Abstract
Many studies have demonstrated that aging is associated with declines in skill acquisition. In the current study, we tested whether older adults could acquire general, transferable knowledge about skill learning processes. Older adult participants learned five different motor tasks. Two older adult control groups performed the same number of trials, but learned only one task. The experimental group exhibited faster learning than that seen in the control groups. These data demonstrate that older adults can learn to learn new motor skills.
The capacity to acquire new motor skills is essential for adaptive motor function throughout the lifespan. Many studies have documented that older adults show reduced rates of skill learning, and in many cases, even when provided with extended practice, their performance levels do not reach those of young adults [9, 20, 22, 31, 33, 34, 36, 38]. In addition to examining the learning process itself, many studies make use of transfer tests to determine the generalizability and flexibility of the acquired representation. Generalization of learning can be tested by having participants adapt to a perturbation and then transfer to either a new effector [29, 40], new workspace [44], or new mode of movement (i.e. from continuous tracking to discrete pointing movements [2]). Savings of learning [28, 32, 45] can be tested by having participants adapt to a perturbation, wash out the effects of learning, and then re-adapt to the same or a similar distortion. This allows the determination of whether subjects can make use of the previously acquired motor memory to learn something new. Recent work has also demonstrated that people can learn to learn new motor skills [7, 35, 37]. In this case, participants acquire multiple unrelated motor tasks (adapt to a gain change, visual rotation, sequence learning, etc.) successively, with the end result that they show faster learning than naïve participants do. This provides evidence that people can acquire something very general and transferable about the learning process itself. Similar findings have been documented for discrimination problem solving in rhesus monkeys [19] and humans [14]. Over multiple experiences with the same problem type, participants gradually acquired a strategy that could then be applied to quickly solve new problems of the same type.
Despite age-related decrements in sensorimotor adaptation [9, 16, 31, 38, 39], older adults are able to generalize adaptive improvements to new modes of movement [3, 4]. Additionally, our work has recently shown that older adults exhibit the same magnitude of savings of learning as young adults when they adapt manual aiming movements to three subsequent rotations of the visual feedback display [39]. There is also evidence that older adults can learn to learn new motor skills [5, 6]. In these studies, older and young adult participants first adapted arm movements to a left-right visual rearrangement, and then transferred to the left-right distortion combined with an up-down reversal. Older adults actually demonstrated stronger learning to learn effects than the younger adults.
It is currently unclear whether this learning to learn phenomenon in older adults extends to different types of skills as well, as has been shown for younger adults [37]. Specifically, it is not known whether multiple visuomotor adaptation experiences would result in enhanced sequence learning for older adults, since this process is both neurally and strategically different from visuomotor adaptation. For example, data suggest that sequence learning relies more heavily on basal ganglia and medial motor cortex circuitry, while adaptation engages the cerebellar and parietal regions to a greater extent [11, 18, 21, 24, 26, 27, 42], although both types of learning may rely on overlapping neural substrates in the earliest minutes of training [12, 13]. Thus, the purpose of these experiments was to determine whether adaptation to multiple visuomotor distortions would enhance adaptation to a new visuomotor distortion, and would also facilitate the acquisition of a movement sequence for older adults. Older adults exhibit greater deficits for visuomotor adaptation tasks than for sequence learning [38], raising the possibility that facilitation between the two classes of learning may also be impaired with advancing age. The current study examined whether this was indeed the case.
We tested thirty-seven older adult participants in this study. They were assigned to one of three groups: multiple learning (ML), gain adaptation only (GL), and sequence learning only (SL). The age and demographic characteristics of the three groups are presented in Table 1. All participants signed an institution-approved consent form prior to partaking in the study. They were compensated for their participation, which took an average of four hours over two testing days.
Table 1.
Group demographics. Mean values are presented, with standard deviations in parentheses. MMSE is the Mini-Mental State Exam score (Folstein et al., 1975); # of meds is the average number of medications taken per day. * indicates a group main effect in mean age at P < .05. Tukey’s HSD follow up comparisons revealed that the ML and SL groups were significantly different (P < .05).
| Group | Age range | Mean age* | MMSE | # of meds | Years education | Hours exercise/wk | Gender ratio |
|---|---|---|---|---|---|---|---|
| ML | 66.0 – 80.0 | 74.9 (3.7) | 29.0 (1.0) | 1.3 (1.1) | 16.4 (2.2) | 4.9 (3.0) | 8F, 11M |
| GL | 65.3 – 76.6 | 72.8 (4.5) | 29.2 (0.4) | 1.3 (1.2) | 15.8 (2.4) | 2.8 (2.2) | 3F, 6M |
| SL | 66.5 – 76.4 | 70.6 (3.6) | 29.8 (0.5) | 1.4 (0.9) | 15.3 (1.5) | 3.6 (2.8) | 6F, 3M |
The procedures have been reported previously [37]. Participants moved a manual joystick device to hit targets presented on a computer screen, with real time feedback display of joystick location. The joystick was secured to the table in front of participants, placed at their body midline. Movements were always initiated from the central start target (0.8 cm in diameter) and made to targets (0.8 cm in diameter) that appeared 4.8 cm up, right, down, or left of the start position. Participants were instructed to move the cursor representing the joystick position into the target as quickly as possible upon target appearance and to hold the cursor within the target until it disappeared (3 sec following its appearance). At this point, participants were instructed to release their grip on the spring-loaded joystick, which returned to the center for the next trial. The subsequent trial began two seconds later.
The ML group learned five motor tasks over two test sessions, conducted within approximately 48 hours of each other. On Day 1, they adapted to three different visuomotor distortions: 15º, 30º, and 45º counterclockwise rotations of the feedback display about the start location. Washout trials were given between each adaptive experience to restore performance to baseline levels. The details of trial presentation, and the results from this portion of the study, are reported in Seidler [38].
On the second testing day, the ML group first adapted to a change in the gain of display of their movements; we increased the size of displayed movements by a factor of 1.5. The ML group then learned a repeating sequence of movements. The sequence blocks consisted of the following repeating target sequence: up, left, right, down. This was not just a simple four-element sequence, however. Participants were required to return to the central start position between each target (passively), increasing the effective sequence length to eight elements. Moreover, the relatively long interval between stimuli (four seconds) in the current study in comparison to other investigations of sequence learning also impacts expression of learning [46]. We selected this longer interval in order to allow time for these older adults to make movements to the targets, even under the distorted visual feedback conditions. The details of block and trial presentation for Day 2 testing are presented in Table 2. The GL and SL groups performed the same number of trials on Day 1 as the ML group, except that they did not receive any trials in which the feedback display was rotated. However, the GL and SL groups performed the baseline aiming task in all other blocks on Day 2.
Table 2.
Day 2 trial and block presentation schedule.
| Learning Stimulus | ||||
|---|---|---|---|---|
| Block # | ML group | GL group | SL group | # of Trials |
| 1 | None (baseline) | None (baseline) | None (baseline) | 24 |
| 2 | None (baseline) | None (baseline) | None (baseline) | 24 |
| 3 | 1.5 gain | 1.5 gain | None (baseline) | 28 |
| 4 | 1.5 gain | 1.5 gain | None (baseline) | 28 |
| 5 | 1.5 gain | 1.5 gain | None (baseline) | 28 |
| 6 | None (baseline) | None (baseline) | None (baseline) | 28 |
| 7 | None (baseline) | None (baseline) | None (baseline) | 28 |
| 8 | Sequence | None (baseline) | Sequence | 28 |
| 9 | Sequence | None (baseline) | Sequence | 28 |
| 10 | Sequence | None (baseline) | Sequence | 28 |
| 11 | None (baseline) | None (baseline) | None (baseline) | 28 |
| 12 | None (baseline) | None (baseline) | None (baseline) | 28 |
Participants were not informed in advance as to whether or not the upcoming block contained a learning stimulus (rotation, gain change, or sequence). They were instructed to hit the target as rapidly as possible, and to attempt to minimize both reaction time (RT) and movement time. Following the first sequence block, ML and SL participants were probed about their awareness of the existence of the sequence (the GL group did not perform the sequence). We asked them the following questions: “Did you notice anything different about the last block? If so, what?” Following the final sequence block, we asked participants: “Did you notice the sequential target presentation over the last three blocks?” They were asked to attempt to report the target sequence regardless of whether they had noticed its existence.
We analyzed the joystick data offline, using custom software routines. We first filtered the data with a dual-pass Butterworth digital filter [48] using a cutoff frequency of 10 Hz. Then we computed the resultant joystick path by taking the square root of the sum of the squared x and y coordinate data at each time point. The tangential velocity profile was computed via differentiation of the resultant path. Movement onset and offset were calculated by applying the optimal algorithm of Teasdale et al. [46] to this velocity profile for each movement. We computed the RT by subtracting the time of the stimulus presentation from the time of the onset of movement. This variable was used to assess sequence learning. In order to assess gain adaptation, we calculated the initial endpoint error (IEE, [37]). This is the distance from the target at the end of the initial ballistic impulse. The algorithm used to identify the end of this initial movement searches for a period of acceleration following a period of deceleration or a change in the sign of the velocity. Thus, the initial preplanned movement has “ended” when there is either a change in movement direction or an additional propulsive action is made [15, 43].
We used repeated measures analyses of variance (RM ANOVA) designs on the variables (RT, IEE) to examine the rate of learning for the three groups (ML, SL, GL). IEE for the ML group was compared to that of the GL group for the gain adaptation period (blocks 1–7 of Table 2), and RT for the ML group was compared to that of the SL group for the sequence learning period (blocks 6–12 of Table 2). The Huynh-Feldt epsilon [21] was evaluated to determine whether the repeated measures data met the assumption of sphericity (Σ > 0.75). In cases where sphericity was met, the univariate tests were used to maintain power. Otherwise, the F statistic was evaluated for significance using the Huynh-Feldt adjusted degrees of freedom.
The demographic characteristics of the three groups are presented in Table 1. The groups did not differ in their Mini Mental State Exam scores [16], years of education, average number of medications taken, or average number of weekly exercise hours (P > .10 in all cases). Participants reported medication use for high blood pressure, high cholesterol, hormone replacement therapy, and thyroid activity. There was a group main effect for age (P < .05), with follow up tests revealing that the ML group was significantly older than the SL group (Tukey’s HSD (honestly significant difference) P < .05).
Figure 1A presents IEE during the gain adaptation (blocks 1–7 of Table 2) for the ML and GL groups. For reference, the mean performance for each block of the young adult GL group from Seidler [36] is also shown. The RM ANOVA including the older adult ML and GL groups resulted in a significant group x block x trial interaction (F73, 1832 = 1.6, P < .01); therefore, follow up tests were conducted. It was first important to determine whether the two groups were equated in terms of baseline performance. The ML group started the first baseline block with higher IEE than the GL group, but their performance improved with practice. By the second baseline block, performance had stabilized for the two groups (group x block x trial interaction across the first two baseline blocks, F23,598 = 2.7, P < .01), and there was no difference between them (absence of group main effect for the initial baseline blocks, P > .10). Errors increased for both groups when the gain change was introduced (performance differed significantly between blocks 2 and 3, F1,25 = 9.1, P < .01). The ML group showed evidence of adapting to the altered gain; that is, their performance improved across trials within the first adaptation block (block 3) and then stabilized. In contrast, the GL group showed little or no evidence of performance change across the three adaptation blocks (blocks 3 – 5; group x block x trial interaction across these three blocks, F36,944 = 1.5, P < .05). Aftereffects of the altered gain were evidenced by an increase in IEE from block 6 to 7 (F1,25 = 18.0, P < .01). The ML group exhibited a faster return towards baseline performance than the GL group during these last two baseline blocks (group x trial effect across blocks 6 and 7, F23,575 = 1.5, P = .01, and group difference in the magnitude of change from block 6 to 7, F1,25 = 5.9, P < .05).
Figure 1.
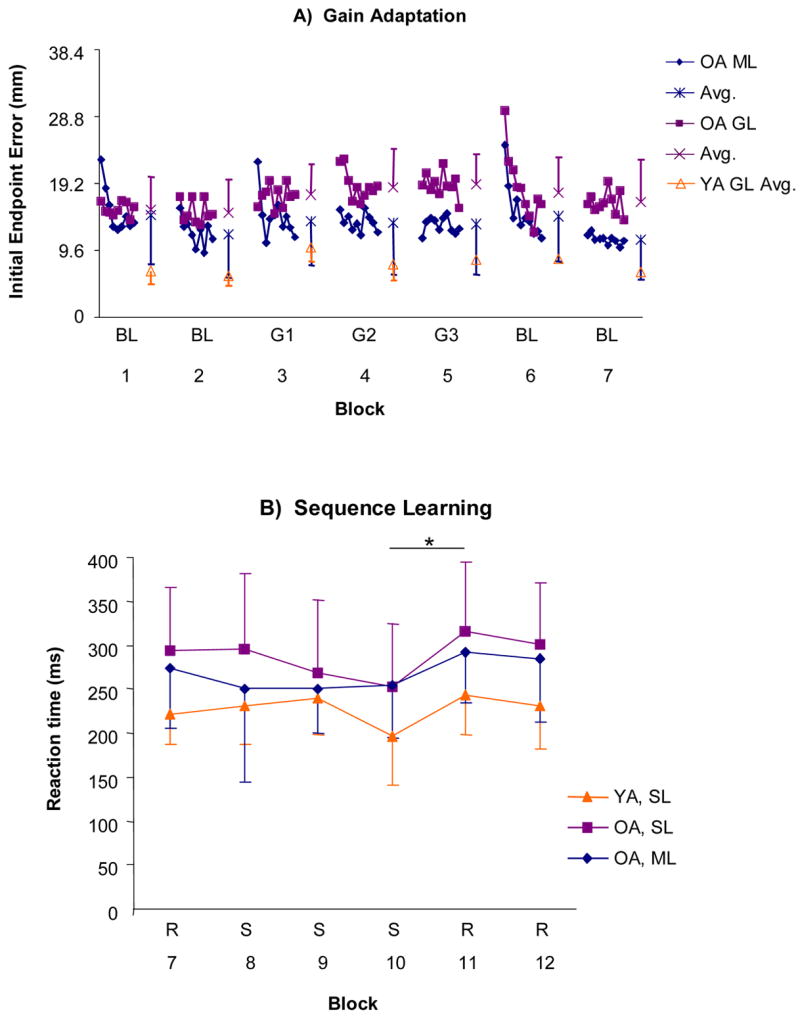
Panel A: Gain adaptation performance is presented for the ML and GL groups; the young adult GL data (averaged for each block) from Seidler (2004) is provided as a reference. BL refers to baseline blocks with the normal feedback display; G refers to the gain adaptation blocks; block numbers are as in Table 2. The first point of each block presents group mean performance for the first trial; subsequent symbols are the mean across three trials. The free floating symbols reflect the mean for each previous block; error bars are standard deviations across participants. Both the older adult GL and ML groups show an increase in error when the gain change is first introduced (block 3 versus 2). The GL group shows little or no performance modulation across trials or blocks, however, while the ML group shows decreasing errors with practice during the first block.
Panel B: Sequence learning performance is presented for the ML and SL groups; the young adult SL data from Seidler (2004) is provided as a reference. R refers to blocks in which the target locations appeared in a random fashion; S refers to the sequence learning blocks; block numbers are as in Table 2. RTs were averaged across trials and participants for each block; error bars are standard deviations across participants. The OA ML group exhibits learning, reflected as faster RTs, within the first sequence learning block while the OA SL group does not. However, both groups exhibit a comparable amount of acquired sequence knowledge (reflected as faster RTs for the last S block than the subsequent R block, comparison indicated by *) by the end of practice.
Figure 1B presents RT during sequence learning (blocks 7 – 12 of Table 2) for the ML and SL groups. For reference, the mean performance for each block of the young adult SL group from Seidler [37] is also shown. The RM ANOVA including the older adult ML and SL groups resulted in a significant group x block interaction (F5,122 = 3.3, P < .01); therefore, follow-up comparisons were performed. The two groups did not differ in terms of performance for the first baseline block (block 7, P > .10). Both groups exhibited evidence of learning the sequence, reflected as a significant increase in RT when transitioning from the last sequence block (block 10) to the subsequent baseline (random) block (block 11, difference between blocks 10 and 11 F1, 25 = 58.5, P < .01). The rate at which the groups acquired the sequence differed however, with the ML group showing significant learning within the first sequence block (block 8) while the SL group did not (group difference in the magnitude of change from block 7 to block 8, F1,25 = 5.1, P < .05). There was no significant group difference in the proportion of subjects that gained explicit awareness of the sequence (88% SL group, 69% ML group).
We found that participating in multiple visuomotor adaptation experiences resulted in a facilitation of movement gain adaptation for older adults (“learning to learn”). This is a notable finding, given that older adults exhibit more profound learning deficits for this type of skill in comparison to adapting to rotated visual feedback or learning a sequence of actions [38]. In fact, the older adult participants with multiple adaptive experiences had a rate of gain adaptation that was more comparable to that of young adults than that of naïve older adult participants (see Figure 1). This enhancement in learning rate was not simply due to a greater facilitation with the joystick device and stimulus display, since the gain only participants had the same quantity of exposure to the experimental setup. Multiple motor learning experiences also led to facilitation in the rate of sequence learning for older adults (see Figure 2). While there was no difference in the overall amount of learning achieved by the older adult group with prior adaptive experiences and the naïve older adult group (assessed at the completion of practice), the former learned at a faster rate, with most of the learning taking place within the first practice block.
The current results suggest that differential mechanisms contribute to skill learning in the naïve state and learning following multiple experiences, with the processes underlying naïve learning negatively impacted by advancing age. It is not currently clear what these differential mechanisms are, however. It may be that participants acquire very general skills during a learning to learn paradigm, which can be applied to a broad range of types of learning. Such general skills might include the detection of repeated patterns in the environment, conflict monitoring, and error detection. These are all functions that have been attributed to the anterior cingulate cortex [2, 8, 10, 30], which has been shown to be active in the early stages of skill learning [25]. Regardless of the underlying mechanisms, the present findings imply that, in order to maximize the rate of learning and degree of transfer, training for older adults should encompass a variety of motor learning experiences.
Acknowledgments
This work was supported by NIA AG24106 and AG20883, and the UM Claude D. Pepper OAIC Research Career Development and Human Subjects Cores (NIA AG08808). I thank the following undergraduate students for their assistance with data acquisition and analysis: M. Zafar, E. Andreae, C. Chase, K. Gustafson, and K. Keen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abeele S, Bock O. Transfer of sensorimotor adaptation between different movement categories. Exp Brain Res. 1993;148:128–32. doi: 10.1007/s00221-002-1317-0. [DOI] [PubMed] [Google Scholar]
- 2.Berns GS, Cohen JD, Mintun MA. Brain regions responsive to novelty in the absence of awareness. Science. 1997;276:1272–1275. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- 3.Bock O. Components of sensorimotor adaptation in young and elderly subjects. Exp Brain Res. 2005;160:259–263. doi: 10.1007/s00221-004-2133-5. [DOI] [PubMed] [Google Scholar]
- 4.Bock O, Girgenrath M. Relationship between sensorimotor adaptation and cognitive functions in younger and older subjects. Exp Brain Res. 2006;169:400–6. doi: 10.1007/s00221-005-0153-4. [DOI] [PubMed] [Google Scholar]
- 5.Bock O, Schneider S. Acquisition of a sensorimotor skill in younger and older adults. Acta Physiol Pharmacol Bulg. 2001;26:89–92. [PubMed] [Google Scholar]
- 6.Bock O, Schneider S. Sensorimotor adaptation in young and elderly humans. Neurosci Biobehav Rev. 2002;26:761–7. doi: 10.1016/s0149-7634(02)00063-5. [DOI] [PubMed] [Google Scholar]
- 7.Bock O, Schneider S, Bloomberg J. Conditions for interference versus facilitation during sequential sensorimotor adaptation. Exp Brain Res. 2001;138:359–365. doi: 10.1007/s002210100704. [DOI] [PubMed] [Google Scholar]
- 8.Botvinick M, Nystrom LE, Fissel K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 9.Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learn Mem. 2003;10:55–63. doi: 10.1101/lm.50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 11.Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, Alexander GE. Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature. 1996;383:618–621. doi: 10.1038/383618a0. [DOI] [PubMed] [Google Scholar]
- 12.Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–7. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–62. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- 14.Duncan CP. Description of learning to learn in human subjects. The American Journal of Psychology. 1960;73:108–114. [PubMed] [Google Scholar]
- 15.Elliott D, Carson RG, Goodman D, Chua R. Discrete vs. continuous visual control of manual aiming. Human Movement Science. 1991;10:393–418. [Google Scholar]
- 16.Fernandez-Ruiz J, Hall C, Vergara P, Diaz R. Prism adaptation in normal aging: slower adaptation rate and larger aftereffect. Cogn Brain Res. 2000;9:223–226. doi: 10.1016/s0926-6410(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state:" A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Research. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 19.Harlow HF. The formation of learning sets. Psychological Review. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- 20.Harrington DL, Haaland KY. Skill learning in the elderly: diminished implicit and explicit memory for a motor sequence. Psych Aging. 1992;7:425–34. doi: 10.1037//0882-7974.7.3.425. [DOI] [PubMed] [Google Scholar]
- 21.Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning and sequential procedures. Trends In Neurosciences. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- 22.Howard JH, Howard DV. Age differences in implicit learning of higher order dependencies in serial patterns. Psych Aging. 1997;12:634–656. doi: 10.1037//0882-7974.12.4.634. [DOI] [PubMed] [Google Scholar]
- 23.Huynh H, Feldt LS. Conditions under which the mean square ratios in repeated measures designs have exact F-distributions. J Am Stat Assoc. 1970;65:1582–9. [Google Scholar]
- 24.Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RSJ, Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning I. Frontal cortex and attention to action. J Neurophys. 1997;17:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- 27.Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning II. Subcortical structures and learning by trial and error. J Neurophys. 1997;77:1325–1337. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- 28.Kojima Y, Iwamoto Y, Yoshida K. Memory of learning facilitates saccadic adaptation in the monkey. J Neurosci. 2004;24:7531–7539. doi: 10.1523/JNEUROSCI.1741-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol. 2006;4:e316. doi: 10.1371/journal.pbio.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNay EC, Willingham DB. Deficit in learning of a motor skill requiring strategy, but not of perceptuomotor recalibration, with aging. Learn Mem. 1998;4:411–420. doi: 10.1101/lm.4.5.411. [DOI] [PubMed] [Google Scholar]
- 32.Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messier J, Adamovich S, Jack D, Hening W, Sage J, Poizner H. Visuomotor learning in immersive 3D virtual reality in Parkinson’s disease and in aging. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0802-2. Dec 5[epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Pratt J, Chasteen AL, Abrams RA. Rapid aimed limb movements: age differences and practice effects in component submovements. Psych Aging. 1994;9:325–334. doi: 10.1037//0882-7974.9.2.325. [DOI] [PubMed] [Google Scholar]
- 35.Roller CA, Cohen HS, Kimball KT, Bloomberg JJ. Variable practice with lenses improves visuomotor plasticity. Cogn Brain Res. 2001;12:341–352. doi: 10.1016/s0926-6410(01)00077-5. [DOI] [PubMed] [Google Scholar]
- 36.Ruch FL. The differentiative effects of age upon human learning. J Gen Psych. 1934;11:261–286. [Google Scholar]
- 37.Seidler RD. Multiple motor learning experiences enhance motor adaptability. J Cogn Neuro. 2004;16:65–73. doi: 10.1162/089892904322755566. [DOI] [PubMed] [Google Scholar]
- 38.Seidler RD. Differential effects of age on sequence learning and sensorimotor adaptation. Brain Research Bulletin. 2006;70:337–346. doi: 10.1016/j.brainresbull.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Seidler RD. Aging affects motor learning but not savings at transfer of learning. Learn Mem. 2007;14:17–21. doi: 10.1101/lm.394707. [DOI] [PubMed] [Google Scholar]
- 40.Seidler RD, Bloomberg JJ, Stelmach GE. Patterns of transfer of adaptation among body segments. Behav Brain Res. 2001;122:145–157. doi: 10.1016/s0166-4328(01)00183-8. [DOI] [PubMed] [Google Scholar]
- 41.Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res. 2006;175:544–555. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- 42.Seidler RD, Purushotham A, Kim S, Ugurbil K, Willingham D, Ashe J. Cerebellum activation associated with performance change but not motor learning. Science. 2002;296:2043–2046. doi: 10.1126/science.1068524. [DOI] [PubMed] [Google Scholar]
- 43.Seidler-Dobrin RD, Stelmach GE. Persistence in visual feedback control by the elderly. Exp Brain Res. 1998;119:467–474. doi: 10.1007/s002210050362. [DOI] [PubMed] [Google Scholar]
- 44.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–24. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLOS Biology. 2006;4:1035–1043. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teasdale N, Bard C, Fleury M, Young D, Proteau L. Determining movement onsets from temporal series. J Motor Behav. 1993;25:97–106. doi: 10.1080/00222895.1993.9941644. [DOI] [PubMed] [Google Scholar]
- 47.Willingham DB, Greenberg AR, Thomas RC. Response-to-stimulus interval does not affect implicit motor sequence learning, but does affect performance. Mem Cognit. 1997;25:534–542. doi: 10.3758/bf03201128. [DOI] [PubMed] [Google Scholar]
- 48.Winter DA. Biomechanics and motor control of human movement. 2. New York: Wiley; 1990. [Google Scholar]


