Abstract
Synthetic vectors represent an attractive alternative approach to viral vectors for gene transfer, in particular into airway epithelial cells for lung-directed gene therapy for cystic fibrosis. Having recently found that guanidinium-cholesterol cationic lipids are efficient reagents for gene transfer into mammalian cell lines in vitro, we have investigated their use for gene delivery into primary airway epithelial cells in vitro and in vivo. The results obtained indicate that the lipid bis(guanidinium)-tren-cholesterol (BGTC) can be used to transfer a reporter gene into primary human airway epithelial cells in culture. Furthermore, liposomes composed of BGTC and dioleoyl phosphatidylethanolamine (DOPE) are efficient for gene delivery to the mouse airway epithelium in vivo. Transfected cells were detected both in the surface epithelium and in submucosal glands. In addition, the transfection efficiency of BGTC/DOPE liposomes in vivo was quantitatively assessed by using the luciferase reporter gene system.
Delivery of genetic material to the airway epithelium is a major goal for gene therapy, as it could offer possible treatment of both inherited and acquired lung diseases. For example, cystic fibrosis (CF), a common genetic disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, is a prime target for lung-directed gene therapy, as CF mortality mainly results from pulmonary complications (1, 2). Lung gene therapy for CF requires in vivo gene delivery, since an ex vivo approach does not seem feasible mainly because of the wide distribution of possible cellular targets. The airway epithelium is an attractive but challenging target for direct gene transfer. Indeed, it is of easy access via the airway route but it is a complex structure that normally has a low level of cell cycling (3).
Several approaches for gene delivery to the airways have been proposed. To date, most of the focus has been on adenoviral vectors because of their natural tropism for the respiratory tract. Adenovirus-based clinical trials have even demonstrated evidence of gene transfer in CF patients (4). However, first-generation adenoviral vectors have severe limitations, including occurrence of an inflammatory immune response, transient expression of the transgene, and practical issues relating to bulk production and quality control.
Nonviral gene delivery systems have therefore been investigated as an alternative approach to viral vectors. Amine-carrying cationic lipids have been used to transfer either reporter genes into the airway epithelium of various species or a normal human CFTR gene into the respiratory epithelium of CF mice (5). These studies paved the way for a liposome-based gene therapy trial for CF, which showed that gene transfer into the human respiratory tract was feasible but had only transient and partial effects (6). Thus, many critical issues, relating especially to the efficiency of liposome-based gene transfer, still need to be addressed.
We have recently found that novel cationic lipids, cholesterol derivatives bearing guanidinium polar headgroups, are efficient and convenient reagents for gene transfection into a wide variety of mammalian cell lines (7). In the present study, we have explored the potential utility of these novel cationic lipids for gene transfer into primary airway epithelial cells in vitro and in vivo. We herein show the feasibility of gene transfer into primary human airway epithelial cells in culture using the lipid bis(guanidinium)-tren-cholesterol [BGTC; tren is tris(2-aminoethyl)amine]. Most importantly, we also demonstrate the possibility and quantitatively assess the efficiency of gene transfection by liposomes composed of BGTC and dioleoyl phosphatidylethanolamine (DOPE) into the mouse respiratory epithelium in vivo.
MATERIALS AND METHODS
Transfection Reagents and Plasmids.
Lipid BGTC was synthesized and BGTC/DOPE liposomes were prepared as previously described (7). The reagent Transfectam was kindly provided by J.-P. Behr (Faculté de Pharmacie, Strasbourg, France).
Plasmids pRSV-nlsLacZ and pRSV-Luc were amplified in Escherichia coli and prepared by double CsCl gradient purification using standard techniques. In plasmid pRSV-nlsLacZ, the E. coli lacZ gene with the simian virus 40 nuclear localization signal is under the transcriptional control of the Rous sarcoma virus (RSV) long terminal repeat (LTR). In plasmid pRSV-Luc, the firefly Photinus pyralis luciferase gene is driven by the RSV LTR.
Recombinant Adenoviruses.
Viral stocks of the replication-deficient recombinant adenoviral vector AdRSVβGal were obtained from M. Perricaudet (Institut Gustave Roussy, Villejuif, Fance). Construction of the vector AdRSVβGal has been described (8). Briefly, AdRSVβGal contains an engineered E. coli lacZ gene encoding a nuclear-targeted β-galactosidase driven by the RSV LTR.
Primary Cultures of Human Airway Epithelial Cells.
Human nasal polyps were obtained from adult patients undergoing nasal polypectomy and were immediately immersed in RPMI-1640 medium (ICN, France) containing antibiotics.
Surface epithelial cells were dissociated by overnight enzymatic treatment at 4°C with 0.15% Pronase E (Boehringer Mannheim) and cultured in a hormonally defined serum-free medium (9). Briefly, the isolated cells were seeded onto type I collagen-coated culture dishes (Corning) and cultured in a humidified 5% CO2-containing atmosphere in serum-free RPMI-1640 medium supplemented with 1 μg/ml insulin (Sigma), 1 μg/ml transferrin (Sigma), 0.5 μg/ml hydrocortisone (Sigma), and 10 ng/ml retinoic acid (Sigma).
In Vitro Transfection Procedure.
Transfection of primary cultures of human airway epithelial cells was performed 48–72 h after plating. For formation of BGTC/DNA aggregates, the conditions were identical to those previously determined to be optimal for gene transfection into cultured cell lines (7). The primary cells were exposed to the transfection mixture for 4 h at 37°C; hormonally defined serum-free medium was then added without removing the transfection mixture. After 48 h, staining with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) was performed as described below. For transfection with Transfectam, an identical transfection procedure was followed, using DNA complexes characterized by an optimal charge ratio ≈6 (10).
Adenovirus Infection of Primary Cultures.
At 48–72 h after plating, human airway epithelial cells were exposed to AdRSVβGal-containing RPMI-1640 medium for 1 h at 37°C and then returned to the defined culture medium for 48 h before X-Gal staining. Primary cultures were exposed to various amounts of recombinant adenovirus (approximate corresponding range of multiplicity of infection: 1 to 100).
In Vivo Gene Delivery to Mouse Airways.
Male Swiss OF1 mice (30 g body weight) were purchased from Iffa-Credo (Lyon, France). BGTC/DOPE (molar ratio 3:2) cationic liposomes were used for the in vivo experiments. The transfection mixture was obtained by mixing 10 μg of plasmid DNA (in 10 μl of water) with 20 μl of cationic liposomes in 20 mM Hepes (pH 7.4) at a total lipid concentration of 5 mg/ml. The resulting lipid/DNA aggregates are characterized by a positive mean charge of ≈6 according to our previous studies (7).
After anesthesia with pentobarbital, the transfection mixture (30 μl) was delivered to the airways by intratracheal instillation via a cannula inserted into the tracheal lumen. At 48 h after instillation, the animals were killed by an i.p. overdose of pentobarbital and the lungs and trachea were removed for analysis.
X-Gal Staining.
To detect E. coli β-galactosidase expression in primary cultures, cells were fixed for 15 min at 4°C in a 4% paraformaldehyde solution and then incubated with the chromogenic β-galactosidase substrate X-Gal (Sigma). Blue staining of the nuclei of the transfected cells was assessed by light microscopy.
For detection of X-Gal-positive cells in mouse airways, lungs and trachea were fixed for 1 h in 4% paraformaldehyde, immersed overnight in phosphate-buffered saline (PBS) containing 30% sucrose, included in congelation medium, and frozen in liquid nitrogen. Cryostat sections of 5 μm were then stained for β-galactosidase activity by overnight incubation with the X-Gal reagent (Sigma).
Luciferase Assay.
The luciferase assay used for determining the luciferase activity of in vitro transfected cells has been previously described (7).
To assess the luciferase activity after in vivo transfection, tissue pieces were placed in 300 μl of lysis buffer [25 mM Tris phosphate, pH 7.8/1 mM dithiothreitol (DTT)/15% (vol/vol) glycerol and 1% Triton X-100], disrupted for about 1 min with a hand-held homogenizer (Polylabo, Strasbourg, France) and kept on ice. After centrifugation, 20 μl of the lysate was used for the luciferase assay.
Data for luciferase activity are expressed as relative light units per mg of protein, the protein concentration being determined by using the Bio-Rad assay.
Immunohistochemistry.
Cryosections of lung and trachea samples were incubated for 1 h either with a mouse monoclonal anti-E. coli β-galactosidase antibody used at 5 μg/ml (Genzyme) or with a rabbit polyclonal anti-luciferase antibody used at a 1:400 dilution (Promega). The E. coli β-galactosidase protein was detected by immunoperoxidase staining employing a biotin-labeled secondary anti-mouse Ig antibody (Boehringer Mannheim) and a streptavidin-peroxidase conjugate (Boehringer Mannheim). The luciferase protein was detected by immunoperoxidase staining using a secondary goat anti-rabbit antibody (ICN) and the rabbit peroxidase-antiperoxidase (PAP) system (Dako). Tissue sections were observed by light microscopy. Negative controls were performed by omitting the primary antibody.
Ki67 Immunolabeling.
Primary airway epithelial cells (grown on collagen I-coated coverslips) were fixed in situ for 1 h in a 4% paraformaldehyde solution and permeabilized by treatment with 0.1% Triton X-100 (Sigma). The cells were then incubated sequentially with the mouse monoclonal anti-Ki 67 antibody (Novocastra Laboratories, Newcastle-upon-Tyne, U.K.) used at a 1:50 dilution and with TRITC-conjugated goat anti-mouse immunoglobulin antibody (Nordic Immunological Laboratories, Tilburg, The Netherlands). Finally, coverslips were mounted in glycerol and observed by use of a Zeiss fluorescence microscope.
RESULTS AND DISCUSSION
BGTC Lipid-Mediated Gene Transfection into Primary Human Airway Epithelial Cells in Vitro.
Airway epithelial cells were isolated from human nasal polyps and cultured as indicated in Materials and Methods. In our cell culture system, dissociated surface epithelial cells attach to a collagen I substratum and form proliferative cell clusters. Staining of all cultured cells with an anti-pan- human cytokeratin antibody confirmed their epithelial origin (data not shown).
For gene transfection into the cultured primary human airway cells, we used lipid BGTC as a micellar solution, since we have shown that compound BGTC without liposomal formulation was efficient for gene transfer into cultured cell lines (7). Transfection conditions were identical to those determined to be optimal for mammalian cell lines—i.e., conditions characterized by DNA/lipid aggregates bearing a strongly positive mean charge. Indeed, they were considered to be satisfactory since, in preliminary experiments with the luciferase reporter gene, they resulted in efficiencies of gene transfection into the primary cells similar to those we had previously obtained for A549 cells, a human lung carcinoma-derived cell line (ref. 7 and data not shown). Furthermore, it has also been reported that positively charged lipid/DNA complexes were effective for gene transfection by the cationic lipid Transfectam into various types of primary cells in vitro, including neurons, glial cells, and endothelial cells (11–13).
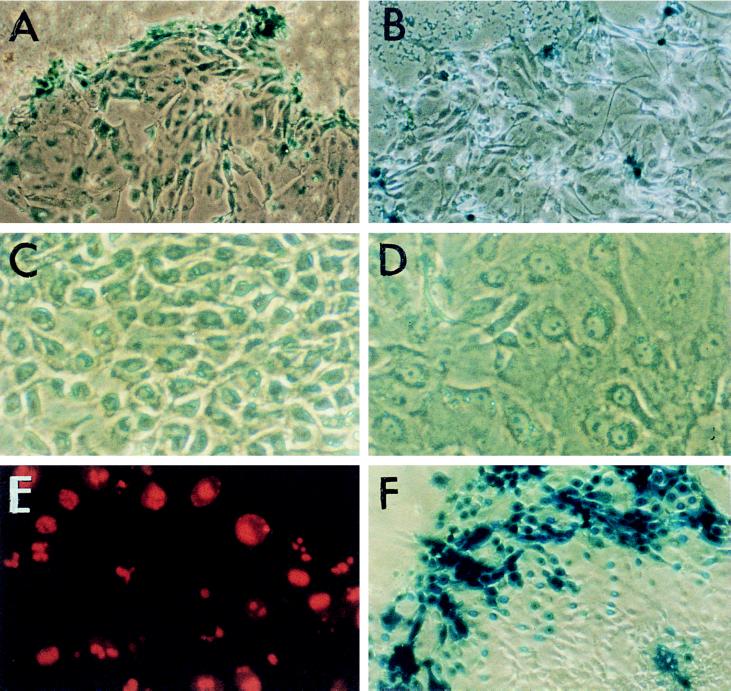
BGTC-mediated gene transfer into primary human airway cells was clearly demonstrated by using an E. coli lacZ-expressing plasmid. Interestingly, X-Gal staining revealed a particular spatial distribution of the transfected cells (Fig. 1A). Indeed, X-Gal-positive cells were preferentially located in the peripheral areas of the cell clusters. A similar distribution pattern of the transfected cells was also observed when the lipopolyamine Transfectam was used (Fig. 1B).
Figure 1.
Gene transfer into primary cultures of human airway epithelial cells. (A and B) Peripheral location of the transfected cells shown by the distribution of the X-Gal-positive cells after transfer of the E. coli lacZ gene by lipid BGTC (A) or by Transfectam (B). (×200.) (C and D) Phase-contrast micrographs showing the morphology of cultured airway epithelial cells located in the center (C) or at the periphery (D) of cell clusters. (×400.) (E) Localization of proliferating cells evidenced by immunofluorescent detection of Ki67 staining. (×400.) (F) Distribution of X-Gal-positive cells when AdRSVβGal adenoviruses were used at a high multiplicity of infection. (×200.)
Thus, we directed our efforts toward the identification of biological features that may account for the higher transfectability of the cells located at the edge of the cellular islands. Microscopic inspection showed that the edge cells were flattened, larger, and much less tightly packed than the central cells (Fig. 1 C and D). Thus, preferential transfection of the peripheral cells could be related to an increase in their accessibility for and their uptake of the DNA/lipid complexes. Accordingly, the low transfectability of the tightly packed central cells may be related to a decreased accessibility and a reduced uptake efficiency. Differences in uptake mechanisms may involve binding as well as internalization.
We also studied the distribution of proliferating cells in the cell clusters by immunofluorescence using a monoclonal antibody against the Ki67 nuclear antigen, which is expressed in all cycling cells. Most of the cells located at the periphery were proliferating cells, but some cycling cells could also be detected throughout the cellular islands (Fig. 1E). The finding that the edge cells, which are easy to transfect, were mainly cycling cells provides additional support to a role of mitosis in cationic lipid-mediated gene transfection. Indeed, it has been reported that transfected DNA does not easily enter the nuclear compartment during interphase (14–16).
A preferential location of the transfected cells at the periphery has also been reported when the cationic lipids Lipofectin and Transfectam were used for gene transfection into explant growth cultures of human nasal polyp tissue (16). Interestingly, in this type of cell culture system, the cells from the periphery of the outgrowth have been characterized as migrating cells, mimicking the poorly differentiated regenerating cells present in vivo during the repair of the airway epithelium (17). These results invited us to define more precisely the differentiation state of the transfected edge cells in our cell culture system. As cytokeratins are good markers of epithelial differentiation, we are at present investigating expression in the transfected cells of various cytokeratins (CK), including CK14 (a basal cell specific marker) and CK18 (a specific marker of differentiated columnar cells).
Interestingly, in explant outgrowth cell cultures, a preferential location of the X-Gal-positive cells at the periphery of the outgrowth has also been reported when using adenovirus vectors containing the lacZ gene under the control of different promoters, including the CFTR promoter (18, 19). These data suggest that transcriptional variation of the promoters driving transgene expression is not the cause for the particular distribution pattern of the X-Gal-positive cells. Thus, regenerating cells may also represent preferential targets for adenovirus vectors. Accordingly, it has been suggested that efficiency of gene transduction by adenoviruses could be higher in damaged airway epithelium than in normal tissue (18). In primary cultures of dissociated cells, we also observed that edge cells were preferentially transduced when AdRSVβGal recombinant viruses were used, even at a high multiplicity of infection (Fig. 1F). Taken together, these results invite elucidation of the mechanisms by which edge cells are more easily gene modified. It is also crucial to examine the relevance of the conclusions of these in vitro studies for gene transfer into the airway epithelium in vivo.
Location of Transgene Expression in Mouse Airways in Vivo After Gene Transfection by BGTC/DOPE Liposomes.
We have assessed the ability of guanidinium-cholesterol cationic lipids to mediate gene transfection into mouse airways in vivo. For these in vivo studies, we chose to use BGTC/DOPE cationic liposomes because the results of previous studies, including our own work with mammalian cell lines in vitro, have supported the role of a DOPE-facilitated step in cationic liposome-mediated transfection, involving in particular the well-known fusogenic properties of DOPE (7, 20). Furthermore, it has also been reported that the addition of DOPE to the cationic lipid Transfectam increased the efficiency of gene transfection into the newborn mouse brain in vivo (12).
Obtaining the DNA/lipid aggregates and administering the transfection mixture in vivo were as indicated in Materials and Methods. Taking into account that the properties of cultured primary cells are close to those of their in vivo counterparts, we chose to deliver DNA/lipid aggregates characterized by a positive mean charge ≈6, as such aggregates were efficient for gene transfection into primary airway epithelial cells in vitro as indicated above. Indeed, it is generally recognized that, when using in vitro studies as a means of establishing starting values for subsequent in vivo studies, it is important to choose a cell culture system mimicking the in vivo target cells as closely as possible (14). It has also been reported that gene transfer into the intact avian embryo could be obtained by directly depositing strongly positive DNA/Transfectam complexes onto embryonic membranes (21). However, it should be stressed that further studies investigating the in vivo transfection efficiencies of various BGTC/DOPE liposomal formulations and various DNA/lipid ratios will be necessary to work out optimal conditions for gene transfection into the airways in vivo.
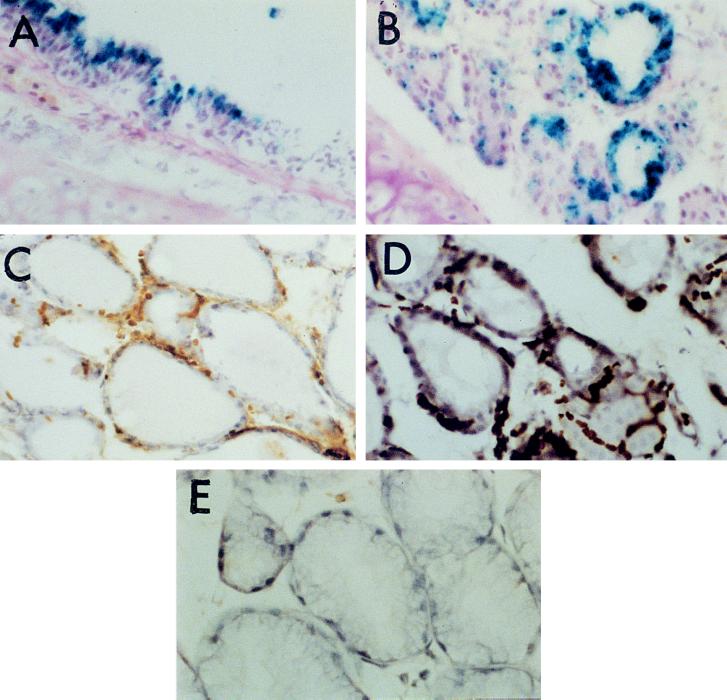
X-Gal-positive cells could be detected in the airway epithelium of the treated mice at 48 h after transfection of a lacZ-expressing plasmid by BGTC/DOPE liposomes (Fig. 2 A and B). Almost all transfected cells were located in the trachea and only a few X-Gal-positive cells were observed in the small airways. A similar distribution of the transfected cells has been reported by others (16, 22, 23). This distribution pattern could be due simply to the intratracheal administration technique, especially because of the small volume (30 μl) of transfection mixture instilled. Occasional observation of nonspecific staining (characterized by staining of virtually all airway cells after prolonged incubation of the cryostat sections with the X-Gal reagent) invited us to also perform immunohistochemistry techniques to detect the reporter gene product (see below).
Figure 2.
Cryosections of mouse trachea, showing reporter gene expression in the respiratory epithelium after gene transfection by BGTC/DOPE liposomes in vivo. (A and B) Detection of β-galactosidase expression in transfected cells located in the surface epithelium (A) and in submucosal glands (B) by X-Gal staining. (×400.) (C) Detection of β-galactosidase-expressing cells in submucosal glands by immunoperoxidase labeling using a monoclonal anti-β-galactosidase antibody. (×400.) (D) Detection of luciferase-expressing cells in submucosal glands by immunoperoxidase labeling with a polyclonal anti-luciferase antibody. (×400.) (E) Immunoperoxidase staining: negative control performed by omitting the primary antibody. (×400.)
Mainly columnar cells were transfected in the surface epithelium (Fig. 2A). This observation is in agreement with data reported by other groups (16, 23). In contrast to the results of the in vitro studies (see above), these data show that cationic liposomes are able to induce gene transfection into fully differentiated, nonproliferating cells of the airway epithelium in vivo. Interestingly, we could also detect transgene expression in the basal nuclei of submucosal glands (Fig. 2B). Immunolabel detection of the transfected cells by using a monoclonal antibody against E. coli β-galactosidase confirmed reporter gene expression in the submucosal glands as well as in the surface epithelium (Fig. 2C and data not shown). Furthermore, we also detected transgene expression in submucosal glands by immunoperoxidase staining with an antibody directed against the luciferase protein after BGTC/DOPE liposome-mediated transfer of the luciferase-expressing plasmid pRSV-Luc (Fig. 2D). Negative controls were performed by omitting the primary antibody (Fig. 2E). These results may be of special interest for lung-directed gene therapy for CF, as it could be required to target the submucosal glands, which are the predominant site of CFTR expression in the human bronchus (24).
Luciferase Activity in Mouse Airway Epithelium in Vivo.
Transfection of the firefly Photinus pyralis luciferase gene into mouse airways allowed us to quantitatively assess the transfection efficiency of BGTC/DOPE liposomes in vivo. Thus, lungs and trachea of the treated mice were harvested at 48 h after transfection and assayed for luciferase activity as indicated in Materials and Methods. Luciferase activity was repeatedly detectable in the trachea homogenates but not in the lung homogenates (Table 1). This observation is in good agreement with the distribution pattern of X-Gal-positive cells in vivo (see above). Luciferase expression was exclusively derived from the luciferase-expressing plasmid, since no luciferase activity could be detected in control mice receiving an irrelevant lacZ plasmid (Table 1). We also included a “naked” (uncomplexed) plasmid DNA control in our study. Indeed, it has been reported that administration of plasmid DNA in the absence of any enhancing reagent can result in gene expression in mouse airways (22, 25). Interestingly, we could not detect luciferase activity in tissue homogenates from mice receiving “naked” luciferase- expressing plasmid DNA under identical conditions (Table 1). This contrasting result may be explained by experimental factors, especially the low amount (10 μg) of plasmid DNA administered to the mice. Thus, our data indicate that BGTC/DOPE liposomes play a pivotal role in gene transfection into the airway epithelium in vivo under our conditions. Of note, it has recently been reported that a novel reagent of the 2,3-dioxypropaniminium class of cationic lipids can also enhance plasmid DNA delivery and expression in mouse lung (25).
Table 1.
Luciferase activity in mouse tracheas after intratracheal administration of either BGTC/DOPE/DNA complexes or “naked” plasmid DNA
| Type of vector | Luciferase activity, RLU/mg protein |
|---|---|
| BGTC/DOPE liposomes | |
| pRSV-Luc | 2.8 × 105 |
| (range: 3 × 104 to 8 × 105) | |
| pRSV-LacZ | 0 |
| “Naked” DNA | |
| pRSV-Luc | 0 |
Intratracheal gene delivery was performed and tracheas were harvested and assayed for luciferase activity as indicated in Materials and Methods. Data are expressed as mean relative light units (RLU)/mg of protein (n ≥ 3 with n = 7 for BGTC/DOPE/Luc complexes).
In mouse trachea homogenates, we could measure levels of luciferase activity similar to those previously obtained with A549 cells (ref. 7 and Table 1). In the rat, preliminary results show that similar levels of luciferase activity can be obtained in the trachea (data not shown). These levels of luciferase expression are apparently higher than those reported by other groups when using Lipofectin or Transfectam (16, 26). Experimental factors may at least partially account for these differences. For example, in the Lipofectin study, luciferase activity was measured in whole lung (trachea plus lungs) homogenates (26). Unknown factors may, however, also be involved, as the mechanisms of cationic liposome-facilitated transfection are still poorly understood. After adenoviral vector-mediated gene transfer into the mouse airways in vivo, luciferase levels greater by one or two orders of magnitude have been reported (16). Although this higher efficiency of adenoviruses may suggest that further progress is still required in the field of cationic liposome-mediated gene transfer, it should also be stressed that it is difficult to extrapolate results obtained with reporter genes. Thus, studies in animal models like the CF mouse should allow to better assess the real usefulness of BGTC/DOPE liposomes for transfection of a therapeutic gene.
In conclusion, the present results indicate that gene transfection mediated by guanidinium-cholesterol lipids is feasible within the mammalian airway epithelium. Positive data with primary human cells in vitro and in the mouse airways in vivo confirm the potential of cationic lipids for lung-directed gene therapy. It will be important to work out optimal formulations for gene transfection in vivo, to investigate delivery by aerosol, to study the duration of transgene expression, and to evaluate quantitatively the in vivo toxicity of these new transfection reagents. Finally, studies in CF mice should allow better assessment of the utility of guanidinium-cholesterol lipids for lung gene therapy for CF.
In a broader perspective, the positive results obtained here and in our earlier work (7) provide the basis for the design of other potential transfection agents, for the investigation of the factors and processes leading to transfection and determining its efficiency, and for the exploration of a broad range of in vitro and in vivo gene transfer systems for therapeutic or preventive as well as biotechnological purposes.
Acknowledgments
We express our gratitude to J. Navarro for his constant support. We also thank the following: J.P. Behr for providing Transfectam; M. Perricaudet for supplying recombinant adenoviruses; P. Arnaud, J.P. Behr, F. Brion, B. Demeneix, D. Escande, S. Fabrega, L. Ferkdadji, E. Puchelle, and M. Scarpa for helpful discussions; M. Fauquet for critical reading of the manuscript; and J. Soupault for secretarial assistance. This work was supported by grants from the Association Française de Lutte contre la Mucoviscidose (AFLM) and the Association Française contre les Myopathies (AFM) and by a special grant from the Centres d’Application et Réseaux de Développement de Thérapies Géniques (CRTG 1995).
ABBREVIATIONS
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- BGTC
bis(guanidinium)-tren-cholesterol [tren, tris(2-aminoethyl)amine]
- DOPE
dioleoyl phosphatidylethanolamine
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Wilson J M. J Clin Invest. 1995;96:2547–2554. doi: 10.1172/JCI118318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alton E W F W, Geddes D M. Gene Ther. 1995;2:88–95. [PubMed] [Google Scholar]
- 3.Ayers M M, Jeffery P K. Eur Respir J. 1988;1:58–80. [PubMed] [Google Scholar]
- 4.Crystal R G. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Huang L. Gene Ther. 1995;2:710–722. [PubMed] [Google Scholar]
- 6.Caplen N J, Alton E W F W, Middleton P G, Dorin J R, Stevenson B J, Gao X, Durham S R, Jeffery P K, Hodson M E, Coutelle C, Huang L, Porteous D J, Williamson R, Geddes D M. Nat Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 7.Vigneron J P, Oudrhiri N, Fauquet M, Vergely L, Bradley J C, Basseville M, Lehn P, Lehn J M. Proc Natl Acad Sci USA. 1996;93:9682–9686. doi: 10.1073/pnas.93.18.9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevillard M, Hinnrasky J, Pierrot D, Zahm J M, Klossek J M, Puchelle E. Epithelial Cell Biol. 1993;2:17–25. [PubMed] [Google Scholar]
- 10.Barthel F, Rémy J S, Loeffler J P, Behr J P. DNA Cell Biol. 1993;12:553–560. doi: 10.1089/dna.1993.12.553. [DOI] [PubMed] [Google Scholar]
- 11.Lezoualc’h F, Hassan A H, Giraud P, Loeffler J P, Lee S L, Demeneix B A. Mol Endocrinol. 1992;6:1797–1804. doi: 10.1210/mend.6.11.1480171. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz B, Benoist C, Abdallah B, Scherman D, Behr J P, Demeneix B A. Hum Gene Ther. 1995;6:1515–1524. doi: 10.1089/hum.1995.6.12-1515. [DOI] [PubMed] [Google Scholar]
- 13.Boussif O, Lezoualc’h F, Zanta M A, Mergny M D, Scherman D, Demeneix B, Behr J P. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheule R K, Cheng S H. In: Artificial Self-Assembling Systems for Gene Delivery. Felgner P L, Heller M J, Lehn P, Behr J P, Szoka F C, editors. Washington, DC: Am. Chem. Soc.; 1996. pp. 177–190. [Google Scholar]
- 15.Zabner J, Fassbender A J, Moninger T, Poellinger K A, Welsh M J. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 16.Fortunati E, Bout A, Zanta M A, Valerio D, Scarpa M. Biochim Biophys Acta. 1996;1306:55–62. doi: 10.1016/0167-4781(95)00217-0. [DOI] [PubMed] [Google Scholar]
- 17.Plotkowski M C, Chevillard M, Altemayer D, Zahm J M, Colliot G, Puchelle E. J Clin Invest. 1991;87:2018–2028. doi: 10.1172/JCI115231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupuit F, Zahm J M, Pierrot D, Brezillon S, Bonnet N, Imler J L, Pavirani A, Puchelle E. Hum Gene Ther. 1995;6:1185–1193. doi: 10.1089/hum.1995.6.9-1185. [DOI] [PubMed] [Google Scholar]
- 19.Imler J L, Dupuit F, Chartier C, Accart N, Dieterlé A, Schultz H, Puchelle E, Pavirani A. Gene Ther. 1996;3:49–58. [PubMed] [Google Scholar]
- 20.Felgner J H, Kumar R, Sridhar C N, Wheeler C J, Tsai Y J, Border R, Ramsey P, Martin M, Felgner P L. J Biol Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- 21.Demeneix B A, Abdel-Taweb H, Benoist C, Seugnet I, Behr J P. BioTechniques. 1994;16:496–501. [PubMed] [Google Scholar]
- 22.Meyer K B, Thompson M M, Levy M Y, Barron L G, Szoka F C., Jr Gene Ther. 1995;2:450–460. [PubMed] [Google Scholar]
- 23.McLachlan G, Davidson D J, Stevenson B J, Dickinson P, Davidson-Smith H, Dorin J R, Porteous D J. Gene Ther. 1995;2:614–622. [PubMed] [Google Scholar]
- 24.Engelhardt J, Yankaskas J, Ernst S, Yang Y, Marino C, Boucher R, Cohn J, Wilson J M. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler C J, Felgner P L, Tsai Y J, Marshall J, Sukhu L, Doh S G, Hartikka J, Nietupski J, Manthorpe M, Nichols M, Plewe M, Liang X, Norman J, Smith A, Cheng S H. Proc Natl Acad Sci USA. 1996;93:11454–11459. doi: 10.1073/pnas.93.21.11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimura K, Rosenfeld M A, Nakamura H, Scherer E M, Pavirani A, Lecoq J P, Crystal R G. Nucleic Acids Res. 1992;20:3233–3240. doi: 10.1093/nar/20.12.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]