Abstract
Background
Arterial stiffness is reported in numerous family studies to be heritable; linkage analysis has identified genomic regions that likely harbor genes contributing to its phenotypic expression. We sought to identify loci contributing to arterial stiffness in a large group of African American hypertensive families.
Methods
We performed a genome scan on 1251 African Americans in families participating in the HyperGEN (Hypertension Genetic Epidemiology Network) study. Children of the hypertensive proband generation were also included in the analysis. Arterial stiffness was estimated as pulse pressure (PP: systolic –diastolic blood pressure) divided by echocardiographically determined stroke volume (SV). PP/SV ratio was adjusted for several non-genetic sources of variation, including demographic and lifestyle factors; the residual phenotype was analyzed using multipoint variance components linkage implemented in SOLAR 2.0.3.
Results
Arterial stiffness was 20% heritable in African Americans. Two regions were highly suggestive of linkage, one between markers D1S1665 and D1S1728 in the 215 cM region of chromosome 1 (LOD = 3.08), and another between D14S588 and D14S606 in the 85 cM region of chromosome 14 (LOD = 2.42). Two candidate genes (GPR-25, SMOC-1) are located in the linked regions. SMOC-1 is of physiological interest because it codes a secreted glycoprotein with five domains, each containing regions homologous to those on other proteins that mediate cell-matrix interactions. GPR-25 is homologous to receptors involved in blood pressure regulation.
Conclusions
At least two chromosomal regions in humans are likely to harbor genes contributing to interindividual variation in PP/SV ratio, an index of arterial stiffness, in African Americans.
Keywords: Blood Pressure, Pulse Pressure, Arterial Stiffness, Genetic Linkage
INTRODUCTION
Stiff arteries are associated with numerous adverse health events. Subjects with cardiovascular disease consistently have stiffer arteries than subjects who are cardiovascular disease-free. Prospective studies have shown arterial stiffness is strongly associated with atherosclerosis, and cross sectional studies have shown associations between stiffness and myocardial infarction and stroke,1 hypercholesterolemia,2 and non-insulin-dependent diabetes mellitus.3 Arterial stiffening is associated with aging;4 gender;5 hypertension;6 and inflammatory, growth, neural, and hormonal factors.
There are two forms of arterial stiffness—acute and sustained. Acute stiffening of arterial tissue is the normal, biomechanical response to temporary increases arterial pressure and is likely mediated by nitric oxide and the renin-angiotensin system. Sustained, pathogenic stiffening of the large arteries is caused by a reduction of the elastin-to-collagen ratio in the arterial elastic matrix and medial smooth muscle cell hypertrophy.7 The structure and composition of the arterial matrix is regulated by the plasminogen activator-plasmin system, matrix metalloproteinases (MMPs), and their tissue-specific inhibitors (TIMPs).8
Evidence suggests regulation of arterial structure and function has a strong genetic component (recently reviewed by Laurent et al.).9 Polymorphisms in MMP-3, MMP-9, fibrillin-1, angiotensin converting enzyme (ACE), angiotensin II type 1 and 2 receptors, nitric oxide synthase (eNOS), and elastin have all been reported to affect various measures of arterial stiffness in different populations.10–14 Pulse pressure is directly related to arterial stiffness,15 although it is considered a fairly crude measure of arterial function. Elevated pulse pressure may be observed in younger individuals due to increased stroke volume rather than a deterioration in arterial function;16 thus, the ratio of pulse pressure to stroke volume has been proposed as an index of total arterial compliance.17
In addition to candidate gene studies, linkage analyses have identified some promising chromosomal regions thought to affect pulse pressure. A genome scan for pulse pressure in Mexican American families showed a LOD score of 2.78 at marker D21S1440 on chromosome 21 and LOD=2.04 at marker D7S1799 on chromosome 7.18 In phase 1 of the Hypertension Genetics (HyperGEN) study, a linkage analysis for arterial stiffness using the PP/SV ratio identified a suggestive linkage (LOD score of 2.15, 231 cM from the p-terminus of chromosome 2) in a sample of 221 African American sibpairs (unpublished). For this analysis, variance components linkage analysis was used to analyze the PP/SV phenotype in the full component of African Americans in the HyperGEN sample.
METHODS
Study Population
Detailed HyperGEN methods have been previously published.19 Briefly, White and African American hypertensive sibships were recruited in four communities (Birmingham, AL; Forsyth County, NC; Minneapolis, MN; and Salt Lake City, UT). Eligible sibships had two or more members meeting at least one of the following criteria: clinical diagnosis or treatment of hypertension before age 60 years, excluding hypertension diagnosed at pregnancy; current SBP greater than or equal to 140 mm Hg; current DBP greater than or equal to 90 mm Hg; current use of antihypertensive medications or historical treatment of hypertension with prescribed medications for at least one year during the previous five years. Parents and adult offspring of these sibships were also recruited. Study procedures were approved by an institutional review board, and all subjects provided informed consent.
Clinical Measurements
All blood pressures were obtained using automated Dinamap devices (model 1846 SX/P, GE Medical Systems, Tampa, FL). For this analysis, arterial stiffness was estimated by the ratio of PP/SV, derived from a 2-element Windkessel model of the arterial tree. To provide a measure of pulse pressure in the central arterial tree to relate to the stroke volume ejected from the left ventricle on each heartbeat, central pulse pressure was estimated using the following age-adjusted formula previously generated in 230 normotensive or hypertensive subjects:20
Stroke volume was calculated using Doppler echocardiography. Doppler echocardiograms were performed following a standardized protocol as previously described.21
Genotyping
Genotyping was done using short tandem repeat polymorphisms by the National Heart, Lung, and Blood Institute Mammalian Genotyping Service (Marshfield, WI). Genotypes were determined by means of an automated technique using polymerase chain reaction and the SCAnning FlUorescence Detector (SCAFUD). The Cooperative Human Linkage Center Screening set 8 was used, which includes 387 microsatellite markers spaced about every 9 cM throughout the genome. The average marker heterozygosity was 0.76. Marker consistency with Mendelian expectations between siblings was tested using affected sib-pair exclusion mapping, a likelihood-based method. Only confirmed sibs with marker compatibility were used in the linkage analysis.
Statistical Methods and Linkage Analysis
To increase the signal-to-noise ratio of genetic variability, SAS v.8.12 was used to regress PP/SV values on potential covariates. Several variables were tested simultaneously for significance. Age, age2, field center, heart rate, height, weight, smoking status, and diabetes diagnosis contributed to variation in arterial stiffness and each explained a significant portion of the variation (significant parameter estimate P ≤ .15 and/or increase in R2 ≥ .01) inthe arterial stiffness index and were therefore retained for the fully adjusted model. Residuals were calculated independently in males and females, but the models contained the same covariates. Residual PP/SV values were analyzed for genetic linkage.
Multipoint identity by descent (IBDs) were computed using MERLIN,22 and were converted to SOLAR format using MER2SOL.23 Variance components linkage analysis was performed on African Americans using SOLAR version 2.0.3 on platforms supported by the Minnesota Supercomputing Institute. In an attempt to adjust for non-genetic sources of variation and to differentiate genetic factors specific to arterial stiffness from those that may act through other pathways (e.g., diabetes and body composition), separate scans were performed using both minimal adjustment (age, age2, field center, heart rate), and full adjustment (age, age2, field center, heart rate, height, weight, smoking status, and diabetes diagnosis), with additive-only inheritance models. Use of antihypertensive medications was given special consideration as a possible covariate. Regression analysis showed no association between PP/SV and antihypertensive medication use in general, nor were there associations between specific use of various classes of these medications (beta blockers, ACE inhibitors, calcium channel blockers, and diuretics), or combinations of these drugs. In addition, the mean systolic and diastolic blood pressures were nearly identical in people who were and were not currently taking antihypertensive medication. Medication status was, therefore, not used in calculating the fully adjusted residual arterial stiffness used for linkage analysis. These findings are consistent with previous research on the effects of antihypertensive treatment on arterial function.24
RESULTS
The analyzed sample consisted of 1251 African American individuals both phenotyped and genotyped. Characteristics of the study sample including all covariates are listed in Table 1. A total of 1209 African-American relative pairs contributed to variance components analysis. The distribution of informative pairs is given in Table 2.
Table 1.
Arterial stiffness covariates in African Americans, reported as mean (standard deviation) or percent of sample.
| Variable | Males | Females |
|---|---|---|
| Age | 48.5 (13.0) | 49.1 (13.4) |
| Height (M) | 1.76 (0.07) | 1.63 (0.06) |
| Weight (Kg) | 91.6 (20.9) | 88.5 (22.3) |
| PP/SV | 0.63 (0.2) | 0.71 (0.2) |
| Heart rate (BPM) | 68.1 (12.1) | 70.8 (11.3) |
| SBP (mm Hg) | 131.0 (21.0) | 128.5 (22.4) |
| DBP (mm Hg) | 77.9 (12.0) | 72.6 (11.0) |
| Diabetic (%) | 19.5 | 25.2 |
| Ever smoke (%) | 68.9 | 43.1 |
| HBP Rx (%) | 79.3 | 70.0 |
PP/SV = pulse pressure/stroke volume ratio; PP = pulse pressure; SBP = systolic blood pressure; DBP = diastolic blood pressure; HBP Rx = current use of any antihypertensive medication.
Table 2.
Distribution of relations among informative pairs in African American families.
| Relationship | Number of pairs |
|---|---|
| Siblings | 581 |
| Parent-offspring | 199 |
| Avuncular | 189 |
| Half siblings | 154 |
| First Cousins | 32 |
| Half Avuncular | 41 |
| Grandparent-Grandchild | 8 |
| Grand Avuncular | 3 |
| Half Grand Avuncular | 2 |
The ratio of polygenic additive variance-to-total variance was used to estimate residual heritability of arterial stiffness. Trait heritability was estimated at 20% in African Americans using the minimally adjusted model. Several loci were suggestive of linkage in African American families. Marker D1S1660 on chromosome 1 gave a LOD score of 3.08, and a locus between markers D14S588 and D14S606 on chromosome 14 yielded a LOD score of 2.42, both using the minimally adjusted model. Peak LOD scores over 1.0, their positions, and marker names are given in Table 3. LOD scores calculated using the fully adjusted phenotype were lower than those from the minimally adjusted model, indicating that part of the observed genetic effect in the minimal model may actually be due to lifestyle factors (e.g., smoking) or may influence arterial function through genetic pathways associated with diabetes and body composition.
Table 3.
Markers, loci, and peak LOD scores suggestive of linkage to arterial stiffness in African Americans.
| Minimally adjusted model* |
Maximally adjusted model† |
||||
|---|---|---|---|---|---|
| Marker | Position | LOD score | P-value | LOD score | P-value |
| D1S1660 | Ch 01 [215 cM] | 3.08 | 0.0002 | 2.41 | 0.0009 |
| D1S1665-D1S1728 | Ch 01 [115 cM] | 1.61 | 0.0065 | 1.43 | 0.0103 |
| D1S3728 | Ch 01 [95 cM] | 1.58 | 0.0070 | 1.40 | 0.0112 |
| D2S1363-D2S427 | Ch 02 [236 cM] | 1.58 | 0.0070 | 1.39 | 0.0115 |
| D10S677 | Ch 10 [120 cM] | 1.41 | 0.0109 | 1.12 | 0.0232 |
| D14S588-D14S606 | Ch 14 [85 cM] | 2.42 | 0.0008 | 1.72 | 0.0049 |
| D14S1426 | Ch 14 [126 cM] | 1.45 | 0.0009 | 1.33 | 0.0134 |
DISCUSSION
There was little evidence for linkage at the locations of MMP-3 (LOD = 0.23), MMP-9 (LOD = 0.0), fibrillin-1 (LOD = 0.0), ACE (LOD = 0.12), angiotensin II type 1 receptor (LOD = 0.0), or eNOS (LOD = 0.0). There was no evidence for linkage in the region previously linked to PP in Mexican Americans on chromosome 21, but there was a small signal (LOD = 0.38) in the region of the linkage peak on chromosome 7 identified in the previous study. Another genome scan for PP in African Americans in the GenNet study found a linkage peak (LOD = 3.1) at 75cM on chromosome 725, and we found a small signal (LOD = 0.44) at that location. The apparent lack of agreement between these findings may be due to the fact this analysis used the PP/SV ratio rather than simple PP. Also, linkage results have typically been difficult to replicate.
However, we did find evidence that other regions are linked to an indirect index of arterial stiffness in African American families ascertained for hypertension. A search for candidates in these regions revealed genes that may be of importance in regulating arterial stiffness. These genes affect arterial physiology and possibly the vascular remodeling process associated with sustained hypertension.
One of the genes with the most promising potential for influencing arterial stiffness is secreted modular calcium binding protein (SMOC-1), characterized by Vannahme et al.26 Located at 64.1 Mb on chromosome 14, it lies 0.1 Mb downstream from D14S588 (our LODmin-adj in this region was 2.42). SMOC-1 is a 51 kDa, secreted glycoprotein that bears 37% homology to the osteonectin protein (BM-40) at the amino acid level. BM-40 has domains that bind basement membrane collagen (collagen IV) and vascular endothelial growth factor. It also inhibits cell proliferation and adhesion and regulates the expression of proteins involved in matrix remodeling.27 SMOC-1 contains five domains, four of which are homologous to domains present in other proteins, as well as a novel domain with unknown functionality.
Two thyroglobulin-like domains are present in SMOC-1. Other proteins, including testican-3, thryoglobulin, equistatin, ECI, saxiphilin, and MHC class II-associated invariant chain p41, also contain this domain. These proteins have in common protease inhibitory properties, and testican-3 inhibits the activity of membrane type 1 matrix metalloproteinase. The follistatin-like domain is present in BM-40 and follistatin, both of which bind growth factors and inhibit proteases and elastase. It is also present in complement proteins C6 and C7, factor-1, agrin, and some transmembrane receptors. The EF-hand calcium binding domain is homologous to that of BM-40, and may bind collagen and contribute to the structural stability of the protein.
SMOC-1 is highly expressed in basement membranes of cardiac and skeletal muscle tissue. Given the similarity between SMOC-1 and other proteins associated with cell-matrix interactions, it is a strong candidate for a gene that mediates the vascular remodeling process associated with sustained arterial stiffness. According to the HapMap database, there are 9 tag SNPs that capture common haplotypes in the gene in a Yoruban population.
The G protein-coupled receptor (GPR-25) gene lies 2.1 Mb downstream from D1S1660 on chromosome 1 (our LODmin-adj in this region was 3.08). G protein-coupled receptors (GPCs) are integral membrane proteins that bind hormones and neurotransmitters. They help initiate signaling cascades that ultimately regulate blood pressure during changes in cardiac output. Within the GPC family of genes, GPR-25 is most similar at the sequence level to angiotensin type II 1A and somatostatin receptor 5. It is an intronless gene with one synonymous coding SNP, two SNPs in flanking regions, and an insertion polymorphism.28 Our peak on chromosome 2 (LODmin-adj was 1.58) is near several genes involved in cell proliferation and vascular function. The C-type natriuretic peptide precursor gene (NPPC) lies at almost the exact location of D2S427. This protein has vasorelaxant and anti-proliferative properties and inhibits the renin-angiotensin system. It has also been implicated in blood pressure control.29 There are three known SNPs; one codes an amino acid change.
The addition of the covariates used in the fully adjusted model reduced the LOD scores observed in the minimally adjusted model at every suggestive locus. It is possible that PP/SV is partially determined by genes that act through pathways related to height, weight, or the development of diabetes. Adjustment for these factors may have removed genetic variation contributing to stiffness and may explain the reduction in LOD scores.
Study limitations
Although highly suggestive linkage was detected, the method used to estimate arterial stiffness was not ideal. Several methods can be used to assess arterial compliance directly or indirectly (reviewed by Asmar et al. 2001).16 Direct methods of measuring arterial compliance based on pulse wave velocity or on the diastolic-decay time constant (tau) and total peripheral resistance of the arterial system (the Windkessel model) are the most accurate.16 PP/SV is highly correlated (r = .85), however, with estimates of arterial compliance measured according to the Windkessel model.30
In conclusion, our data indicate that arterial stiffness is heritable in African Americans. In addition, there are likely at least two chromosomal regions containing genes that affect inter-individual variation on the trait. Finally, candidate genes in both regions have functions related to blood pressure regulation or vascular remodeling.
Figure 1.
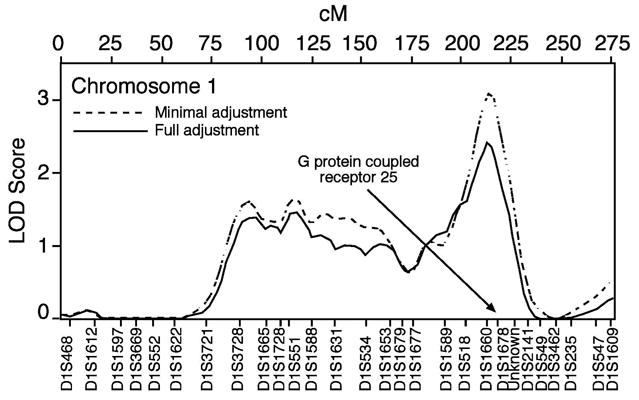
LOD plot for chromosome 1, African Americans, minimal and full adjustment models.
Figure 2.
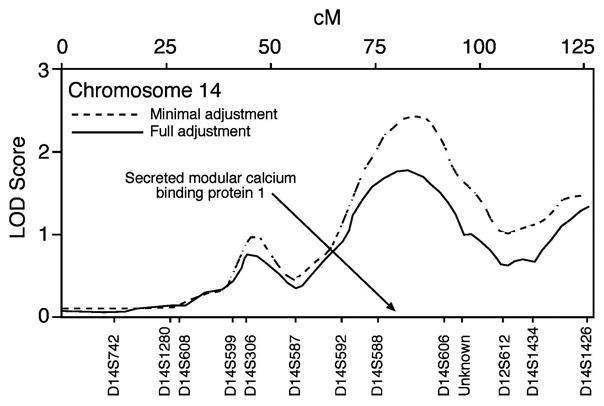
LOD plot for chromosome 14, African Americans, minimal and full adjustment models.
Acknowledgments
We thank the University of Minnesota Supercomputing Institute for use of their facilities. This research was supported be the NHLBI Cardiovascular Disease Genetics Training Grant (5 T32 HL007972).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millar JA, Lever AF, Burke V. Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens. 1999;17:1065–1072. doi: 10.1097/00004872-199917080-00004. [DOI] [PubMed] [Google Scholar]
- 2.Virkola K, Pesonen E, Akerblom HK, Siimes MA. Cholesterol and carotid artery wall in children and adolescents with familial hypercholesterolaemia: a controlled study by ultrasound. Acta Paediatr. 1997;86:1203–1207. doi: 10.1111/j.1651-2227.1997.tb14847.x. [DOI] [PubMed] [Google Scholar]
- 3.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 4.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 6.Ting CT, Brin KP, Lin SJ, Wang SP, Chang MS, Chiang BN, Yin FC. Arterial hemodynamics in human hypertension. J Clin Invest. 1986;78:1462–1471. doi: 10.1172/JCI112737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman MJ, Saba PS, Pini R, Spitzer M, Pickering TG, Rosen S, Alderman MH, Devereux RB. Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86:1909–1918. doi: 10.1161/01.cir.86.6.1909. [DOI] [PubMed] [Google Scholar]
- 8.Kingwell BA, Medley TL, Waddell TK, Cole TJ, Dart AM, Jennings GL. Large artery stiffness: structural and genetic aspects. Clin Exp Pharmacol Physiol. 2001;28:1040–1043. doi: 10.1046/j.1440-1681.2001.03580.x. [DOI] [PubMed] [Google Scholar]
- 9.Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45:1050–1055. doi: 10.1161/01.HYP.0000164580.39991.3d. [DOI] [PubMed] [Google Scholar]
- 10.Medley TL, Cole TJ, Dart AM, Gatzka CD, Kingwell BA. Matrix metalloproteinase-9 genotype influences large artery stiffness through effects on aortic gene and protein expression. Arterioscler Thromb Vasc Biol. 2004;24:1479–1484. doi: 10.1161/01.ATV.0000135656.49158.95. [DOI] [PubMed] [Google Scholar]
- 11.Benetos A, Gautier S, Ricard S, Topouchian J, Asmar R, Poirier O, Larosa E, Guize L, Safar M, Soubrier F, Cambien F. Influence of angiotensin-converting enzyme and angiotensin II type 1 receptor gene polymorphisms on aortic stiffness in normotensive and hypertensive patients. Circulation. 1996;94:698–703. doi: 10.1161/01.cir.94.4.698. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Li S, Boerwinkle E, Berenson GS. Nitric oxide synthase gene polymorphism (G894T) influences arterial stiffness in adults: The Bogalusa Heart Study. Am J Hypertens. 2004;17:553–559. doi: 10.1016/j.amjhyper.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Hanon O, Luong V, Mourad JJ, Bortolotto LA, Jeunemaitre X, Girerd X. Aging, carotid artery distensibility, and the Ser422Gly elastin gene polymorphism in humans. Hypertension. 2001;38:1185–1189. doi: 10.1161/hy1101.096802. [DOI] [PubMed] [Google Scholar]
- 14.Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ. Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res. 2003;92:1254–1261. doi: 10.1161/01.RES.0000076891.24317.CA. [DOI] [PubMed] [Google Scholar]
- 15.Safar ME. Epidemiological aspects of pulse pressure and arterial stiffness. J Hypertens Suppl. 1999;17:S37–40. [PubMed] [Google Scholar]
- 16.Asmar R. Arterial stiffness and pulse wave velocity: clinical applications. Oxford: Elsevier; 1999. [Google Scholar]
- 17.Ferguson JJ, Julius S, Randall OS. Stroke volume--pulse pressure relationships in borderline hypertension: a possible indicator of decreased arterial compliance. J Hypertens Suppl. 1984;2:S397–399. [PubMed] [Google Scholar]
- 18.Atwood LD, Samollow PB, Hixson JE, Stern MP, MacCluer JW. Genome-wide linkage analysis of blood pressure in Mexican Americans. Genet Epidemiol. 2001;20:373–382. doi: 10.1002/gepi.7. [DOI] [PubMed] [Google Scholar]
- 19.Williams RR, Rao DC, Ellison RC, Arnett DK, Heiss G, Oberman A, Eckfeldt JH, Leppert MF, Province MA, Mockrin SC, Hunt SC. NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann Epidemiol. 2000;10:389–400. doi: 10.1016/s1047-2797(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 20.Palmieri V, Dahlof B, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 21.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Wood EA, Howard BV, Welty TK, Lee ET, Fabsitz RR. Relations of Doppler stroke volume and its components to left ventricular stroke volume in normotensive and hypertensive American Indians: the Strong Heart Study. Am J Hypertens. 1997;10:619–628. doi: 10.1016/s0895-7061(97)00059-9. [DOI] [PubMed] [Google Scholar]
- 22.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 23.Miller M. MER2SOL: translating MERLIN or Loki IBD data to SOLAR format. Genet Epidemiol. 2003;25:261–262. [Google Scholar]
- 24.Asmar R. Effect of antihypertensive agents on arterial stiffness as evaluated by pulse wave velocity: clinical implications. Am J Cardiovasc Drugs. 2001;1:387–397. doi: 10.2165/00129784-200101050-00008. [DOI] [PubMed] [Google Scholar]
- 25.Bielinski SJ, Lynch AI, Miller MB, Weder A, Cooper R, Oberman A, Chen YD, Turner ST, Fornage M, Province M, Arnett DK. Genome-wide linkage analysis for loci affecting pulse pressure: the Family Blood Pressure Program. Hypertension. 2005;46:1286–1293. doi: 10.1161/01.HYP.0000191706.41980.29. [DOI] [PubMed] [Google Scholar]
- 26.Vannahme C, Smyth N, Miosge N, Gosling S, Frie C, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem. 2002;277:37977–37986. doi: 10.1074/jbc.M203830200. [DOI] [PubMed] [Google Scholar]
- 27.Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. Faseb J. 1994;8:163–173. [PubMed] [Google Scholar]
- 28.Jung BP, Nguyen T, Kolakowski LF, Jr, Lynch KR, Heng HH, George SR, O’Dowd BF. Discovery of a novel human G protein-coupled receptor gene (GPR25) located on chromosome 1. Biochem Biophys Res Commun. 1997;230:69–72. doi: 10.1006/bbrc.1996.5828. [DOI] [PubMed] [Google Scholar]
- 29.Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. II: Natriuretic peptide receptors. J Hypertens. 1992;10:1111–1114. doi: 10.1097/00004872-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Randall OS, Westerhof N, van den Bos GC, Alexander B. Reliability of stroke volume to pulse pressure ratio for estimating and detecting changes in arterial compliance. J Hypertens Suppl. 1986;4:S293–296. [PubMed] [Google Scholar]


