observed in tubulointerstitial damage which plays a pivotal role in progressive renal injury.
Take‐home messages
Renal proximal tubular cell lines (HKC‐5 cells) constitutively make cell bound tissue factor (TF) activity and secrete TF activity and antigen into the milieu in vitro.
Secretion is up‐regulated by stimulating the cells with LPS and is also controlled by FCS in the medium.
Serum starvation or growth in high serum medium but with cell cycle arrest using hydroxyurea results in reduced TF levels.
This in vitro model offers a simple and reproducible system for the investigation of the procoagulant nature of TF and its role in inflammatory or neoplastic disease.
The coagulation process balances activation and inactivation of coagulation factors. In a separate series of experiments, we found that the HKC‐5 also express TFPI. It is common in such protease activated systems that the trigger and the inhibitor are produced by the same source,29,30,31 probably to regulate cellular procoagulant activity in their milieu. In kidneys TFPI has been found in a normal and in a glomerulonephritis crescentic rabbit model,32,33 where fibrin deposition could be a key mediator of injury,32 through TF‐mediated coagulation pathways.34 Indeed, TFPI protects renal function in experimental crescentic glomerulonephritis.32,33
In conclusion, the human kidney proximal tubule cell line, HKC‐5, has been shown to constitutively express and secrete TF in vitro. TF secretion increased in a time‐dependent manner and coincided with a decline in the intracellular levels. TF expression was up‐regulated by FCS and by LPS stimulation. The model supports the concept that proximal tubule cells may be a source of uTF and in disease conditions is reflected in the excreted uTF. Further studies will exploit the model to examine the effect of a range of inflammatory mediators on TF synthesis and secretion.
Abbreviations
FCS - fetal calf serum
HKC‐5 - human kidney proximal tubular cell line
LPS - lipopolysaccharide
ODN - oligodeoxynucleotide
TF - tissue factor
TFPI - tissue factor pathway inhibitor
uTF - urinary tissue factor
Footnotes
Competing interests: None declared.
References
- 1.Grunke W. Studien über die Blutgerinnung mit besonderer Berücksichtigung der Haemophilie. I. Mitteilung: Einfluss des Harnes auf die Gerinnung haemophilen Blutes. Blutes Z Ges Exp Med 183596512–516. [Google Scholar]
- 2.Carty N, Taylor I, Roath O S.et al Urinary tissue factor activity in malignancy. Thromb Res 199057473–478. [DOI] [PubMed] [Google Scholar]
- 3.Carty N, Taylor I, Roath O S.et al Urinary tissue factor activity in colorectal disease. Br J Surg 1990771091–1094. [DOI] [PubMed] [Google Scholar]
- 4.Adamson A S, Francis J L, Roath O S.et al Urinary tissue factor levels in transitional cell carcinoma of the bladder. J Urol 1992148449–452. [DOI] [PubMed] [Google Scholar]
- 5.Adamson A S, Francis J L, Witherow R O ' N.et al Urinary tissue factor levels in prostatic carcinoma: a potential marker of metastatic spread. Br J Urol 199371587–592. [DOI] [PubMed] [Google Scholar]
- 6.Lwaleed B A, Chisholm M, Francis J L. Urinary tissue factor levels in patients with breast and colorectal cancer. J Pathol 1999187291–294. [DOI] [PubMed] [Google Scholar]
- 7.Lwaleed B A, Bass P S, Chisholm M.et al Urinary tissue factor levels in glomerulonephritis: a potential marker of glomerular injury. J Clin Pathol 199750336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neale T J, Carson S D, Tipping P G.et al Participation of cell‐mediated immunity in deposition of fibrin in glomerulonephritis. Lancet 19882421–424. [DOI] [PubMed] [Google Scholar]
- 9.Drake T A, Morrissey J H, Edgington T A. Selective cellular expression of tissue factor in human tissues: implication for disorders of haemostasis and thrombosis. Am J Pathol 19891341087–1097. [PMC free article] [PubMed] [Google Scholar]
- 10.Fleck R A, Vijaya Mohan Rao L, Rapaport S I.et al Localization of human tissue factor antigen by immuno‐staining with mono‐specific, polyclonal anti‐human tissue factor antibody. Thromb Res 199059421–437. [DOI] [PubMed] [Google Scholar]
- 11.Adamson A S. Pro‐coagulants, coagulopathy and prostate cancer. Master of Surgery Thesis, University of Southampton 1992
- 12.Bukovsky A, Labarrere C A, Haag B.et al Tissue factor in normal and transplanted human kidney. Transplantation 199254644–650. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura T, Hutt M P, von Kulla K N. Urinary pro‐coagulant excretion in experimental renal disease. Proc Soc Exp Biol Med 1968127147–153. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, Aoki N, Kawaoi A. Localization of urinary pro‐coagulant in human kidney. Kidney Int 197915612–617. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura T, Hutt M P, von Kulla K N. Urinary pro‐coagulant excretion and its relation to kidney function. Nephron 19707165–177. [DOI] [PubMed] [Google Scholar]
- 16.Lwaleed B A, Bass P S, Francis J L.et al Functional and structural properties of urinary tissue factor. Nephrol Dial Transplant 199914588–596. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara T, Yamabe H, Osawa H.et al Tissue factor pathway inhibitor production by human proximal tubular epithelial cells in culture. Thromb Res 2003110141–147. [DOI] [PubMed] [Google Scholar]
- 18.Nestoridi E, Kushak R I, Duguerre D.et al Up‐regulation of tissue factor activity on human proximal tubular epithelial cells in response to Shiga toxin. Kidney Int 2005672254–2266. [DOI] [PubMed] [Google Scholar]
- 19.Racusen L C, Monteil C, Sgrignoli A.et al Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducer, and comparison with established cell lines. J Lab Clin Med 1997129318–329. [DOI] [PubMed] [Google Scholar]
- 20.Racusen L C, Wilson P D, Hartz P A.et al Renal proximal tubular epithelium from patients with nephropathic cystinosis: immortalized cell lines as in vitro model systems. Kidney Int 199548536–543. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Hartz P A, Philip E.et al Cell lines from kidney proximal tubules of a patient with Lowe syndrome lack OCRL inositol polyphosphate 5‐phosphatase and accumulate phosphatidylinositol 4,5‐bisphosphate. J Biol Chem 19982731574–1582. [DOI] [PubMed] [Google Scholar]
- 22.Detrisac C J, Sens M A, Garvin A J.et al Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int 198425383–390. [DOI] [PubMed] [Google Scholar]
- 23.Wilson P D, Dillingham M A, Breckon R.et al Defined human renal tubular cells in culture growth, characterization, and hormonal response. Am J Physiol 1985248436–443. [DOI] [PubMed] [Google Scholar]
- 24.Stephens A C, Rivers R P. Suppression of human monocyte tissue factor synthesis by antisense oligodeoxynucleotide. Thromb Res 199785387–398. [DOI] [PubMed] [Google Scholar]
- 25.Francis J L. Haemostasis and cancer. Med Lab Sci 198946331–346. [PubMed] [Google Scholar]
- 26.Brauner A, Soderhall M, Jacobson S H.et al Escherichia coli‐induced expression of IL‐1 alpha, IL‐1 beta, IL‐6 and IL‐8 in normal human renal tubular epithelial cells. Clin Exp Immunol 2001124423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enestrom S, Druid H, Rammer L. Fibrin deposition in the kidney in post‐ischaemic renal damage. Br J Exp Pathol 198869387–394. [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto K, Loskutoff D J. The kidneys of mice with autoimmune disease acquire a hypo‐fibrinolytic/pro‐coagulant state that correlates with the development of glomerulonephritis and tissue microthrombosis. Am J Pathol 1997151725–734. [PMC free article] [PubMed] [Google Scholar]
- 29.Rana S V, Reimers H J, Pathikonda M S.et al Expression of tissue factor and factor VIIa/tissue factor inhibitor activity in endotoxin or phorbol ester stimulated U937 monocyte‐like cells. Blood 198871259–262. [PubMed] [Google Scholar]
- 30.McGee M P, Foster S, Wang X. Simultaneous expression of tissue factor and tissue factor pathway inhibitor by human monocytes. A potential mechanism for localized control of blood coagulation. J Exp Med 19941791847–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopp C W, Robson S C, Siegel J B.et al Regulation of monocyte tissue factor activity by allogeneic and xenogeneic endothelial cells. Thromb Haemost 199879529–538. [PubMed] [Google Scholar]
- 32.Erlich J H, Apostolopoulos J, Wun T C.et al Renal expression of tissue factor pathway inhibitor and evidence for a role in crescentic glomerulonephritis in rabbits. J Clin Invest 199698325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham M A, Ono T, Hewitson T D.et al Tissue factor pathway inhibitor expression in human crescentic glomerulonephritis. Kidney Int 1999551311–1318. [DOI] [PubMed] [Google Scholar]
- 34.Lwaleed B A. Is tissue factor a mediator of fibrin deposition in glomerular pathology? Nephrol Dial Transplant 1999142533. [DOI] [PubMed] [Google Scholar]