Abstract
Neurokinins released by capsaicin-responsive (C-R) dorsal root ganglia neurons (DRG) may control firing in these neurons by an autofeedback mechanism. Here we used patch clamp techniques to examine the effects of neurokinins on firing properties of dissociated DRG neurons of male rats. In C-R neurons that generated only a few action potentials (APs, termed phasic) in response to long depolarizing current pulses (600 ms), substance P (SP, 0.5 μM) lowered the AP threshold by 11.0±0.3 mV and increased firing from 1.1±0.7 APs to 5.2±0.6 APs. In C-R tonic neurons that fire multiple APs, SP elicited smaller changes in AP threshold (6.0±0.1 mV reduction) and the number of APs (11±1 vs. 9±1 in control). The effects of SP were similar to the effect of heteropodatoxin II (0.05 μM) or low concentrations of 4-aminopyridine (50 μM) that block A-type K+ currents. A selective NK2 agonist, [βAla8]-neurokinin A (4–10) (0.5 μM), mimicked the effects of SP. The effects of SP in C-R phasic neurons were fully reversed by an NK2 receptor antagonist (MEN10376, 0.5 μM) but only partially by a protein kinase C (PKC) inhibitor (bisindolylmaleimide, 0.5 μM). An NK3 selective agonist ([MePhe7]-neurokinin B, 0.5 μM), an NK1 selective agonist ([Sar9, Met11]-substance P, 0.5 μM) or activation of PKC with phorbol 12, 13-dibutyrate (0.5 μM) did not change firing. Our data suggest that the excitability of C-R phasic afferent neurons is increased by activation of NK2 receptors and intracellular signaling mediated only in part by PKC.
Keywords: dorsal root ganglia, nociception, hyperexcitability, K+ channels, neurokinins, autofeedback
Introduction
Substance P and neurokinin A (NKA) are synthesized in dorsal root ganglia (DRG) neurons and released from afferent terminals in the spinal cord and in the target organs (skin, visceral organs, Maggi, 1997b). Since DRG neurons express functional neurokinin receptor types 1, 2 and 3 (Brechenmacher et al., 1998, Sculptoreanu and de Groat, 2003), it has been proposed that neurokinins may act in an autofeedback manner to regulate afferent terminal excitability (Morrison et al., 1999; Sculptoreanu and de Groat, 2003; Sculptoreanu et al., 2004). Saban et al., (1997) and Morrison et al., (1999) have presented evidence for such an action at afferent terminals in the urinary bladder of the rat.
Substance P has also been implicated as a neurotransmitter mediating bladder hyperactivity induced by chemical irritation (Maggi, 1997b). Neurokinin receptor antagonists administered peripherally or intrathecally suppress the increased frequency of voiding induced by instillation of acetic acid into the bladder (Andersson, 1997) or bacterial toxin-induced bladder hyperactivity (Lecci et al., 1998). Furthermore disruption of the preprotachychynin gene which encodes for substance P, leads to an impaired response to chemical irritation of the urinary tract in mice (Kiss et al., 2001). Neurokinin A and substance P also have an excitatory effect on autonomic ganglia in the bladder (Kawatani et al., 1989; Shinkai et al., 1993) and on bladder smooth muscle (Maggi, 1997b). Thus Substance P or a related neurokinin may be an excitatory transmitter in bladder afferent pathways in the peripheral and central nervous system.
Patch clamp studies demonstrated two main types of bladder afferent neurons (Yoshimura et al., 1994, 1996, 1999). One group consisted of capsaicin-sensitive, neurofilament negative neurons exhibiting primarily high threshold, tetrodotoxin-resistant (TTX-R) Na+ channel currents and action potentials (AP), phasic firing (ie., one or two action potentials in response to prolonged depolarizing current pulses) and low threshold inactivating K+ currents (A-type currents). The second group consisted of capsaicin-resistant, neurofilament positive neurons with TTX-sensitive Na+ currents and APs and tonic firing to depolarizing current pulses. Substance P increases excitability in DRG neurons and converts phasic to tonic firing (Abdulla et al., 2001, Sculptoreanu et al, 2004) and also increases voltage–dependent Ca2+ currents by activating NK2 receptors (Sculptoreanu et al., 2003). The effect on Ca2+ currents was mediated by a protein kinase C signaling pathway. However, the receptors and signaling mechanism underlying the effect of SP on firing in capsaicin-sensitive DRG neurons has not been studied in detail.
Acute or chronic chemical irritation of the urinary bladder is associated with an increased excitability of capsaicin-sensitive bladder DRG neurons (Kiss et al., 2001; Shea et al., 2000; Yoshimura et al., 2001). Patch clamp studies of capsaicin-sensitive bladder DRG neurons have revealed that chronic bladder irritation reduces the low threshold A-type K+ currents, reduces the threshold for initiating action potentials and induces tonic firing (Yoshimura et al., 1999). Other investigators have reported similar changes in K+ currents in visceral afferents following chemical irritation of the colon or stomach (Dang et al., 2004) and by administration of a 4-aminopyridine (4-AP) a K+ channel blocking agent or substance P (Sculptoreanu et al., 2004). Conversely, KW-7158, a K+ channel opener that activates a 4-AP sensitive K+ current, antagonizes the increase in firing triggered by substance P in phasic, capsaicin-responsive DRG neurons (Sculptoreanu et al., 2004). Systemic administration of KW-7158 also reduces the bladder hyperactivity induced by chemical irritation of the bladder (Lu et al., 2002).
In the present experiments, we examined the mechanisms and neurokinin receptors responsible for the change in excitability in dissociated lumbo-sacral dorsal root ganglion cells from adult rats using whole cell patch clamp techniques.
Methods
Preparation of dissociated neurons
Neurons were isolated from L4-S3 DRG of adult rats (200–250 g) using methods previously described (Sculptoreanu and de Groat, 2003). Briefly, freshly dissected ganglia were minced and washed in cold, oxygenated DMEM (Sigma). This was followed by a brief, 10 min dissociation at 37°C in DMEM containing 0.5 mg/ml trypsin (Sigma). At the end of this step, DMEM containing 1 mg/ml collagenase B (Boehringer-Mannheim) and 0.5 mg/ml trypsin inhibitor type 1S (Sigma) were added and the dissociation was continued for another 25–60 min. During dissociation the neurons were gently triturated with siliconized Pasteur pipettes every 20 min. After the ganglia were dissociated into individual neurons the cell suspension was layered on 25 ml of 50% adult bovine serum (Sigma) and DMEM in centrifuge tubes and centrifuged again at 800 rpm. This step removed most of the debris and broken cells. The pellet was resuspended in DMEM containing 10% heat inactivated horse serum and 5% fetal bovine serum (Sigma), and plated on collagen coated 35 mm petridishes (Collaborative Research, Biocoat). Neurons were plated at low density (2000–3000 per dish). Primary cultures were kept in a 95% air, 5% CO2 incubator at 37°C.
Patch clamp
Gigaohmseal whole-cell recordings of capsaicin (CAPS) currents, and evoked action potentials were recorded in DRG neurons 3–5 days in culture using whole cell patch clamp techniques. Immediately before recording, the serum containing media was replaced with Dulbecco’s phosphate buffered saline (Invitrogen) of the following composition (in mM): 138 NaCl, 2.6 KCl, 0.9 CaCl2, 0.5 MgCl2, 1.5 KH2PO4, 8.1 Na2HPO4, pH 7.2. The long and short axes of the neurons were measured in some experiments with an eyepiece micrometer. In these neurons the membrane capacitance (pF) varied linearly as a function of average cell diameter (μM). CAPS currents were recorded in voltage clamp as previously described (Sculptoreanu et al., 2005b). Action potentials in response to current injections were recorded using an Axopatch 200A (Axon Instruments, Foster City, California) amplifier. Pulse generation, membrane potential recording and data analysis used pClamp software (Axon Instruments). Voltage changes were sampled at 50–500 μs intervals, and filtered at 2 kHz. Action potentials were generated by rectangular current pulse injections 5 ms long and 50–500 pA in intensity, followed by a 100 ms interpulse at the holding potential and a second pulse, 600 ms long. In general, the sequence consisted of at least two control recordings of activation and inactivation and evoked APs followed by pharmacological studies in which drugs were tested on the firing. The effects of drugs reached steady state within <3 minutes of application and were stable throughout the duration of the experiments (15–50 min.). Various AP parameters were measured including threshold, overshoot, duration of APs and number of APs during the 600 ms depolarizing current pulse. Recording was done at room temperature in static chambers fitted with a rapid mixing setup for addition of drugs.
The pipette (intracellular) solution contained (mM): KCl 120, K2HPO4 10, NaCl 10, MgCl2 2, EGTA 1, HEPES 10, pH adjusted to 7.4 with HCl. To this solution Mg-ATP (3 mM), cyclic AMP (0.3 mM) and tris-GTP (0.5 mM) were added just prior to the experiments. Neurokinin agonists (Regoli et al., 1994) substance P, NK2 selective agonist [βAla8]-neurokinin A (4–10) (Calbiochem), and selective NK3 agonist [MePhe7]-neurokinin B (Calbiochem), NK1 agonist [Sar9, Met11]-substance P (Calbiochem), NK2 antagonist (MEN 10,376, Sigma), NK3 antagonist (SB 235,375, gift from SmithKline Beecham) and the Kv4 channel blocker heteropodatoxin II (Alomone Labs) were prepared in external solutions. Capsaicin (Calbiochem), phorbol 12, 13-dibutyrate (Research Biochemicals), and the PKC inhibitor bisindolylmaleimide I HCl (Calbiochem) were dissolved in DMSO (100 mM) and used at less than 0.01% of their stock concentration. At these dilutions, DMSO alone had no effect on currents. Stock solutions in 10–100 mM were stored at −20° C and diluted in the external recording solution just before experiments. Extracellularly applied drugs were pipetted from stock solutions at 50 times the final concentration and rapidly mixed in the recording chamber as described previously (Sculptoreanu et al. 1995). Steady-state effects of each drug concentration were measured for at least 2–3 minutes before changing the drug concentration or adding a new drug. Results are reported as mean ± SEM. Statistical analysis used t-test, 2 tail, and unequal variance. Data are considered to be statistically different if P<0.05.
Results
Properties of phasic and tonic neurons. The cultured dorsal root ganglion neurons isolated from lumbo-sacral ganglia ranged in size from 18–50 μm diameter (average of long and short axes) and had capacitances of 20–90 pF. The cells could be subgrouped based on firing patterns (phasic and tonic) and capsaicin responsiveness (Tables 1 and 2). At a near maximal stimulus intensity (0.45 nA) tonic cells (n=24) fired 5–12 action potentials (APs, mean, 10.4±1.2 APs/600ms) in response to depolarizing current injections 600 ms in duration (Fig. 1D, Table 2). Phasic firing cells (n=106) were smaller (44±2 pF) than tonic firing cells (70±8 pF) and characteristically fired at most 4 APs (mean, 1.5±0.3 APs/600 ms, at 0.45 nA stimulus intensities) in response to depolarizing current injections 600 ms in duration (Fig. 1A, and Fig. 2A, C, Table 1), but were otherwise comparable with the tonic cells with respect to resting potentials and membrane resistances (Tables 1 and 2).
Table 1.
Effect of substance P (SP) and [βAla8]-neurokinin A (4–10) (NKA) on passive membrane properties and firing parameters in phasic firing neurons.
| Phasic cells | control (n=30) | SP (n=30) | Percent change | Control (n=9) | NKA (n=9) | Percent change |
|---|---|---|---|---|---|---|
| Cm (pF) | 44±2 | 42±3 | ||||
| RP (mV) | −53±1 | −57±1 (P<0,001) | +7 | −55±1 | −58±1 | +7 |
| Rm (MΩ) | 198±14 | 266±15 (P<0.05) | +34 | 176±14 | 268±13 (P<0.001) | +52 |
| AP Threshold (mV) | −23±1 | −34±1 (P<0.001) | +53 | −22±1 | −33±1 (P<0.001) | +48 |
| dV/dtmax (V/s) | 27±1 | 33±2 (P<0.05) | +22 | 25±1 | 30±2 (P<0.05) | +18 |
| OS (mV) | 23±1 | 28±2 (P<0.05) | +20 | 18±2 | 24±2 (P<0.05) | +30 |
| AP50(ms) | 3.7±0.2 | 4.1±0.2 (ns) | +10 | 3.4±0.2 | 3.7±0.3 (ns) | +8 |
| rise time (ms) | 1.2±0.1 | 1.11±0.04 (ns) | −7 | 1.07±0.07 | 1.02±0.04 (ns) | −5 |
| decay time (ms) | 3.0±0.2 | 3.4±0.2 (ns) | +13 | 2.8±0.2 | 3.5±0.3 (ns) | +24 |
| tauAHP (ms) | 36±2 | 90±7 (P<0.001) | 149 | 44±3 | 130±6 (P<0.001) | 194 |
Cm, membrane capacitance; RP, resting potential; Rm, membrane resistance. The action potential (AP) parameters were determined for single spike responses, just above threshold, to current injections 5 ms in duration and of 50 to 200 pA intensities: dV/dtmax, maximum rate of rise; OS, overshoot; AP50, duration of AP at 50% maximum amplitude; tauAHP is the time constant of hyperpolarizing afterpotential decay. Averages; standard error of the mean (SEM); t-test relative to values before application of drugs (control), 2 tailed, unequal variance, level of significance: ns-not significant, p < 0.05, or p < 0.001.
Table 2.
Effect of substance P (SP) on passive membrane properties and firing parameters in tonic firing neurons.
| Tonic cells | control | SP (n=19) | Percent change |
|---|---|---|---|
| Cm (pF) | 70±8 | ||
| RP (mV) | −53±1 | −58±1 (P<0.05) | +7 |
| Rm (MΩ) | 200±11 | 242±11 (P<0.05) | +17 |
| AP Threshold (mV) | −34±1 | −40±1 (P<0.05) | +14 |
| dV/dtmax (V/s) | 35±2 | 38±2 (P<0.05) | +9 |
| OS (mV) | 30±1 | 32±1 (ns) | +4 |
| AP50 (ms) | 3.5±0.3 | 3.8±0.3 (ns) | +8 |
| rise time (ms) | 1.1 ±0.4 | 1.06±0.05 (ns) | −4 |
| decay time (ms) | 3.0±0.2 | 3.3±0.3 (ns) | +9 |
| tauAHP (ms) | 59±4 | 122±13 (P<0.001) | 52 |
Cm, membrane capacitance; RP, resting potential; Rm, membrane resistance. The action potential (AP) parameters were determined for single spike responses, just above threshold, to current injections 5 ms in duration and of 50 to 200 pA intensities: dV/dtmax, maximum rate of rise; OS, overshoot; AP50, duration of AP at 50% maximum amplitude; tauAHP is the time constant of hyperpolarizing afterpotential decay. Averages; standard error of the mean (SEM); t-test relative to values before application of drugs (control), 2 tailed, unequal variance, level of significance: ns-not significant, p < 0.05, or p < 0.001.
Fig 1.
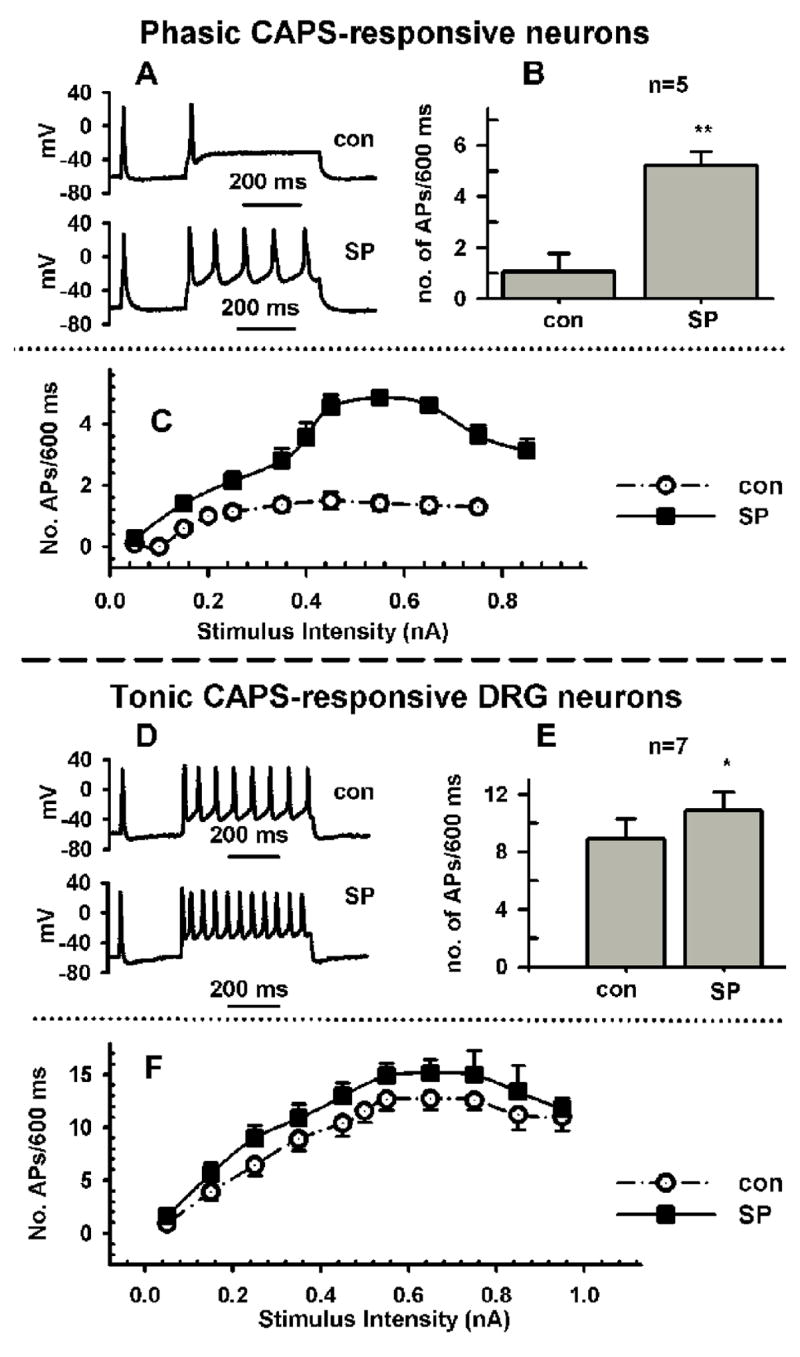
Effect of substance P (SP, A–F) in phasically firing neurons (A–C) and tonically firing (D–F) adult rat DRG neurons. A, SP (0.5 μM) increases firing in response to a current injection 600 ms long and 250 pA in intensity in a phasic neuron from 1 action potential (AP, control trace, con) to 5 AP. B, Average increase in APs in response to SP in 5 neurons. C, Dependence of firing on stimulus intensity for experiment in B, before (Control, empty circles) and after SP (0.5 μM, filled squares). D, SP (0.5 μM) increases firing in response to a current injection 600 ms long and 250 pA in intensity in a tonic firing neuron from 8 APs (control trace, con) to 11 APs. E, Average increase in APs in response to SP in 7 neurons. C, Dependence of firing on stimulus intensity for experiment in E, before (Control, empty circles) and after SP (0.5 μM, filled squares).
Fig 2.
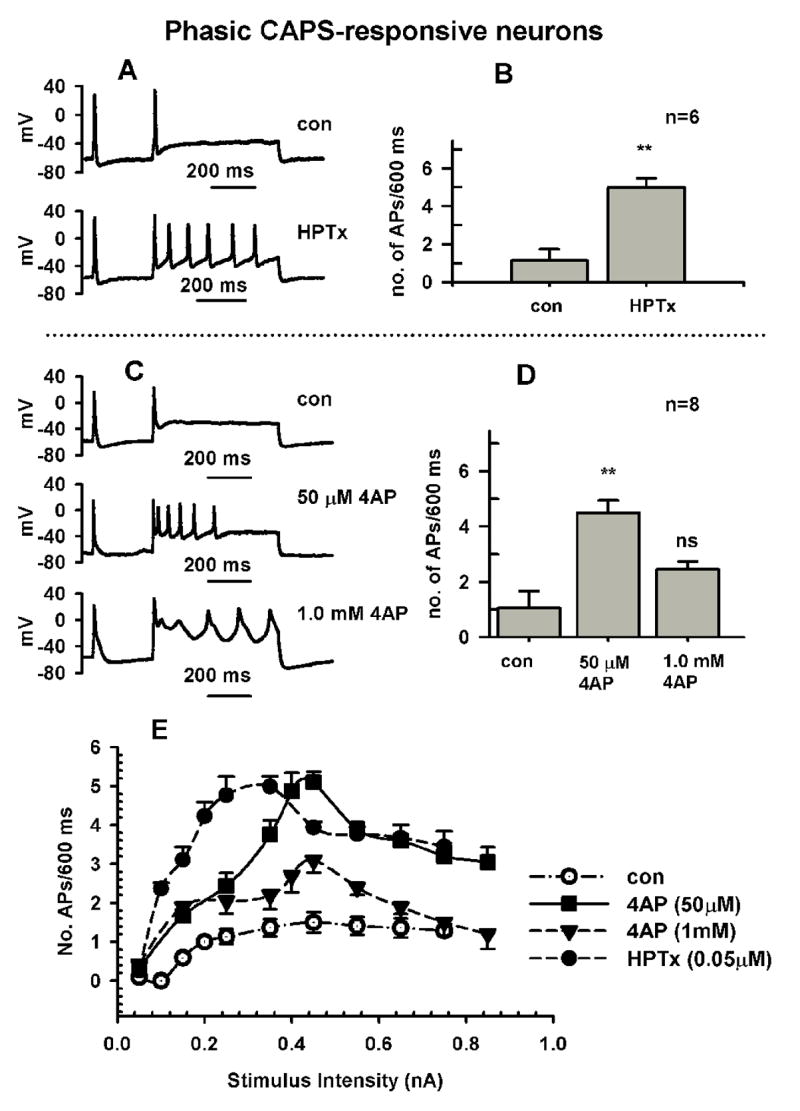
Effect of A-type K+ channel blockers in phasically firing neurons (A–E) adult rat DRG neurons. A, The Kv4 K+ channel blocker heteropodatoxin (HPTx, 0.05 μM) increases firing in response to a current injection 600 ms long and 250 pA in intensity in a phasic neuron. B, Average increase in firing for experiment as shown in A in 6 neurons. C, The A-type K+ channel blocker 4-aminiopyridine (4AP, 50 μM, middle trace) increases firing in response to a current injection 600 ms long and 250 pA in intensity in a phasic neuron. Further increasing 4AP to 1 mM (lower trace), prolongs the action potentials and inhibits the increase in firing in response to lower concentrations of the drug, D, Average increase in firing for experiment as shown in C in 8 neurons. Dependence of firing on stimulus intensity for experiment in C and D before (Control, empty circles) and after HPTx (0.05 μM, filled circles), and 4AP (50 μM, filled squares, 1 mM, filled triangles).
Capsaicin applied in a concentration (0.5 μM) that is near the ED50 of the concentration response curve, induced inward currents (0.05–5 nA) in 62 of 106 phasic neurons tested and 16 of 24 tonic neurons. Phasic firing, CAPS-responsive (C-R) neurons (n=62) fired up to 4 APs at increasing stimulus intensities; whereas the majority of phasic firing CAPS-unresponsive (C-U) cells (n=44) fired only one AP regardless of stimulus intensity (50–800 pA). The C-U phasic neurons tended to be smaller in size (41.7±0.3 pF) than the C-R phasic firing neurons (50±2 pF, P<0.001). C-U tonic firing DRG neurons were larger (72±6 pF, n=8) than the C-R tonic neurons (57±5 pF, n=16) and exhibited a slightly larger maximum number of APs per 600 ms depolarizing current pulse (>10 APs vs. 5–8 APs).
Comparison of effects of neurokinins and K+ channel blockers on passive properties and firing in CAPS-responsive and CAPS-unresponsive DRG neurons. Neurokinins were tested in a concentration (0.5 μM) that was shown previously to produce a nearly maximal activation of NK2 receptors in DRG neurons (Sculptoreanu and de Groat, 2003). DRG neurons responded to substance P (0.5 μM) and an NK2-selective agonist ([βAla8]-neurokinin A (4–10), 0.5 μM) with a rapid increase in input resistance (Rm, Tables 1 and 2) and a modest 3–5 mV hyperpolarization. These effects were comparable in phasic (Table 1) and tonic neurons (Table 2) and reached steady state within 3 minutes of drug application.
Substance P (SP, 0.5 μM) significantly increased firing in 100% of C-R phasic (Fig. 1A–C, n=5) and C-R tonic neurons (Fig. 1D–F, n=7), but increased firing in only 2 of 12 capsaicin-unresponsive (C-U) phasic neurons and none of the C-U tonic cells (n=4). SP (0.5 μM) also lowered the firing threshold by 11 mV in C-R phasic neurons (from −23±1 mV to −34±1 mV after SP, Table 1) and by 6 mV in C-R tonic neurons (from −34±1 mV to −40±1 mV after SP, Table 2). The maximum rate of rise (dV/dtmax), overshoot (OS) and rise time of APs were also increased in phasic neurons, but these increases were smaller in tonic neurons (Tables 1 and 2). SP increased the AP duration (50% repolarization- AP50 and full repolarization- decay time) in both phasic (Table 1) and tonic (Table 2) neurons, however, the changes were not statistically significant. The decay of the hyperpolarizing after potential fitted by a single exponential (tauAHP) was significantly prolonged (slowed) in both phasic and tonic firing cells in the presence of SP and an NK2 selective agonist (Table 1 and 2).
Role of K+ channels in the control of firing. Heteropodatoxin II (HPTx, 0.05 μM, Fig. 2AB), a blocker of low threshold inactivating K+ currents (Kv4) (Zarayskiy et al, 2005), mimicked the effects of SP in phasic neurons significantly increasing the number of APs (from 1.2/600ms to 10/600ms, n=4) elicited by a prolonged depolarizing current pulse. Similarly, low concentrations of 4-aminopyridine (4-AP, 50 μM, Fig. 2C–E) which are known to selectively block low threshold A-type K+ channels (Gold et al., 1996, Sculptoreanu et al., 2004) induced a similar response in phasic firing neurons (n=8). However, a higher concentration of 4-AP (1 mM, n=5) had a smaller effect in the same cells (Fig. 1C–E). At 1 mM 4-AP also increased AP duration, suggesting that higher voltage activated K+ currents responsible for the repolarizing phase of AP were also blocked (Fig. 2C, lower trace).
Neurokinin receptor types mediating the effects of SP. Since SP activates all neurokinin receptors with different potencies it was important to determine which of the three known neurokinin receptors contributes to the changes in firing seen in C-R phasic neurons. The effects of SP on firing in phasic, C-R neurons was reversed by a selective NK2 antagonist (MEN10376, 0.5 μM, n=7 cells, Fig. 3A, B) but unaffected by an NK3 antagonist (SB 235,375, 0.5 μM, n=4 cells, not shown).
Fig. 3.
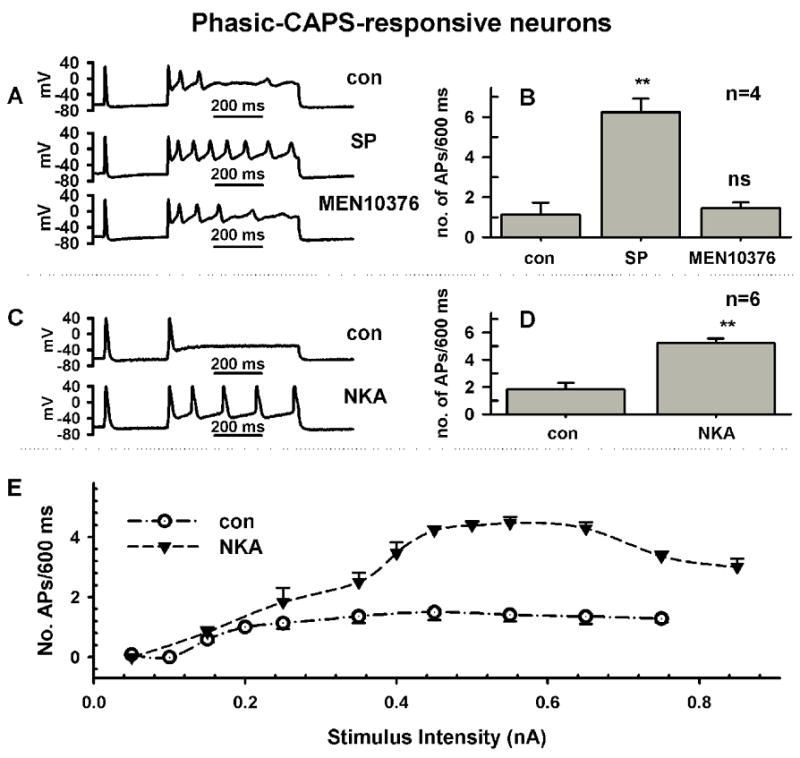
The neurokinin-mediated increase in firing in phasic DRG neurons requires activation of NK2 receptors. A, Substance P (0.5 μM, middle trace) increased firing from 3 APs (top trace, control) to 8 APs. Application of an NK2 antagonist, MEN10376 (0.5μM, bottom trace) reduced the increase in firing to near control rates. Firing in response to a current injection 600 ms long and 250 pA in intensity. B, Average increase in APs in response to SP and MEN10376 after SP in 4 neurons. C, A NK2-selective agonist, [βAla8]-neurokinin A (4–10) (NKA, 0.5 μM, bottom trace) increased firing from 1 APs (top trace, control) to 5 APs. Firing in response to a current injection 600 ms long and 250 pA in intensity. D, Average increase in APs in response to NKA in 6 neurons. E, Dependence of firing on stimulus intensity for experiment in D, before (Control, empty circles) and after NKA (0.5 μM, filled triangles).
The NK2-selective agonist [βAla8]-neurokinin A (4–10) mimicked the ability of SP to lower the threshold of APs and increase the number of APs in response to depolarizing pulses in C-R phasic firing neurons (NKA, n=9 cells, Table 1, Fig. 3C–E). In these neurons, NKA changed other parameters including the upstroke and repolarization phase of APs and the after hyperpolarizing potential in ways similar to SP (Table 1). In contrast, neither action potentials nor evoked firing were changed by an NK3-selective agonist, [MePhe7]-neurokinin B (NKB, 0.5 μM, Fig. 4A, B, n=5 cells) or an NK1-selective agonist, [Sar9, Met11]-substance P (SarMetSP, 0.5 μM, Fig. 4, C, D, n=4 cells). These concentrations were well above EC50 previously reported for these potent and selective agonists (Regoli et al., 1994).
Fig. 4.
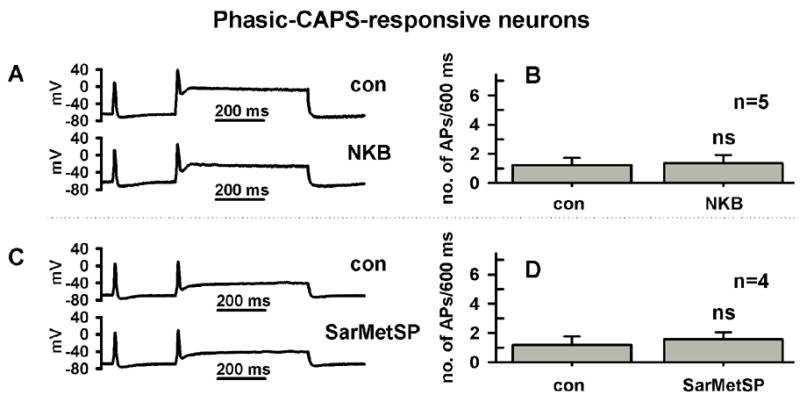
The NK1 and NK3 agonists do not increase firing in phasic DRG neurons. A, A NK3-selective agonist, [MePhe7]-neurokinin B (NKB, 0.5 μM, bottom trace) did not change firing (top trace, control). Firing in response to a current injection 600 ms long and 250 pA in intensity. B, Average firing before and after NKB in 5 neurons. C, An NK1-selective agonist, [Sar9, Met11]-substance P (SarMetSP, 0.5 μM, bottom trace) did not change firing (top trace, control). Firing in response to a current injection 600 ms long and 250 pA in intensity. D, Average firing before and after SarMetSP in 5 neurons.
Role of PKC in the effect of SP on firing. Because previous studies (Sculptoreanu and de Groat, 2003) showed that the facilitatory effect of SP on Ca+2 currents was mediated by a PKC signaling pathway, the role of PKC in SP induced facilitation of firing was also tested. Fig. 5 (A, B) shows that in phasic firing C-R neurons (n=5), a broad spectrum PKC inhibitor, bisindolylmaleimide (BIM, 0.5μM), only partially reversed (15–20% recovery) the effect of SP on firing. This partial reversal was not statistically significant (P>0.05). In addition, in phasic firing C-R DRG neurons, PDBu (0.5μM, n=5, Fig. 5, C-E), a PKC activator, had no significant effect on firing and had an inhibitory action in tonic C-R neurons (Fig. 6, A–C, n=4).
Fig. 5.
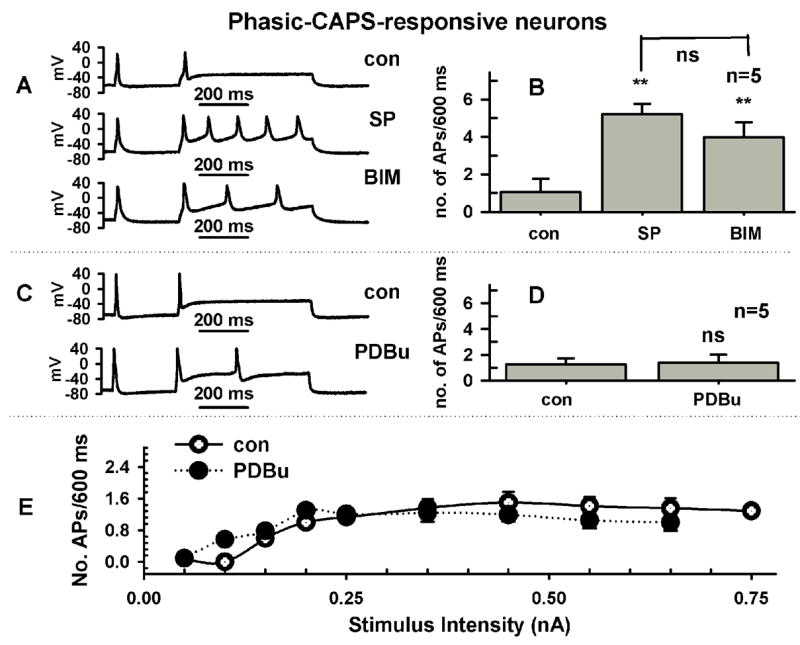
Neurokinin mediated increase in firing is not reversed by inhibition of PKC or mimicked by direct activation of PKC with phorbol ester. A, In a phasic firing neuron SP (0.5 μM, middle trace) increased firing from 1 AP (top trace, control) to 5 APs. Application of a PKC inhibitor, bisindolylmaleimide I HCl (BIM, 0.5μM, bottom trace), partially reduced the increase in firing to 3APs. Firing in response to a current injection 600 ms long and 250 pA in intensity. B, Average increase in APs in response to SP and BIM after SP in 5 neurons. C, Direct activation of PKC with phorbol 12,13-dibutyrate (PDBu, 0.5 μM, bottom trace) did not alter significantly (top trace, control) firing. Firing in response to a current injection 600 ms long and 250 pA in intensity. D, Average firing before and after PDBu in 5 neurons. E, Dependence of firing on stimulus intensity for experiment in D, before (Control, empty circles) and after PDBu (0.5 μM, filled circles).
Fig. 6.
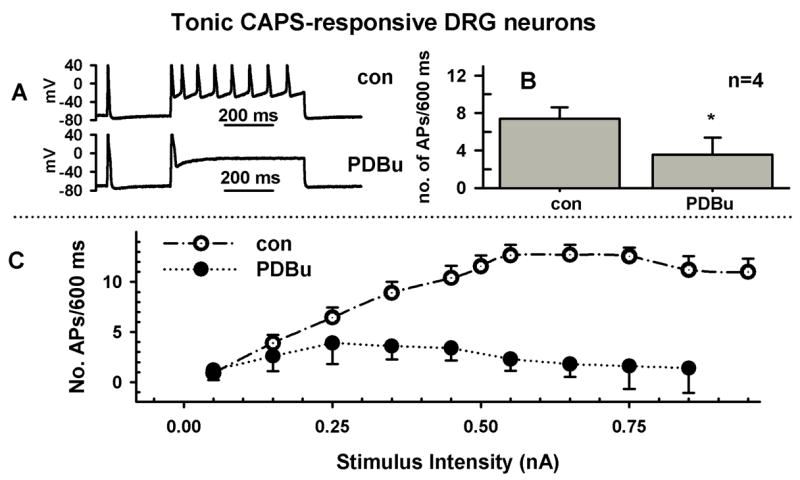
The phorbol ester PDBu reduces firing in tonic neurons. A, Direct activation of PKC with phorbol 12,13-dibutyrate (PDBu, 0.5 μM, bottom trace) prolonged the APs and reduced firing (top trace, control) in tonic firing neurons. Firing in response to a current injection 600 ms long and 250 pA in intensity. B, Average firing before and after PDBu in 4 neurons. C, Dependence of firing on stimulus intensity for experiment in B, before (Control, empty circles) and after PDBu (0.5 μM, filled circles).
Discussion
The major finding in this study was that substance P (SP) and the NK2 selective agonist, [βAla8]-neurokinin A (4–10), increase firing in C-R DRG neurons. We conclude in C-R phasic DRG neurons, neurokinins unmask tonic firing. This action is mediated by activation of NK2 receptors and may be due to inhibition of low voltage activated K+ channels and require an unidentified intra-cellular signaling pathway. PKC phosphorylation was excluded as a potential mechanism.
Selective activation of NK2 receptors followed by blockade of low threshold K+ currents by neurokinins may be required to enhance firing in DRG neurons. Our present data suggests that neurokinins sensitize nociceptive C-R neurons. The increase in firing is accompanied by a significant (>10 mV) lowering of the threshold of AP generation and an increase in the rate of rise of APs. Both phasic and tonic firing neurons which were C-R were affected, although, the increase in firing was substantially greater in phasic neurons. We believe that the increase in firing is due to a selective activation of NK2 receptors. This conclusion is based on a number of observations: (1) an NK2 selective agonist ([βAla8]-neurokinin A (4–10) as well as SP, increased firing; (2) the increase in firing induced by SP, a non-selective agonist, was fully reversed by a NK2 antagonist (MEN10376); (3) neither an NK1 ([Sar9, Met11]-substance P) nor an NK3 ([MePhe7]-neurokinin B, NKB) selective agonist increased firing in C-R phasic neurons. It is noteworthy that SP and NKA did not change the firing in the majority of C-U phasic neurons which were otherwise remarkably similar to their C-R counterparts in terms of the AP parameters. These data suggest that the mechanism responsible for induction of tonic firing is only present in C-R neurons.
We have previously shown that a selective low threshold K+ channel agonist, KW-7158, reversed the increase in firing caused by either low concentrations of 4-aminopyridine (50 μM) or substance P in C-R phasic firing neurons from adult rat dorsal root ganglia (Sculptoreanu et al., 2004). The low threshold K+ current, enhanced by KW-7158, was insensitive to concentrations of TEA as high as 60 mM which presumably block high threshold A-type K+ channels (Gold et al, 1996, Kv1.4) but not low threshold A-type channels presumed to be of the Kv4 family (Sculptoreanu et al, 2004). The effect of neurokinins was mimicked by heteropodatoxin, an agent known to selectively inhibit the Kv4 family of K+ channels suggesting that the effect of neurokinins may be mediated by similar mechanisms. In addition to effects on firing, SP and NKA increased the input resistance and induced modest hyperpolarizations of membrane potential. Mechanisms responsible for these effects are not known. While an increase in potassium conductance could account for a hyperpolarization in responses to SP and a selective NK2 agonist, it would produce a concomitant decrease in membrane resistance rather that the observed increase. For this reason we favor as an explanation neurokinin-mediated inhibition of a constitutively active non-selective cation channel (EREV = −10 mV, Yang et al, 2003), which would normally drive the membrane away from EK in the depolarized direction and therefore when blocked by neurokinins would result in a membrane hyperpolarization.
A-type K+ currents are important modulators of neuronal firing (Sculptoreanu et al, 2004, Vydyanathan et al., 2005, Yoshimura and de Groat, 1999). A reduction of A-type K+ currents has been implicated in the induction of tonic firing in phasic firing DRG neurons, induced by chemical irritation of the bladder (Yoshimura and de Groat, 1999), by gastric ulcers (Dang et al, 2003), following axotomy (Abdulla et al, 2001, Everill and Kocsis, 1999, Yang et al, 2004) and in cats with feline interstitial cystitis (Sculptoreanu et al., 2005a). Rat DRG neurons express a diverse population of K+ channel types, exhibiting as many as 6 subtypes of currents distinguishable both by voltage dependence and pharmacology (Gold et al., 1996). Three of these currents, exhibit rapid to slow rates of activation (Fedulova et al, 1998) and inactivation (Fedulova et al, 1998, Everill et al, 1998) and correspond to Kv4 subtypes (Pongs, 1992; Tkatch et al., 2000), Kv1.2 and Kv1.4 types of K+ channels (Ishikawa et al., 1999). The remaining currents are non-inactivating, delayed rectifier type (Gold et al., 1996; Ishikawa et al., 1999). The transient K+ currents are all sensitive to 4-aminopyridine (4-AP), but only one (Kv1.4, Pongs, 1992, Rasband et al, 2001) is also sensitive to TEA blockade (Gold et al., 1996). Each voltage dependent K+ current may contribute differently to the threshold of APs (Vydyanathan et al., 2005), repolarization of the AP as well as after hyperpolarizing potentials, as shown in the present experiments and earlier reports (Sculptoreanu et al., 2005a, Weinreich and Wonderlin, 1997).
It is tempting to speculate that the neurokinin-induced reduction in AP threshold and increase in upstroke velocity which accompanied the increase in firing was due to a large extent to the inhibition of low threshold, rapidly activating K+ channels. Recently, Vydyanathan et al. (2005) concluded that 4-AP, which mimicked the effect of SP on firing in our study, increased firing only in IB4 positive neurons, (the majority of which are believed to be capsaicin unresponsive). It was suggested that this was due to selective expression of Kv1.4 channels in these DRG neurons and their inhibition by 4-AP. However, the conclusion that Kv1.4 would be the sole contributor to firing in DRG neurons is inconsistent with our findings that HPTx, a Kv4 channel blocker (Zarayskiy, 2005), also induced tonic firing in C-R phasic DRG neurons. The Kv4 family of K+ channels is also widely expressed in DRG neurons (Winkelman et al, 2005). The majority of C-R phasic neurons in our work responded to both 4-AP and neurokinins with an increase in firing. The present knowledge suggests that only a subpopulation of small to medium size IB4 neurons are also sensitive to CAPS and contain neuropeptides SP and CGRP (Chen et al., 1995, Petruska et al., 2003). The neurokinin unresponsive phasic neurons in our study could belong to the population of C-U IB4 positive neurons which are non-peptidergic but respond to 4-AP in a manner similar to those reported by Vydyanathan et al. (2005). In our study, >90% of C-R phasic and tonic neurons tested responded to either SP or NKA with an increase in firing in contrast to C-U neurons which did not respond to these agents with an increase in firing. These neurons could belong to a mix of IB4 positive (Petruska et al., 2003) and IB4 negative neurons (this study).
Activation of PKC is not sufficient for the induction of hyperexcitability by neurokinins in DRG neurons. The family of neurokinin receptors NK1, NK2 and NK3 are G-protein coupled receptors which activate phospholipase C (Maggi, 1995, 1997b). The breakdown of phospholipids into diacylglycerol by PLC, activates PKC (Torrens et al, 1997) to phosphorylate and inhibit a number of K+ channels (Boland and Jackson, 1999, Hagiwara et al., 2003, Richardson and Vasko, 2002). We have shown that direct activation of PKC with PDBu did not increase firing in DRG neurons. In addition, the PKC inhibitor bisindolylmaleimide, only partially reversed the increase in firing caused by NK2 activation. Taken together, these data suggest that while phosphorylation of K+ channels may contribute to the increase in firing it is not sufficient alone for the induction of hyperexcitability in DRG neurons.
The suggestion that a mechanism in addition to phosphorylation may contribute to the neurokinin effect on firing is not restricted to our findings. Yang et al. (2003) concluded that the activation of non-selective ion channels by neurokinins in DRG neurons was independent of G-protein coupling to receptors as it was insensitive to GDP-βS, unlike the modulation of GABA evoked responses by neurokinins. Koike-Tani et al. (2005) reported that SP-induced inhibition of Kir3 (GIRK) channels was due to direct binding of Gαq to the channels.
Neurokinins enhance firing in nociceptive neurons by an autofeedback mechanism. There is growing evidence that neurokinin auto-receptors influence the properties of C-R nociceptive neurons (Hu et al., 1997, Maggi, 1997b, Richardson and Vasko, 2002). In the urinary bladder, neurokinin A released from sensory nerves seems to be involved in both the normal voiding function induced by bladder distension as well as the hyperactive voiding induced by noxious stimuli (Morrison et al, 1999). Here we show that neurokinins influence several parameters that may act in concert to increase excitability in C-R neurons. Changes include a lowering of the firing threshold, an increase in membrane resistance and a resting potential change in the hyperpolarized direction. Hyperpolarization could increase firing by enhancing repriming of Na+ channels and thus lowering the firing threshold. However, while inhibition of low threshold K+ currents may be essential for the induction of hyperexcitability, we cannot exclude the possible contribution of changes in Na+ or other voltage-dependent K+ currents. For example, the increase in overshoot and rate of rise of action potentials in response to SP and NKA is also suggestive of changes in Na+ currents. In addition, neurokinins which increase firing also slow the decay of hyperpolarizing afterpotential in C-R neurons. This effect which is presumably due to an increase in Ca2+-activated K+ currents (Scholz et al., 1998), may be secondary to a NK2-mediated Ca2+-current enhancement (Sculptoreanu and de Groat, 2003). It is tempting to speculate that the increase in hyperpolarizing afterpotential together with the membrane hyperpolarization may allow for more rapid recovery from inactivation of both TTX-resistant and TTX-sensitive currents (Blair and Bean, 2002, 2003) thus contribute to the increase in firing we report here.
In conclusion, the increase in firing in response to neurokinins coupled with the enhancement of voltage-dependent Ca2+ currents (Sculptoreanu and de Groat, 2003) in a subpopulation of primary afferents (Petruska et al., 2003), would enhance the release of neurokinins from afferent terminals and thus contribute to a neurokinin-mediated autofeedback mechanism to acutely sensitize nociceptive neurons and increase the input of signals from the periphery to the spinal cord.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulla FA, Stebbing MJ, Smith PA. Effects of substance P on excitability and ionic currents of normal and axotomized rat dorsal root ganglion neurons. Eur J Neurosci. 2001;13:545–652. doi: 10.1046/j.0953-816x.2000.01429.x. [DOI] [PubMed] [Google Scholar]
- Andersson KE. The overactive bladder: pharmacologic basis of drug treatment. Urol. 1997;50:74–84. doi: 10.1016/s0090-4295(97)00595-5. [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci. 2002;22:10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Role of tetrodotoxin-resistant Na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons. J Neurosci. 2003;23:10338–10350. doi: 10.1523/JNEUROSCI.23-32-10338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland LM, Jackson KA. Protein kinase C inhibits Kv1.1 potassium channel function. Am J Physiol. 1999;277:C100–C110. doi: 10.1152/ajpcell.1999.277.1.C100. [DOI] [PubMed] [Google Scholar]
- Brechenmacher C, Larmet Y, Feltz P, Rodeau JL. Cultured rat sensory neurones express functional tachykinin receptor subtypes 1, 2 and 3. Neurosci Lett. 1998;241:159–162. doi: 10.1016/s0304-3940(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilloti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol. 2004;286:G573–G579. doi: 10.1152/ajpgi.00258.2003. [DOI] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- Everill B, Rizzo MA, Kocsis JD. Morphologically identified cutaneous afferent DRG neurons express three different potassium currents in varying proportions. J Neurophysiol. 1998;79:1814–1824. doi: 10.1152/jn.1998.79.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulova SA, Vasilyev DV, Veselovsky NS. Voltage-operated potassium currents in the somatic membrane of rat dorsal root ganglion neurons: ontogenetic aspects. Neurosci. 1998;85:497–508. doi: 10.1016/s0306-4522(97)00600-3. [DOI] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996;5:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Nunoki K, Ishii K, Abe T, Yanagisawa T. Differential inhibition of transient outward currents of Kv1.4 and Kv4.3 by endothelin. Biochem Biophys Res Comm. 2003;310:634–640. doi: 10.1016/j.bbrc.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Li ZW, Si JQ. Evidence for the existence of substance P autoreceptor in the membrane of rat dorsal root ganglion neurons. Neurosci. 1997;77:535–541. doi: 10.1016/s0306-4522(96)00451-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Tanaka M, Black JA, Waxman SG. Changes in expression of voltage- gated potassium channels in dorsal root ganglion neurones following axotomy. Muscle Nerve. 1999;22:502–507. doi: 10.1002/(sici)1097-4598(199904)22:4<502::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Whitney T, Booth AM, de Groat WC. Excitatory effect of substance P in parasympathetic ganglia of cat urinary bladder. Am J Physiol. 1989;257:R1450– R1456. doi: 10.1152/ajpregu.1989.257.6.R1450. [DOI] [PubMed] [Google Scholar]
- Kiss S, Yoshiama M, Cao YQ, Basbaum AI, de Groat WC, Lecci A, Maggi CA, Birder LA. Impaired response to chemical irritation of the urinary tract in mice with disruption of the preprotachychynin gene. Neurosci Lett. 2001;313:57–60. doi: 10.1016/s0304-3940(01)02255-8. [DOI] [PubMed] [Google Scholar]
- Koike-Tani M, Collins JM, Kawano T, Zhao P, Zhao Q, Kozasa T, Nakajima S, Nakajima Y. Signal transduction pathway for the substance P-induced inhibition of rat Kir3 (GIRK) channel. J Physiol (London) 2005;564:489–500. doi: 10.1113/jphysiol.2004.079285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci A, Tramontana M, Giuliani S, Criscuoli M, Maggi CA. Effect of tachykinin NK2 receptor blockade on detrusor hyperreflexia induced by bacterial toxin rats. J Urol. 1998;160:206–209. [PubMed] [Google Scholar]
- Lu SH, Yamagata T, Atsuki K, Sun L, Smith CP, Yoshimura N, Chancellor MB, de Groat WC. Effect of KW-7158, a putative afferent nerve inhibitor, on bladder and vesico-vascular reflexes in rats. Brain Res. 2002;946:72–78. doi: 10.1016/s0006-8993(02)02828-7. [DOI] [PubMed] [Google Scholar]
- Maggi CA. Tachykinins and calcitonin gene-related peptide CGRP as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regul Pept. 1997a;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- Maggi CA. Tachykinins as peripheral modulators of primary afferent nerves and visceral sensitivity. Pharmacol Res. 1997b;36:153–169. doi: 10.1006/phrs.1997.0219. [DOI] [PubMed] [Google Scholar]
- Morrison J, Wen J, Kibble A. Activation of pelvic afferent nerves from the rat bladder during filling. Scand J Urol & Nephrol Suppl. 1999;201:73–75. [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Cooper BY. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neurosci. 2002;115:15–30. doi: 10.1016/s0306-4522(02)00409-8. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992;72:S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci (USA) 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D, Nguyen QT, Jukic D. Neurokinin receptor subtypes characterized by biological assays. Life Sci. 1994;54:2035–2047. doi: 10.1016/0024-3205(94)00712-8. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- Saban MR, Saban R, Bjorling DE. Kinetics of peptide-induced release of inflammatory mediators by the urinary bladder. Br J Urol. 1997;80:742–747. doi: 10.1046/j.1464-410x.1997.00415.x. [DOI] [PubMed] [Google Scholar]
- Scholz A, Gruβ M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol (London) 1998;513:55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC. Protein kinase C is involved in neurokinin receptor modulation of N- and L-type Ca2+ channels in DRG neurons of the adult rat. J Neurophysiol. 2003;90:21–31. doi: 10.1152/jn.00108.2003. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol. 2005a;193:437–443. doi: 10.1016/j.expneurol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neurosci Lett. 2005b;381:42–46. doi: 10.1016/j.neulet.2005.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, Figourov A, de Groat WC. Voltage-dependent potentiation of neuronal L-type calcium channels due to state-dependent phosphorylation. Am J Physiol. 1995;269:C725–C732. doi: 10.1152/ajpcell.1995.269.3.C725. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, Yoshimura N, de Groat WC. KW-7158 [(2S)-(+)-3,3,3-trifluoro-2- hydroxy-2-methyl-N-(5,5,10-trioxo-4,10-dihydrothieno[3,2-c][1]benzothiepin-9-yl) propanamide] enhances A-type K+ currents in neurons of the dorsal root ganglion of the adult rat. J Pharmacol Exp Ther. 2004;310:159–168. doi: 10.1124/jpet.104.065409. [DOI] [PubMed] [Google Scholar]
- Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the Rat’s urinary bladder. J Neurophysiol. 2000;84:1924–1933. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- Shinkai M, Takayanagi I, Kato T. Tachykinin receptors of the NK2 type involved in the acetylcholine release by nicotine in guinea-pig bladder. Br J Pharmacol. 1993;108:759–762. doi: 10.1111/j.1476-5381.1993.tb12874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatch T, Baranauskas G, Surmeier J. Kv4.2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci. 2000;20:579–588. doi: 10.1523/JNEUROSCI.20-02-00579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens Y, Saffroy M, Glowinski J, Beaujouan JC. Substance P(6–11) and natural tachykinins interact with septide-sensitive tachykinin receptors coupled to a phospholipase C in the rat urinary bladder. Neuropeptides. 1997;31:243–251. doi: 10.1016/s0143-4179(97)90055-x. [DOI] [PubMed] [Google Scholar]
- Vydyanathan A, Wu Z-Z, Chen S-R, Pan H-L. A-type voltage-gated K+ currents influence firing properties of isolectin B 4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol. 2005;93:3401–3409. doi: 10.1152/jn.01267.2004. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Wonderlin WF. Inhibition of calcium-dependent spike after- hyperpolarization increases excitability of rabbit visceral sensory neurons. J Physiol (London) 1987;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman DL, Beck CL, Ypey DL, O’Leary ME. Inhibition of the A-type K+ channels of dorsal root ganglion neurons by the long-duration anesthetic butamben. J Pharmacol Exp Ther. 2005;314:1177–1186. doi: 10.1124/jpet.105.087759. [DOI] [PubMed] [Google Scholar]
- Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neurosci. 2004;123:867–874. doi: 10.1016/j.neuroscience.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Yang YL, Yao KH, Li ZW. Similarities of SP-, NKA- and NKB-induced currents in rat dorsal root ganglion neurons. Brain Res. 2003;991:18–25. doi: 10.1016/s0006-8993(03)03451-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Novakovic SD, Tzoumaka E, Erickson VL, Erickson KA, Chancellor MB, de Groat WC. The involvement of the tetrodotoxin-resistant sodium channel Nav1.8 (PN3/SNS) in a rat model of visceral pain. J Neurosci. 2001;21:8690–8696. doi: 10.1523/JNEUROSCI.21-21-08690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, White G, de Groat WC. Patch clamp recordings from subpopulations of autonomic and afferent neurons identified by axonal tracing techniques. J Auton Nerv Syst. 1994;49:85–92. doi: 10.1016/0165-1838(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, White G, Weight FF, de Groat WC. Different types of Na+ and A- type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J Physiol (London) 1996;494:1–16. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarayskiy VV, Balasubramanian G, Bondarenko VE, Morales MJ. Heteropoda toxin 2 is a gating modifier toxin specific for voltage-gated K+ channels of the Kv4 family. Toxicon. 2005;45:431–442. doi: 10.1016/j.toxicon.2004.11.015. [DOI] [PubMed] [Google Scholar]


