Abstract
Genetic correction of inherited muscle diseases, such as Duchenne muscular dystrophy, will require long term expression of the recombinant protein following gene transfer. We have shown previously that a new adenoviral vector that lacks all viral genes expressed both full-length dystrophin and β-galactosidase in mdx (dystrophin-deficient) mouse muscle. We observed a significant histologic improvement of vector-transduced mdx muscle before the eventual loss of vector-encoded transgene expression. In this study, we investigated whether an immunological response against vector-encoded β-galactosidase contributed to the loss of vector expression and affected vector persistence in muscle. Intramuscular vector injection in control normal mice resulted in an early and complete loss of β-galactosidase expression accompanied by predominantly CD4+ and CD8+ lymphocytic infiltration and a significant loss of vector DNA. In contrast, intramuscular vector injection in lacZ transgenic mice resulted in persistent expression of β-galactosidase for at least 84 days with no evidence of inflammation or significant loss of vector DNA. Our studies demonstrate that, in the absence of an immune response induced by β-galactosidase expression, an adenoviral vector lacking all viral genes is stably maintained in muscle.
Keywords: Duchenne muscular dystrophy, gene transfer, lacZ transgenic mice, vector persistence
Duchenne muscular dystrophy (DMD) is an X-linked, lethal disorder of skeletal muscle in which muscle fiber damage and degeneration results from the absence of dystrophin protein at the muscle membrane (1, 2). Somatic gene transfer of the dystrophin cDNA has the potential to provide functional dystrophin protein to muscle fibers, thus rescuing them from repeated cycles of degeneration. The treatment of inherited deficiency disorders, such as DMD, with somatic gene transfer will require persistent expression of the therapeutic protein.
Adenoviral vectors are thought to be good candidates for somatic gene transfer to muscle because of their ability to transduce postmitotic cells (3, 4). In animal studies, replication-defective adenoviral vectors have been used for the transfer of genes encoding marker proteins (5), secretory proteins (6–8), and truncated forms of dystrophin to muscle (9–14). T cell-mediated and B cell antibody immune responses to first generation adenoviral vectors have been observed in a variety of tissues, including skeletal muscle (7). These immune responses are induced by both viral proteins and transgene targets. Studies of first generation adenoviral vectors in genetically modified, immune-deficient mice or systemically immunosuppressed mice have supported the hypothesis that the major limitation to the persistence of the first generation adenoviral vector in muscle is the immune response to the vector and the proteins it produces (7, 15, 16). However, the relative importance of the immune response to the transgene as compared with viral proteins expressed from the first and second generation adenoviral vectors had not been determined. A further limitation to the use of first or second generation adenoviral vectors for transfer of the 14-kb dystrophin cDNA is their insert capacity maximum of only 8 kb (17).
To address the issues of limited insert capacity and the production of potentially immunogenic viral proteins by first or second generation adenoviral vectors, we and others have developed adenoviral vectors that package 30 kb of foreign DNA and have no viral genes (18–21). Our vector, AdDYSβgal, has a foreign DNA insert consisting of the full-length murine dystrophin cDNA driven by a muscle-specific promoter and the Escherichia coli lacZ gene driven by the human cytomegalovirus (CMV) immediate early promoter (18). AdDYSβgal is produced with a helper virus that provides necessary viral functions by trans-complementation. Vector purification by cesium chloride banding leaves a helper virus level remaining within the purified AdDYSβgal stock of 1% or less.
We have demonstrated the efficient transduction of muscle cells with AdDYSβgal providing dystrophin and β-galactosidase in vitro (18) and in vivo (22). Our in vivo study showed that dystrophin and β-galactosidase expression from AdDYSβgal increased with time after intramuscular injection of the vector in mdx mice and reached a peak 28 days after injection. The histological phenotype of transduced muscle was significantly improved, with a reduced number of fibers harboring centralized nuclei (22). However, by 42 days after injection, the proportion of muscle fibers expressing both genes was diminished. In this report, we address whether an immune response induced by the marker gene product, β-galactosidase, contributed to the decrease of vector expression and affected vector persistence in muscle.
MATERIALS AND METHODS
Animals.
Homozygous male lacZ transgenic mice expressing nuclear-localized β-galactosidase under the control of the muscle-specific myosin light chain 3F promoter and enhancer (23) were bred with female Swiss Webster normal mice. The heterozygous lacZ transgenic offspring, which were used for AdDYSβgal injections, expressed β-galactosidase in muscle fiber nuclei, which should provide immune tolerance to the foreign marker protein. Control normal mice, which were coisogenic to lacZ transgenic mice except for the lacZ transgene, did not express β-galactosidase and were expected to be immunoreactive to marker protein. These mice were bred with female Swiss Webster mice to generate control mice for our studies.
Preparation of the Adenoviral Vector, AdDYSβgal.
AdDYSβgal (18) contained a two-part expression cassette: (i) the full-length murine dystrophin cDNA under the control of the mouse muscle creatine kinase promoter including the first intron (24) and (ii) the E. coli lacZ gene under the control of human CMV immediate early promoter (25). After propagation in 293 cells with helper virus, AdDYSβgal was purified on three successive equilibrium cesium chloride gradients. The purified band was dialyzed against two changes of PBS2+ (0.137 M NaCl/2.7 mM KCl/10.1 mM Na2HPO4/1.7 mM KH2PO4/0.4 mM CaCl2/0.5 mM MgCl2, pH 7.4) containing 5% glycerol. Vector titer was determined by infection of 293 cells to limiting dilution and staining 18 h later for β-galactosidase. Contamination of the purified vector preparation with helper virus, as determined by plaque assay and by Southern blot analysis of isolated DNA, was 1% or less.
Intramuscular Injection of AdDYSβgal.
Four-day-old lacZ transgenic mice or nontransgenic mice were each given a single 5 μl intramuscular injection of 2 × 107 AdDYSβgal infectious particles in the posterior compartment of the right hind leg. The same volume of PBS2+ was injected in the posterior compartment of the left hind leg. Mice were sacrificed at 14, 28, 42, 63, and 84 days after injection. After removal, the left and right gastrocnemius muscles were immersed in 4% paraformaldehyde in PBS (0.137 M NaCl/2.7 mM KCl/10.1 mM Na2HPO4/1.7 mM KH2PO4, pH 7.4) at 4°C for 8 h, rinsed with PBS, cryoprotected with 30% sucrose, and then snap frozen in liquid nitrogen-cooled isopentane.
Detection of β-Galactosidase in Muscle Cryosections.
Muscle blocks were cryosectioned at 10 μm thickness. Sections were rinsed with PBS at room temperature for 15–30 min with two changes and histochemically stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) (0.1% X-Gal in 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2,) at 37°C for 12–16 h. Cryosections, taken from the middle of each muscle block to limit variability due to vector injection, was analyzed with mcid image analysis software (Imaging Research, St. Catherine’s, ON, Canada) linked to a Zeiss axiophot microscope for quantitation of the ratio of the cross-sectional area expressing β-galactosidase to the total cross-sectional area of the muscle section. Serial sections, stained with hematoxylin and eosin, were analyzed to determine the total muscle cross-sectional area. The ratios were expressed as percentages.
Detection of CD4+ and CD8+ T Cells in Muscle Cryosections.
Muscle cryosections were rinsed with PBS at room temperature for 15–30 min, and endogenous peroxidase was blocked with peroxidase blocking reagent (DAKO) for 5 min at room temperature. The slides were rinsed in PBS, blocked with 10% goat serum in PBS for 1 h, and then incubated with rat anti-mouse CD4 (clone number RM4–5, PharMingen) and CD8a (clone number 53–6.7, PharMingen) monoclonal antibodies overnight at room temperature. The sections were rinsed with 10% goat serum in PBS and then incubated with biotinylated goat anti-rat secondary antibody for 1 h. The sections were rinsed with 10% goat serum in PBS as before, incubated with Avidin DH and biotinylated horseradish peroxidase H reagents (Vectastain Elite ABC kit, Vector Laboratories) for 30 min, and then rinsed with PBS. Incorporated peroxidase activity was localized with diaminobenzidine tetrahydrochloride (DAB substrate kit for peroxidase, Vector Laboratories).
Detection of Macrophages in Muscle Cryosections.
Slides of muscle cryosections were rinsed twice with PBS at room temperature for 15–30 min. The sections were blocked with 10% goat serum in PBS for 1 h and then incubated with fluorescein isothiocyanate-conjugated rabbit anti-mouse macrophage antibody (Axell) for 1 h at room temperature.
Isolation of Muscle DNA.
Each sample, consisting of 20 cryosections of paraformaldehyde-fixed muscle tissue (10 μm per section), was digested with 200 μg of proteinase K per ml in 50 mM Tris·HCl (pH 8.0)/10 mM EDTA (pH 8.0)/0.5% SDS at 55°C for 6–16 h. The sample DNA was purified by phenol-chloroform extraction and ethanol precipitation.
PCR Quantitation of Vector DNA.
The standard PCR using Taq DNA polymerase was modified for semiquantitation of AdDYSβgal DNA (26). Briefly, 100 ng of DNA sample, extracted from lacZ transgenic and nontransgenic mouse muscle after intramuscular injection of AdDYSβgal, was amplified with two pairs of primers. One pair of primers, 5′-TTGGAAAGCAACACATAGACAACC-3′ and 5′-TTCACTGTTGGTTTGCTGCAATCC-3′, was used to amplify DNA between nucleotides 315 and 608 of the murine dystrophin cDNA (GenBank accession number M68859M68859) in AdDYSβgal, giving a 317-bp PCR product. The other pair, 5′-TGAGACCCCTACCCTTGCAATACG-3′ and 5′-CATGTTAATGGTGACTACCCCGTC-3′ (26), was specific for the single-copy mouse adipsin gene, giving a 191-bp PCR product. In control experiments, 100 ng of uninjected mouse muscle DNA was mixed with purified pDYSβgal plasmid DNA (18) at a concentration corresponding to 0.5, 0.1, 0.05, 0.01, 0.005 copy per cell and amplified in parallel, to generate a standard curve. PCR reactions were performed in 50 μl containing 10 mM Tris·HCl (pH 8.3), 500 mM KCl, 200 μM each deoxynucleotide triphosphate, and 2.5 units of Taq DNA polymerase, with 50 pmol of each primer described above. Amplification was carried out for 25 cycles at 95°C for 30 s, 55°C for 1 min, 72°C for 1 min, followed by a single extension at 72°C for 8 min. Aliquots of 7 μl from the PCR amplification were separated by electrophoresis on a 1.5% agarose gel, transferred to a Nytran plus nylon membrane (Schleicher & Schuell), and UV cross-linked using a stratalinker (Stratagene). The blot was analyzed for dystrophin and adipsin by Southern blotting. The standard curve of pDYSβgal plasmid DNA was linear over the range of 0.01–0.5 copy per nucleus. Vector band detection was done with a random-primed 32P-labeled SpeI fragment of the murine dystrophin cDNA containing nucleotides 1–1441. Adipsin band detection was achieved with 32P-labeled PCR product generated with the primers described above. The membrane was prehybridized at 65°C for 3 h in 6× SSPE (0.9 M NaCl/60 mM NaH2PO4, pH 7.4/6 mM EDTA, pH 8.0)/0.1% SDS/5× Denhardt’s solution/200 μg of denatured salmon sperm DNA per ml. The membrane was then hybridized in the same solution with the 32P-labeled dystrophin probe. The membrane was washed twice at room temperature (5 min each time) with 2× SSPE/1% SDS and once at 50°C with 0.2× SSPE/0.1% SDS for 30 min. The membrane was then exposed to Kodak XAR film to produce a photographic image, and the Southern blot was quantitated by PhosphorImager analysis on a scanner (Molecular Dynamics). The blot was then stripped and analyzed in an analogous way for adipsin.
RESULTS
Prolonged Expression of β-Galactosidase from the Injected AdDYSβgal Vector in lacZ Transgenic Mice.
To test the hypothesis that expression of β-galactosidase from the vector could induce an immune response resulting in the elimination of muscle fibers expressing this protein, we injected the vector into the gastrocnemius muscle of 4-day-old mice tolerized to β-galactosidase. These mice have a lacZ transgene modified with a nuclear localization signal under the control of the muscle-specific myosin light chain 3F promoter and enhancer (23). The nuclear localized β-galactosidase expressed from the transgene was easily distinguished from the cytoplasmic β-galactosidase expressed from the viral vector (Fig. 1). Parallel intramuscular vector injections were performed in coisogenic, age-matched nontransgenic mice that were not tolerized to β-galactosidase.
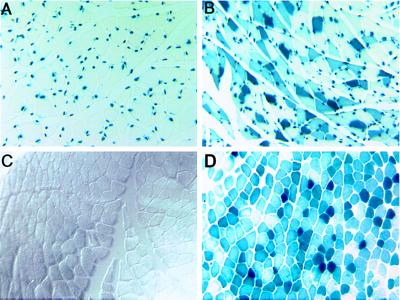
Figure 1.
Expression and localization of β-galactosidase in lacZ transgenic and nontransgenic control mouse muscle. (A) Cross-section of a sham-injected gastrocnemius muscle from a lacZ transgenic mouse shows β-galactosidase localized to the nuclei of muscle fibers. In these mice, a lacZ transgene with the nuclear-localizing signal is expressed under the control of the muscle-specific myosin light chain 3F promoter and enhancer. (B) Cross-section of a gastrocnemius muscle from a lacZ transgenic mouse that received an intramuscular injection of AdDYSβgal. Vector-encoded β-galactosidase localizes to the cytoplasm. (C) Cross-section of a sham-injected gastrocnemius muscle from a control nontransgenic mouse. (D) Cross-section of a gastrocnemius muscle from a control mouse that received an intramuscular injection of AdDYSβgal. Vector-encoded β-galactosidase localizes to the cytoplasm. (×80).
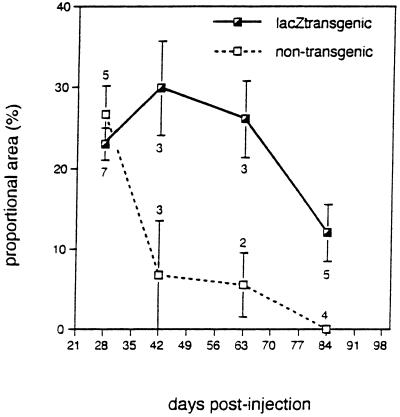
Muscle expression of cytoplasmic β-galactosidase was followed in lacZ transgenic and nontransgenic mice for up to 84 days after intramuscular administration of AdDYSβgal. Representative sections of both lacZ transgenic and nontransgenic muscle 14, 28, and 42 days after injection are shown in Fig. 2. The level of β-galactosidase expression was evaluated as the ratio of the muscle cross-sectional area expressing β-galactosidase to the total muscle cross-sectional area and was reported as the percentage (Fig. 3). At 28 days, β-galactosidase expression was observed in 23–27% of muscle cross-sectional area in both lacZ transgenic and nontransgenic mice. In lacZ transgenic mice, the cytoplasmic level of β-galactosidase expression remained constant up to 63 days after injection; however, a decrease to 10% of the cross-sectional area was observed at 84 days. In contrast, nontransgenic mice showed a significant loss in β-galactosidase expression as early as 42 days after injection. In two of three mice, complete loss of β-galactosidase expression was observed at 42 days. No β-galactosidase expression from the vector was detected in any nontransgenic mice examined 84 days after injection.
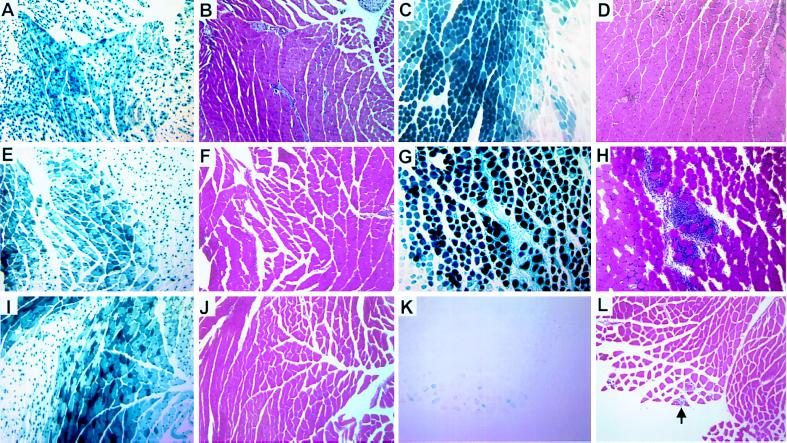
Figure 2.
Expression of vector-encoded β-galactosidase is associated with inflammation in nontransgenic, but not lacZ transgenic mouse muscle. (A, E, and I) lacZ transgenic mouse muscle 14, 28, and 42 days after an intramuscular injection of AdDYSβgal, stained with X-Gal. (B, F, and J) Serial sections of A, E, and I, stained with hematoxylin and eosin show no inflammatory cellular infiltrate. (C, G, and K) Nontransgenic mouse muscle 14, 28, and 42 days after an intramuscular injection of AdDYSβgal, stained with X-Gal. (D, H, and L) Serial sections of C, G, and K, stained with hematoxylin and eosin show an inflammatory cellular infiltrate correlated with β-galactosidase expression. The arrow in L indicates a scant inflammatory infiltrate in proximity to rare β-galactosidase expressing nontransgenic muscle fibers at 42 days after injection. (×20).
Figure 3.
Time course of vector-encoded β-galactosidase expression in muscle of lacZ transgenic and nontransgenic mice. The proportional area of muscle sections expressing vector-encoded β-galactosidase after intramuscular AdDYSβgal injection was quantitated. The means at each time-point with their standard errors are shown. The numbers of transgenic and nontransgenic animals are indicated below and above the standard error bars, respectively.
Extensive Inflammatory Cellular Infiltration in AdDYSβgal-Injected Nontransgenic Mice.
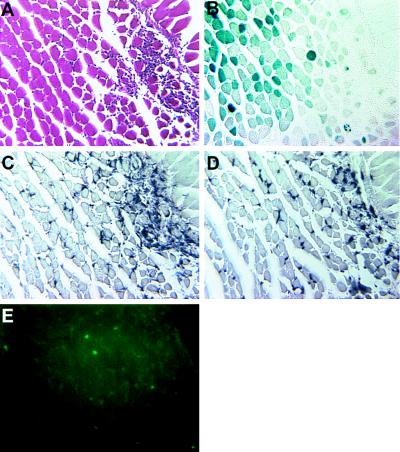
An inflammatory cellular infiltrate was observed in all AdDYSβgal-injected nontransgenic muscles that had detectable β-galactosidase expression. In general, the degree of inflammation was correlated with the level of β-galactosidase expression (compare Fig. 2 D, H, and L with C, G, and K). At 14 and 28 days after injection, extensive inflammation was observed at sites of high β-galactosidase expression. At 42 days after injection, the proportion of the muscle cross-sectional area with β-galactosidase expression decreased and the extent of cellular infiltrate detected around the remaining β-galactosidase expressing fibers also decreased (Fig. 2L). No muscle inflammation (Fig. 2 B, F, and J) was observed in any lacZ transgenic mice administered an equivalent amount of AdDYSβgal, despite the consistently high level of β-galactosidase expression over the period of study (Fig. 2 A, E, and I). These results suggested that the immunological response observed in vector-transduced nontransgenic muscle caused a decrease of vector-encoded β-galactosidase expression. The majority of the infiltrating cells in nontransgenic mice were CD4+ and CD8+ T cells as shown by immunostaining (Fig. 4). Therefore, it is likely that both MHC class I and class II antigen presentation mediated the immune response observed in AdDYSβgal-transduced nontransgenic muscles. Scant macrophages, as detected with an antibody specific for mouse macrophages, were also observed in AdDYSβgal-transduced nontransgenic muscle (Fig. 4).
Figure 4.
Detection of CD4+, CD8+ T cells, and macrophages at the site of vector-encoded β-galactosidase expression in nontransgenic mice. Serial sections of a nontransgenic mouse muscle 28 days after an intramuscular injection of AdDYSβgal. (A) Stained with hematoxylin and eosin; (B) stained with X-Gal; (C) immunostained with anti-CD4 monoclonal antibody; (D) immunostained with anti-CD8 monoclonal antibody; (E) immunostained with anti-macrophage antibody. (×80).
Loss of β-Galactosidase Expression in Nontransgenic Mice Is only Partly Explained by Loss of Viral Vector DNA.
Our original hypothesis predicted that viral vector DNA should be eliminated from nontransgenic mice due to the immune-mediated elimination of muscle fibers harboring the β-galactosidase expressing vector. To test this hypothesis, we compared the stability of vector DNA in lacZ transgenic and nontransgenic mice by a semiquantitative PCR assay detected by Southern blot analysis (26). DNA samples were from the same lacZ transgenic and nontransgenic muscles used to test β-galactosidase expression. Each DNA sample was amplified by Taq DNA polymerase and two primer sets. One primer set was specific for amplification of the AdDYSβgal vector and the other primer set was specific for the mouse adipsin gene that served as an internal control of input DNA amount (100 ng, equivalent to approximately 20,000 nuclei). Uninjected mouse muscle DNA was mixed with known copy numbers of pDYSβgal plasmid DNA (18) and amplified in parallel to generate a standard curve for quantitation of vector DNA. The resulting PCR products were analyzed by Southern blot analysis. Using this method, we could detect one copy of viral vector DNA per 200 nuclei.
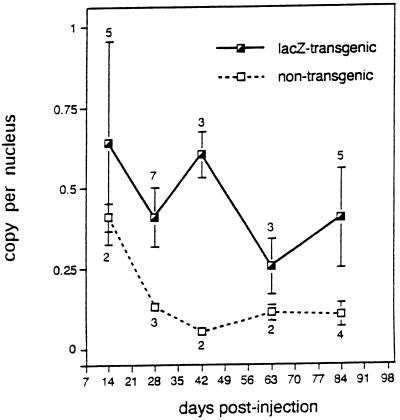
The lacZ transgenic mouse muscle contained an average of 0.4 copy of vector per nucleus over the duration of the study (Fig. 5). There was a trend toward a reduced copy number of vector per nucleus between 14 and 84 days, but this was not significant (P > 0.1). Despite this persistence of vector DNA, however, the proportion of muscle cross-sectional area expressing vector-encoded β-galactosidase decreased between the 63 and 84 day time points. In contrast to our findings in transduced lacZ transgenic muscle, a decrease in vector copy number in transduced nontransgenic muscle was detected as early as 28 days after injection (from 0.4 copy per nucleus at day 14 to 0.1 copy per nucleus at day 28) (Fig. 5). This decrease in vector copy number in transduced nontransgenic muscle was also reflected in a decrease in β-galactosidase expression at 42 days after injection. The time lag between loss of viral vector and loss of β-galactosidase expression likely reflects the slow degradation of β-galactosidase in muscle fibers. Surprisingly, vector DNA could still be detected in transduced nontransgenic muscle at approximately 0.1 copy per nucleus 84 days after injection. Therefore, the immune response triggered by vector-encoded β-galactosidase expression in nontransgenic muscle did not eliminate all of the vector DNA.
Figure 5.
Viral vector DNA stability in lacZ transgenic and nontransgenic mice after intramuscular AdDYSβgal injection. Total muscle DNA samples extracted from vector-injected lacZ transgenic, and nontransgenic mouse muscles were assayed for viral vector DNA using PCR. Uninjected mouse muscle DNA was mixed with known copy numbers of pDYSβgal plasmid and amplified in parallel reactions to generate a standard curve for vector DNA quantitation. The data shown represent the result of PhosphorImager scanning of the PCR Southern blot analysis after normalizing input DNA to the adipsin PCR product. The means of vector copy number per nucleus are shown with their standard errors at each time point. The numbers of transgenic and nontransgenic animals are indicated above and below the standard error bars, respectively. Animals studied here were the same groups that were studied for the expression of vector-encoded β-galactosidase protein (see Fig. 3).
DISCUSSION
There were two principal goals driving the development of adenoviral vectors for DMD gene therapy: (i) enlargement of the insert capacity to accommodate the entire dystrophin cDNA and (ii) elimination of the immune response induced by first-generation adenoviral vector-encoded viral proteins. The inclusion of full-length dystrophin cDNA was achieved in the development of the large capacity adenoviral vector, AdDYSβgal (18). Our previous in vivo studies in mdx mice demonstrated the full-length dystrophin expression from the vector that was accompanied by restoration of dystrophin-associated proteins and histologic improvement of dystrophic muscle. However, vector expression in mdx muscle peaked at 28 days and decreased by 42 days after injection. Here, we addressed whether the decrease in expression was due to the loss of vector DNA and whether an immunological response against the marker gene product contributed to the loss of expression.
Persistent expression from the AdDYSβgal vector for at least 84 days was observed in lacZ transgenic mice tolerized to β-galactosidase. No evidence of a T cell-mediated immune response was observed in β-galactosidase-tolerized mice. In contrast, AdDYSβgal-transduced control nontransgenic muscle showed a significant decrease of β-galactosidase expression as early as 42 days after injection, and no β-galactosidase was detected at 84 days after injection. In addition, an extensive inflammatory cellular infiltration consisting primarily of CD4+ and CD8+ lymphocytes was associated with the expression of β-galactosidase in nontransgenic mice. These results support the hypothesis that an immune response induced by the foreign protein, β-galactosidase, was responsible for the loss of viral vector expression in nontransgenic mice. Tripathy and colleagues have also reported that a foreign transgene expressed in muscle from a first-generation adenoviral vector was the major determinant inducing an immune response and limiting the stability of vector expression (6).
DNA extracted from AdDYSβgal-injected nontransgenic muscle showed a decrease in vector copy number per nucleus as early as 28 days after injection but remained at a detectable level for as long as 84 days. These results demonstrate that the immune response triggered by vector-encoded β-galactosidase expression did not result in complete elimination of vector from muscle and suggested that β-galactosidase expression either fell below the detection threshold or was shut off in those muscle fibers where viral vector DNA persisted. β-Galactosidase expressed from AdDYSβgal is under the control of the CMV immediate early promoter. Other investigators have shown that interferon-γ, which would be produced in the course of T cell-mediated immune response, can inhibit the CMV promoter (27). Although we did not measure cytokine production directly, the extensive T cell infiltration observed in vector-transduced nontransgenic muscle is suggestive of local cytokine release. Repression of β-galactosidase expression from AdDYSβgal may allow those muscle fibers harboring viral vector DNA to escape immune surveillance and to retain residual vector 84 days after injection. Thus, the observed loss of β-galactosidase expression in nontransgenic mice is only partly explained by loss of viral vector DNA and potentially also reflects an immune-mediated repression of the CMV promoter.
In lacZ transgenic mice, vector-encoded β-galactosidase expression was still present 84 days after intramuscular injection of AdDYSβgal, but at a lower level than at 63 days. Despite this significant reduction in β-galactosidase expression, there was not a corresponding significant reduction in the levels of vector DNA. Therefore the decrease in β-galactosidase expression cannot be explained solely by a loss of vector DNA. Loss of transgene expression in muscle from the CMV promoter, in the absence of an immune response, has been observed by others. When a similar CMV immediate early promoter was used to control canine factor IX cDNA expression from a recombinant retrovirus, infected primary mouse myoblasts, and their derived myotubes secreted factor IX (28). However, following transplantation of the infected primary myoblasts into nude mice, the in vivo expression of canine factor IX increased for 2–4 days and then ceased, suggesting that transcriptional regulation of the CMV promoter differed in vitro and in vivo. Alternatively, loss of transgene expression in lacZ-transgenic mice might reflect a gradual degradation of viral vector. Since most of the wild-type adenoviral genome has been replaced in the construction of AdDYSβgal, one might expect a different nucleosomal structure in AdDYSβgal that might affect the susceptibility of vector DNA to endo- and exonucleases. Although we have demonstrated persistence of vector DNA in the absence of an immune response to β-galactosidase, further studies will be required to determine the complete integrity of AdDYSβgal DNA in muscle fibers. In this regard, it will also be important to compare transgene expression and vector stability of AdDYSβgal with that of prior generation adenoviral vectors that retain more adenoviral genome in addition to a recombinant transgene.
It is possible that administration of an adenoviral vector early in life might result in immune tolerance to the foreign proteins expressed from the vector. However, the ability of the animals in our experiments to mount an immune response to foreign proteins was reflected by the extensive cellular infiltrate observed in nontransgenic control muscle despite vector administration at 4 days of age. The absence of a cellular immune response to AdDYSβgal in β-galactosidase-tolerized mouse muscle suggested that AdDYSβgal vector components other than β-galactosidase were not sufficient to elicit a cellular immune response. In particular, helper virus remaining in the vector preparation (approximately 1%) did not appear to elicit a cellular immune response in vector-transduced lacZ transgenic muscle. Because both the lacZ transgenic and nontransgenic mice express wild-type dystrophin in muscle, the studies presented here could not address whether vector-encoded dystrophin expression would induce an immune response in dystrophin-deficient mdx or DMD muscle.
In summary, large capacity adenoviral vectors hold considerable promise for the delivery of large genes, such as the dystrophin cDNA, to muscle. We demonstrate that transgenes delivered by an adenoviral vector lacking all viral genes are stably maintained in skeletal muscle. Our studies clearly show that evaluation of the therapeutic potential of AdDYSβgal will require testing in dystrophin-deficient muscle that is tolerized to β-galactosidase. Ultimately, the development of new vectors expressing dystrophin, but not a marker protein, will be required to test the efficacy of dystrophin gene transfer for DMD.
Acknowledgments
We thank Drs. Alexandre F. R. Stewart, Eric P. Hoffman, and Susan A. McCarthy, for reviewing the manuscript and Dr. Simon P. Watkins and David S. Turner of the Structural Biology Imaging Center for assistance with photography of the histological sections. We thank Steven Chan for assistance with data anyalsis. This work was supported by grants from the Muscular Dystrophy Association (to P.R.C.), the National Institutes of Health (Grants NS01611 to P.R.C. and AR36294 to M.O.) and the European Community Biotechnology (Grant PL950228 to R.K.).
ABBREVIATIONS
- DMD
Duchenne muscular dystrophy
- CMV
cytomegalovirus
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Hoffman E P, Brown J R H, Kunkel L M. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Zubrzycka-Gaarn E E, Bulman D E, Karpati G, Burghes A H M, Belfall B, Klamut H J, Talbot J, Hodges R S, Ray P N, Worton R G. Nature (London) 1988;333:466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]
- 3.Kozarsky K F, Wilson J M. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- 4.Berkner K L. Biotechniques. 1988;6:616–629. [PubMed] [Google Scholar]
- 5.Quantin B, Perricaudet L D, Tajbakhsh S, Mandel J-L. Proc Natl Acad Sci USA. 1992;89:2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 7.Dai Y, Schwartz E M, Gu D, Zhang W-W, Sarvetnick N, Verma I M. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripathy S K, Goldwasser E, Lu M-M, Barr E, Leiden J M. Proc Natl Acad Sci USA. 1994;91:11557–11561. doi: 10.1073/pnas.91.24.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acsadi G, Lochmuller H, Jani A, Huard J, Massie B, Prescott S, Simoneau M, Petrof B J, Karpati G. Hum Gene Ther. 1996;7:129–140. doi: 10.1089/hum.1996.7.2-129. [DOI] [PubMed] [Google Scholar]
- 10.Deconinck N, Ragot T, Marechal G, Perricaudet M, Gillis J M. Proc Natl Acad Sci USA. 1996;93:3570–3574. doi: 10.1073/pnas.93.8.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens P R, Krause T L, Chan S, Korb K E, Graham F L, Caskey C T. Hum Gene Ther. 1995;6:1477–1485. doi: 10.1089/hum.1995.6.11-1477. [DOI] [PubMed] [Google Scholar]
- 12.Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D, Cartaud J, Briand P, Kaplan J-C, Perricaudet M, Kahn A. Nature (London) 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- 13.Vincent N, Ragot T, Gilgenkrantz H, Couton D, Chafey P, Gregoire A, Briand P, Kaplan J-C, Kahn A, Perricaudet M. Nat Genet. 1993;5:130–134. doi: 10.1038/ng1093-130. [DOI] [PubMed] [Google Scholar]
- 14.Alameddine H S, Quantin B, Cartaud A, Dehaupas M, Mandel J L, Fardeau M. Neuromusc Disord. 1994;4:193–203. doi: 10.1016/0960-8966(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 15.Lochmuller H, Petrof B J, Allen C, Prescott S, Massie B, Karpati G. Biochem Biophys Res Commun. 1995;213:569–574. doi: 10.1006/bbrc.1995.2169. [DOI] [PubMed] [Google Scholar]
- 16.Vilquin J-T, Guerette B, Kinoshita I, Roy B, Goulet M, Gravel C, Roy R, Tremblay J P. Hum Gene Ther. 1995;6:1391–1401. doi: 10.1089/hum.1995.6.11-1391. [DOI] [PubMed] [Google Scholar]
- 17.Bett A J, Haddara W, Prevec L, Graham F L. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochanek S, Clemens P R, Mitani K, Chen H-H, Chan S, Caskey C T. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar-Singh R, Chamberlain J S. Hum Mol Genet. 1996;5:913–921. doi: 10.1093/hmg/5.7.913. [DOI] [PubMed] [Google Scholar]
- 20.Haecker S E, Stedman H H, Balice-Gordon R-J, Smith D B J, Greelish J P, Mitchell M A, Wells A, Sweeney H L, Wilson J M. Hum Gene Ther. 1996;7:1907–1914. doi: 10.1089/hum.1996.7.15-1907. [DOI] [PubMed] [Google Scholar]
- 21.Fisher K J, Choi H, Burda J, Chen S-J, Wilson J M. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 22.Clemens P R, Kochanek S, Sunada Y, Chan S, Chen H-H, Campbell K P, Caskey C T. Gene Ther. 1996;3:965–972. [PubMed] [Google Scholar]
- 23.Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M. J Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaynes J B, Chamberlain J S, Buskin J N, Johnson J E, Hauschka S D. Mol Cell Biol. 1986;6:2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacGregor G R, Caskey C T. Nucleic Acids Res. 1989;17:2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz J P, Bodin E T, Coen D M. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harms J S, Splitter G A. Hum Gene Ther. 1995;6:1291–1297. doi: 10.1089/hum.1995.6.10-1291. [DOI] [PubMed] [Google Scholar]
- 28.Dai Y, Roman M, Naviaux R K, Verma I M. Proc Natl Acad Sci USA. 1992;89:10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]