Abstract
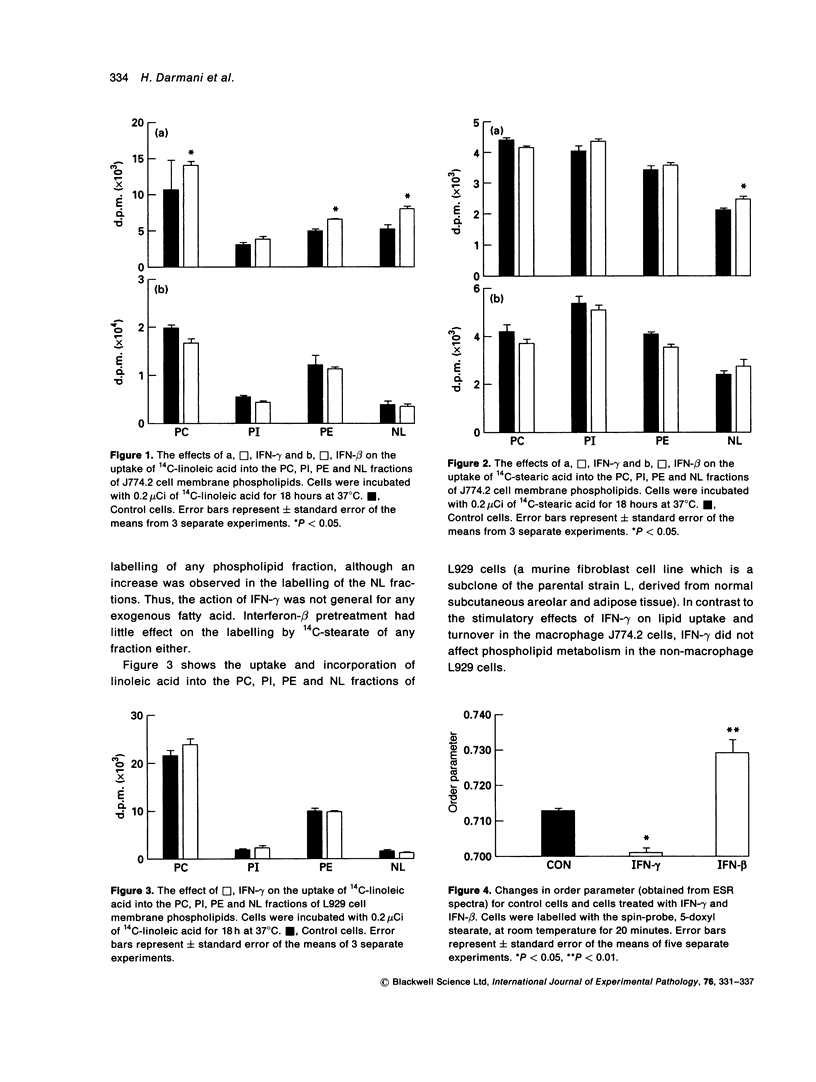
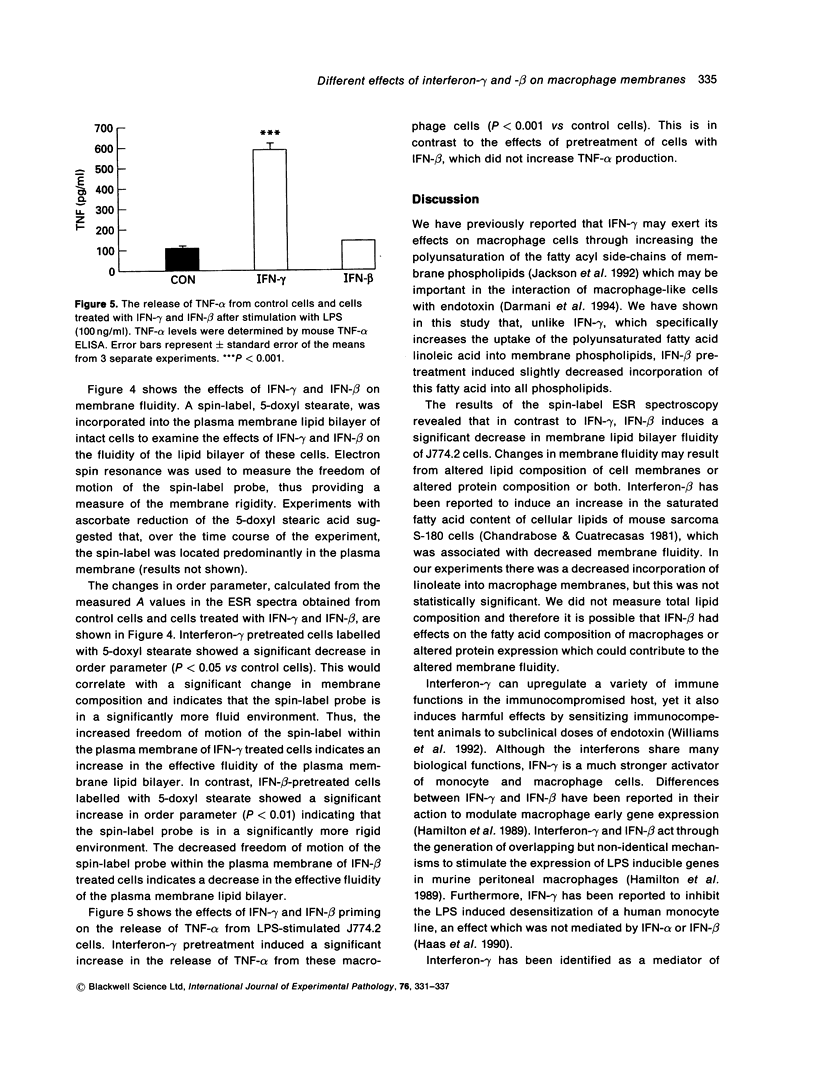
The effects of interferon (IFN)-gamma and IFN-beta on the incorporation of 14C-linoleic acid into J774.2 cell membrane phospholipids were examined. Interferon-gamma induced a statistically significant increase in incorporation of 14C-linoleic acid into all the major phospholipid classes. In contrast, IFN-beta induced a slightly reduced incorporation of this fatty acid into the phospholipids. Neither IFN-gamma nor IFN-beta had any effect on the incorporation of the saturated fatty acid 14C-stearic acid into the cellular phospholipids. Interferon-gamma had no effect on the metabolism of 14C-linoleic acid in the fibroblast cell line L929. Macrophage membrane fluidity was assessed by spin-label ESR spectroscopy after incubation with either IFN-gamma or IFN-beta. Interferon-gamma significantly increased membrane fluidity whereas IFN-beta significantly decreased the fluidity. The findings of this study reveal that IFN-gamma might act on the enzymes controlling the labelling of the sn2 position of phospholipids (linoleic acid) but not the sn1 position (stearic acid), and this increases the polyunsaturated fatty acid content of macrophage membranes. This increase in polyunsaturation is reflected in the increased membrane fluidity. We also conclude that IFN-beta and IFN-gamma have different mechanisms of action on macrophage membrane lipid metabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba A., Tatsuno T., Iwata H. Modulation by unsaturated fatty acids of norepinephrine- and adenosine-induced formation of cyclic AMP in brain slices. J Neurochem. 1984 Jan;42(1):192–197. doi: 10.1111/j.1471-4159.1984.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Chandrabose K., Cuatrecasas P., Pottathil R. Changes in fatty acyl chains of phospholipids induced by interferon in mouse sarcoma S-180 cells. Biochem Biophys Res Commun. 1981 Feb 12;98(3):661–668. doi: 10.1016/0006-291x(81)91165-7. [DOI] [PubMed] [Google Scholar]
- Darmani H., Harwood J. L., Jackson S. K. Interferon-gamma-stimulated uptake and turnover of linoleate and arachidonate in macrophages: a possible pathway for hypersensitivity to endotoxin. Cell Immunol. 1993 Nov;152(1):59–71. doi: 10.1006/cimm.1993.1267. [DOI] [PubMed] [Google Scholar]
- Darmani H., Parton J., Harwood J. L., Jackson S. K. Interferon-gamma and polyunsaturated fatty acids increase the binding of lipopolysaccharide to macrophages. Int J Exp Pathol. 1994 Oct;75(5):363–368. [PMC free article] [PubMed] [Google Scholar]
- GARBUS J., DELUCA H. F., LOOMANS M. E., STRONG F. M. The rapid incorporation of phosphate into mitochondrial lipids. J Biol Chem. 1963 Jan;238:59–63. [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A. Mechanisms of endotoxin shock and endotoxin hypersensitivity. Immunobiology. 1993 Apr;187(3-5):346–356. doi: 10.1016/S0171-2985(11)80349-9. [DOI] [PubMed] [Google Scholar]
- Haas J. G., Meyer N., Riethmüller G., Ziegler-Heitbrock H. W. Inhibition of lipopolysaccharide-induced in vitro desensitization by interferon-gamma. Eur J Immunol. 1990 May;20(5):1181–1184. doi: 10.1002/eji.1830200535. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Bredon N., Ohmori Y., Tannenbaum C. S. IFN-gamma and IFN-beta independently stimulate the expression of lipopolysaccharide-inducible genes in murine peritoneal macrophages. J Immunol. 1989 Apr 1;142(7):2325–2331. [PubMed] [Google Scholar]
- Hayes M. P., Zoon K. C. Priming of human monocytes for enhanced lipopolysaccharide responses: expression of alpha interferon, interferon regulatory factors, and tumor necrosis factor. Infect Immun. 1993 Aug;61(8):3222–3227. doi: 10.1128/iai.61.8.3222-3227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Jackson S. K., Darmani H., Stark J. M., Harwood J. L. Interferon-gamma increases macrophage phospholipid polyunsaturation: a possible mechanism of endotoxin sensitivity. Int J Exp Pathol. 1992 Dec;73(6):783–791. [PMC free article] [PubMed] [Google Scholar]
- Kamijo R., Le J., Shapiro D., Havell E. A., Huang S., Aguet M., Bosland M., Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guérin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993 Oct 1;178(4):1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katschinski T., Galanos C., Coumbos A., Freudenberg M. A. Gamma interferon mediates Propionibacterium acnes-induced hypersensitivity to lipopolysaccharide in mice. Infect Immun. 1992 May;60(5):1994–2001. doi: 10.1128/iai.60.5.1994-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Kwok B. C., Landsberger F. R., Tamm I. Interferon stimulates cholesterol and phosphatidylcholine synthesis but inhibits cholesterol ester synthesis in HeLa-S3 cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2417–2421. doi: 10.1073/pnas.82.8.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., KIRSANOW E. M. Hyperreactivity to endotoxin in mice infected with mycobacteria. Induction and elicitation of the reactions. Immunology. 1961 Oct;4:354–365. [PMC free article] [PubMed] [Google Scholar]
- Schade U. F. Involvement of lipoxygenases in the activation of mouse macrophages by endotoxin. Biochem Biophys Res Commun. 1986 Jul 31;138(2):842–849. doi: 10.1016/s0006-291x(86)80573-3. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Jurkovich G. J., Hahnel G. B., Maier R. V. Macrophage priming by interferon gamma: a selective process with potentially harmful effects. J Leukoc Biol. 1992 Dec;52(6):579–584. doi: 10.1002/jlb.52.6.579. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Ulevitch R. J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993 Mar;14(3):121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]


