Abstract
Environmentally benign insecticides derived from the soil bacterium Bacillus thuringiensis (Bt) are the most widely used biopesticides, but their success will be short-lived if pests quickly adapt to them. The risk of evolution of resistance by pests has increased, because transgenic crops producing insecticidal proteins from Bt are being grown commercially. Efforts to delay resistance with two or more Bt toxins assume that independent mutations are required to counter each toxin. Moreover, it generally is assumed that resistance alleles are rare in susceptible populations. We tested these assumptions by conducting single-pair crosses with diamondback moth (Plutella xylostella), the first insect known to have evolved resistance to Bt in open field populations. An autosomal recessive gene conferred extremely high resistance to four Bt toxins (Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F). The finding that 21% of the individuals from a susceptible strain were heterozygous for the multiple-toxin resistance gene implies that the resistance allele frequency was 10 times higher than the most widely cited estimate of the upper limit for the initial frequency of resistance alleles in susceptible populations. These findings suggest that pests may evolve resistance to some groups of toxins much faster than previously expected.
Insecticidal crystal proteins from the cosmopolitan soil bacterium Bacillus thuringiensis (Bt) are becoming a cornerstone of insect pest management (1). These toxins kill insects by binding to and creating pores in midgut membranes (2). Unlike many conventional insecticides, Bt toxins do not harm people, arthropod natural enemies, or most other nontarget organisms (1). Evolution of resistance to insecticides has occurred in more than 500 species of arthropods (3), but so far only one crop pest, the diamondback moth (Plutella xylostella), has evolved resistance to Bt in open field populations (4). Nonetheless, laboratory selection experiments show that many insects can become resistant to Bt (4). The risk of resistance has increased, because sprays of Bt are being used more often to control pests of crops and forests as well as insect vectors of human disease (1). Also, 1996 was the first year that transgenic crops producing insecticidal proteins from Bt were grown commercially in the United States. During 1996, U.S. growers planted approximately 1 million hectares of transgenic corn, cotton, and potatoes that produce Bt toxins Cry1Ab, Cry1Ac, and Cry3A, respectively. Better understanding of the genetic basis of resistance is essential for devising strategies to delay resistance (5).
Many strategies proposed for managing resistance to pesticides incorporate two or more toxins either in combination or sequence (5–9). It also has been suggested that the naturally occurring combinations of toxins produced by Bt and by plants have slowed counteradaptation by insects (2, 9). Nonetheless, insects have shown the ability to overcome such combinations of toxins (4, 10). The putative value of multiple-toxin strategies, whether devised by people or molded by natural selection, depends on the assumption that the gene or genes conferring resistance to one toxin segregate independently from those conferring resistance to other toxins (6–8). Conversely, cross-resistance occurs when selection with one toxin or set of toxins reduces susceptibility to other toxins. Evaluation of field- and laboratory-selected strains of insects shows that narrow to broad cross-resistance to Bt is possible (4, 9, 11–15).
The genetic basis of insect resistance to single Bt toxins and to multiple-toxin strains of Bt has been studied (4, 9, 12–14), yet most previous investigations have not determined if resistance to groups of toxins is conferred by corresponding groups of independently segregating genes. However, laboratory selection with one Bt toxin produced cross-resistance to other Bt toxins in a strain of tobacco budworm (Heliothis virescens) (13), which suggests that one gene or set of genes conferred resistance to more than one toxin. Compared with the mass crosses used in most previous studies of resistance (16), single-pair crosses (14, 17) have greater power to elucidate the number of loci influencing resistance and to reveal genetic variation within strains. Our results from single-pair crosses with diamondback moth show that one gene can confer resistance to four Bt toxins. We also found that a surprisingly high proportion (21%) of individuals from a susceptible strain were heterozygous for the multiple-toxin resistance gene.
MATERIALS AND METHODS
Insects.
We used two strains of diamondback moth from Hawaii to analyze the genetic basis of resistance. The susceptible LAB-P strain had been reared in the laboratory for more than 100 generations without exposure to Bt or any other insecticide (18). The resistant NO-QA strain was isolated from a field population that had evolved resistance in response to intensive treatments with multiple-toxin formulations of Bt subspecies kurstaki such as Dipel (18). Dipel contains five toxins (Cry1Aa, Cry1Ab, Cry1Ac, Cry2A, and Cry2B), spores, and formulation ingredients (19). To remove susceptible individuals, the NO-QA strain was selected repeatedly in the laboratory with Dipel (20). After field and laboratory selection, the NO-QA strain showed >3000-fold resistance to Dipel relative to the LAB-P strain (21). Previous work showed that NO-QA was resistant to the three toxins in Dipel that are most potent against susceptible diamondback moth larvae (Cry1Aa, Cry1Ab, and Cry1Ac) and cross-resistant to toxins Cry1F and Cry1J, to which NO-QA had not been exposed (21–23). NO-QA showed little or no crossresistance to Cry1B, Cry1C, Cry1D, or Cry1I (21–23).
Toxins.
We focused on four toxins: Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F. Amino acid sequence homology of the activated form of these toxins ranges from 75% to 89% among the three Cry1A toxins and from 49% to 52% between the Cry1A toxins and Cry1F (24). Although Cry1A and Cry1F toxins have relatively low overall similarity, they are closely related in terms of their amino acid sequences in the region known as domain II, which is thought to be critical for binding of toxin (23). Each of the toxins studied here has a unique spectrum of insecticidal activity within the Lepidoptera (25–29). All four are extremely potent against susceptible diamondback moth larvae (11, 20–23). Against the gypsy moth (Lymantria dispar) Cry1Ab is 100 times more toxic than Cry1Ac, but against corn earworm (Helicoverpa zea) Cry1Ac is 10 times more toxic than Cry1Ab (26, 27). Bioassays with the Japanese silkworm (Bombyx mori) showed that Cry1Aa was 400 times more potent than Cry1Ac (28), whereas with tobacco budworm (Heliothis virescens) Cry1Ac was 2000 times more potent than Cry1Aa (29). From the moth Manduca sexta, Vadlamudi et al. (30) cloned a Cry1Ab receptor (a cadherin-like protein) that is distinct from the Cry1Ac receptor (aminopeptidase N) (31, 32). In the gypsy moth, Cry1Ac, but not Cry1Aa, binds to aminopeptidase N from the brush border membrane (33). We obtained Cry1Aa, Cry1Ab, and Cry1Ac as inclusion bodies from three strains of Escherichia coli, each of which had been genetically engineered to express only one of the three Cry1A toxins (21). We used liquid formulation MYX837–446 of Cry1F from Mycogen (San Diego, CA), which contains Cry1F that has been expressed in and is encapsulated by transgenic Pseudomonas fluorescens (22).
Crosses and Bioassays.
To determine if genes responsible for resistance to Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F segregate independently in the NO-QA strain, we used bioassays in conjunction with three sets of single-pair crosses and selection experiments. In every single-pair cross, one virgin female and one male were caged together for mating and egg production (17). Each resulting family was reared separately on cabbage (17). Larvae were tested in bioassays by letting groups of 8–12 (usually 10) third instars eat cabbage leaf disks that had been dipped in distilled water dilutions of the protoxin form of Cry1Aa, Cry1Ab, Cry1Ac, or Cry1F (18, 21). In all tests, we added a surfactant (0.2% Triton AG98, Rohm & Haas). Mortality was recorded after 5 days and adjusted for mortality of larvae that ate control disks dipped in distilled water only. As internal standards, larvae from the LAB-P strain, the NO-QA strain, or both were tested simultaneously with larvae from hybrid crosses.
RESULTS
Results of the present study confirmed that larvae from the NO-QA strain were extremely resistant to Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F (Table 1). Concentrations of 10 or 100 mg of toxin per liter killed 94–100% (mean = 98%) of LAB-P larvae, but only 0–11% (mean = 4%) of NO-QA larvae. For each toxin, mortality of NO-QA larvae caused by 100 mg of toxin per liter was not higher than mortality caused by 10 mg of toxin per liter, which suggests that the highest concentration tested here was not even close to the concentration required to kill 50% (LC50) of the resistant larvae. Thus, calculation of resistance ratios was not possible, but comparison with related data (21, 22) suggests that the resistance ratio for each toxin was probably greater than 1000-fold.
Table 1.
Responses of diamondback moth larvae from parental susceptible (LAB-P) and resistant (NO-QA) strains to four Bt toxins
| Strain | Toxin | 10 mg/liter
|
100 mg/liter
|
||
|---|---|---|---|---|---|
| n | Mortality,* % | n | Mortality,* % | ||
| LAB-P | Cry1Aa | 86 | 98 | 197 | 99 |
| Cry1Ab | 118 | 97 | 61 | 100 | |
| Cry1Ac | 50 | 94 | 109 | 100 | |
| Cry1F† | 91 | 100 | 220 | 97 | |
| NO-QA | Cry1Aa | 86 | 11 | 168 | 4 |
| Cry1Ab | 139 | 2 | 164 | 1 | |
| Cry1Ac | 140 | 10 | 212 | 3 | |
| Cry1F† | 91 | 0 | 120 | 0 | |
Mortality after 5 days was adjusted for control mortality.
Concentrations for Cry1F were 1 and 10 ml of formulated Cry1F per liter.
Bioassays of the F1 progeny of the first set of single-pair crosses between NO-QA and LAB-P suggested that resistance to Cry1Ab was genetically linked to resistance to Cry1Ac (Table 2). Cry1Ab or Cry1Ac killed all larvae from five of six F1 families tested, but only 37–62% (mean = 56%) of the larvae from the sixth family (QL4) (Table 2). The simplest explanation for these results is that resistance to Cry1Ab and Cry1Ac was conferred by an autosomal recessive allele (r) at a single locus, and the LAB-P parent in each of the first five F1 crosses was homozygous susceptible at this locus (ss), but the LAB-P father of F1 family QL4 was heterozygous (rs).
Table 2.
Resistance to four Bt toxins produced by exposure of a hybrid isofemale line (QL4) to either toxin Cry1Ab or Cry1Ac
| Source | Generation | Toxin | 10 mg/liter
|
100 mg/liter
|
||
|---|---|---|---|---|---|---|
| n | Mortality,* % | n | Mortality,* % | |||
| QL4 | F1† | Cry1Ab | 21 | 62 | 17 | 65 |
| Cry1Ac | 19 | 63 | 19 | 37 | ||
| Others‡ | F1 | Cry1Ab | 100 | 100 | 96 | 100 |
| Cry1Ac | 99 | 100 | 96 | 100 | ||
| QL4 | F2§ | Cry1Ab | 100 | 4 | 100 | 0 |
| Cry1Ac | 100 | 0 | 100 | 4 | ||
| QL4 | F4 | Cry1Aa | 100 | 0 | 96 | 0 |
| QL4 | F8 | Cry1Ab | 40 | 0 | 40 | 0 |
| Cry1Ac | 40 | 0 | 41 | 0 | ||
| QL4 | F10 | Cry1F¶ | 50 | 2 | 50 | 0 |
Mortality after 5 days was adjusted for control mortality.
F1 progeny from family QL4 were produced by one NO-QA female and one LAB-P male; pooled mortality of F1 (56%) was not significantly different from 50% (χ2 = 1.32, df = 1, P > 0.2).
Five other F1 families from single-pair crosses of NO-QA × LAB-P were tested simultaneously with QL4.
Thirty-one F1 survivors of exposure to Cry1Ab or CryAc were pooled and mated among themselves to produce F2.
Concentrations for Cry1F were 1 and 10 ml of formulated Cry1F per liter.
To test this hypothesis, the F1 survivors of exposure to Cry1Ab or Cry1Ac from family QL4 (putative rr homozygotes) were allowed to mate among themselves to produce F2 progeny. According to the single-locus hypothesis, F2 progeny would be entirely homozygous resistant (rr) and thus would show no greater mortality than the parental NO-QA strain. If alleles at more than one locus were required for resistance or the resistance was not completely recessive, mortality of the F2 progeny in bioassays would be significantly greater than mortality of NO-QA. In comparable tests with Cry1Ab and Cry1Ac, mortality of QL4 F2 progeny (mean = 2%) (Table 2) was not greater than mortality of NO-QA (mean = 4%) (Table 1). These results support the single-locus hypothesis.
Subsequent generations of QL4 progeny (F3–F10) were produced by inter se matings and reared without selection. Bioassays of these QL4 progeny showed that the gene conferring resistance to Cry1Ab and Cry1Ac also conferred resistance to Cry1Aa (F4) and Cry1F (F10) (Table 2). Tests with F8 progeny showed that resistance to Cry1Ab and Cry1Ac was stable and fixed in QL4 (Table 2).
To test further the single-locus hypothesis, we performed two additional sets of single-pair crosses between NO-QA and LAB-P. Unlike the first set of crosses in which one family (QL4) had only 56% mean mortality, exposure of the five F1 families in the second set of crosses to Cry1Aa caused mortality per family ranging from 84% to 100% (mean = 92%) (Table 3). F1 survivors of exposure to Cry1Aa mated among themselves to produce F2. The F2 progeny of this hybrid multifamily strain were reared without exposure to toxin and mated to produce F3 progeny. If survival of the few F1 individuals was caused by environmental, rather than genetic variation, mortality of the F3 predicted by the single-locus hypothesis would be 75% (based on the Mendelian ratio of 1 ss:2 rs:1 rr). If survival of the few F1 individuals was genetically based, mortality would be less than 75% in the F3. Bioassays of F3 progeny of the hybrid multifamily strain showed 77% mortality in response to Cry1Aa (Table 3), which is consistent with the single-locus hypothesis and suggests that survival of F1 individuals in this set of crosses was caused by environmental rather than genetic variation.
Table 3.
Resistance to four Bt toxins produced by exposing a hybrid multifamily strain (HMF) to toxin Cry1Aa
| Source | Generation | n | Toxin | Conc., mg/liter | Mortality,* % |
|---|---|---|---|---|---|
| 5 families (pooled) | F1 | 80 | Cry1Aa | 10 | 93 |
| 83 | Cry1Aa | 100 | 91 | ||
| HMF | F3† | 280 | Cry1Aa | 100 | 77 |
| HMF | F5‡ | 102 | Cry1Aa | 100 | 0 |
| F5 | 100 | Cry1Ac | 100 | 6 | |
| HMF | F7 | 100 | Cry1Ab | 100 | 2 |
| F7 | 100 | Cry1F | 10§ | 0 |
Mortality after 5 days was adjusted for control mortality.
Eleven F1 survivors of exposure to Cry1Aa were pooled and mated among themselves for two generations to produce F3; mortality of F3 was not significantly different from 75% (χ2 = 2.45, df = 1, P > 0.1).
Fifty-eight F3 survivors of exposure to Cry1Aa were pooled and mated among themselves for two generations to produce F5.
Milliliters of formulated Cry1F per liter.
From F3 survivors, we produced generations F4–F7 of the hybrid multifamily strain by inter se matings without selection. Bioassays of the strain’s progeny showed that selection for resistance by exposure of the F3 progeny to Cry1Aa produced resistance to Cry1Aa (F5), Cry1Ac (F5), Cry1Ab (F7), and Cry1F (F7) (Table 3). These results confirm the single-locus hypothesis.
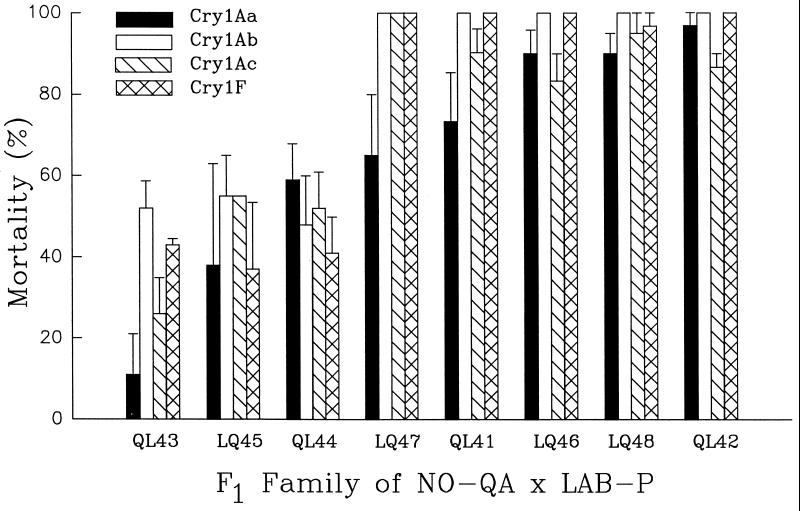
In the third and final experiment, we divided the F1 offspring from each of eight single-pair crosses from NO-QA × LAB-P into four sets, each of which was exposed to one of the four toxins. Resistance again was genetically correlated across the four toxins (Fig. 1). For Cry1Ab, Cry1Ac, and Cry1F, mortality was close to 50% in three families (range = 26–52% per family, mean = 45%) and close to 100% in the other five families (range = 83–100%, mean = 99%). These data fit the single-locus hypothesis and imply that the LAB-P parent of each of the first three families was rs, whereas the other five were ss. Lower than expected mortality to Cry1Aa in three families (QL43, LQ47, and QL41), which is reflected in a significant family by toxin interaction (Fig. 1), suggests that at least one locus that segregates independently from the multiple-toxin resistance gene influenced susceptibility to Cry1Aa but not to the other toxins. Although environmental factors contribute to variation in mortality, environmental variation is unlikely to be the sole explanation in this experiment, because lower than expected mortality in response to Cry1Aa was consistent across replicates within families.
Figure 1.
Responses of split broods of F1 progeny to four toxins. The first letter of each family name denotes the strain of the female parent (Q = NO-QA, L = LAB-P). Families are arranged from lowest (left) to highest mortality caused by Cry1Aa. Two or three groups of 10 or 11 larvae from each family were tested against each toxin (n = 80–121 larvae per family). Concentrations were 100 mg of Cry1A toxin per liter and 10 ml of formulated Cry1F per liter. Bars show mean mortality plus one standard error. Mortality was genetically correlated between all six pairs of toxins as determined by analysis of arcsine-transformed data (Cry1Aa/Cry1Ab: r = 0.80, P = 0.016; Cry1Aa/Cry1Ac: r = 0.71, P = 0.047; Cry1Aa/Cry1F: r = 0.79, P = 0.021; Cry1Ab/Cry1Ac: r = 0.89, P = 0.0032; Cry1Ab/Cry1F: r = 0.99, P = 5 × 10−6; Cry1Ac/Cry1F: r = 0.86, P = 0.0056; df = 6 for each correlation). Two-way ANOVA of the arcsine-transformed data revealed significant effects of family (df = 7, 88, F = 46.4, P < 0.0001), toxin (df = 3, 88, F = 9.3, P < 0.0001), and family by toxin interaction (df = 21, 88, F = 2.15, P = 0.012).
Results from bioassays of F1 progeny suggest that 21% (4/19) of the LAB-P parents were heterozygous for multiple-toxin resistance (1/6, Table 2; 0/5, Table 3; and 3/8, Fig. 1).
DISCUSSION
The data from all three sets of crosses suggest that a single autosomal recessive gene conferred extremely high resistance to Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F. Fine-scale mapping will be needed to determine if the genetic factor conferring multiple-toxin resistance is a single locus or a set of tightly linked loci. In either case, the evidence refutes the idea that resistance to this set of toxins requires mutations at independently segregating loci. One implication is that a polyphagous pest, such as Helicoverpa zea, attacking transgenic cotton that produces Cry1Ac might be selected for cross-resistance to transgenic corn that produces Cry1Ab.
Although one gene can confer resistance to at least four toxins, genes that confer resistance to fewer toxins also occur in insect populations. For example, resistance to Cry1Ab, but not to Cry1Aa or Cry1Ac, was found in a field-selected strain of diamondback moth from the Philippines (34) and in a laboratory-selected strain of the cabbage looper (Trichoplusia ni) (35). Also, a strain of Indian meal moth (Plodia interpunctella) selected with Dipel in the laboratory showed only 6-fold resistance to Cry1Aa compared with 260-fold resistance to Cry1Ab and 2800-fold resistance to Cry1Ac (15). The results of the present study also suggest the presence of a gene that affects responses to Cry1Aa but not to Cry1Ab, Cry1Ac, or Cry1F (Fig. 1). In populations with genes for both single- and multiple-toxin resistance, evolutionary responses will depend on initial frequencies of each resistance allele, the dominance of the alleles, and how the toxins are used.
The simplest explanation for one mutation conferring resistance to four toxins is disruption of binding to a single insect protein that acts as the receptor for all four toxins. Some evidence suggests that reduced binding of toxin to midgut membranes confers diamondback moth resistance to Cry1Ab and Cry1Ac (4, 9, 11, 12, 20, 34, 36). Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F have some similar sequences in putative binding regions (23) and may share a common binding site in diamondback moth (34, 37).
Unlike the relatively low, broad crossresistance in one laboratory-selected strain of the moth Heliothis virescens (13), extremely high resistance in the NO-QA strain is limited to a subset of the toxins that kill susceptible diamondback moth larvae (21–23). The number of independent mutations required for resistance probably depends on the pest, the type of mutation, and the particular combination of toxins.
Results reported here and related data suggest that the frequency of the multiple-toxin resistance allele has remained close to 0.10 in the LAB-P strain for at least 3 years. Bioassays of F1 progeny (Tables 2 and 3, Fig. 1) imply that 21% (4/19) of the LAB-P parents were heterozygous for multiple-toxin resistance, which, assuming Hardy–Weinberg equilibrium, translates to an r allele frequency of 0.12. This estimate is 10 times higher than the most widely cited upper limit for estimated initial frequency of resistance alleles in susceptible populations (38). Based on the data from 19 families reported here and 22 additional families from related experiments, estimates of the r allele frequency in the LAB-P strain are 0.15 for 1992–1993, 0.05 for 1994, 0.13 for 1995, and 0.11 overall; n = 8, 11, 22, and 41 families, respectively.
Although stringent measures were used to keep susceptible and resistant strains separated, a few resistant individuals might have occasionally contaminated LAB-P. Nonetheless, more than 500 individuals of LAB-P were reared per generation, and gene flow into LAB-P probably was not sufficient to maintain the observed frequency of heterozygotes. Repetitive gene flow would create temporary disequilibria with an excess of resistant homozygotes, but no such excess has been observed in tests of thousands of LAB-P larvae. In the present study, survival of LAB-P larvae exposed to the highest toxin concentrations tested was 1.6% (Table 1, n = 587), which is not significantly greater than the expected equilibrium frequency of rr homozygotes of 1.4% (0.122) (χ2 = 0.075, df = 1, P > 0.5). Analysis of randomly amplified polymorphic DNA fragments (39) and allozymes (ref. 40, B.E.T. unpublished data) also indicates little or no gene flow into LAB-P from resistant strains.
Extended maintenance of a resistance allele frequency close to 0.10 without exposure to Bt implies that in the absence of Bt heterozygotes have little or no fitness disadvantage relative to susceptibles. Like the resistance to Bt conferred by the multiple-toxin resistance gene, fitness costs associated with this resistance (20, 41) appear to be recessive.
If selection against heterozygotes in the absence of insecticide is weak, initial frequencies of resistance alleles may be much higher than previously thought (38), even if mutation rates are low. Responses to selection by small laboratory colonies of several species of moths suggest that initial frequencies of alleles conferring resistance to Bt may be greater than 0.001 (4, 9, 11, 13–15). On the basis of recent comparisons among worldwide populations of mosquitoes resistant to organophosphate insecticides, Raymond et al. (42) concluded that low rates of mutation have limited the evolution of resistance. This inference overlooks, however, that initial frequencies of resistance alleles are determined by selection as well as mutation (38).
Most genetic analyses of insecticide resistance rely on bioassays of progeny pooled from crosses between many individuals from susceptible and resistant strains (16). Such analyses cannot readily detect genetic variation within strains and must rely on the assumption that susceptible strains are composed entirely of homozygous susceptible individuals. If resistance is recessive and heterozygous individuals are not rare in susceptible strains (as found here), this approach will generate mortality of pooled F1 offspring that is slightly less than that of the susceptible strain. Such results would tend to produce the misleading conclusion that resistance is “partially recessive.” The most common conclusion from previous studies is that inheritance of resistance to Bt is partially recessive (4). The methods described here can be used to determine the extent to which this pattern reflects unexpectedly high frequencies of heterozygotes in susceptible strains.
Intense selection and the surprisingly common presence of a multiple-toxin resistance gene may explain the diamondback moth’s rapid evolution of resistance to multiple-toxin formulations of Bt. The frequencies of multiple-toxin resistance genes in other populations of diamondback moth and in other pests remain to be measured. Compared with adaptation based on independent mutations that each confer resistance to one toxin, multiple-toxin resistance genes, such as the one identified here, can greatly hasten evolution of resistance to groups of toxins used in combination or sequence.
Acknowledgments
We thank D. Borthakur, C. Chilcutt, J. Ferré, F. Gould, G. Roderick, and J. Rosenheim for their comments and B. Artelt, T. Gonsalves, and B. Helvig for technical assistance. Support was provided by the U.S. Department of Agriculture through Cooperative State Research Service Special Grant 95-34135-1771, National Research Initiative Competitive Grant 96-35302-3470, and a Western Regional Pesticide Impact Assessment Program grant.
ABBREVIATION
- Bt
Bacillus thuringiensis
References
- 1.Entwistle P, Bailey M J, Cory J, Higgs S, editors. Bacillus thuringiensis: An Environmental Biopesticide. New York: Wiley; 1993. [Google Scholar]
- 2.Gill S S, Cowles E A, Pietrantonio P V. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 3.Georghiou G P, Lagunes-Tejeda A. The Occurrence of Resistance to Pesticides in Arthropods. Rome: Food Agric. Org. UN; 1991. [Google Scholar]
- 4.Tabashnik B E. Annu Rev Entomol. 1994;39:47–79. [Google Scholar]
- 5.National Research Council. Pesticide Resistance: Strategies and Tactics for Management. Washington, DC: Natl. Acad. Press; 1986. [Google Scholar]
- 6.Mani G S. Genetics. 1985;109:761–783. doi: 10.1093/genetics/109.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roush R T. Pestic Sci. 1989;26:423–441. [Google Scholar]
- 8.Tabashnik B E. J Econ Entomol. 1989;82:1263–1269. doi: 10.1093/jee/82.5.1263. [DOI] [PubMed] [Google Scholar]
- 9.McGaughey W H, Whalon M E. Science. 1992;258:1451–1455. doi: 10.1126/science.258.5087.1451. [DOI] [PubMed] [Google Scholar]
- 10.Neal J, Berenbaum M. J Chem Ecol. 1989;15:439–446. doi: 10.1007/BF02027803. [DOI] [PubMed] [Google Scholar]
- 11.Ferré J, Real M D, Van Rie J, Jansens S, Peferoen M. Proc Natl Acad Sci USA. 1991;88:5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferré J, Escriche B, Bel Y, Van Rie J. FEMS Microbiol Lett. 1995;132:1–7. [Google Scholar]
- 13.Gould F, Martinez-Ramirez A, Anderson A, Ferré J, Silva F J, Moar W J. Proc Natl Acad Sci USA. 1992;89:7986–7990. doi: 10.1073/pnas.89.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould F, Anderson A, Reynolds A, Bumgarner L, Moar W. J Econ Entomol. 1995;88:1545–1559. [Google Scholar]
- 15.McGaughey W H, Johnson D E. J Econ Entomol. 1994;87:535–540. [Google Scholar]
- 16.Tabashnik B E. J Econ Entomol. 1991;84:703–712. doi: 10.1093/jee/84.3.703. [DOI] [PubMed] [Google Scholar]
- 17.Tabashnik B E, Cushing N L. J Econ Entomol. 1989;82:5–10. doi: 10.1093/jee/82.5.1263. [DOI] [PubMed] [Google Scholar]
- 18.Tabashnik B E, Cushing N L, Finson N, Johnson M W. J Econ Entomol. 1990;83:1671–1676. [Google Scholar]
- 19.Abbott Laboratories. Bt Products Manual. North Chicago, IL: Abbott; 1992. [Google Scholar]
- 20.Tabashnik B E, Finson N, Groeters F R, Moar W J, Johnson M W, Luo K, Adang M J. Proc Natl Acad Sci USA. 1994;91:4120–4124. doi: 10.1073/pnas.91.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabashnik B E, Finson N, Johnson M W, Moar W J. Appl Environ Microbiol. 1993;59:1332–1335. doi: 10.1128/aem.59.5.1332-1335.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabashnik B E, Finson N, Johnson M W, Heckel D G. Appl Environ Microbiol. 1994;60:4627–4629. doi: 10.1128/aem.60.12.4627-4629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabashnik B E, Malvar T, Liu Y-B, Finson N, Borthakur D, Shin B-Y, Park S-H, Masson L, de Maagd R A, Bosch D. Appl Environ Microbiol. 1996;62:2839–2844. doi: 10.1128/aem.62.8.2839-2844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devereaux J, Haeberli P, Smithies O. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacIntosh S C, Stone T B, Sims S R, Hunst P L, Greenplate J T, Marrone P G, Perlak F J, Fischhoff D, A, Fuchs R L. J Invert Pathol. 1990;56:258–266. doi: 10.1016/0022-2011(90)90109-j. [DOI] [PubMed] [Google Scholar]
- 26.van Frankenhuyzen K, Gringorten J L, Milne R E, Gauthier D, Pusztai M, Brousseau R, Masson L. Appl Environ Microbiol. 1991;57:1650–1655. doi: 10.1128/aem.57.6.1650-1655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers J A, Jelen A, Gilbert M P, Jany C S, Johnson T B, Gawron-Burke C. J Bacteriol. 1991;173:3966–3976. doi: 10.1128/jb.173.13.3966-3976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge A Z, Shivarova N I, Dean D H. Proc Natl Acad Sci USA. 1989;86:4037–4041. doi: 10.1073/pnas.86.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M K, Rajamohan F, Gould F, Dean D H. Appl Environ Microbiol. 1995;61:3836–3842. doi: 10.1128/aem.61.11.3836-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vadlamudi R K, Weber E, Ji I, Ji T H, Bulla L., Jr J Biol Chem. 1995;270:5490–5494. doi: 10.1074/jbc.270.10.5490. [DOI] [PubMed] [Google Scholar]
- 31.Sangadala S, Walters F S, English L H, Adang M J. J Biol Chem. 1994;269:10088–10092. [PubMed] [Google Scholar]
- 32.Knight P J K, Knowles B H, Ellar D J. J Biol Chem. 1995;270:17765–17770. doi: 10.1074/jbc.270.30.17765. [DOI] [PubMed] [Google Scholar]
- 33.Lee M K, Young B A, Dean D H. Biochem Biophys Res Commun. 1995;216:306–312. doi: 10.1006/bbrc.1995.2625. [DOI] [PubMed] [Google Scholar]
- 34.Ballester V, Escriche B, Ménsua J L, Riethmacher G W, Ferré J. Biocontrol Sci Tech. 1994;4:437–443. [Google Scholar]
- 35.Estada U, Ferré J. Appl Environ Microbiol. 1994;60:3840–3846. doi: 10.1128/aem.60.10.3840-3846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang J D, Shelton A M, Van Rie J, de Roeck S, Moar W J, Roush R T, Peferoen M. Appl Environ Microbiol. 1996;62:564–569. doi: 10.1128/aem.62.2.564-569.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granero F, Ballester V, Ferré J. Biochem Biophys Res Commun. 1996;224:779–783. doi: 10.1006/bbrc.1996.1099. [DOI] [PubMed] [Google Scholar]
- 38.Roush R T, McKenzie J A. Annu Rev Entomol. 1987;32:361–380. doi: 10.1146/annurev.en.32.010187.002045. [DOI] [PubMed] [Google Scholar]
- 39.Heckel D G, Gahan L, Tabashnik B E, Johnson M W. Ann Entomol Soc Am. 1995;88:531–537. [Google Scholar]
- 40.Caprio M A, Tabashnik B E. J Econ Entomol. 1992;21:808–816. [Google Scholar]
- 41.Groeters F, Tabashnik B E, Finson N, Johnson M W. Evolution. 1994;48:197–201. doi: 10.1111/j.1558-5646.1994.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 42.Raymond M, Callaghan A, Fort P, Pasteur N. Nature (London) 1991;350:151–153. doi: 10.1038/350151a0. [DOI] [PubMed] [Google Scholar]