Abstract
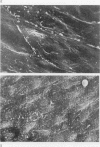
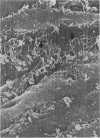
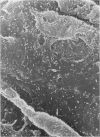
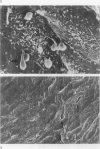
Endothelial monolayer integrity is a critical factor limiting vascular permeability of solid organs in transplantation. Several in vitro, ex vivo and in vivo studies suggest that damage to endothelial cells (EC) due to hypothermia and ischaemia-reperfusion injury causes morphological and functional damage to the endothelium leading to parenchymal oedema and haemorrhage. Aiming to study morphological changes to arterial pulmonary EC subjected to transplantation procedures, random scanning electron micrographs of vascular endothelium of rat lungs were taken. Forty-eight rat lungs were hypothermically stored for 48 or 72 hours in two different preservation solutions and studied either at the end of the cold storage period, or 5 min, 24 h or 4 weeks following transplantation. After 5 minutes of revascularization, micrographs showed EC shape variations, bleb formation and cell retraction with intercellular gap formation. Twenty-four hours after transplantation loss of monolayer continuity was widely extended. Four weeks of revascularization resulted in either well preserved specimens with nearly normal endothelium, or badly preserved arteries with fibrotic degeneration of the luminal vessel wall. The morphological disruptions found in this study help to explain the alterations in permeability control and vascular dysfunction observed in lung transplantation.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athanassakis-Vassiliadis I., Papamatheakis J., Vassiliadis S. Specific CSF-1 binding on murine placental trophoblasts and macrophages serves as a link to placental growth. J Recept Res. 1993;13(1-4):739–751. doi: 10.3109/10799899309073690. [DOI] [PubMed] [Google Scholar]
- Cai W. Q., Bodin P., Loesch A., Sexton A., Burnstock G. Endothelium of human umbilical blood vessels: ultrastructural immunolocalization of neuropeptides. J Vasc Res. 1993 Nov-Dec;30(6):348–355. doi: 10.1159/000159017. [DOI] [PubMed] [Google Scholar]
- Fratté S., Gendrault J. L., Steffan A. M., Kirn A. Comparative ultrastructural study of rat livers preserved in Euro-Collins or University of Wisconsin solution. Hepatology. 1991 Jun;13(6):1173–1180. [PubMed] [Google Scholar]
- Frim J., Kruuv J., Frey H. E., Raaphorst G. P. Survival of unprotected, mammalian plateau-phase cells following freezing in liquid nitrogen. Cryobiology. 1976 Aug;13(4):475–483. doi: 10.1016/0011-2240(76)90104-8. [DOI] [PubMed] [Google Scholar]
- Gertler J. P., Abbott W. M. Prothrombotic and fibrinolytic function of normal and perturbed endothelium. J Surg Res. 1992 Jan;52(1):89–95. doi: 10.1016/0022-4804(92)90284-7. [DOI] [PubMed] [Google Scholar]
- Gertler J. P., Weibe D. A., Ocasio V. H., Abbott W. M. Hypoxia induces procoagulant activity in cultured human venous endothelium. J Vasc Surg. 1991 Mar;13(3):428–433. doi: 10.1067/mva.1991.25767. [DOI] [PubMed] [Google Scholar]
- Goldman G., Welbourn R., Alexander S., Klausner J. M., Wiles M., Valeri C. R., Shepro D., Hechtman H. B. Modulation of pulmonary permeability in vivo with agents that affect the cytoskeleton. Surgery. 1991 Apr;109(4):533–538. [PubMed] [Google Scholar]
- Hall S. M., Odom N., McGregor C. G., Haworth S. G. Transient ultrastructural injury and repair of pulmonary capillaries in transplanted rat lung: effect of preservation and reperfusion. Am J Respir Cell Mol Biol. 1992 Jul;7(1):49–57. doi: 10.1165/ajrcmb/7.1.49. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. A., Manek S., Fryer P. R., Fuller B. J., Green C. J. Morphological changes in rat single lung isografts after long-term survival. Int J Exp Pathol. 1995 Feb;76(1):43–54. [PMC free article] [PubMed] [Google Scholar]
- Hinshaw D. B., Burger J. M., Armstrong B. C., Hyslop P. A. Mechanism of endothelial cell shape change in oxidant injury. J Surg Res. 1989 Apr;46(4):339–349. doi: 10.1016/0022-4804(89)90199-6. [DOI] [PubMed] [Google Scholar]
- Inauen W., Payne D. K., Kvietys P. R., Granger D. N. Hypoxia/reoxygenation increases the permeability of endothelial cell monolayers: role of oxygen radicals. Free Radic Biol Med. 1990;9(3):219–223. doi: 10.1016/0891-5849(90)90031-d. [DOI] [PubMed] [Google Scholar]
- Kumar S., West D. C., Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987;36(1):57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Kurzawinski T. R., Appleby J. A., Hardy S. C., Fuller B., Cheetham K., Haswell D., Davidson B., Rolles K. A prospective randomized clinical trial of liver preservation using high-sodium versus high-potassium lactobionate/raffinose solution. Transpl Int. 1994;7 (Suppl 1):S489–S492. doi: 10.1111/j.1432-2277.1994.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Lampugnani M. G., Resnati M., Dejana E., Marchisio P. C. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991 Feb;112(3):479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A. B., Lynch J. J., Cooper J. A. Endothelial barrier function. J Invest Dermatol. 1989 Aug;93(2 Suppl):62S–67S. doi: 10.1111/1523-1747.ep12581072. [DOI] [PubMed] [Google Scholar]
- Manolopoulos V. G., Lelkes P. I. Cyclic strain and forskolin differentially induce cAMP production in phenotypically diverse endothelial cells. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1379–1385. doi: 10.1006/bbrc.1993.1370. [DOI] [PubMed] [Google Scholar]
- Marzi I., Zhong Z., Lemasters J. J., Thurman R. G. Evidence that graft survival is not related to parenchymal cell viability in rat liver transplantation. The importance of nonparenchymal cells. Transplantation. 1989 Sep;48(3):463–468. doi: 10.1097/00007890-198909000-00023. [DOI] [PubMed] [Google Scholar]
- McKeown C. M., Edwards V., Phillips M. J., Harvey P. R., Petrunka C. N., Strasberg S. M. Sinusoidal lining cell damage: the critical injury in cold preservation of liver allografts in the rat. Transplantation. 1988 Aug;46(2):178–191. [PubMed] [Google Scholar]
- Mills A. N., Hooper T. L., Hall S. M., McGregor C. G., Haworth S. G. Unilateral lung transplantation: ultrastructural studies of ischemia-reperfusion injury and repair in the canine pulmonary vasculature. J Heart Lung Transplant. 1992 Jan-Feb;11(1 Pt 1):58–67. [PubMed] [Google Scholar]
- Momii S., Koga A. Time-related morphological changes in cold-stored rat livers. A comparison of Euro-Collins solution with UW solution. Transplantation. 1990 Nov;50(5):745–750. doi: 10.1097/00007890-199011000-00003. [DOI] [PubMed] [Google Scholar]
- Novick R. J., Menkis A. H., McKenzie F. N. New trends in lung preservation: a collective review. J Heart Lung Transplant. 1992 Mar-Apr;11(2 Pt 1):377–392. [PubMed] [Google Scholar]
- Pickford M. A., Gower J. D., Doré C., Fryer P. R., Green C. J. Lipid peroxidation and ultrastructural changes in rat lung isografts after single-passage organ flush and 48-hour cold storage with and without one-hour reperfusion in vivo. Transplantation. 1990 Aug;50(2):210–218. doi: 10.1097/00007890-199008000-00008. [DOI] [PubMed] [Google Scholar]
- Rauen U., Hanssen M., Lauchart W., Becker H. D., de Groot H. Energy-dependent injury to cultured sinusoidal endothelial cells of the rat liver in UW solution. Transplantation. 1993 Mar;55(3):469–473. doi: 10.1097/00007890-199303000-00002. [DOI] [PubMed] [Google Scholar]
- Rogers K. R., Morris C. J., Blake D. R. The cytoskeleton and its importance as a mediator of inflammation. Ann Rheum Dis. 1992 Apr;51(4):565–571. doi: 10.1136/ard.51.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savion N., Vlodavsky I., Greenburg G., Gospodarowicz D. Synthesis and distribution of cytoskeletal elements in endothelial cells as a function of cell growth and organization. J Cell Physiol. 1982 Feb;110(2):129–141. doi: 10.1002/jcp.1041100205. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Shepard J. M., Goderie S. K., Brzyski N., Del Vecchio P. J., Malik A. B., Kimelberg H. K. Effects of alterations in endothelial cell volume on transendothelial albumin permeability. J Cell Physiol. 1987 Nov;133(2):389–394. doi: 10.1002/jcp.1041330226. [DOI] [PubMed] [Google Scholar]
- Shreeniwas R., Ogawa S., Cozzolino F., Torcia G., Braunstein N., Butura C., Brett J., Lieberman H. B., Furie M. B., Joseph-Silverstein J. Macrovascular and microvascular endothelium during long-term hypoxia: alterations in cell growth, monolayer permeability, and cell surface coagulant properties. J Cell Physiol. 1991 Jan;146(1):8–17. doi: 10.1002/jcp.1041460103. [DOI] [PubMed] [Google Scholar]
- Siflinger-Birnboim A., Cooper J. A., del Vecchio P. J., Lum H., Malik A. B. Selectivity of the endothelial monolayer: effects of increased permeability. Microvasc Res. 1988 Nov;36(3):216–227. doi: 10.1016/0026-2862(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Sims J. R., Karp S., Ingber D. E. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J Cell Sci. 1992 Dec;103(Pt 4):1215–1222. doi: 10.1242/jcs.103.4.1215. [DOI] [PubMed] [Google Scholar]
- Solberg S., Larsen T., Jørgensen L., Sørlie D. Cold induced endothelial cell detachment in human saphenous vein grafts. J Cardiovasc Surg (Torino) 1987 Sep-Oct;28(5):571–575. [PubMed] [Google Scholar]
- Solberg S., Larsen T., Småbrekke A., Brox J. H., Bertheussen K., Sørlie D., Osterud B., Jørgensen L. A new protective solution for hypothermic storage of free vein grafts in cardiovascular surgery. Scand J Clin Lab Invest. 1992 Apr;52(2):73–82. doi: 10.3109/00365519209088769. [DOI] [PubMed] [Google Scholar]
- Speiser W., Anders E., Preissner K. T., Wagner O., Müller-Berghaus G. Differences in coagulant and fibrinolytic activities of cultured human endothelial cells derived from omental tissue microvessels and umbilical veins. Blood. 1987 Mar;69(3):964–967. [PubMed] [Google Scholar]
- Toledo-Pereyra L. H., Rodríguez F. J. Scientific basis and current status of organ preservation. Transplant Proc. 1994 Feb;26(1):309–311. [PubMed] [Google Scholar]
- Unruh H. Pulmonary endothelial cell function after modified Eurocollins solution infusion. J Heart Lung Transplant. 1993 Jul-Aug;12(4):700–705. [PubMed] [Google Scholar]
- Vidal-Vanaclocha F., Rocha M., Asumendi A., Barberá-Guillem E. Isolation and enrichment of two sublobular compartment-specific endothelial cell subpopulations from liver sinusoids. Hepatology. 1993 Aug;18(2):328–339. [PubMed] [Google Scholar]
- Ward B. J., Donnelly J. L. Hypoxia induced disruption of the cardiac endothelial glycocalyx: implications for capillary permeability. Cardiovasc Res. 1993 Mar;27(3):384–389. doi: 10.1093/cvr/27.3.384. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W. Regulatory functions of the coronary endothelium. Mol Cell Biochem. 1992 Oct 21;116(1-2):163–169. doi: 10.1007/BF01270584. [DOI] [PubMed] [Google Scholar]