Abstract
For meadow voles, Microtus pennsylvanicus, seasonal differences exist in self-grooming and in odor preferences for conspecifics, two behaviors which facilitate sexual interactions in this species. Both behaviors are mediated by photoperiodically-induced changes in circulating gonadal steroid hormone titers which, in turn, can be transduced by the duration of the melatonin signal that a seasonally breeding animal receives. The goal of this study was to determine whether exogenous melatonin administration affects circulating gonadal steroid hormone titers in meadow voles, and whether it influences their odor preferences and self-grooming behavior to same-and opposite-sex conspecifics. Long-photoperiod voles that did not receive exogenous melatonin had higher testosterone (males) and estradiol (females) titers than did short-photoperiod voles and long-photoperiod voles treated with melatonin for 12 weeks; the latter had similar estradiol and testosterone titers. Long-photoperiod voles that did not receive melatonin preferred the scent marks of long-photoperiod opposite-sex conspecifics and spent more time self-grooming in response to their odors than those of either long-photoperiod same-sex, short photoperiod same-sex, or short-photoperiod opposite-sex conspecifics. Long-photoperiod voles that received melatonin, however, no longer preferred the marks of long-photoperiod opposite-sex conspecifics and no longer spent more time self-grooming in response to their odors, not unlike the odor preferences and self-grooming behavior of short-photoperiod voles. As a whole, the data suggest that the duration of the melatonin signal is likely involved in mediating the photoperiodically-induced changes in gonadal steroid hormones that mediate a meadow vole’s odor preferences for opposite-sex conspecifics and its self-grooming response to those marks.
1. Introduction
For many seasonally breeding mammals, seasonal shifts exist in the behaviors that facilitate and surround reproduction (18, 26, 34, 36, 41). Seasonal differences in these behaviors that are directed at opposite-sex conspecifics are generally mediated by photoperiodically-induced changes in the endocrine milieu of individuals (2, 25, 40, 41, 45–47). For example, high titers of the gonadal steroid hormones characterize long-day breeders such as voles and Syrian hamsters housed under long photoperiod, whereas low titers of these hormones are characteristic of individuals housed under short photoperiod (48, 54). In voles, high titers of gonadal steroids support reproductive activity and related behaviors directed at opposite-sex conspecifics, whereas low titers of gonadal steroids induce reproductive quiescence and related behaviors not directed at opposite-sex conspecifics (19–21, 33–35).
This photoperiodically-mediated change in hormonal control underlying seasonal shifts in the behaviors that facilitate interactions with opposite-sex conspecifics, may be transduced by the duration of the melatonin signal that a seasonally breeding animal receives (46, 47). However, numerous studies that have examined the effects of melatonin on the behaviors that support interactions between opposite-sex conspecifics show that there is no general pattern as to whether melatonin duration inhibits, stimulates, or has negligible effects on such behavior (26, 28, 48, 53, 54). Some studies on Syrian and Siberian hamsters report that short-day patterns of melatonin during long photoperiod maintain or stimulate the production of gonadal steroid hormone titers that in turn induce or maintain the behaviors directed at opposite-sex conspecifics. These studies also point out that long-day patterns of melatonin during short-photoperiod inhibit or suppress gonadal steroid hormone titers that maintain or stimulate behavior directed at opposite-sex conspecifics (26, 47). In contrast, other studies on Syrian and Siberian hamsters report that short-day patterns of melatonin during long-day lengths are either independent of the gonadal steroid hormones, independent of melatonin, or both (1, 5, 49), and that sexual behavior and other behaviors directed at opposite-sex conspecifics are not affected by melatonin treatment (2, 7). Still other studies on male Syrian hamsters, meadow voles, and rats found that exogenous melatonin has mixed effects on behavior, some of which are stimulatory (6, 11, 29) and some of which are inhibitory (16, 25, 31, 39, 40). The results of these previous studies are somewhat intriguing in that they suggest that the effects of exogenous melatonin on circulating gonadal steroid hormones and the behaviors that surround reproduction differ among species of long-day seasonal breeding mammals.
In the present study, we evaluated the effects of exogenous melatonin on testosterone and estradiol titers, odor preferences, and self-grooming behavior of meadow voles to same- and opposite-sex conspecifics. Meadow voles are an excellent species to study in this regard because they display striking seasonal differences in their responses to odors of conspecifics, an indication of their interest in the opposite sex (17, 18). During the breeding season or under long photoperiod (LP), meadow voles spend more time investigating the scent marks of opposite-sex conspecifics relative to those of same-sex conspecifics (15, 18–21), and spend more time self-grooming when they encounter opposite-sex conspecifics relative to those of same-sex conspecifics (34). In contrast, during the non-breeding season or under short photoperiod (SP), meadow voles have fewer interactions with opposite-sex conspecifics and generally become reproductively quiescent (18, 22, 36). At this time of year, male and female voles no longer maintain preferences for the odors and scent marks of opposite-sex conspecifics (15, 19, 21), and they no longer spend more time self-grooming in response to odors of opposite-sex conspecifics than to those of same-sex conspecifics (34). In addition, seasonal differences exist in the gonadal steroid hormone titers of male and female meadow voles. Under long photoperiod, voles have high titers of gonadal steroid hormones, which are necessary to support the behaviors that surround reproduction. In contrast, short photoperiod voles have low titers of gonadal steroid hormones, which induce reproductive quiescence (15, 18–21). Many other seasonally breeding mammals show similar seasonal variations in gonadal steroid hormones and the behaviors that surround reproduction (46, 47).
The first hypothesis we tested was that 12 weeks of a constant melatonin signal is sufficient to maintain low gonadal steroid hormone titers in male and female meadow voles. Specifically, LP male and female voles implanted with exogenous melatonin will maintain lower testosterone and estradiol titers as compared to LP male and female voles not treated with melatonin, but similar titers of such hormones as compared to SP male and female voles, respectively. The second hypothesis we tested was that 12 weeks of a constant melatonin signal is sufficient to alter the odor preferences and self-grooming responses of LP male and female meadow voles such they no longer reflect those displayed by LP male and female meadow voles not receiving melatonin. That is, LP voles treated with exogenous melatonin will spend similar amounts of time self-grooming in response to odors of opposite-sex conspecifics and those of same-sex conspecifics, and spend similar amounts of time investigating the odors of opposite-sex conspecifics and same-sex conspecific. Specifically, LP voles treated with melatonin will behave in manner similar to that of SP voles.
2. Methods
2.1 Animals
The LP and SP meadow voles used in this study were third generation captive animals, trapped originally in northern Kentucky and southern Ohio. Long-photoperiod voles were conceived, born, and raised under a 14L:10D regime (lights on at 0700 h, CST, and off at 2100 h, CST). Long-photoperiod voles attain sexual maturity at about 60 d of age, which coincides with that reported for free-living voles during the spring and summer months (16). Long-photoperiod voles, like their free-living counterparts during the breeding season, readily mate within a few minutes of pairing with opposite-sex conspecifics (37); these voles also maintain high titers of circulating gonadal sex steroids (19). Thus, we considered voles born and reared in long-photoperiod as being reproductively active. Short-photoperiod voles were conceived, born, and raised under a 10L:14D regime (lights on at 0700 h, CST, and off at 1700 h, CST). Short-photoperiod voles do not reach sexual maturity until 160–180 d of age, and like most free-living adult voles during the non-breeding season, these voles do not readily mate with opposite-sex conspecifics (37). Short-photoperiod voles have lower titers of circulating gonadal sex steroids than that of LP voles (37). Thus, we considered voles born and reared in short photoperiod as being reproductively quiescent. Both LP and SP voles were weaned at 21 d of age and then housed with littermates in plastic cages (26 × 32 × 31 cm) until they were 40 days of age. At 40 d of age, voles were housed singly in a plastic cage (13 × 16 × 13 cm). Animals were provided ad libitum with food (Rodent Diet 5008, PMI Feeds Inc., Brentwood, Missouri, USA) and water. Cages were cleaned once a week and cotton-nesting material was replaced every two weeks.
2.2 Melatonin Treatment
Forty-d-old LP voles were implanted with either melatonin-filled capsules or empty capsules for 12 consecutive weeks following the procedures detailed in Ferkin and Kile (16). Briefly, capsules were constructed from Silastic tubing (Dow Corning, Midland, MI; OD 0.077 in, ID 0.058 in) cut into 20-mm lengths. Capsules were either filled with 10-mm active length of crystalline melatonin (N-Acetyl-5-methoxytryptamine, Sigma Chemical Co., St. Louis, MO) or left empty. The ends of all of the capsules were sealed with silicon rubber cement (5-mm of silicon rubber cement on each end). Melatonin-filled and empty capsules were incubated separately in sterile 0.9% saline solution for 24-h before insertion into the voles. Voles were anesthetized with isofluorane vapors prior to being implanted with a single capsule subcutaneously in the interscapular region. Although endogenous melatonin is not released in a steady amount as in Silastic capsules (3), we chose this technique as opposed to pumps or daily injections for several reasons. First, capsules reduce handling stress and the need for cannulas, which may affect self-grooming (17). Second, identical melatonin implants reduce the attractiveness of the anogenital area scent marks of LP voles to opposite-sex conspecifics (16). All subjects were tested 12 weeks after they were implanted with either a capsule containing melatonin or an empty capsule. The same subjects were not used the self-grooming and odor preference studies.
2.3 Gonadal Steroid Hormone Measurement
In this study, the circulating testosterone titers of LP male voles treated with melatonin (n=10), LP males not treated with melatonin (n=10), and SP males (n=10), and the circulating estradiol titers of LP female voles treated with melatonin (n=10), LP females not treated with melatonin (n=10), and SP females were measured (n=10). Blood samples were taken from male and females subjects 24 h after they completed their behavioral test. The subjects were anesthetized with isofluorane vapors and a blood sample was obtained via cardiac puncture. All sampling took place between 0800 and 0900 CST. Plasma was isolated and stored at −80°C until analyzed via an enzymatic immunoassay (EIA) using commercial assay kits from Diagnostic Systems Laboratories (DSL, Webster, TX, USA).
We compared the circulating testosterone titers of LP male voles treated with melatonin, LP males not treated with melatonin, and SP males. We also compared the circulating estradiol titers of LP female voles treated with melatonin, LP females not treated with melatonin, and SP females. In that the data were not normally distributed, we used separate one-way ANOVA’s to determine if significant differences existed in estradiol concentrations among females and testosterone concentrations among males. When significant main effects were detected, Holm-Sidak post hoc tests were used to determine significance differences between the pairwise comparisons. Statistical significance was accepted at α ≤ 0.05.
2.4 Self-grooming Procedure
Subjects in the self-grooming tests were melatonin-treated LP male voles, melatonin-treated LP voles, blank-treated LP male voles, blank-treated LP female voles, intact SP male voles, and intact SP female voles. Subjects were given a choice between the anogenital scents of either: a) LP males and LP females, b) SP males and SP females, c) SP females and LP females, or d) SP males and LP males. There were 10 different subjects used for each self-grooming test. Although each subject underwent a single self-grooming test, the scent donors were used in multiple self-grooming tests. Scent donors were 80 similarly aged LP and SP males and females (n = 20 donors per group). Scent donors were not implanted with Silastic capsules and were not used as subjects.
At the beginning of each self-grooming test, the subject’s cotton bedding was removed from its cage. Next, approximately 8 g of vole-scented cotton bedding from either a LP male, a LP female, a SP male, or a SP female donor was placed into the subject’s cage. This scented bedding had been in a cage of a scent donor for 14 d. Two min after the cotton bedding was placed into the cage of a subject, we recorded continuously over the next 5 min, the amount of time that it self-groomed as described elsewhere (23, 24, 35, 43). Briefly, in voles, the general pattern of self-grooming consists of a cephalocaudal progression that begins with rhythmic movements of the paws around the mouth and face, over the ears, descending to the ventrum, flank, anogenital area, and tail (23). After each test, the scent donor’s cotton bedding was removed from the subject’s cage and discarded. The experimenter wore latex gloves at all times to prevent transfer of human odor. The experimenter was blind to the treatment condition and the identity of the scent donors. The amount of time voles self-groomed in response to unscented cotton bedding were not measured because previous studies have shown that voles spend less than 2–3 seconds self-grooming when presented for 5-min with unscented cotton (23, 24, 35, 43).
A two-way ANOVA (sex of donor × photoperiod of donor) was used to determine if significant differences existed in the amount of time that subjects spent self-grooming in response to each odor stimulus. When significant main effects were detected, Holm-Sidak post hoc tests were used to determine significance differences between the pairwise comparisons. Statistical significance was accepted at α ≤ 0.05.
2.5 Odor Preference Testing
Subjects in the odor preference tests were melatonin-treated LP male voles, melatonin-treated LP voles, blank-treated LP male voles, blank-treated LP female voles, intact SP male voles, and intact SP female voles. Subjects were given a choice between two pairs of anogenital scents from either: a) LP males and LP females, b) SP males and SP females, c) SP females and LP females, or d) SP males and LP males. There were 12 different subjects used for each odor preference test. Each subject underwent a single odor preference test with a unique pair of odor donors. However, the same scent donors were not used in multiple odor tests.
We chose anogenital scent marks because they convey sex-specific information to conspecifics (15, 22), and because the attractiveness to of these scent marks to conspecifics is affected by the length of time that scent donors are treated with exogenous melatonin (16). Collection of anogenital scent marks followed procedures detailed elsewhere (15). Briefly, scent marks from the anogenital area were collected by rubbing a clean glass microscope slide against this area of the donor for 5–10 s. The resulting 1.0 × 0.2 cm streak (scent mark) from each donor was placed randomly on either the right or left-side of the slide. Sixty seconds elapsed between placement of the scent marks from the first and second donor. The slide was suspended by a clasp and wire hanger approximately 1 cm above the substrate and against the wall opposite the nest in the subject’s home cage. Then, during the 3-min test, the total amount of time that subjects investigated the marks from that pair of scent donors was recorded. Subjects had to lick or sniff both scent marks on the slide for the data to be included in the analyses. The observer was blind to the position of the scent donor’s marks on the slide. Each slide was used only once and then discarded.
We used paired t-tests to determine if significant differences existed in the amount of time that subjects spent investigating the scent marks of LP male and LP female donors and time spent investigating the scent marks of SP male and SP female conspecifics. Statistical significance was accepted at α ≤ 0.05
3. Results
3.1 Estradiol and Testosterone titers
Treatment with melatonin affected the circulating titers of testosterone and estradiol in male voles (F2,29 = 153.7, p < 0.001) and female voles (F2,29 = 872.6, p < 0.001), respectively. Briefly, melatonin treatment LP male and LP female voles treated with melatonin capsules for 12 weeks had lower circulating titers of testosterone and estradiol titers than did LP male and female voles treated with an empty capsule for 12 weeks, respectively (Holm-Sidak test, p < 0.05 for each comparison, Fig. 1a, b). The testosterone and estradiol titers of LP male and LP female voles treated with melatonin were similar to those of SP male and SP female voles (p > 0.05 for each comparison, Fig. 1a, b). The average intra-assay and the inter-assay coefficients of variation were 7.3 % and 12.1%, respectively.
Figure 1.
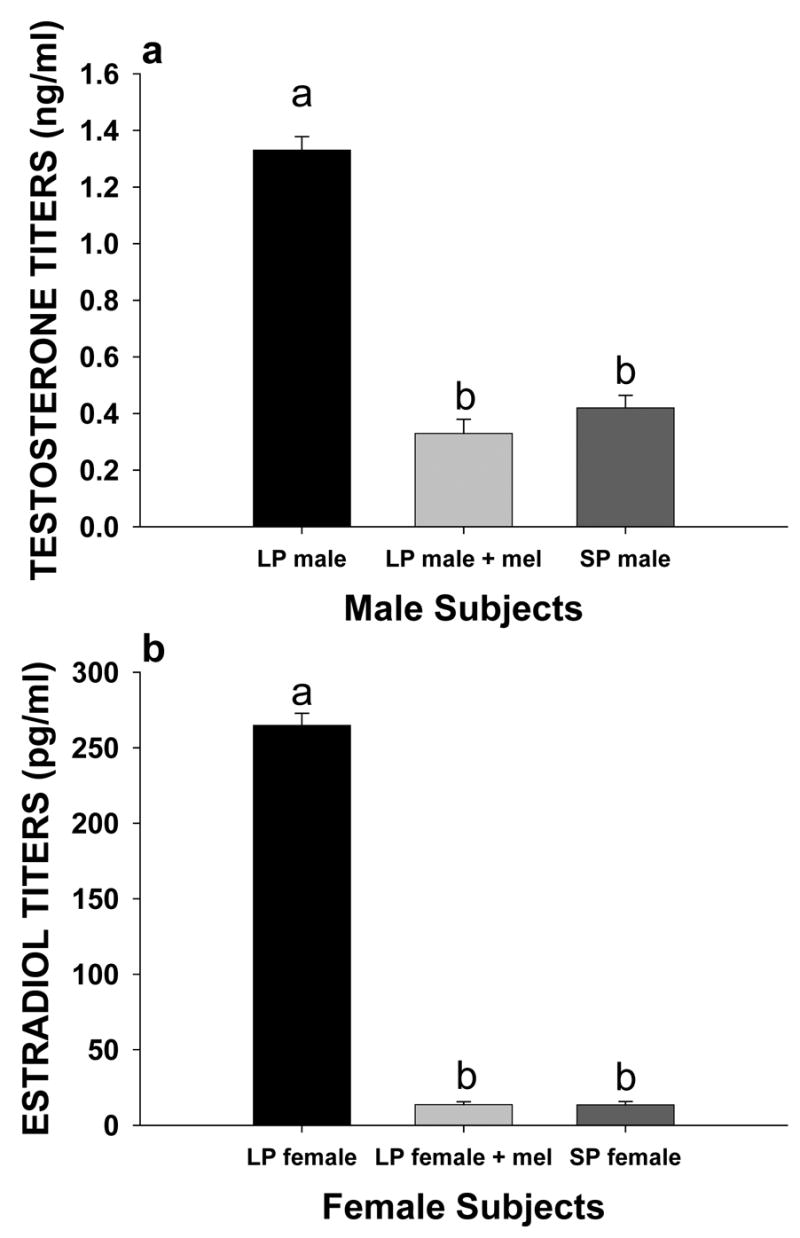
Mean titers ± SEM of circulating (A) testosterone of LP male voles not treated with melatonin, LP male voles treated with melatonin, and SP male voles and (B) estradiol of LP female voles not treated with melatonin, LP female voles treated with melatonin, and SP female voles.
3.2 Self-grooming Tests
The amount of time that male voles and female voles self-groomed in response to the scented bedding of conspecifics was affected by the condition of the scent donor and whether subjects received melatonin capsules. A two-way ANOVA revealed significant main effects for condition of the subject (F 8, 239 = 12.13, P < 0.001) and for condition of the donor (F3, 239 = 5.53, P = 0.001). However, there was also a significant interaction between condition of the subject and condition of the donor (F24, 2 39 = 15.79, P < 0.001), indicating that the effect of the donor’s sex varied across the photoperiod of the donors. To investigate this interaction, data for each of the conditions was analyzed via a one-way ANOVA. The analysis revealed a significant main effect for condition of the subject (F8, 239 = 4.57, P < 0.001) and for condition of the donor (F3, 2 39 = 3.42, P < 0.018). Post hoc comparisons indicated that LP females more time self-grooming in response to the bedding of LP males than to that of LP males, SP males, and SP females (Holm-Sidak, p < 0.05; Fig, 2). In addition, LP females spent more time self-grooming in response to the bedding of LP males than to that of LP females and SP females (p < 0.05; Fig. 2). However, they spent a similar amount of time self-grooming in response to the bedding of SP females than to that of LP females (p > 0.05; Fig. 2). Long-photoperiod males treated with melatonin, LP females treated with melatonin, and SP female spent a similar amount of time self-grooming in response to the odors of LP males, LP females, SP males, and SP females (each comparison, p > 0.05; Fig. 2). Short-photoperiod males spent more time self-grooming in response to odors of SP males and LP females relative to odors of LP males and SP females (each comparison, p < 0.05; Fig. 2).
Figure 2.
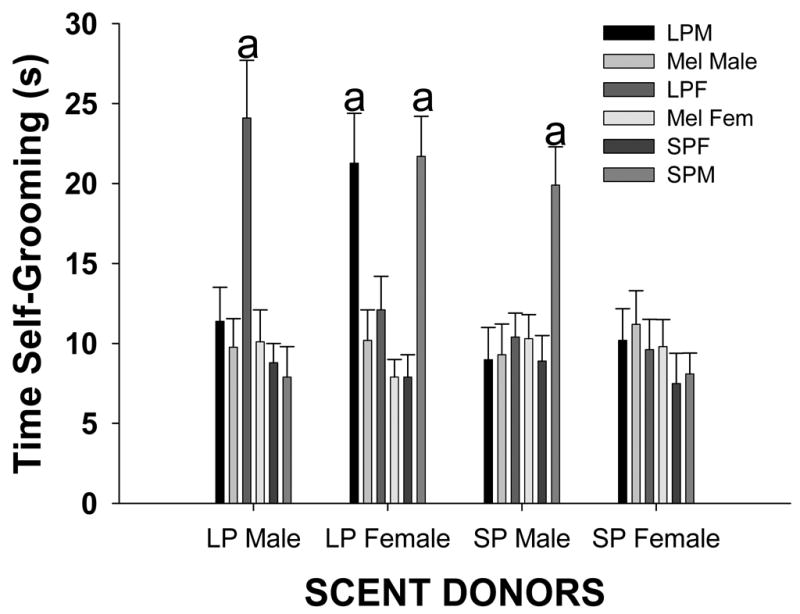
Mean time (s) ± SEM that LP male voles not treated with melatonin, LP male treated with melatonin, LP female voles not treated with melatonin, LP female voles treated with melatonin, SP males, and SP females spent self-grooming when they were exposed to bedding scented by LP males, LP females, SP males, and SP females. A two-way ANOVA was used to test for significant differences in the amount of time self-grooming across each odor condition (α ≤ 0.05). Histograms capped with different letters indicate significant difference between odor conditions (Holm-Sidak test, p <0.05).
3.3 Odor Preference Tests
The amount of time that male voles and female voles spent investigating the scent marks of conspecifics depended on whether the subject received an exogenous melatonin capsule, and on the sex and photoperiod of the scent donor. Specifically, LP males not treated with exogenous melatonin spent more time investigating the odors of LP females than those of LP males (t = 6.12, df = 11, p < 0.001), but spent a similar amount of time investigating the odors of SP males and those of SP females (t = 0.17, df = 11, p = 0.86; Fig. 3a). Untreated LP females spent more time investigating the odors of LP males than those of LP females (t = 5.66, df = 11, p < 0.001), and they also spent a similar amount of time investigating the odors of SP males and those of SP females (t = 0.379, df = 11, p = 0.71; Fig. 3b). In contrast, LP males that received melatonin capsules spent a similar amount of time investigating the odor of opposite and same-sex conspecifics regardless of photoperiod (t = 1.27, df = 11, p = 0.320, for LP donors and t = 0.10, df = 11, p = 0.91 for SP donors; Fig. 3c). Long-photoperiod females that received melatonin capsules spent similar amounts of time investigating the odors of LP opposite and same-sex conspecifics (t = 1.03, df = 11, p = 0.32), but they spent more time investigating the odors of SP females than those of SP males (t = 2.7, df = 11, p = 0.01; Fig. 3d).
Figure 3.
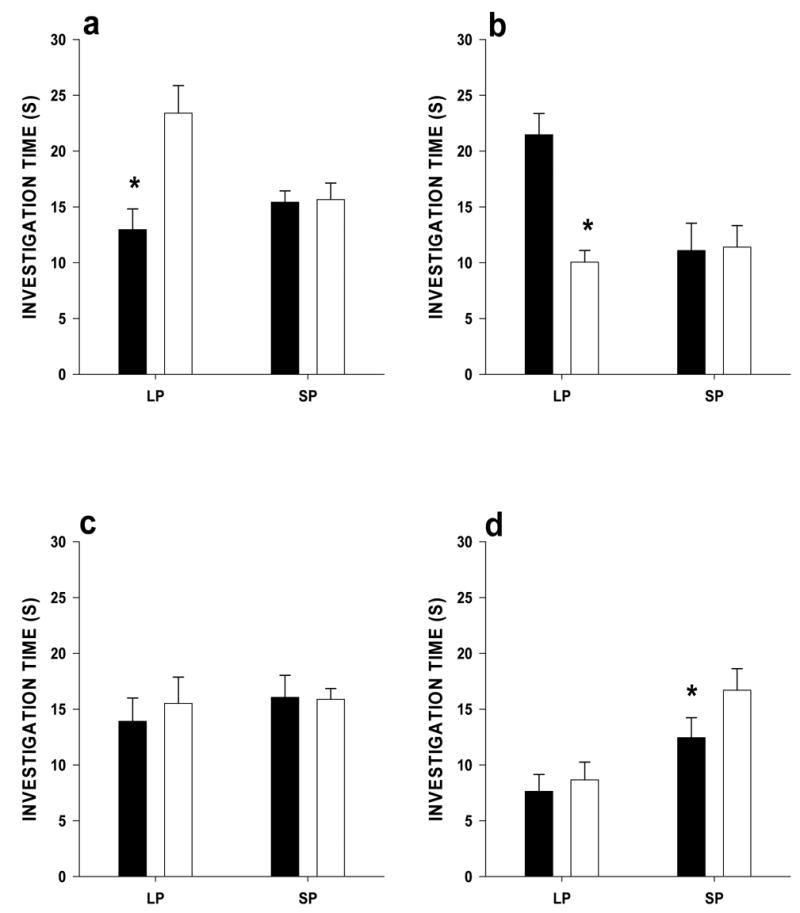
Mean time (s) ± SEM that a) LP male voles not treated with melatonin, b) LP female voles not treated with melatonin, c) LP male voles treated with melatonin, and d) LP female voles treated with melatonin spent investigating the scent marks of LP males versus those of LP females and the scent marks of SP males versus those of SP females. Paired t-tests with a Bonferroni correction was used to test for significant differences in time spent investigating each pair of scent marks. Asterisks indicate significant difference between the scent mark pairs (p <0.0127).
Short-photoperiod males spent similar amounts of time investigating the odors of LP males and those of LP females (t = 0.006, df = 11, p = 0.99), and investigating those of SP males and those of SP females (t = 0.1.05, df = 11, p = 0.31; Fig. 4a). Short-photoperiod females spent similar amounts of time investigating the odors of LP males and those of LP females (t = 0.46, df = 11, p = 0.65), but spent more time investigating the odors of SP females than those of SP males (t = 4.06, df = 11, p = 0.002; Fig. 4b).
Figure 4.
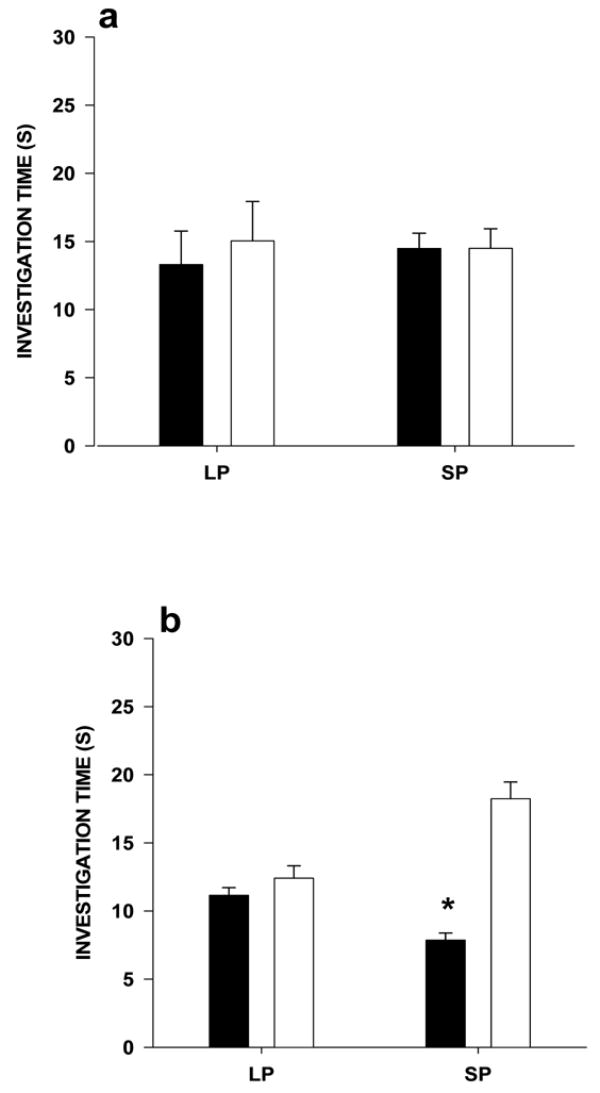
Mean time (s) ± SEM that a) SP male voles not treated with melatonin and b) SP female voles not treated with melatonin spent investigating the scent marks of LP males versus those of LP females and the scent marks of SP males versus those of SP females. Paired t-tests with a Bonferroni correction was used to test for significant differences in time spent investigating each pair of scent marks. Asterisks indicate significant difference between the scent mark pairs (p <0.0127).
4. Discussion
The present study assessed the interactive effects of photoperiod and exogenous melatonin on self-grooming and odor preferences, two behaviors in a suite of those that facilitate sexual interactions between opposite-sex conspecifics (4, 17, 18, 23, 24, 27, 30, 41, 44), in male and female meadow voles. Our results are consistent with the hypothesis that 12 weeks of a constant melatonin signal is sufficient to alter the odor preferences of LP meadow voles such that they no longer reflect those displayed by voles that did not receive exogenous melatonin. That is, LP male and LP female voles implanted with melatonin capsules spent similar amounts of time investigating the odors of LP opposite- and same-sex conspecifics. Thus, exogenous melatonin treatment was sufficient to abolish the opposite-sex odor preferences of LP voles. In contrast, LP voles not treated with melatonin spent more time investigating the scent marks of LP opposite-sex conspecifics as compared to those of LP same-sex conspecifics. These latter findings were in agreement with previous studies. These studies reported that voles not treated with melatonin preferred the odors of LP opposite-sex conspecifics compared to those of LP same-sex conspecifics (18–21).
Our results also demonstrate that 12 weeks of a constant melatonin signal was sufficient to alter the self-grooming behavior of LP male and female meadow voles such they no longer reflect those displayed by LP male and female meadow voles not treated with melatonin. We found that 12 weeks after receiving a melatonin implant, LP meadow voles no longer spent more time self-grooming in response to bedding scented by a LP opposite-sex conspecific as compared to a LP same-sex conspecific. Similarly, gonadectomized LP voles that did not receive replacement gonadal steroids, LP voles that were treated with bromocriptine, and intact SP voles no longer spent more time self-grooming when they were exposed to bedding scented by a LP opposite-sex as compared to a LP same-sex conspecific (34, 35). Previous work has also indicated that treating LP voles with melatonin is sufficient to suppress aspects of the attractivity of LP voles (16). In contrast, LP voles not treated with melatonin spent more time self-grooming in response to scents of LP opposite-sex conspecifics than to scents of LP same-sex conspecifics (this study, 23, 34, 35). Similarly, exogenous melatonin reduced chin marking, a behavior associated with attracting males in rabbits (26). Interestingly, exogenous melatonin reduced or inhibited the sexual behavior and investigation of female vaginal secretions of LP male Syrian hamsters in some studies (25, 31, 32, 39, 40, 45), but exogenous melatonin had no effect on their sexual behavior in other studies (6, 11). For example, exogenous melatonin had no effect on the sexual behavior of LP female Syrian hamsters (2).
We also determined whether exogenous melatonin was sufficient to alter the odor preferences and self-grooming responses of LP male and female meadow voles such that they reflect those displayed by SP male and female meadow voles, respectively. A short-day pattern of melatonin in LP voles was sufficient to induce treated males to no longer display preferences for the odors of LP and SP conspecifics, and for females to prefer the odors of SP females to those of other conspecifics; these odor preferences match those of SP meadow voles (18, 20, 22). In contrast, exogenous melatonin was not sufficient to induce LP-treated males to self-groom in response to odors of LP conspecifics and those of SP conspecifics at rates similar to those of SP male and SP female voles exposed to the odors of LP and SP conspecifics. Short-photoperiod male voles spent more time self-grooming in response to odors of SP males than did LP male voles treated with melatonin. This suggests that some other hormones may, in combination with melatonin, be involved in the seasonal shift in self-grooming behavior in meadow voles, e.g., prolactin and the gonadal steroid hormones (34, 35), however, this speculation requires further testing. Alternatively, it is possible that the self-grooming behavior of melatonin-treated LP male voles differs from that of SP male voles because the duration of the melatonin signal differs. Long-photoperiod male voles received essentially a constant melatonin signal, whereas SP males received a pulsatile melatonin signal that is characteristic of short day length (3, 5).
We found that voles treated with 10-mm implants of melatonin for 12 weeks had lower circulating titers of testosterone and estradiol as compared to voles treated with empty implants for similar amounts of time; the melatonin treated voles had titers of estradiol and testosterone that were similar to those of intact SP voles of similar age. Thus, our data support the predictions of the first hypothesis tested. Our results echo those reported in other studies, showing that a short-day pattern of melatonin induces a decrease in gonadal steroid titers in LP male and LP female voles and deer mice and a suppression of reproductive responsive, which is characteristic of SP voles and deer mice (8, 9, 16). Thus, the current data on meadow voles suggest that melatonin may be involved in the transduction of the photoperiodic effects on circulating gonadal hormone titers, which in turn, mediate odor preferences and self-grooming responses, which may be used by animals to communicate sexual interest and the attractiveness of their odors to conspecifics (16, 18, 20, 33, 34, 35, 39, 41). However, some studies on Syrian and Siberian hamsters report that short-day patterns of melatonin during long-day lengths are either independent of the gonadal hormones, independent of melatonin, or both (1, 5, 7, 49, 54).
It appears that there may not be a general pattern to explain the effects of exogenous melatonin on the signals and behaviors associated with reproduction (e.g., 2, 3, 10; 28, 42, 48, 50–54). In fact, a number of studies have pointed out that melatonin produces bimodal, dose-dependent effects. Administration of low doses of melatonin stimulated sexual behavior in rats (6, 10). Systemic injection of small doses of melatonin (10, 50 and 100 μg/kg) facilitated sexual activity of male rats, whereas a greater dose (1 mg/kg) of melatonin induced sexual inhibition (13), barbiturate-induced narcosis (14), reduced gut motility (12), and antidepressant effects in rats (38). In contrast, the inhibitory effects of melatonin have been reported on the attractivity and proceptive behaviors of female Syrian hamsters and male and female voles (16, 25, 32, 35, 39, 40). Finally, in some animals changes in behavior seem to be independent of melatonin’s affects on gonadal steroid titers. In such cases, melatonin may not necessarily act as an anti-gonadal hormone (7, 49, 54), and may not modulate all seasonally varying signals and behaviors (2, 28, 48). However, it is the duration of the melatonin signal that is important, not the concentration (3). We are not aware of any study showing that the amplitude of the melatonin peak had additional effects over and above what the duration does (i.e., it is all-or-none---if the duration is sufficient, you induce gonadal regression at 10ng/day and 100ng/day has no further effect) (3). In our study, treatment with melatonin capsules for 12 weeks was sufficient to induce LP treated voles to have gonadal hormone titers that were similar to those of SP voles. More importantly, the dose of melatonin used in this study elicited the short-day phenotype with regard to odor preferences and self-grooming. Our data support the speculation that melatonin treatment had inhibitory effects on the gonads and/or its inhibitory action on prolactin (33, 35, 42, 50, 52). However, we urge some caution in this interpretation as our capsules did not simulate the circadian release of melatonin. Implants and timed infusions of melatonin may have different effects on the endogenous pineal melatonin rhythms and subsequent proceptive behavior (42, 51).
Based on our results, we infer that a constant melatonin signal may be sufficient to induce melatonin-treated LP voles to no longer display behaviors that are directed at attracting opposite-sex conspecifics. If this inference is correct, our results may augment those suggesting that melatonin may be involved in the transduction of the photoperiodic effects on circulating gonadal hormone and prolactin titers, which, in turn, mediate odor preferences, which may be used by animals to communicate sexual interest and the attractiveness of their odors to conspecifics (16,18, 21, 33, 39, 40). Thus, it may be likely that for meadow voles, and for perhaps for some other seasonal breeding mammals, seasonal differences in behavior may allow individuals to coordinate sexual behavior, and maximize their reproductive success by mating at a time of year that is propitious for their survival and that of their offspring (1, 5, 17, 18, 26, 47, 53).
Acknowledgments
We thank J. delBarco-Trillo, D. Freeman, L. LaDage, and A. Pierce for commenting on earlier drafts of this manuscript. The work described in this study was supported by NSF grants IBN 9421529, and IOB 0444553 and NIH grants AG 16594 and HD O49525 to MHF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arendt J. Role of the pineal gland and melatonin in seasonal reproductive function in mammals. In: Clarke JR, editor. Oxford Review of Reproductive Biology. New York: Oxford University Press; 1986. pp. 267–320. [PubMed] [Google Scholar]
- 2.Badura LL, Nunez AA. Photoperiodic modulation of sexual and aggressive behavior in female golden hamsters (Mesocricetus auratus): role of the pineal gland. Horm Behav. 1989;23:27–42. doi: 10.1016/0018-506x(89)90072-x. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Goldman BD. Peak duration of serum melatonin and short-day responses in adult Siberian hamsters. Am J Physiol. 1988;255:R812–222. doi: 10.1152/ajpregu.1988.255.5.R812. [DOI] [PubMed] [Google Scholar]
- 4.Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 5.Bittman EL. Melatonin and photoperiodic time measurement: evidence from rodents and ruminants. In: Reiter RJ, editor. The Pineal Gland. New York: Raven Press; 1984. pp. 155–192. [Google Scholar]
- 6.Brotto LA, Gorzalka BB. Melatonin enhances sexual behavior in the male rat. Physiol Behav. 2000;68:483–486. doi: 10.1016/s0031-9384(99)00204-8. [DOI] [PubMed] [Google Scholar]
- 7.Carter D, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- 8.Demas GE, Nelson RJ. Exogenous melatonin enhances cell-mediated, but not humoral, immune function in adult male deer mice (Peromsycus maniculatus) J Biol Rhythms. 1998;13:242–252. doi: 10.1177/074873098129000084. [DOI] [PubMed] [Google Scholar]
- 9.Demas GE, Klein SL, Nelson RJ. Reproductive and immune responses to photoperiod and melatonin are linked in Peromyscus maniculatus. J Comp Physiol (A) 1996;179:819–825. doi: 10.1007/BF00207360. [DOI] [PubMed] [Google Scholar]
- 10.Donham RS, Horton TH, Rollag MD, Stetson MH. Age, photoperiodic responses, and pineal function in meadow voles, Microtus pennsylvanicus. J Pineal Res. 1989;7:243–252. doi: 10.1111/j.1600-079x.1989.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 11.Drago F, Busa L. Acute low doses of melatonin restore full sexual activity in impotent male rats. Brain Res. 2000;878:98–104. doi: 10.1016/s0006-8993(00)02715-3. [DOI] [PubMed] [Google Scholar]
- 12.Drago F, Macauda S, Salehi S. Small doses of melatonin increases intestinal motility in rats. Digest Diseases Sci. 2002;47:1969–1974. doi: 10.1023/a:1019696006677. [DOI] [PubMed] [Google Scholar]
- 13.Drago F, Busa L, Benella A, Bertolini A. Acute low doses of melatonin stimulate rat sex behavior: the role of serotonin neurotransmission. Eur J Pharmacol. 1999;385:1–6. doi: 10.1016/s0014-2999(99)00701-3. [DOI] [PubMed] [Google Scholar]
- 14.Drago F, Frisina M, Grech M, Nicolosi A, Micale V, Nicosia A, Medico M, Foti F. Dual effects of melatonin on barbiturate-induced narcosis in rats. J Neurosci Lett. 2001;16:176–178. doi: 10.1016/s0304-3940(01)01578-6. [DOI] [PubMed] [Google Scholar]
- 15.Ferkin MH, Johnston RE. Meadow voles, Microtus pennsylvanicus, use multiple sources of scent for sex recognition. Anim Behav. 1995;49:37–44. [Google Scholar]
- 16.Ferkin MH, Kile JR. Melatonin treatment affects the attractiveness of the anogenital area scent in meadow voles (Microtus pennsylvanicus) Horm Behav. 1996;30:227–235. doi: 10.1006/hbeh.1996.0027. [DOI] [PubMed] [Google Scholar]
- 17.Ferkin MH, Leonard ST. Self-grooming by rodents in social and sexual contexts. Acta Zool Sinica. 2005;51:772–779. [Google Scholar]
- 18.Ferkin MH, Seamon JO. Odor preferences and social behavior in meadow voles, Microtus pennsylvanicus: seasonal differences. Can J Zool. 1987;65:2931–2937. [Google Scholar]
- 19.Ferkin MH, Zucker I. Seasonal control of odour preferences of meadow voles (Microtus pennsylvanicus) by photoperiod and ovarian hormones. J Reprod Fert. 1991;92:433–441. doi: 10.1530/jrf.0.0920433. [DOI] [PubMed] [Google Scholar]
- 20.Ferkin MH, Gorman MR, Zucker I. Ovarian hormones influence odor cues emitted by female meadow voles, Microtus pennsylvanicus. Horm Behav. 1991;25:572–581. doi: 10.1016/0018-506x(91)90022-a. [DOI] [PubMed] [Google Scholar]
- 21.Ferkin MH, Gorman MR, Zucker I. Gonadal hormones influence odor cues emitted by male meadow voles, Microtus pennsylvanicus. J Reprod Fert. 1992;95:729–736. doi: 10.1530/jrf.0.0950729. [DOI] [PubMed] [Google Scholar]
- 22.Ferkin MH, Sorokin ES, Johnston RE. Seasonal changes in scents and responses to them in meadow voles: evidence for the co-evolution of signals and response mechanisms. Ethology. 1995;100:89–98. [Google Scholar]
- 23.Ferkin MH, Sorokin ES, Johnston RE. Self-grooming as a sexually dimorphic communicative behaviour in meadow voles, Microtus pennsylvanicus. Anim Behav. 1996;51:801–810. [Google Scholar]
- 24.Ferkin MH, Leonard ST, Heath LA, Paz-y-Miño C. Self-grooming as a tactic used by prairie voles, Microtus ochrogaster, to enhance sexual communication. Ethology. 2001;107:939–949. [Google Scholar]
- 25.Fleming AS, Phillips A, Rydall A, Levesque L. Effects of photoperiod, the pineal gland and the gonads on agonistic behavior in female golden hamsters (Mesocricetus auratus) Physiol Behav. 1988;44:227–234. doi: 10.1016/0031-9384(88)90143-6. [DOI] [PubMed] [Google Scholar]
- 26.Goldman BD, Nelson RJ. Melatonin and seasonality in mammals. In: Yu H-S, Reiter RJ, editors. Melatonin Biosynthesis, Physiological Effects, and Clinical Applications. Boca Raton, Florida: CRC Press; 1993. pp. 226–252. [Google Scholar]
- 27.Harriman AE, Thiessen DD. Harderian letdown in male Mongolian gerbils (Meriones unguiculatus) contributes to proceptive behavior. Horm Behav. 1985;19:213–219. doi: 10.1016/0018-506x(85)90020-0. [DOI] [PubMed] [Google Scholar]
- 28.Hudson R, Melo AI, Gonzalez-Mariscal G. Effect of photoperiod and exogenous melatonin on correlates of estrus in the domestic rabbit. J Comp Physiol. 1994;A 175:573–579. doi: 10.1007/BF00199478. 1994. [DOI] [PubMed] [Google Scholar]
- 29.Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short days and exogenous melatonin increase aggression of male Syrian hamsters (Mesocricetus auratus) Horm Behav. 2002;42:13–20. doi: 10.1006/hbeh.2002.1797. [DOI] [PubMed] [Google Scholar]
- 30.Johnston RE. Olfactory preferences, scent marking, and “proceptivity” in female hamsters. Horm Behav. 1979;13:21–39. doi: 10.1016/0018-506x(79)90032-1. [DOI] [PubMed] [Google Scholar]
- 31.Karp JD, Powers JB. Photoperiodic and pineal influences on estrogen-stimulated behaviors in female Syrian hamsters. Physiol Behav. 1993;54:19–28. doi: 10.1016/0031-9384(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 32.Karp JD, Dixon ME, Powers JB. Photoperiod history, melatonin, and reproductive responses of male Syrian hamsters. J Pineal Res. 1990;8:137–152. doi: 10.1111/j.1600-079x.1990.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 33.Leonard ST, Ferkin MH. Prolactin and testosterone affect seasonal differences in male meadow vole, Microtus pennsylvanicus, odor preferences for female conspecifics. Physiol Behav. 1999;68:139–143. doi: 10.1016/s0031-9384(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 34.Leonard ST, Ferkin MH. Seasonal differences in self-grooming in meadow voles, Microtus pennsylvanicus. Acta Ethologica. 2005;8:86–91. doi: 10.1016/j.physbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Leonard ST, Naderi R, Stokes K, Ferkin MH. The role of prolactin and testosterone in mediating seasonal differences in the self-grooming behavior of male meadow voles, Microtus pennsylvanicus. Physiol Behav. 2005;85:461–468. doi: 10.1016/j.physbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Madison DM, McShea WJ. Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles: a microtine model. Amer Zool. 1987;22:899–908. [Google Scholar]
- 37.Meek LR, Lee TM. Prediction of fertility by mating latency and photoperiod in nulliparous and primiparous meadow voles (Microtus pennsylvanicus) J Reprod Fert. 1993;97:353–357. doi: 10.1530/jrf.0.0970353. [DOI] [PubMed] [Google Scholar]
- 38.Micale V, Arezzi A, Drago F. Melatonin affects the immobility time of rats in the forced swim test: the role of serotonin neurotransmission. Eur Neuropsychopharmacol. 2006;16:538–545. doi: 10.1016/j.euroneuro.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Miernicki M, Karp JD, Powers JB. Pinealectomy prevents short photoperiod inhibition of male hamster sexual behavior. Physiol Behav. 1990;47:293–299. doi: 10.1016/0031-9384(90)90145-t. [DOI] [PubMed] [Google Scholar]
- 40.Miernicki M, Pospichal MW, Powers JB. Short photoperiods affect male hamster sociosexual behaviors in the presence and absence of testosterone. Physiol Behav. 1990;47:95–106. doi: 10.1016/0031-9384(90)90046-7. [DOI] [PubMed] [Google Scholar]
- 41.Moffatt CA, Nelson RJ. Day length influences proceptive behavior of female prairie voles (Microtus ochrogaster) Physiol Behav. 1994;55:1163–1165. doi: 10.1016/0031-9384(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 42.Nagy TR, Gower BA, Stetson MH. Response of collared lemmings to melatonin: I. Implants and photoperiod. J Pineal Res. 1994;17:177–184. doi: 10.1111/j.1600-079x.1994.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 43.Paz-y-Miño C, Leonard ST, Ferkin MH, Trimble JF. Self-grooming and sibling recognition in meadow voles (Microtus pennsylvanicus) and prairie voles (M. ochrogaster) Anim Behav. 2002;63:331–338. [Google Scholar]
- 44.Pierce AA, Ferkin MH, Williams TK. Food-deprivation-induced changes in sexual behavior of meadow voles, Microtus pennsylvanicus. Anim Behav. 2005;70:339–348. [Google Scholar]
- 45.Powers JB, Bergondy ML, Matochik MA. Male hamster sociosexual behaviors: effects of testosterone and its metabolites. Physiol Behav. 1985;35:607–616. doi: 10.1016/0031-9384(85)90149-0. [DOI] [PubMed] [Google Scholar]
- 46.Prendergast BJ, Kriegsfeld LJ, Nelson RJ. Photoperiodic polyphenism in rodents: neuroendocrine mechanisms, costs, and functions. Quart Rev Biol. 2001;76:293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- 47.Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pffaf D, editor. Hormones, Brain, and Behavior. New York: Elsevier Science; 2002. pp. 93–156. [Google Scholar]
- 48.Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. 1980. [DOI] [PubMed] [Google Scholar]
- 49.Reiter RJ, Vaughan MK, Waring PJ. Studies on the minimal dosage of melatonin required to inhibit pineal antigonadotrophic activity in male golden hamsters. Horm Research. 1975;6:258–267. doi: 10.1159/000178699. [DOI] [PubMed] [Google Scholar]
- 50.Smale L, Dark J, Zucker I. Pineal and photoperiodic influences on fat deposition, pelage, and testicular activity in male meadow voles. J Biol Rhythms. 1988;3:349–355. doi: 10.1177/074873048800300404. [DOI] [PubMed] [Google Scholar]
- 51.Stetson MH, Rollag MD, Watson-Whitmyre M, Tate-Ostroff B. The effect of daily injections and constant release implants of melatonin on the endogenous pineal melatonin rhythm in golden hamsters. Proc Soc Exp Biol Med. 1983;174:119–122. doi: 10.3181/00379727-174-41713. [DOI] [PubMed] [Google Scholar]
- 52.Stirland JA, Johnston JD, Cagampang FR, Morgan PJ, Castro MG, White MR, Davis JR, Loudon AS. Photoperiodic regulation of prolactin gene expression in the Syrian hamster by a pars tuberalis-derived factor. J Neuroendocrinology. 2001;13:147–157. doi: 10.1046/j.1365-2826.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- 53.Tamarkin L, Baird CJ, Almeida OFX. Melatonin a coordinating signal for mammalian reproduction? Science. 1985;227:714–720. doi: 10.1126/science.3881822. [DOI] [PubMed] [Google Scholar]
- 54.Turek FW, Desjardins C, Menaker M. Melatonin: antigonadal and progonadal effects in male hamsters. Science. 1975;190:280–282. doi: 10.1126/science.1179207. [DOI] [PubMed] [Google Scholar]


