Cell growth, differentiation, and survival responses are the result of integration of numerous chemical and biophysical cues from the cell’s surrounding environment. Interactions between the cell and the ECM are a major source of these environmental signals. The ECM is an intricate arrangement of glycoproteins, collagens, proteoglycans, and growth factors that act not only as a physical scaffold for the attachment and organization of cellular structures, but also as a mediator of intracellular signaling through cell surface receptors that recognize these ECM molecules. Most ECM glycoproteins promote cell adhesion and cause cytoskeletal reorganization, leading to signals that direct differentiation and promote cell survival. Examples include fibronectin, laminin, collagen, and vitronectin; cell adhesion and signaling through these substrates has been studied extensively. However, there exists another class of ECM proteins, termed “matricellular” proteins, that function as adaptors and modulators of cell-matrix interactions (1, 2). These structurally diverse proteins include thrombospondins (TSPs) 1 and 2, the tenascins, and SPARC (secreted protein, acidic and rich in cysteine), all of which exhibit highly regulated expression during development and following cellular injury. One key feature of matricellular proteins is that they function as both soluble and insoluble proteins. As substrates, these proteins are only capable of supporting the initial and intermediate stages of cell adhesion — attachment and spreading. Focal adhesion and stress fiber formation, characteristic of strong cell adhesion, are rarely observed when cells are plated on these substrates. When presented in mixed substrata, the matricellular proteins can also antagonize the pro-adhesive activities of other matrix proteins (3, 4). Interestingly, these matricellular proteins actually have de-adhesive effects when presented as soluble proteins to cells in a strong adhesive state. TSP1, tenascin-C, and SPARC stimulate reorganization of actin stress fibers and disassembly of focal adhesion complexes but have only minimal or negligible effects on cell shape.
The ability of cells to adhere to the ECM is a critical determinant of cytoskeletal organization and thus of cellular morphology (5). In addition to regulating cell shape, cell-ECM interactions also regulate the ability of a cell to proliferate, migrate, and differentiate (6). Furthermore, cell-matrix interactions that support cytoskeletal organization of focal adhesions are essential for survival of anchorage-dependent, nontransformed cells (7–9). This wide range of activities suggests that the ECM is a key contributor to overall cellular physiology. Correspondingly, the ability of matricellular proteins to modulate cell adhesion and cytoskeletal organization suggests an important role for these proteins in essential processes.
Adhesion and de-adhesion
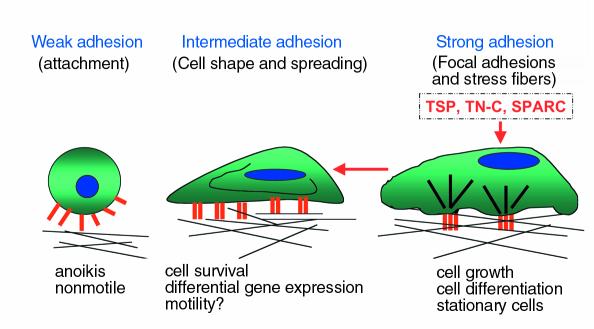
Cell adhesion occurs in three stages: attachment, spreading, and formation of focal adhesions and stress fibers (Figure 1). The first stage of cell attachment involves the interaction between integrins, along with accessory receptors such as syndecans, and their ECM substrates. Binding of ECM components to integrins induces integrin clustering and increases integrin affinity for the ligand, a process known as integrin activation. Following these initial cell receptor–ECM ligand interactions, cells increase their surface contact area with the ECM substrate through formation of actin microfilaments and cell spreading. This stage of attachment is considered an intermediate state between that of weak contact and strong adhesion. If the appropriate signals are provided by the matrix, cells then proceed to organize their cytoskeleton as characterized by the formation of focal adhesions and actin-containing stress fibers. Focal adhesions consist of receptors for ECM proteins and a scaffolding of structural and signaling components that link the termini of actin-containing stress fibers to the membrane and the ECM (10). As such, focal adhesions transduce both mechanical and biochemical signals. This state constitutes a stage of strong adherence.
Figure 1.
The stages of cell adhesion and induction of the intermediate adhesive state by matricellular proteins. During the process of adhesion, a cell undergoes attachment, spreading, and the formation of stress fibers and focal adhesions. With each stage the adhesive strength of the cell increases. We define de-adhesion as the transition from strong adherence to intermediate adherence, as characterized by the disassembly of stress fibers
Cell adhesion is a reversible process: tissue remodeling during morphogenesis and wound healing, cellular metaplasia, cell proliferation, and tumor cell metastasis are events in which the adhesive state undergoes modulation. While the process of cell adhesion has been well characterized, there is much less known about the process of cellular de-adhesion. De-adhesion refers to a reversal of the adhesive process in which a cell moves from a state of stronger adherence to a state of weaker adherence (11). This can involve the transition from a strongly adherent state with focal adhesions and stress fibers to an intermediate state of adherence, characterized by a restructuring of focal adhesions and stress fibers, while maintaining a spread cell shape. This is the type of de-adhesion mediated by the matricellular proteins TSP1, tenascin-C, and SPARC. The biological significance of this cellular state is not currently appreciated. In this article, I will discuss possible biological roles for this adhesive state and its induction by matricellular proteins. De-adhesion can also involve the transition from the spread intermediate state of adherence to a state of weak adherence characterized by the attachment of a round cell to a substrate. Prolonged exposure to SPARC induces cell rounding, as does disruption of ECM-integrin interactions by proteolysis or integrin antagonists (1). This state might be physiologically relevant during cytokinesis or the induction of apoptosis during tissue remodeling.
Induction of focal adhesion restructuring and intermediate cell adhesion by matricellular proteins
In 1989, we reported that TSP1 stimulates the loss of focal adhesions and stress fibers in spread, adherent bovine aortic endothelial cells plated on fibronectin substrates (12). This activity of TSP1 occurs in fibroblasts and smooth muscle cells as well and is independent of the substrate used to support strong adherence. Treatment of adherent cells with TSP1 results in focal adhesion restructuring and alterations in the stress fibers but has no effect on cell spreading or integrin clustering (11). Loss of focal adhesions under these conditions occurs uniformly across the central region of the cell. Although soluble TSP1 can also prevent focal adhesion formation, in this system, the loss of focal adhesions is due to the rapid disassembly of these structures and not due to preventing reformation of adhesion plaques during the course of normal turnover. The effects of TSP1 on tightly adherent cells are discernible by time-lapse interference reflection microscopy (IRM) after 8–10 minutes of treatment, with a complete response by 20 minutes. These changes are persistent, lasting 4–16 hours, but are fully reversible in 2–4 hours. Reversion to the strong adhesive state does not require protein synthesis, although focal adhesions reform slightly more rapidly in the absence of protein synthesis inhibitors. TSP1 selectively stimulates the loss of certain structural proteins, including vinculin and α-actinin, from the focal adhesion plaque, without affecting the localization of other focal adhesion proteins, such as talin and integrins. The link between the actin stress fibers and the submembranous focal adhesion plaque is effectively disrupted without visibly affecting the integrin-ECM protein link. Time-lapse IRM shows that, as a consequence, the bundling of the actin stress fibers is disrupted and actin microfilaments redistribute to the cell periphery rather than terminating at plaques (11, 13). We recently showed that this altered organization of the cytoskeleton in response to TSP1 (as well as other mediators, such as PDGF) occurs at least in part though binding of phosphoinositide 3,4,5-trisphosphate (PIP3) to α-actinin, which disrupts binding of α-actinin to the cytoplasmic tail of the integrin β subunit (14). Because the integrin-matrix link remains, the cell is still attached and spread, although the actin stress fibers are no longer linked to the integrin. This condition is termed “intermediate adherence.” The actions of tenascin-C and SPARC on the cytoskeleton and focal adhesions of adherent cells are basically indistinguishable from those of TSP1 (15, 16). However, as will be discussed, these three proteins each have apparently unique receptors and employ both common and distinct signaling pathways to produce this state of intermediate adhesion.
Active sites of the matricellular proteins
The active site of each of these matricellular proteins has been localized. The NH2-terminal heparin-binding domain (HBD) of TSP1 contains the focal adhesion reorganizing activity (17), consistent with earlier data showing that TSP1 activity could be blocked by either heparin or a monoclonal antibody specific for the amino-terminal HBD (12). Subsequently, we identified a sequence from the HBD comprising amino acids 17–35 that is sufficient to stimulate focal adhesion disassembly when expressed as a peptide. This 19–amino acid sequence, termed hep I, stimulates focal adhesion and stress fiber disassembly to approximately the same degree as does intact TSP1. The hep I sequence has lysine residues present at positions 24 and 32 that are critical for activity. The corresponding sequence from TSP2, although identical to TSP1 at only 7 of 19 residues, is also active, and it appears that the conserved basic amino acids at residues 24 and 32 are important for stress fiber disassembly. However, it is not clear that TSP2 is actually de-adhesive, since the reduced adhesion seen in TSP2-null fibroblasts might be attributed to an increase in matrix metalloproteinase 2 activity (ref. 18; also see Bornstein, this Perspective series, ref. 19).
The active site of tenascin-C was originally mapped through the use of monoclonal antibodies raised against different domains of tenascin-C (15). These studies showed that only antibodies recognizing fibronectin type III repeats in the alternatively spliced domain blocked the ability of tenascin-C to stimulate focal adhesion disassembly. A recombinant form of TNfnA-D (as the alternatively spliced domain is now known) is sufficient for focal adhesion disassembly. Consistent with the identification of this domain as the active site of tenascin-C, only alternatively spliced forms of this protein expressing the TNfnA-D domain are able to stimulate focal adhesion disassembly. This suggests that the ability of tenascin-C to induce intermediate cell adhesion is restricted to tissues or cellular conditions in which this form of the protein is expressed. Interestingly, forms of tenascin-C expressing the variable repeats are present at sites of tissue remodeling and cell migration (20).
Two sequences in SPARC that are located in different domains each can stimulate focal adhesion reorganization. Peptides derived from the COOH-terminal calcium-binding EF hand (peptide 4.2) and from the cationic, cysteine-rich follistatin-like domain (peptide 2.1) each have activity (16). Each sequence appears to be sufficient for activity since anti-peptide antibodies to each sequence can fully block focal adhesion disassembly by SPARC protein. Crystallographic data indicate that these two sites are in close proximity in the native protein and may form a binding pocket.
Receptors
Consistent with the structural diversity of the active sites of these three matricellular proteins, the receptors for these domains are similarly distinct. Annexin II, a calcium-binding peripheral membrane protein that has phospholipid-binding activity, mediates this activity of tenascin-C (21): this protein only recognizes the active isoform of tenascin-C that contains the alternatively spliced TNfnA-D domains. Similarly, another calcium-binding protein, calreticulin, is the receptor for TSP (22). Although calreticulin is best known as a protein of the endoplasmic reticulum lumen, it is expressed on the surface of cells that respond to TSP1, and blocking TSP1-calreticulin interactions at the cell surface prevents TSP1-mediated signaling and focal adhesion disassembly (22). These receptors are specific for their respective ligands, since antibodies to annexin II do not block TSP1 (hep I) activity and antibodies to calreticulin do not inhibit TNfnA-D signaling. It is not clear how these peripheral membrane proteins signal changes to the cytoskeleton. Both of these proteins have been localized to caveolae (23, 24), which are membrane subdomains that are enriched in kinases, heterotrimeric G proteins, and other signaling components. It is possible that localization of these receptors to caveolae facilitates transduction of signals from this class of membrane-associated molecules. Signaling of focal adhesion disassembly through calreticulin signaling is blocked by pertussis toxin, suggesting the involvement of heterotrimeric G proteins (our unpublished data). A receptor for SPARC has not been identified, and it is currently thought that SPARC may function as an antagonist of other ligand-receptor interactions (see Bradshaw and Sage, this Perspective series, ref. 25).
Signaling
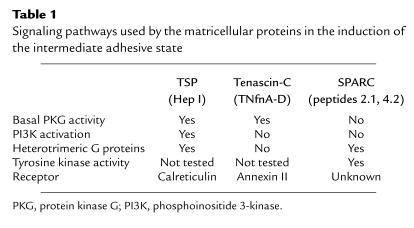
Following identification of the active sites of these de-adhesive matrix proteins, we sought to determine the signaling pathways involved in stimulation of focal adhesion disassembly. Despite the fact that the intermediate adhesive states induced by TSP1, tenascin-C, or SPARC are morphologically indistinguishable, each of these proteins appears to employ a unique array of signaling events to achieve this common state (Table 1).
Table 1.
Signaling pathways used by the matricellular proteins in the induction of the intermediate adhesive state
Basal cGMP-dependent protein kinase (PKG) activity is essential for both TSP- and tenascin-C–induced focal adhesion disassembly (26). SPARC-mediated focal adhesion disassembly occurs independent of PKG activity. TSP/hep I does not stimulate PKG, and PKG activity in the absence of hep I does not stimulate focal adhesion disassembly, suggesting that, while PKG activity is necessary, it is not sufficient in itself to induce focal adhesion disassembly. The role of PKG in this process is unknown, although it is likely to be acting at a point distal to the receptors for either protein. It may be that PKG phosphorylates a component of the focal adhesion complex, thus activating a protein involved in focal adhesion restructuring. VASP, a profilin-binding protein at focal adhesions, is a major substrate of PKG (27). Although we failed to detect changes in VASP phosphorylation in response to hep I, basal VASP activity may yet be important for this process.
TSP1/hep I–mediated interactions with cells stimulate activation of the p85/p110 isoform of phosphoinositide 3-kinase (PI3K) (13). Activation of this lipid kinase is necessary for TSP1- and hep I–mediated focal adhesion disassembly. Two independent inhibitors of PI3K, wortmannin and Ly294002, block TSP1-induced focal adhesion disassembly and stress fiber disruption (13). Hep I–stimulation of PI3K activity occurs as early as 2 minutes and persists for at least 2 hours after stimulation, closely following the time course for hep I–induced focal adhesion disassembly (11, 13). Hep I stimulates an increase in cellular levels of PIP3, the product of PI3K. Recently, Greenwood et al. showed that PIP3 directly alters the structure of the adhesion plaque by binding α-actinin and disrupting interactions between the integrin β subunit and α-actinin (14). This finding is consistent with the increase in soluble α-actinin and the unbundling of the actin stress fibers observed upon stimulation with TSP1/hep I. Although cells loaded with PIP3 show cytoskeletal rearrangements similar to those induced by TSP1, tenascin-C, or SPARC, other factors such as the expression of alternate PI3K isoforms and the intracellular localization or activity of other signaling molecules might also regulate cytoskeletal reorganization, since insulin stimulation activates PI3K but fails to stimulate focal adhesion disassembly in endothelial cells. Furthermore, phosphoinositide-protein interactions are apparently not the only mechanism capable of stimulating focal adhesion reorganization, since tenascin-C and SPARC activities are not blocked by PI3K inhibitors (11).
Signaling by the Rho family of small GTPases may be involved in mediating focal adhesion disassembly by soluble forms of these matricellular proteins. However, the involvement of Rac, Rho, and Cdc42 has only been investigated for insoluble matrix forms of TSP1 and tenascin-C. Recently, it was shown that TSP1 substrates stimulate prolonged activation of Rac, consistent with cell spreading in the absence of focal adhesion formation (i.e., the intermediate type of cell adhesion) (3). It was not reported whether Rho activity was inhibited on TSP1 substrates; however, tenascin-C in matrices prevents Rho activation (4). As with TSP1, we also observed that soluble tenascin-C prevents focal adhesion formation in addition to stimulating disassembly (our unpublished observations). It will be interesting to determine whether soluble versions of these matricellular proteins also modulate the balance between Rac and Rho in driving these cells to the intermediate adhesive state.
In confluent endothelial cells, the ability of SPARC to mediate cytoskeletal reorganization is blocked by tyrosine kinase inhibitors (28), implicating this class of kinase in the process. Under the conditions (80% confluence) in which we perform our assays, focal adhesions are labile in the presence of tyrosine kinase inhibitors. Therefore, we have not been able to determine how they might be involved in TNfnA-D or hep I signaling.
Biological roles for intermediate adhesion
De-adhesion in cell motility.
It has been suggested that the intermediate state of adhesion favors cell motility (29, 30). Cell migration is highest in areas of remodeling, such as during embryogenesis, wound healing, and inflammation. The matricellular proteins exhibit increased expression during development and in response to injury, suggesting that one of their functions might be to promote this intermediate adhesive state to facilitate cell migration.
Cell migration is diminished in cells exhibiting strong adhesion, as characterized by abundant stress fibers and focal adhesions, and the absence of focal adhesions has long been associated with a motile phenotype. However, completely round cells do not migrate (31). The intermediate state of adhesion is most favorable for cell migration. DiMilla et al. developed a mathematical model, which predicts that maximal migration occurs at an intermediate ratio of cellular force (cytoskeletal contractility) to adhesive strength (integrin-matrix interactions) (29). Strong adhesion prevents the cell from releasing its cytoskeleton-ECM linkages, whereas weak adhesion does not generate the contractile force necessary for directed cell movement (30).
It is not entirely clear how focal adhesion restructuring by the matricellular proteins might influence cell motility. The domain of tenascin-C that stimulates focal adhesion disassembly also increases endothelial cell motility in a wound scratch assay (21). This response can be blocked with an antibody to annexin II, which serves as the receptor for tenascin-C–mediated focal adhesion disassembly, suggesting that focal adhesion disassembly correlates with increased motility. In addition, TSP stimulates endothelial cell motility in a Boyden chamber assay through its interactions with the NH2-terminal HBD (our unpublished data). These data are consistent with recent reports that this domain of TSP1 stimulates endothelial cell chemotaxis, although possibly through stimulation of matrix metalloproteinase (32). Preliminary data show both chemotactic and chemokinetic responses to hep I in a Dunn chamber assay, although the extent of endothelial cell migration in response to hep I in a wound scratch assay is more modest (our unpublished data). The maturity of the focal adhesion plaque and the presence of cell-cell junctions may modulate the ability of hep I to stimulate cell motility. In addition, the untethering of the actin microfilament network from clustered integrin receptors by hep I could lessen the contractile forces of cells and thus impede motility under certain conditions. It also remains to be determined whether the adhesive strength is similarly reduced by these matricellular proteins.
Unlike tenascin-C, SPARC inhibits endothelial cell chemotaxis in response to FGF-2, albeit through a domain that does not affect focal adhesion stability (33). Prolonged exposure to SPARC does induce a further transition from intermediate to weak adherence, and the inability of rounded cells to migrate is consistent with the previously described models of cell motility. Clearly, a systematic analysis of the effects of the antiadhesive domains of these matricellular proteins on cytoskeletal contractility and on force generation, requisite for motility, is needed.
De-adhesion and cell survival: the concept of anoikis.
Anchorage-dependent cells require cell adhesion for survival (7–9). When ECM-integrin interactions are disrupted, cells undergo apoptotic cell death. Adhesion-dependent cell death is termed “anoikis,” and it is proposed as a mechanism for preventing cell growth in inappropriate locations and for cavitation during embryogenesis. Some integrin isoforms preferentially mediate survival of specific cell types. However, there is also clear evidence that integrin signaling alone is not sufficient to prevent anoikis (8). Cell shape — in particular an extended spread morphology — is essential for survival (8). Cell attachment and spreading involve activation of focal adhesion kinase (FAK) and PI3K, and both of these mediators may act in adhesion-dependent antiapoptotic signaling (34, 35). FAK may be the primary mediator of survival under serum-free conditions. PI3K through activation of PDK1 activates the antiapoptotic serine-threonine kinase Akt/PKB. Akt is thought to block apoptosis through phosphorylation and inhibition of Bad, Forkhead transcription factors, and caspase-9.
The intermediate adhesive state therefore may not only favor cell migration, but, by maintaining the extended morphology and signaling through antiapoptotic mediators, may also support cell survival. If so, cells that become motile in response to injury would be expected to enjoy protection from apoptosis. Preliminary studies in our lab show that hep I induces a transient phosphorylation of both Akt and FAK and that hep I–treated cells do not become apoptotic. It will be interesting to determine whether tenascin-C and SPARC, which also promote the intermediate state, have similar antiapoptotic effects.
De-adhesion and cellular differentiation.
The composition and organization of the ECM profoundly influences the synthetic profile and thus the differentiation state of cells (6). One of the mechanisms whereby ECM proteins regulate specific protein expression is through stimulation of mitogen-activated protein kinase downstream of integrin activation. In addition, matricellular proteins such as SPARC and TSP1 modulate growth factors such as TGF-β that regulate transcriptional activity. Preliminary data from cells treated with hep I support this idea: expression of specific proteins is altered by 6 hours following treatment with hep I (our unpublished data). It is possible that the intermediate adhesive state engages a program of gene transcription and protein expression distinct from that of cells in a state of strong adherence. This would be consistent with the notion of “tensegrity” as developed by Ingber and colleagues, who suggest that the mechanical forces of the cytoskeleton regulate nuclear shape and organization (36). On the other hand, although SPARC has been shown to regulate matrix protein and protease expression, this activity is localized to domains outside those identified as being active in focal adhesion disassembly (33). The effects of the TNfnA-D domain on gene expression are unknown.
Summary
The process of cellular de-adhesion is potentially important for the ability of a cell to participate in morphogenesis and to respond to injurious stimuli. Cellular de-adhesion is induced by the highly regulated matricellular proteins TSP1 and 2, tenascin-C, and SPARC. These proteins induce a rapid transition to an intermediate state of adhesiveness characterized by loss of actin-containing stress fibers and restructuring of the focal adhesion plaque that includes loss of vinculin and α-actinin, but not of talin or integrin. This process involves intracellular signaling mediators, which are engaged in response to matrix protein–receptor interactions. Each of these proteins employs different receptors and signaling pathways to achieve this common morphologic endpoint. What is the function of this intermediate adhesive state and what is the physiologic significance of this action of the matricellular proteins? Given that matricellular proteins are expressed in response to injury and during development, one can speculate that the intermediate adhesive state is an adaptive condition that facilitates expression of specific genes that are involved in repair and adaptation. Since cell shape is maintained in weakly adherent cells, this state might induce survival signals to prevent apoptosis due to loss of strong cell adhesion, but yet allow for cell locomotion. The three matricellular proteins considered here might each preferentially facilitate one or more aspects of this adaptive response rather than all of these equally. Currently, we have only preliminary data to support the specific ideas proposed in this article. It will be interesting in the next several years to continue to elucidate the biological roles of the intermediate adhesive state induced by these matricellular proteins.
Acknowledgments
This work was supported by NIH grant HL-44575 and by an American Heart Association Established Investigator award to J.E. Murphy-Ullrich (grant 9640228N). The author wishes to acknowledge the essential contributions of Manuel Antonio Pallero, Jeffrey Greenwood, Silvia Goicoechea, Anthony Wayne Orr, and Claudio Pedraza to published and unpublished work discussed in this article. She also acknowledges the good fortune to have collaborators such as Magnus Höök, William Frazier, Harold Erickson, Trudy Cornwell, Tom Lincoln, Paul Eggleton, Helene Sage, and Anne Woods during the course of these studies. The author also wishes to apologize for the omission of some specific references due to space limitations.
References
- 1.Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions: SPARC, tenascin, thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- 2.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JC, Schwartz MA. Stimulation of fascin spikes by thrombospondin-1 is mediated by the GTPases rac and cdc42. J Cell Biol. 2000;150:807–822. doi: 10.1083/jcb.150.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenk MB, Midwood KS, Schwarzbauer JE. Tenascin-C suppresses Rho activation. J Cell Biol. 2000;150:913–919. doi: 10.1083/jcb.150.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 6.Lelievre S, Bissell MJ, Pujuguet P. Cell nucleus in context. Crit Rev Eukaryot Gene Expr. 2000;10:13–20. doi: 10.1615/critreveukargeneexpr.v10.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meredith JE, Jr, Schwartz MA. Integrins, adhesion, and apoptosis. Trends Cell Biol. 1997;7:146–150. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 9.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 10.Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood JA, Murphy-Ullrich JE. Signaling of de-adhesion in cellular regulation and motility. Microsc Res Tech. 1998;43:420–432. doi: 10.1002/(SICI)1097-0029(19981201)43:5<420::AID-JEMT8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Murphy-Ullrich JE, Höök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989;109:1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwood JA, Pallero MA, Theibert AB, Murphy-Ullrich JE. Thrombospondin signaling of focal adhesion disassembly requires activation of phosphoinositide 3-kinase. J Biol Chem. 1998;273:1755–1763. doi: 10.1074/jbc.273.3.1755. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood JA, Theibert AB, Prestwich GD, Murphy-Ullrich JE. Restructuring of focal adhesion plaques by PI 3-kinase. Regulation of PtdIns (3, 4, 5)-P3 binding to alpha-actinin. J Cell Biol. 2000;150:627–642. doi: 10.1083/jcb.150.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy-Ullrich JE, et al. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991;115:1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the calcium-binding EF-hand. J Cell Biochem. 1995;57:341–350. doi: 10.1002/jcb.240570218. [DOI] [PubMed] [Google Scholar]
- 17.Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Höök M. Heparin-binding peptides from thrombospondin-1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268:26784–26789. [PubMed] [Google Scholar]
- 18.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bornstein, P. 2001. Thrombospondins as matricellular modulators of cell function. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 20.Derr LB, Chiquet-Ehrismann R, Grandour-Edwards R, Spence J, Tucker RP. The expression of tenascin-C with the AD1 variable repeat in embryonic tissues, cell lines and tumors in various vertebrate species. Differentiation. 1998;62:71–82. doi: 10.1046/j.1432-0436.1997.6220071.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996;7:883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goicoechea S, Orr AW, Pallero MA, Eggleton P, Murphy-Ullrich JE. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J Biol Chem. 2000;275:36358–36368. doi: 10.1074/jbc.M005951200. [DOI] [PubMed] [Google Scholar]
- 23.Fielding PE, Fielding CJ. Plasma membrane caveolae mediate the efflux of cellular free cholesterol. Biochemistry. 1995;34:14288–14292. doi: 10.1021/bi00044a004. [DOI] [PubMed] [Google Scholar]
- 24.Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1226–L1235. doi: 10.1152/ajplung.2000.279.6.L1226. [DOI] [PubMed] [Google Scholar]
- 25.Bradshaw, A.D., and Sage, E.H. 2001. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 26.Murphy-Ullrich JE, et al. Cyclic GMP-dependent protein kinase is required for thrombospondin and tenascin mediated focal adhesion disassembly. J Cell Sci. 1996;109:2499–2508. doi: 10.1242/jcs.109.10.2499. [DOI] [PubMed] [Google Scholar]
- 27.Reinhard M, et al. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 1995;14:1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motamed K, Sage EH. SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem. 1998;70:543–552. doi: 10.1002/(sici)1097-4644(19980915)70:4<543::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palacek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 31.Huttenlocher A, Sandborg RB, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 32.Taraboletti G, et al. The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. FASEB J. 2000;14:1674–1676. doi: 10.1096/fj.99-0931fje. [DOI] [PubMed] [Google Scholar]
- 33.Yan Q, Sage EH. SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem. 1999;47:1495–1505. doi: 10.1177/002215549904701201. [DOI] [PubMed] [Google Scholar]
- 34.Khwaja A, Rodriquez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilic D, et al. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]