Abstract
Parathyroid hormone (PTH) secretion is regulated by a cell surface Ca2+ receptor that detects small changes in the level of plasma Ca2+. Because this G protein-coupled receptor conceivably provides a distinct molecular target for drugs useful in treating bone and mineral-related disorders, we sought to design small organic molecules that act on the Ca2+ receptor. We discovered that certain phenylalkylamine compounds, typified by NPS R-568 and its deschloro derivative NPS R-467, increased the concentration of cytoplasmic Ca2+ ([Ca2+]i) in bovine parathyroid cells and inhibited PTH secretion at nanomolar concentrations. These effects were stereoselective and the R enantiomers were 10- to 100-fold more potent than the S enantiomers. NPS R-568 potentiated the effects of extracellular Ca2+ on [Ca2+]i and PTH secretion but was without effect in the absence of extracellular Ca2+. Both compounds shifted the concentration–response curves for extracellular Ca2+ to the left. Presumably, these compounds act as positive allosteric modulators to increase the sensitivity of the Ca2+ receptor to activation by extracellular Ca2+. Both NPS R-467 and NPS R-568 increased [Ca2+]i in HEK 293 cells expressing the human parathyroid Ca2+ receptor but were without effect in wild-type HEK 293 cells. Neither compound affected the cytoplasmic Ca2+ responses elicited by several other G protein-coupled receptors in HEK 293 cells or in bovine parathyroid cells. Significantly, these compounds did not affect responses elicited by the homologous metabotropic glutamate receptors, mGluR1a, mGluR2, or mGluR8. These compounds therefore act selectively on the Ca2+ receptor. Compounds that mimic or potentiate the effects of extracellular Ca2+ at the Ca2+ receptor are termed calcimimetics. The discovery of calcimimetic compounds with potent and selective activity enables a pharmacological approach to regulating plasma levels of PTH. Calcimimetic compounds could conceivably provide a specific medical therapy for primary hyperparathyroidism.
Keywords: cytoplasmic Ca2+, hyperparathyroidism, parathyroid cells, NPS R-568, NPS R-467
The concentration of ionized calcium (Ca2+) in plasma is regulated largely by parathyroid hormone (PTH), which acts on the kidney and on bone to increase the level of plasma Ca2+. Elevated levels of plasma Ca2+, in turn, depress the secretion of PTH. The parathyroid cell is therefore somewhat unusual because it can sense small changes in the concentration of extracellular Ca2+ and alter hormone secretion appropriately. Studies based on measurements of cytoplasmic Ca2+ concentrations ([Ca2+]i) gave rise to the hypothesis that parathyroid cells express a cell surface Ca2+ receptor that enables these cells to detect and respond to small changes in the concentration of extracellular Ca2+ (1, 2). Subsequent studies supported the existence of a Ca2+ receptor (for review, see ref. 3), and the hypothesis has now been confirmed by the recent cloning of a cDNA encoding a Ca2+ receptor from bovine (4) and human (5) parathyroid cells.
The Ca2+ receptor is a member of the G protein-coupled receptor superfamily and possesses an unusually large extracellular domain, the characteristic seven transmembrane domain, and a relatively long cytoplasmic tail. In these topological aspects, the Ca2+ receptor is similar to metabotropic glutamate receptors (mGluR), although the sequence homology between these receptors is only about 25%. The human (1,078 amino acids) and bovine (1,085 amino acids) parathyroid cell Ca2+ receptors are glycosylated proteins of ∼120 kDa and are 93% identical (5). The Ca2+ receptor expressed on authentic parathyroid cells or in heterologous cellular systems couples to phospholipase C and, when activated by increased concentrations of extracellular Ca2+, elicits rapid increases in inositol 1,4,5-trisphosphate and [Ca2+]i (2, 4, 6). Thus, in its functional and structural properties, the parathyroid Ca2+ receptor is akin to other cell surface receptors that initially transduce extracellular signals into functional cellular responses. The difference is that the physiological ligand for the Ca2+ receptor is an inorganic ion, rather than an organic molecule.
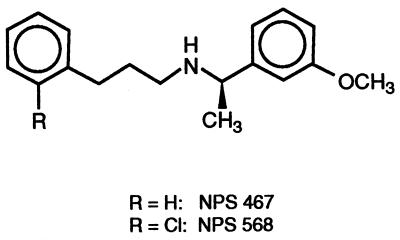
G protein-coupled receptors have been a classic site of action for drugs useful in treating various diseases. As a member of the G protein-coupled receptor superfamily, the Ca2+ receptor is seemingly an ideal target for new pharmaceuticals useful in treating disorders of bone and mineral metabolism, such as hyperparathyroidism and osteoporosis. At present, however, the only ligands known to act on the Ca2+ receptor are other inorganic di- and trivalent cations (2, 7) and organic polycations (8–10) that are nonselective and lack utility as systematic therapeutics. The present report describes the results of our initial efforts to devise a small organic compound that selectively acts on the parathyroid Ca2+ receptor yet lacks a polycationic structure. NPS R-467 and NPS R-568 (Fig. 1) are two compounds that emerged from this effort. They are potent and selective activators of the Ca2+ receptor and inhibit PTH secretion in vitro. Such an action defines a new class of pharmacological agents and suggests an entirely novel approach to treating hyperparathyroidism.
Figure 1.
Structures of NPS 467 and NPS 568 shown as the R enantiomer.
MATERIALS AND METHODS
Buffers.
Parathyroid cell buffer (PCB) contained 126 mM NaCl, 4 mM KCl, 1 mM MgSO4, 0.7 mM K2HPO4/KH2PO4, 20 mM Hepes⋅NaOH (pH 7.45), and variable amounts of CaCl2 as indicated. PCB was supplemented with glucose (1 mg/ml) and BSA (Miles; fraction V) as specified. Measurements of [Ca2+]i were performed in PCB lacking phosphate and sulfate but containing 1 mM MgCl2.
Studies with Bovine Parathyroid Cells.
Dissociated bovine parathyroid cells were prepared by collagenase digestion of minced glands and purified on Percoll gradients (11). After extensive washing, the cells were resuspended in a 1:1 mixture of Ham’s F-12 and DMEM (F12/DMEM) supplemented with penicillin (10 units/ml), streptomycin (10 μg/ml), gentimicin (4 μg/ml), and ITS+ (Collaborative Research) and incubated at 37°C in a humidified atmosphere of 95% air-5% CO2. These concentrations of streptomycin and gentimicin are below those required to activate the Ca2+ receptor. After overnight culture, the cells were washed and resuspended in PCB for measurements of [Ca2+]i (11) or PTH secretion (12).
Studies with HEK 293 Cells.
A 4.0-kb NotI–HindIII fragment of the human parathyroid cell Ca2+ receptor (hPCaR) cDNA was subcloned into the mammalian expression vector pCEP4 (Invitrogen) containing the hygromycin-resistance gene as a selectable marker. This plasmid was transfected into HEK 293 cells by calcium phosphate precipitation. Transfected cells were grown in DMEM containing 10% fetal bovine serum and hygromycin (200 μg/ml). Hygromycin-resistant colonies were subcloned and assayed for hPCaR mRNA by solution hybridization using a 32P-labeled RNA probe complementary to the (4.0 kb) hPCaR sequence (5). In the present study, clone 7 was used to assess the effects of compounds on [Ca2+]i. This stably transfected cell line is termed HEK 293 4.0–7.
An XhoI(blunted)–BamHI fragment of the rat mGluR1a cDNA was subcloned into the mammalian expression vector pCEP4 containing hygromycin as a selectable marker. This plasmid was transfected into HEK 293 cells by using Lipofectamine (GIBCO/BRL). Cells were grown in DMEM (without l-glutamine) supplemented with 10% dialyzed fetal bovine serum and hygromycin (200 μg/ml).
For measurements of [Ca2+]i, the cells were recovered from tissue culture flasks by brief treatment with 0.02% EDTA and then washed and resuspended in PCB containing 1 mM CaCl2 and 0.1% BSA. The cells were loaded with fluo-3 by incubation for 30 min at 37°C, with PCB containing 0.5% BSA in 1 mM CaCl2 and 2 μM fluo-3 acetoxymethyl ester. The cells were subsequently washed and fluorescence was recorded by using excitation and emission wavelengths of 485 and 530 nm, respectively.
Studies with Xenopus Oocytes.
Oocytes were isolated from adult female Xenopus laevis toads and injected with 5 ng of cRNA encoding either the human mGluR8 or human mGluR2 plus 1 ng of G protein-coupled inwardly rectifying K+ channel (GIRK) and 1 ng of cardiac inwardly rectifying K+ channel (CIR) cRNA and incubated at 16°C in MBS for 3 or 4 days prior to electrophysiological recording (13). MBS contained 88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, 10 mM Hepes, and 2.4 mM NaCO3 (pH, 7.5). Oocytes were voltage-clamped at a holding potential of −90 mV by using standard two-electrode voltage-clamp techniques, and currents were recorded on a chart recorder. The standard control buffer was ND96 (96 mM NaCl/4 mM KCl/5 mM Hepes/1 mM CaCl2/1 mM MgCl2, pH 7.5). All substances were added by bath superfusion at a flow rate of about 5 ml/min. Prior to application of test substances, the oocytes were allowed to equilibrate in a high potassium solution (HK25) containing 25 mM KCl and 75 mM NaCl. Activation of mGluR2 or mGluR8 through GIRK/CIR was quantified by measuring the peak inward current, relative to the holding current at −90 mV.
Synthetic Chemistry.
NPS 568 was prepared by the condensation of 3′-methoxyacetophenone with 3-(2′-chlorophenyl)-1-propylamine in the presence of titanium in isopropoxide. Reduction of the intermediate imine was accomplished with sodium cyanoborohydride in ethanol. Standard work-up followed by silica gel chromatrography afforded NPS 568 in 96% yield. NPS 467 was prepared in a similar fashion starting with 3-phenyl-1-propylamine. Enantiomers of both compounds were resolved with chiral HPLC (ChiralCel OD; 25 × 2 cm), and the hydrochloride salt of each enantiomer was prepared by treatment of the free base with anhydrous HCl in diethyl ether.
Structural analysis was by GC–MS, 1H NMR, 13C NMR, and combustion analysis. Optical purity (>99.5%) was assessed by chiral HPLC. The structure and absolute configuration of NPS R-568⋅HCl were confirmed by x-ray crystallography.
Data Presentation and Analysis.
The concentrations reported are for the free base of NPS R-467 and NPS R-568. To determine EC50 or IC50 values, concentration–response data were fit to the measured [Ca2+]i or PTH level with the Levenberg–Marquardt algorithm by using the kaleidagraph program (Synergy Software).
RESULTS
To screen for agonist-like activity on the Ca2+ receptor, we assessed the ability of test substances to evoke an increase in [Ca2+]i in bovine parathyroid cells. Screening of phenylalkylamine-type compounds revealed that fendiline caused a large increase in [Ca2+]i (EC50 = 12 μM); and this compound served as the starting point for structural modifications. Out of a number of analogs synthesized and tested, NPS 568 and its deschloro derivative NPS 467 (Fig. 1) were selected for detailed pharmacological profiling.
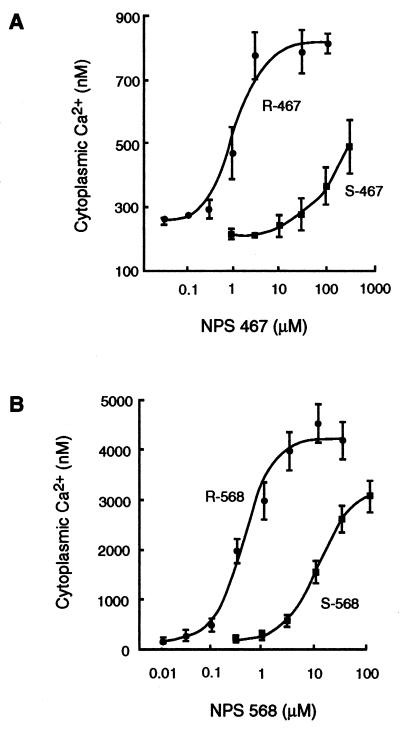
The racemic mixtures of NPS 568 and NPS 467 were resolved into the R and S enantiomers and each isomer was tested for its ability to increase [Ca2+]i in bovine parathyroid cells. Both isomers caused concentration-dependent increases in [Ca2+]i. The R enantiomer of NPS 467 was about 100-fold more potent than the corresponding S enantiomer (Fig. 2A), and the R enantiomer of NPS 568 was about 10-fold more potent than its corresponding enantiomer (data not shown). When tested on HEK 293 cells expressing the human parathyroid Ca2+ receptor (HEK 293 4.0–7 cells), both isomers caused increases in [Ca2+]i. Again, the R enantiomers were 10- to 100-fold more potent than the S enantiomers (Fig. 2B). In both cell types, NPS R-568 was about twice as potent as NPS R-467.
Figure 2.
NPS 467 and NPS 568 increase [Ca2+]i in a concentration-dependent and stereoselective manner. Bovine parathyroid cells loaded with fura-2 (A) were suspended in PCB containing 0.5 mM CaCl2 and treated with the indicated concentration of the R or S enantiomer of NPS 467. HEK 293 cells expressing the human parathyroid cell Ca2+ receptor and loaded with fluo-3 (B) were suspended in medium containing 1 mM CaCl2 and treated with the indicated concentration of NPS R-568 or NPS S-568. For both cell types, the peak transient increase in [Ca2+]i evoked by NPS 467 or NPS 568 is plotted. Each point is the mean ± SEM of three or four experiments.
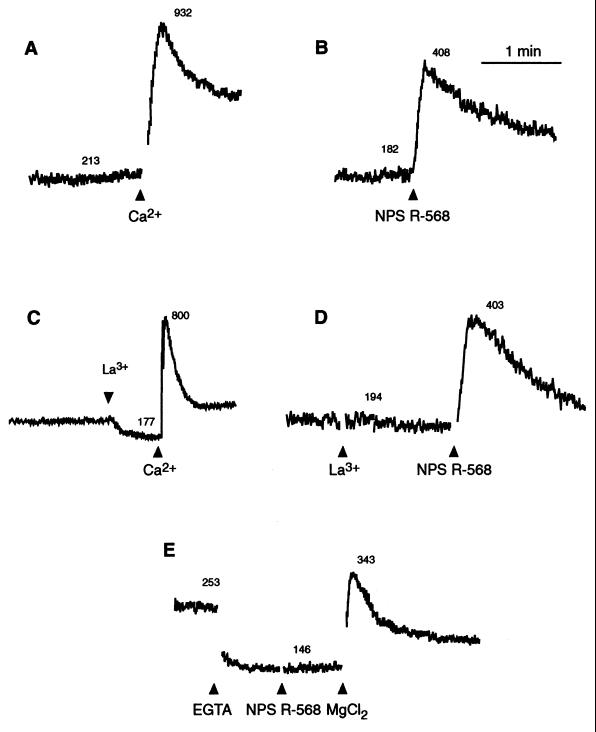
In parathyroid cells, the increase in [Ca2+]i elicited by extracellular Ca2+ arises from the mobilization of intracellular Ca2+ and the influx of extracellular Ca2+ through voltage-insensitive channels (2, 14). The pattern of change of [Ca2+]i elicited by NPS R-467 or NPS R-568 in bovine parathyroid cells was similar to that evoked by extracellular Ca2+ and consisted of a rapid and transient increase followed by a lower yet sustained increase in [Ca2+]i (Fig. 3 A and B). When influx of extracellular Ca2+ was blocked by pretreatment with a low concentration of La3+ (1 μM), the transient phase elicited by extracellular Ca2+ or the compounds remained, but the sustained steady-state increase in [Ca2+]i was blocked (Fig. 3 C and D). This suggests that the transient increase in [Ca2+]i elicited by NPS R-467 or NPS R-568, observed in the presence of a low concentration of extracellular Ca2+ (0.5 mM), results from the mobilization of intracellular Ca2+. To test this directly, extracellular Ca2+ was reduced to <1 μM by the addition of EGTA. Surprisingly, in the nominal absence of extracellular Ca2+, neither NPS R-467 nor NPS R-568 caused an increase in [Ca2+]i although extracellular Mg2+ (a known Ca2+ receptor agonist) evoked a large increase in [Ca2+]i under these conditions (Fig. 3E). The phenylalkylamine compounds were similarly without effect on [Ca2+]i in HEK 293 4.0–7 cells when extracellular Ca2+ was omitted (data not shown). These findings indicate that the effects of NPS R-467 or NPS R-568 on cytoplasmic Ca2+ are dependent on the presence of extracellular Ca2+.
Figure 3.
Increases in [Ca2+]i elicited by NPS R-568 are refractory to inhibition by La3+ but are blocked by removal of extracellular Ca2+. Bovine parathyroid cells were suspended in medium containing 0.5 mM CaCl2. In all traces, the final concentration of NPS R-568 was 1 μM. La3+ (1 μM, final), EGTA (1 mM, final), or Mg2+ (3 mM, final) was added as indicated. The numbers accompanying each trace are estimates of [Ca2+]i in nM. Each trace is from a single preparation of cells and is representative of the pattern seen in three other experiments.
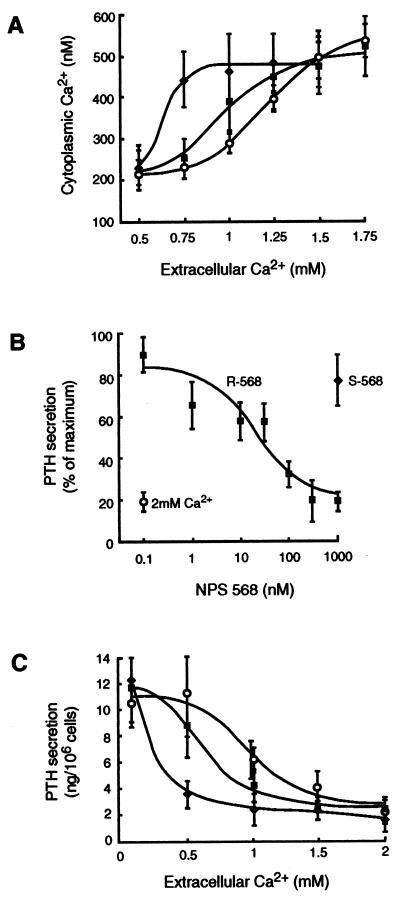
To further investigate the mechanism of action of these compounds, concentration–response curves to extracellular Ca2+ in the presence and absence of NPS R-467 or NPS R-568 were determined in bovine parathyroid cells. In the absence of NPS R-568, increasing the concentration of extracellular Ca2+ caused progressive increases in [Ca2+]i and did so with an EC50 of 1.66 ± 0.34 mM. The addition of NPS R-568 (100 nM) shifted the concentration–response curve for extracellular Ca2+ to the left without affecting the maximal response and, thereby, decreased the EC50 value for extracellular Ca2+ to 0.61 ± 0.04 mM (Fig. 4A). At these concentrations, NPS R-568 failed to elicit increases in [Ca2+]i when extracellular Ca2+ was ≤0.5 mM or have further stimulating effects when levels of extracellular Ca2+ were ≥1.5 mM. Essentially identical results were obtained with NPS R-467 or when either compound was studied under the same experimental conditions with HEK 293 4.0–7 cells. These compounds therefore potentiate the cytoplasmic Ca2+ response to extracellular Ca2+.
Figure 4.
NPS R-568 potentiates the effects of extracellular Ca2+ on [Ca2+]i and PTH secretion. In A, bovine parathyroid cells loaded with fura-2 and suspended in buffer containing 0.5 mM CaCl2 were pretreated with 100 nM (⧫) or 10 nM (▪) NPS R-568 or vehicle control (○) for 1 min before extracellular Ca2+ was increased to the final concentration indicated. In B, bovine parathyroid cells were incubated in buffer containing 0.5 mM CaCl2 for 30 min in the presence or absence of the indicated NPS R-568 (▪) or NPS S-568 (⧫). For comparison, the effects of increasing the concentration of CaCl2 to 2 mM (○) are shown. In C, bovine parathyroid cells were incubated for 30 min in buffer containing the indicated concentration of CaCl2 in the absence (○) or presence of 10 nM (⧫) or 100 nM (⧫) NPS R-568. Each point is the mean ± SEM of three experiments.
Both compounds inhibited secretion of PTH in a concentration-dependent and stereoselective manner. In bovine parathyroid cells, the inhibitory effects of NPS R-568 on PTH secretion were seen at concentrations as low as 3 nM and the IC50 was 27 ± 7 nM. NPS R-467 also inhibited PTH secretion at concentrations ≥10 nM. The S enantiomer of NPS R-467 or NPS R-568 did not affect secretion of PTH even when tested at concentrations as high as 1 μM. This is shown for NPS 568 in Fig. 4B. Significantly, the magnitude of inhibition (70–80%) achieved with NPS R-568 was just as great as that caused by the physiological ligand, extracellular Ca2+ (Fig. 4B).
As with the effect of these compounds on [Ca2+]i, their effects on the secretion of PTH was abolished at low levels of extracellular Ca2+. Rather, both compounds augmented the inhibitory effects of extracellular Ca2+, thereby shifting the concentration–response curve for extracellular Ca2+ to the left (Fig. 4C). The IC50 value for inhibition of PTH secretion by extracellular Ca2+ was decreased by NPS R-568 from 0.97 ± 0.02 to 0.36 ± 0.09 mM. Thus, these compounds increase the potency of extracellular Ca2+ on both cytoplasmic Ca2+ and secretory responses.
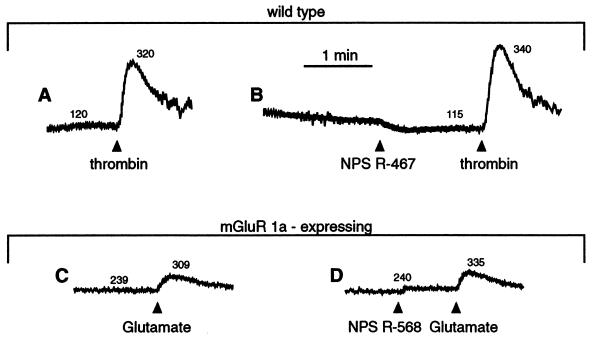
To assess the selectivity of these compounds, we determined their effects on wild-type HEK 293 cells and on the cytoplasmic Ca2+ responses elicited by other agonists acting on several different G protein-coupled receptors. Neither compound altered [Ca2+]i in wild-type HEK 293 cells, even when tested at concentrations as high as 10 μM (Fig. 5A). Because the wild-type HEK 293 cells do not express the Ca2+ receptor, but HEK 293 4.0–7 cells do, these results indicate that expression of the Ca2+ receptor is necessary for these compounds to affect [Ca2+]i in HEK 293 cells. The wild-type HEK 293 cell line, however, does express other G protein-coupled receptors, specifically those for bradykinin, thrombin, and ATP. These ligands cause a rapid increase in [Ca2+]i in wild-type HEK 293 cells, similar to that caused by extracellular Ca2+ in HEK 293 4.0–7 cells. However, the cytoplasmic Ca2+ responses elicited by submaximal concentrations of bradykinin, thrombin, or ATP were neither augmented nor depressed by high concentrations (10 μM) of NPS R-467 or NPS R-568 (Fig. 5 A and B). Nor did these compounds affect the cytoplasmic Ca2+ response to ATP in bovine parathyroid cells. This P2Y-purinergic receptor, like the Ca2+ receptor, is coupled to the mobilization of intracellular Ca2+ in parathyroid cells (15). Yet ATP (100 μM) elicited essentially identical increases in [Ca2+]i in both the presence (156 ± 33% increase over basal [Ca2+]i) and absence of 100 nM NPS R-568 (163 ± 37% increase; n = 3). Moreover, the concentration–response curve to ATP (3–100 μM) in parathyroid cells was similar in the presence and the absence of 300 nM NPS R-467.
Figure 5.
NPS R-467 and NPS R-568 act selectively on the parathyroid Ca2+ receptor. All traces are from either wild-type HEK 293 cells (A and B) or HEK 293 cells expressing the mGluR1a receptor (C and D).
Although the above findings indicate that NPS R-467 and NPS R-568 do not affect a number of other structurally diverse G protein-coupled receptors, a more rigorous test of selectivity would be to examine the effects of these compounds on G protein-coupled receptors that are structurally related to the Ca2+ receptor. The G protein-coupled receptors that share significant homology with the Ca2+ receptor are the mGluRs. Group I mGluRs (mGluR1 and mGluR5), like the Ca2+ receptor, couple to phospholipase C and, when activated, elicit the mobilization of intracellular Ca2+ (16). We therefore tested for the effects of these compounds on the cytoplasmic Ca2+ responses elicited by glutamate in HEK 293 cells expressing the rat mGluR1a. In these cells, glutamate elicits a rapid increase in [Ca2+]i that arises from the mobilization of intracellular Ca2+ (Fig. 5 C and D). Treatment with NPS R-568 did not evoke an increase in [Ca2+]i nor did it affect the cytoplasmic Ca2+ responses elicited by the subsequent addition of glutamate in these mGluR1a-expressing cells.
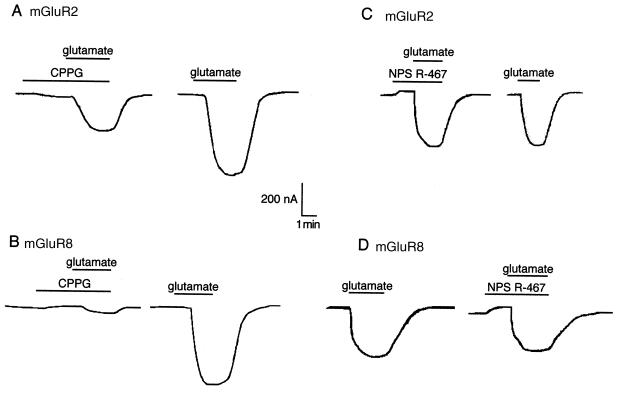
Group II (mGluR2 and mGluR3) and group III receptors (mGluR4, mGluR6, mGluR7, and mGluR8) do not couple effectively to the mobilization of intracellular Ca2+ but do activate GIRK when expressed in Xenopus oocytes (13). Phenylalkylamine compounds were therefore examined for effects on glutamate-evoked currents in Xenopus oocytes expressing mGluR2 or mGluR8 and GIRK/CIR. Maximal responses to l-glutamate were obtained at 30 μM in oocytes expressing mGluR2 and at 10 μM in those expressing mGluR8. Submaximal concentrations of glutamate elicited an inward K+ current in oocytes that was either partially (for mGluR2) or totally blocked (for mGluR8) by the glutamate receptor antagonist (R,S)-α-cyclopropyl-4-phosphonophenylglycine (Fig. 6 A and B). By itself, 10 μM NPS R-467 caused a small inhibition of the basal K+ current (10 ± 2% inhibition, n = 6) that was not dependent on expression of either mGluR2 or mGluR8. However, even this relatively high concentration of NPS R-467 was without effect on glutamate-evoked responses in oocytes expressing mGluR2 and only slightly inhibited evoked responses in those expressing mGluR8 (10 ± 6% inhibition, n = 4; Fig. 6 C and D). Similar results were obtained with 10 μM NPS R-568. The phenylalkylamine compounds thus appear to lack activity at mGluRs.
Figure 6.
NPS R-467 does not affect responses mediated by mGluR2 (A and C) or mGluR8 (B and D). Oocytes were equilibrated in HK25 buffer for 3–5 min before the application of NPS R-467 (10 μM) or (R,S)-α-cyclopropyl-4-phosphonophenylglycine (CPPG; 10 μM) and l-glutamate (3 or 10 μM) as indicated by the horizontal bars above each trace.
DISCUSSION
Although extracellular Ca2+ is the primary physiological ligand activating the Ca2+ receptor, a variety of di- and trivalent cations and organic polycations can also act as ligands at this receptor and decrease secretion of PTH (2, 7–10). Polycationic ligands, however, are of limited utility as pharmacological probes of the Ca2+ receptor because they lack specificity and/or potency. Inorganic and organic polycations are clearly unsuitable for altering plasma levels of PTH in disease states. Indeed, it has been uncertain whether pharmaceutically useful compounds acting on the Ca2+ receptor could be designed, because the atomic nature of the physiological ligand provides essentially no structural insight into the type of compounds that might act on this receptor. The present results provide evidence that potent and selective small organic compounds that lack a polycationic structure yet retain activity on the Ca2+ receptor can be devised.
Phenylalkylamine compounds like NPS R-467 or NPS R-568 do not elicit cytoplasmic Ca2+ responses in wild-type HEK 293 cells but do so in HEK 293 cells expressing the Ca2+ receptor. Either these compounds directly activate the Ca2+ receptor or they affect some transmembrane signaling mechanism peculiar to the Ca2+ receptor. This putative signaling mechanism cannot couple to receptors for thrombin, bradykinin, or ATP because the phenylalkylamine compounds neither elicit nor block cytoplasmic Ca2+ responses in wild-type cells expressing these receptors. Similarly, this presumed signaling mechanism is not peculiar to expression of a nonnative receptor because cells transfected with mGluR1a also do not respond to the phenylalkylamine compounds. The most parsimonious view supposes that NPS R-467 and NPS R-568 act directly on the Ca2+ receptor.
To the extent studied so far, the effects of the phenylalkylamine compounds on parathyroid cell function parallel those of extracellular Ca2+; i.e., they elicit increases in [Ca2+]i that arise from the mobilization of intracellular Ca2+ and the influx of extracellular Ca2+ and they inhibit the secretion of PTH. Preliminary results suggest that these phenylalkylamine compounds also block β-adrenergic receptor-induced increases in cAMP formation in a pertussis toxin-sensitive manner (M.E.S. and E.F.N., unpublished data) as does extracellular Ca2+ (17). Despite these parallels, the molecular mechanism of action of the phenylalkylamine compounds differs from that of extracellular Ca2+ and other polycations. Unlike the inorganic and organic polycations, the activity of the phenylalkylamine compounds is dependent on the presence of extracellular Ca2+ or some substitute polycation. The ability of the phenylalkylamine compounds to shift the concentration–response curves for extracellular Ca2+ without affecting the maximal responses suggests that these compounds act as allosteric effectors and not as conventional receptor agonists.
Phenylalkylamine compounds are known to interact with a number of different molecular targets. Nonetheless, NPS R-467 and NPS R-568, despite their relatively simple structure, do not affect responses to a variety of other G protein-coupled receptors. Particularly noteworthy is their failure to affect responses to a representative receptor from each of the three groups of mGluRs. Of all structurally characterized G protein-coupled receptors, these are the most homologous to the Ca2+ receptor. In testing for activity on other G protein-coupled receptors, submaximal concentrations of agonist (usually an EC80) were used, so it is unlikely that these phenylalkylamines acted as agonists, antagonists, or allosteric modulators at these other receptors.
The structure of the phenylalkylamine compounds, their potency, selectively, and mechanism of action are features that define a new class of pharmacological compounds. These structural and pharmacodynamic properties are significant improvements over existing Ca2+ receptor ligands. Ligands that mimic or potentiate the effects of extracellular Ca2+ at the Ca2+ receptor have been termed calcimimetics (18). The phenylalkylamine compounds define a distinct subclass called type II calcimimetics. They are allosteric effectors that seemingly increase the sensitivity of the Ca2+ receptor to activation by extracellular Ca2+. In contrast, type I calcimimetics act as conventional receptor agonists. At present, all known type I calcimimetics are inorganic or organic polycations.
Although it is clear that type II calcimimetics effectively lower the concentrations of extracellular Ca2+ required for activating the Ca2+ receptor, the molecular details of this effect are far from certain. It is not known, for example, whether phenylalkylamine compounds bind to the receptor in the absence of extracellular Ca2+ or whether binding of extracellular Ca2+ unmasks a cryptic binding site for these type II calcimimetics. It is also unknown whether type II calcimimetics cause shifts in the concentration–response curves for extracellular Ca2+ by increasing the binding affinity for extracellular Ca2+, increasing the efficiency of Ca2+ receptor–G protein coupling, or affecting some other mechanism proximal to the measured variables of [Ca2+]i or secretion of PTH. Small organic compounds that act as potent and selective type I calcimimetics would facilitate answers to these questions.
Type II calcimimetics, typified by NPS R-467 and NPS R-568, suggest a fundamentally new therapeutic approach to the treatment of bone and mineral-related disorders. Compounds of this sort might provide a novel means of controlling plasma levels of PTH in disease states. Because they decrease the secretion of PTH, type II calcimimetic compounds conceivably provide a new therapeutic approach to the management of both primary and secondary hyperparathyroidism. Indeed, a small study has recently demonstrated that orally administered NPS R-568 lowers plasma levels of PTH and Ca2+ in postmenopausal women with mild primary hyperparathyroidism (19). Thus, calcimimetic compounds may provide the first medical therapy for primary hyperparathyroidism, a disease that has so far resisted pharmacological intervention.
Acknowledgments
We thank Dr. Kimberly V. Rogers and Christine Dunn for constructing HEK 293 4.0–7 cells and Karen Krapcho for constructing HEK cells expressing mGluR1a. The research in this article was funded primarily by National Institutes of Health Small Business Innovative Research Program Grant no. 5 R44 43636-03.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PTH, parathyroid hormone; [Ca2+]i, concentration of cytoplasmic Ca2+; mGluR, metabotropic glutamate receptor; GIRK, G protein-coupled inwardly rectifying K+ channel; CIR, cardiac inwardly rectifying K+ channel.
References
- 1.Nemeth E F, Scarpa A. FEBS Lett. 1986;203:15–19. doi: 10.1016/0014-5793(86)81427-2. [DOI] [PubMed] [Google Scholar]
- 2.Nemeth E F, Scarpa A. J Biol Chem. 1987;262:5188–5196. [PubMed] [Google Scholar]
- 3.Brown E M. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 4.Brown E M, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger M A, Lytton J, Hebert S C. Nature (London) 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 5.Garrett J E, Capuano I V, Hammerland L G, Hung B C P, Brown E M, Hebert S C, Nemeth E F, Fuller F H. J Biol Chem. 1995;270:12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- 6.Shoback D M, Membreno L A, McGhee J G. Endocrinology. 1988;123:382–389. doi: 10.1210/endo-123-1-382. [DOI] [PubMed] [Google Scholar]
- 7.Brown E M, Butters R, Katz C, Kifor O. Endocrinology. 1991;128:3047–3054. doi: 10.1210/endo-128-6-3047. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E F. In: Calcium-Binding Proteins in Health and Disease. Norman A W, Vanaman T C, Means A R, editors. San Diego: Academic; 1987. pp. 36–38. [Google Scholar]
- 9.Brown E M, Fuleihan Ge-H, Chen C J, Kifor O. Endocrinology. 1990;127:1064–1071. doi: 10.1210/endo-127-3-1064. [DOI] [PubMed] [Google Scholar]
- 10.Brown E M, Katz C, Butters R, Kifor O. J Bone Miner Res. 1991;6:1217–1225. doi: 10.1002/jbmr.5650061112. [DOI] [PubMed] [Google Scholar]
- 11.Racke F K, Nemeth E F. J Physiol. 1993;468:141–162. doi: 10.1113/jphysiol.1993.sp019764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Racke F K, Nemeth E F. J Physiol. 1993;468:163–176. doi: 10.1113/jphysiol.1993.sp019765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saugstad J A, Segerson T P, Westbrook G L. J Neurosci. 1996;16:5979–5985. doi: 10.1523/JNEUROSCI.16-19-05979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muff R, Nemeth E F, Haller-Brem S, Fischer J A. Arch Biochem Biophys. 1988;265:128–135. doi: 10.1016/0003-9861(88)90378-5. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E F, Kosz L M. Am J Physiol. 1989;257:E505–E513. doi: 10.1152/ajpendo.1989.257.4.E505. [DOI] [PubMed] [Google Scholar]
- 16.Knöpfel T, Gasparini F. DDT. 1996;1:103–108. [Google Scholar]
- 17.Chen C J, Barnett J V, Congo D A, Brown E M. Endocrinology. 1989;124:233–239. doi: 10.1210/endo-124-1-233. [DOI] [PubMed] [Google Scholar]
- 18.Nemeth E F. Principles of Bone Biology. San Diego: Academic; 1996. pp. 1019–1035. [Google Scholar]
- 19.Silverberg S J, Bone H G, III, Marriott T B, Locker F G, Thys-Jacobs S, Dziem G, Kaatz S, Sanguinetti E L, Bilezkian J P. N Engl J Med. 1997;337:1506–1510. doi: 10.1056/NEJM199711203372104. [DOI] [PubMed] [Google Scholar]