Abstract
Brain processing of acupuncture stimuli in chronic neuropathic pain patients may underlie its beneficial effects. We used fMRI to evaluate verum and sham acupuncture stimulation at acupoint LI-4 in Carpal Tunnel Syndrome (CTS) patients and healthy controls (HC). CTS patients were retested after 5 weeks of acupuncture therapy. Thus, we investigated both the short-term brain response to acupuncture stimulation, as well as the influence of longer-term acupuncture therapy effects on this short-term response. CTS patients responded to verum acupuncture with greater activation in the hypothalamus and deactivation in the amygdala as compared to HC, controlling for the non-specific effects of sham acupuncture. A similar difference was found between CTS patients at baseline and after acupuncture therapy. For baseline CTS patients responding to verum acupuncture, functional connectivity was found between the hypothalamus and amygdala – the less deactivation in the amygdala, the greater the activation in the hypothalamus, and vice versa. Furthermore, hypothalamic response correlated positively with the degree of maladaptive cortical plasticity in CTS patients (inter-digit separation distance). This is the first evidence suggesting that chronic pain patients respond to acupuncture differently than HC, through a coordinated limbic network including the hypothalamus and amygdala.
Introduction
Acupuncture is a component of traditional Chinese medicine, and has evolved empirically over thousands of years to treat a multitude of ailments (Kaptchuk 2002). While the efficacy of acupuncture for many conditions is still under debate, recent evaluation of this treatment modality has lent credence to the hypothesis that the brain and nervous system plays a leading role in processing acupuncture stimuli. Neuroimaging studies of acupuncture have noted stimulus-associated response in several limbic structures including the amygdala, cingulate cortex (Wu et al. 1999; Hui et al. 2000; Napadow et al. 2005), and hypothalamus (Wu et al. 1999; Hui et al. 2000; Hsieh et al. 2001). However, the majority of acupuncture neuroimaging studies have been performed on healthy adults. Conversely, an interesting PET study which controlled for expectancy, found verum acupuncture-specific response in the insula (Pariente et al. 2005). However, this study did not directly contrast these results with healthy controls. Historically, it has been posited that acupuncture plays a homeostatic role (Zhu 1954; Kaptchuk 2000) and thus may have a greater effect on patient populations with a pathological imbalance, compared to healthy individuals. Hence, it remains to be seen if past acupuncture neuroimaging results in healthy adults will also apply to patients with chronic pain, for which a direct comparison is necessary. Chronic neuropathic and inflammatory pain alters processing in several limbic brain regions (e.g. amygdala, insula, ACC) and may initiate and be maintained by sensitization in brain processing. Evidence for this effect comes from both animal (Neugebauer et al. 2004) and human (Stern et al. 2006) studies. Acupuncture may alter brain function through neuroplasticity mechanisms by a combination of afferent somatosensory stimulus and affective evaluation, thereby modulating centrally maintained chronic pain states.
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy, with a prevalence of 3.72% in the United States (Papanicolaou et al. 2001). CTS etiology is characterized by compression of the distal median nerve due to an elevated interstitial fluid pressure in the carpal tunnel. Ischemic injury to the median nerve leads to anoxic capillary damage, which then leads to increased membrane permeability, exudative edema and subsequent fibrosis (Keir and Rempel 2005; Sud and Freeland 2005). CTS is associated with a range of symptoms primarily in the first through fourth digit, including paresthesias, pain, and weakness.
We have previously demonstrated that CTS is associated with cortical hyperactivation to simple (non-acupuncture) somatosensory stimuli and maladaptive somatotopic plasticity in contralateral primary somatosensory cortex (Napadow et al. 2006a). This pathological central correlate of the peripheral CTS lesion was found to be altered after successful acupuncture treatment (Napadow et al. 2006b). In the current study, we explored brain processing of acupuncture stimulation in CTS patients compared to healthy controls (HC), controlling for cutaneous somatosensory/cognitive effects with sham acupuncture. Our hypothesis was that chronic pain patients would demonstrate greater response to verum acupuncture stimulation in limbic brain regions which have been associated with the maintenance of a persistent pain state.
Methods
Subject Recruitment and Evaluation
All participants in the study provided written informed consent in accordance with the Human Research Committee of the Massachusetts General Hospital and also took part in a study of cortical plasticity and somatotopy. A total of 25 subjects were enrolled in this study; 13 adults affected by CTS, and 12 age and gender-matched HC. Of the 13 CTS patients initially studied, one was removed for excessive motion during fMRI scanning, and two were not imaged during acupuncture stimuli due to claustrophobia. Of the 12 healthy adults recruited, one subject was removed for suspected sub-clinical neuropathy, while 2 subjects had scans removed for excessive motion artifact. Clinical evaluation was completed for both groups by an experienced physician [JA] at the Spaulding Rehabilitation Hospital, and has been previously described (Audette et al. 2006). Subjects were screened and excluded if they were pregnant or had a history of diabetes mellitus, rheumatoid arthritis, thyroid dysfunction, wrist fracture or direct trauma to median nerve, atrophy of the thenar eminence, carpal tunnel surgery, current use of prescriptive opioid pain medications, psychiatric and neurological disorders, head trauma with loss of consciousness, or other serious cardiovascular, respiratory or renal illness. Subjects were also excluded if they had any contraindication to undergoing MRI (e.g. pacemaker), or any contraindication for acupuncture (e.g. anticoagulation therapy). CTS patients were included if they had experienced pain and/or paresthesias for greater than 3 months in the median nerve distribution of the affected hand – digits 1 to 3, and the radial aspect of digit 4. Further, Nerve Conduction Study (NCS) findings needed to be consistent with mild to moderate CTS. Mild CTS was defined by delayed distal latency of median sensory nerve conduction across the wrist (>3.7ms and/or median – ulnar > 0.5ms) with normal motor nerve conduction. Moderate CTS was defined by mild CTS and with delayed distal latency of median motor nerve conduction across the wrist (>4.2ms), but with normal motor amplitudes. Subjects with severe CTS, defined by prolonged median sensory and motor latencies with either absent sensory nerve action potentials and/or reduced (50%) median motor amplitudes, were excluded. Patients were also excluded if they demonstrated any evidence on NCS of generalized peripheral neuropathy or localized ulnar nerve entrapment.
Acupuncture Treatment
Acupuncture was performed by experienced practitioners on CTS patients over a 5 week period, after baseline clinical and fMRI evaluation. Treatments were provided 3 times per week for three weeks and 2 times per week for the remaining two weeks. A semi-individualized approach was used wherein every subject was treated for 10 minutes with 2Hz electro-acupuncture at common acupoints - unilateral TW-5 (triple-warmer 5, dorsal aspect of forearm) to PC-7 (pericardium 7, 1st wrist crease). This was followed by manual needling at acupoints chosen by the practitioner that were based on the individual symptoms of the presenting patient. Three acupoints were chosen out of the following six: HT-3 (heart 3, medial aspect of elbow), PC-3 (pericardium 3, medial aspect of elbow crease), SI-4 (small intestine 4, ulnar aspect of wrist), LI-5 (large intestine 5, radial aspect of wrist), LI-10 (large intestine 10, lateral aspect of forearm), LU-5 (lung 5, lateral aspect of elbow crease). These acupoints were stimulated with a manual even needle technique where a deqi response was obtained.
FMRI Image Acquisition and Stimulation Protocol
FMRI test-retest scanning was completed on 10 CTS patients (6 female; mean age: 51.1, range 31–60) before (baseline) and following 5 weeks of acupuncture, with an interval of 40.6±4.4 days (μ±SD) between scan sessions. FMRI scanning was also completed on 9 age and gender-matched HC subjects (6 female; mean age: 46.9, range 32–59) spaced at least 5 weeks apart, with an interval of 44.0±7.7 days between scan sessions. Five patients presented with CTS symptoms in both hands, while 5 presented with only unilateral symptomatology. For patients with bilateral CTS symptoms, testing was done on the more affected hand (self report). In all cases, the more affected hand was also the patient’s dominant hand. The chronicity of symptoms (self reported) ranged from 4 months to 10 years, with 10 of 11 patients having symptoms for longer than 1 year.
Functional scans were acquired using a 3.0 Tesla Siemens Allegra MRI System equipped for echo planar imaging with quadrature head coil. The subject lay supine in the scanner with the head immobilized using cushioned supports. Two sets of structural images were collected using a T1-weighted MPRAGE sequence (TR/TE = 2.73/3.19 ms, flip angle = 7°, FOV = 256 × 256 mm; slice thickness = 1.33 mm). Blood oxygenation level-dependent (BOLD) functional imaging was performed using a gradient echo T2*-weighted pulse sequence (TR/TE = 3000/30 ms, flip angle = 90°, FOV = 200 × 200 mm, 38 sagittal slices, slice thickness = 3.0 mm with 0.6 mm interslice gap, matrix = 64 × 64, 140 time points). Image collection was preceded by 4 dummy scans to allow for equilibration of the MRI signal.
During an fMRI session, two different types of acupuncture intervention were performed. Both interventions adopted a 7-minute block paradigm including a 2-minute rest, 1-minute stimulation, 2-minute rest, 1-minute stimulation, 1-minute rest block (Figure 1). Both interventions were performed at acupoint LI-4 (hegu, see Figure 1) over the 1st interosseus m. on the affected hand for CTS patients (right hand: 9/10) and dominant hand for HC subjects (right hand: 8/9). This acupoint was chosen because it is distal to the CTS lesion, is a very common acupoint used clinically for many chronic pain conditions (Napadow et al. 2004), and has been used in several neuroimaging studies (including our own group) in the past (Hui et al. 2000; Pariente et al. 2005). This acupoint is innervated over the skin surface by the superficial branch of the radial nerve, and at its depth by the deep branch of the ulnar nerve (dorsal interosseus, adductor pollicis muscles) and, if the needle is inserted deep enough, the recurrent branch of the median nerve (flexor pollicis brevis muscle). Our insertion depth was chosen for stimulation of deep muscle afferents, as these ulnar and median nerve fibers have been implicated in experimental analgesia (Chiang et al. 1973). The first two scan runs evaluated sham acupuncture (see below), while the next two scan runs evaluated verum acupuncture at LI-4.
Figure 1.
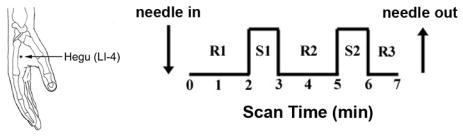
Verum and sham acupuncture during fMRI scanning was accomplished at LI-4 on the hand. The stimulation paradigm consisted of two stimulus blocks and three rest blocks.
Verum acupuncture was performed by first inserting a non-magnetic (pure silver), 0.23mm diameter, 30mm long acupuncture needle (MAEDA Toyokichi Shoten, Tokyo, Japan) into LI-4 before the fMRI scan run began. The insertion depth was approximately 1.5cm. The needle was briefly stimulated (twirled) until the subjects felt a sensation that was not described as sharp pain to minimize the risk of inducing sharp pain during the scan run (see below). During the fMRI scan, the needle was manually stimulated (twirled, ±90°) at 1Hz during the stimulation blocks. In all scan runs, subjects were instructed to keep their eyes closed, and to pay close attention to the sensations felt at the stimulated hand.
Sham acupuncture consisted of a non-insertive cutaneous stimulation over the acupoint. Sham controls for acupuncture represent one of the most controversial topics in acupuncture research. We chose to impart a sham acupuncture control via repetitive tactile stimulation (tapping at 1Hz) at acupoint LI-4 using a 5.88 von Frey monofilament. Subjects were acupuncture-naive and were not informed of a sham acupuncture condition, only that there would be “different forms” of acupuncture during fMRI. Subjects lay supine in the scanner with their eyes closed and their vision of distal body regions blocked by the volumetric head coil. Thus, they could not see the intervention occurring at their periphery. Sham acupuncture was always performed before verum acupuncture to keep subjects from speculating as to whether the control stimulation was truly acupuncture. As order effects would most likely be the same for all subgroups, we felt it was more important to keep subjects from suspecting that a sham procedure was being used, and did not randomize the order of sham and verum stimulation. In sum, our sham acupuncture stimulation controlled for both superficial, cutaneous somatosensory effects over the acupoint, as well as the cognitive processing induced by subjects expecting an “acupuncture” stimulation.
In order to conduct a concurrent psychophysical analysis, we gathered data by verbal analog scale after each scan run as to the existence of acupuncture sensation, or deqi. The sensations included aching, soreness, pressure, heaviness, fullness, warmth, cool, numbness, tingling, spreading and dull pain. The perception of sharp pain was also recorded, though this sensation is not considered to be characteristic of deqi. We also asked subjects if they were anxious or relaxed during the scan run. This procedure has been successfully used by our group in the past to decipher psychophysical response in conjunction with neuroimaging (Hui et al. 2005; Napadow et al. 2005). Frequency counts of different sensations were compared between different groups with a Chi-squared test, significant at p<0.05. The intensity of different sensations was analyzed with a 2×2 ANOVA for factors GROUP and TIME.
Single Subject fMRI Data Analysis
Analysis was carried out using a combination of software including FEAT (FMRI Expert Analysis Tool) Version 5.1, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl), and AFNI (Cox 1996). Data collected from left-hand dominant HC subjects (1 subject) or predominant left-handed CTS patients (1 patient), were mirror reversed across the mid-sagittal plane. The following pre-statistics processing was applied: motion correction using MCFLIRT; non-brain removal using Brain Extraction Tool, BET and FMRIB’s Automated Segmentation Tool, FAST; spatial smoothing using a Gaussian kernel of FWHM 5mm; mean-based intensity normalization; and highpass temporal filtering (f=1/180.0s). Time-series statistical analysis was carried out using FILM (FMRIB’s Improved Linear Model) with local autocorrelation correction. The hemodynamic response function utilized in the general linear model (GLM) analysis was defined by the block design paradigm convolved with a prescribed gamma function (standard deviation = 3s, mean lag = 6s).
Group fMRI Data Analysis
Functional images from each subject were co-registered to their averaged T1-weighted MPRAGE structural volume, which was co-registered to standard MNI space using affine transformation with FMRIB’s Linear Image Registration Tool (FLIRT). Standard space parameter estimates from individual subjects were imported to a 2nd level analysis with FLAME (FMRIB’s Local Analysis of Mixed Effects, stage 1 only) in order to average multiple runs for the same subject. Parameter estimates from this 2nd level analysis were then used in a full 3rd level Markov Chain Monte Carlo (MCMC)-based mixed effects analysis with FLAME, which takes into account inter-subject variance.
In order to test for cross-sectional differences between CTS patients and HC at baseline, the 3rd level analysis was set up as a 2×2 mixed effects ANOVA model with fixed factors GROUP (levels include CTS and HC) and STIM (levels include verum and sham acupuncture) and subjects treated as a nested random factor. The interaction from this analysis was defined as [CTS(acup) – CTS(sham)] – [HC(acup) – HC(sham)]. Thus the interaction tests for whether there were differences between CTS patients and HC subjects in how they respond to verum acupuncture, controlling for somatosensory/cognitive stimulation. In order to test for longitudinal differences in brain response for CTS patients before versus after 5 weeks of acupuncture treatment, we performed a 2×2 mixed effects ANOVA 3rd level analysis with factors TIME (levels include baseline and post-acupuncture) and STIM (levels include verum and sham acupuncture). Here, the interaction was defined as [CTS.baseline(acup) –CTS.baseline(sham)] – [CTS.postacup(acup) – CTS.postacup(sham)]. Thus the interaction tests for whether there were differences for CTS patients before versus after 5 weeks of acupuncture treatment in how their brain responds to verum acupuncture, controlling for somatosensory/cognitive stimulation. A confounding issue in presenting a 2×2 ANOVA interaction formed by a difference of differences is that a significant interaction effect may arise from many different permutations in super or sub-threshold response on the part of any of the 4 sub-groups tested. In order to clarify the origin of a significant interaction effect, imaging results were presented with interaction plots and tables in order to denote which sub-group(s) in the interaction produced the largest %signal change.
Resultant statistical parametric maps were corrected for multiple comparisons at a false discovery rate (FDR) of 0.01 (Genovese et al. 2002). Data were then clustered with a minimum volume of 5 image voxels.
Functional connectivity in CTS patients’ baseline fMRI signal response to verum acupuncture was explored by calculating a within-subject correlation matrix. Brain regions in the correlation matrix were limited to those with significant modulation under the baseline ANOVA interaction (Table 1). In other words, we asked the following question: when a patient responds with greater activation in one brain region, is there also a greater activation or deactivation in another brain region? A correlation matrix was computed (MATLAB, Mathworks Inc., Natick, MA) and resultant p-values Bonferroni corrected for multiple comparisons. Regions that demonstrated significant correlation were then correlated to other metrics of clinical and brain response collected from our CTS patient cohort.
Table 1.
Summary of the baseline GROUP × STIM interaction which defines the difference between healthy adults and CTS patients in their different fMRI responses to verum and sham acupuncture stimulation. (HC: healthy controls; pgACC: pre-genual subdivision of anterior cingulate cortex; amACC: anterior-middle subdivision of anterior cingulate cortex,; rspPCC: retrosplenial posterior cingulate cortex; Amyg: amygdala; DLPFC: dorsolateral prefrontal cortex; LatHypothA: lateral hypothalamic area; aInsula: anterior Insula; SI: primary somatosensory cortex; Z-score: most significant voxel in cluster; %Δ: percent signal change expressed as mean ± standard deviation in cluster)
| Baseline GROUP × STIM Interaction:
(CTSbase.acup – CTSbase.sham) - (HC.acup – HC.sham) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region(Brodmann Area) | Talairach | CTSbase %Δ Acup (μ±σ) | CTSbase %Δ Sham (μ±σ) | Healthy %Δ Acup (μ±σ) | Healthy %Δ Sham (μ±σ) | ||||
| Side | X (mm) | Y (mm) | Z (mm) | Z-score | |||||
| pgACC (24) | R | 13 | 40 | 6 | −3.30 | −0.12±0.09 | 0.21±0.09 | 0.00±0.06 | −0.20±0.04 |
| amACC (24) | L | −3 | 13 | 34 | −3.70 | −0.09±0.08 | 0.29±0.07 | 0.28±0.06 | 0.03±0.05 |
| (24) | R | 8 | 26 | 27 | −3.42 | −0.18±0.02 | 0.11±0.03 | 0.28±0.07 | −0.06±0.04 |
| rspPCC(31) | L | −7 | −53 | 25 | −3.30 | −0.21±0.03 | 0.24±0.12 | −0.07±0.02 | −0.23±0.04 |
| Amyg | L | −21 | −8 | −13 | −4.33 | −0.37±0.09 | 0.11±0.08 | 0.06±0.03 | −0.11±0.08 |
| DLPFC | L | −20 | 49 | 9 | −3.00 | −0.31±0.06 | 0.14±0.06 | 0.01±0.07 | −0.01±0.03 |
| VMPFC | R | 8 | 41 | −6 | −4.15 | −0.36±0.02 | 0.17±0.01 | −0.28±0.01 | −0.60±0.02 |
| LatHypothA | L | −7 | −6 | −8 | 2.88 | 0.38±0.03 | −0.18±0.05 | −0.17±0.22 | 0.20±0.05 |
| aInsula | L | −34 | 7 | −4 | −3.16 | −0.04±0.06 | 0.16±0.06 | 0.15±0.09 | −0.04±0.09 |
| Septal Area | R | 2 | 0 | 8 | −3.57 | −0.31±0.03 | 0.10±0.02 | 0.28±0.19 | −0.17±0.12 |
| SMA | L | −6 | −19 | 51 | −4.15 | −0.04±0.03 | 0.30±0.08 | 0.25±0.07 | −0.02±0.05 |
| SI (3b/1) | L | −42 | −32 | 45 | −4.08 | −0.14±0.07 | 0.16±0.09 | −0.01±0.04 | −0.20±0.05 |
| Thalamus | R | 13 | −12 | 13 | −2.85 | −0.03±0.03 | 0.25±0.04 | 0.14±0.03 | 0.07±0.05 |
Results
Our neuroimaging results demonstrate differences in how chronic pain patients with CTS process acupuncture compared to HC. The clinical results of acupuncture intervention in our CTS cohort have been presented elsewhere (Audette et al. 2006; Napadow et al. 2006b), and demonstrated improvement in subjective (Boston CTS questionnaire) as well as objective (nerve conduction studies, grip strength) measures.
In order to derive the differences in how CTS patients process verum deep intramuscular acupuncture compared to HC, controlling for non-specific cutaneous somatosensory and cognitive elements of acupuncture stimulation (sham), we performed a mixed effects 2×2 ANOVA whose interaction was defined as: [CTS(acup) –CTS(sham)] – [HC(acup) – HC(sham)]. A significant interaction was found in the contralateral amygdala and lateral hypothalamic area (LHA), in addition to other limbic, somatosensory, and cognitive processing brain regions. These regions included pregenual and anterior-middle anterior, and retrosplenial posterior cingulate cortices (pgACC, amACC, rspPCC), dorsolateral and ventromedial prefrontal cortices (DLPFC, VMPFC), anterior insula, septal area, S1, supplementary motor area (SMA), and thalamus (Table 1). All of the interactions were negative except for the LHA. An analysis of fMRI signal response in each of the four interaction subgroups found that the negative interaction in the left amygdala was mostly due to fMRI signal decrease in CTS patients in response to verum acupuncture (Figure 2, Table 1). Similarly, the positive interaction in the LHA was mainly due to fMRI signal increase in CTS patients in response to verum acupuncture (Figure 2, Table 1). This was due to the fact that the greatest effect size (%signal change) in the interaction was by the subgroup: CTS patients at baseline with verum acupuncture stimulation.
Figure 2.
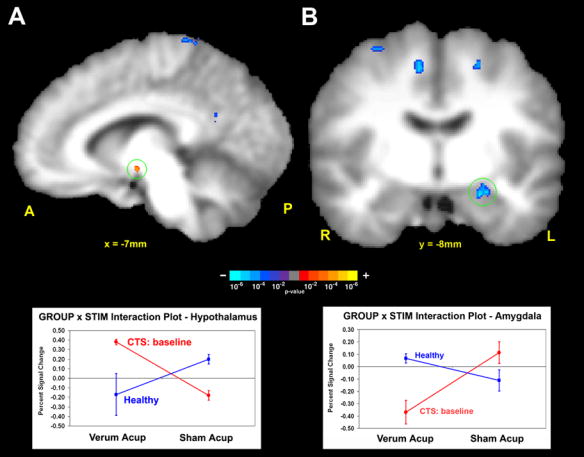
Cross-sectional fMRI analysis of CTS patients at baseline and healthy controls (HC). The ANOVA interaction tests: (CTSbase.acup – CTSbase.sham) –(HC.acup – HC.sham). A positive interaction was found in the hypothalamus, while a negative interaction was found in the amygdala. Interaction plots demonstrated that the greatest %-signal change in the interaction was by the subgroup: CTS patients at baseline with verum acupuncture stimulation.
CTS patients and healthy adults respond differently to sham acupuncture. We found that CTS patients respond with greater fMRI signal increase in somatosensory regions such as contralateral S1, SII, and posterior insula; cognitive regions such as DLPFC; and affective regions such as pre-genual ACC (pgACC, BA24/32) and VMPFC (Figure 3, Table 2).
Figure 3.
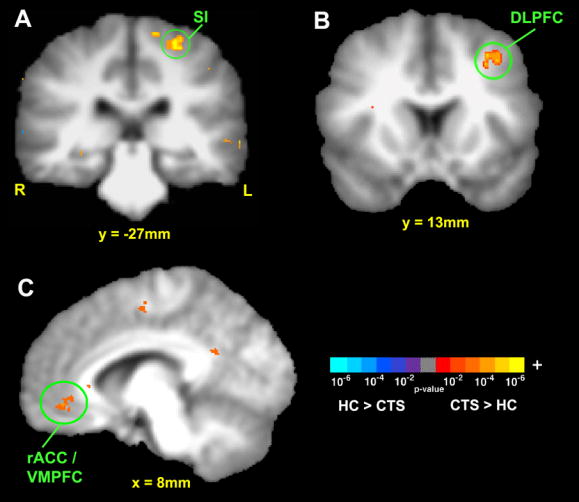
Cross-sectional fMRI contrast between CTS patients and healthy controls at baseline in processing sham acupuncture. CTS patients responded with greater fMRI signal increase in [A] primary sensory, SI; [B] cognitive, dorsolateral prefrontal cortex (DLPFC); and [C] affective/motivational, rostral ACC (rACC) and ventromedial prefrontal cortex (VMPFC).
Table 2.
Summary of significant clusters of activation difference between CTS patients and healthy controls in processing sham acupuncture. (pgACC: pre-genual subdivision of anterior cingulate cortex; pInsula: posterior insula; SI: primary somatosensory cortex; SII: secondary somatosensory cortex; VMPFC: ventromedial prefrontal cortex; Z-score: most significant voxel in cluster; %Δ: percent signal change expressed as mean ± standard deviation in cluster).
| Sham Acupuncture Processing at Baseline | |||||||
|---|---|---|---|---|---|---|---|
| Talairach | |||||||
| Region (Brodmann Area) | Side | X (mm) | Y (mm) | Z (mm) | Z-score | %Δ CTS (μ±σ) | %Δ HC (μ±σ) |
| CTS > Healthy Controls | |||||||
| pgACC (24/32)/VMPFC | L | −3 | 34 | −3 | 3.14 | 0.13±0.07 | −0.43±0.08 |
| Hippocampus | L | −30 | −21 | −9 | 3.17 | −0.00±0.02 | −0.27±0.02 |
| pInsula | L | −38 | −10 | 10 | 2.83 | 0.50±0.09 | 0.16±0.07 |
| SI | L | −25 | −27 | 59 | 4.80 | 0.19±0.08 | −0.23±0.07 |
| SII | L | −54 | −16 | 14 | 3.66 | 0.88±0.15 | 0.31±0.08 |
| Healthy Controls > CTS | |||||||
| none | |||||||
In order to derive the differences in how CTS patients process acupuncture stimuli before versus after 5 weeks of clinical acupuncture treatments (again controlling for non-specific somatosensory and cognitive elements), we performed another mixed effects 2×2 ANOVA whose interaction was defined as: [CTS.baseline(acup) – CTS.baseline(sham)] – [CTS.postacup(acup) – CTS.postacup(sham)]. A significant interaction was again found in the left amygdala and LHA, in addition to ACC, insula, and SI (Table 3). The greatest %-signal change in the interaction was again by the subgroup: CTS patients at baseline with verum acupuncture stimulation for both the LHA and amygdala (Figure 4, Table 3). While the longitudinal CTS analysis yielded significant interactions in limbic and primary somatosensory cortical regions, the HC test-retest analysis yielded significant ANOVA interactions in mainly higher associative and cognitive cortical regions (Table 3). These regions included the cuneus, superior parietal lobule (BA 7), supramarginal gyrus (BA 40), and ventromedial prefrontal cortex (VMPFC).
Table 3.
Summary of the STIM × TIME interaction for CTS patients and HC, which defines the difference between baseline and post-acupuncture CTS patients (or re-test for HC) in their different fMRI responses to verum and sham acupuncture. (amACC: anterior-middle subdivision of anterior cingulate cortex; LatHypothA: lateral hypothalamic area; aInsula: anterior insula; a/pInsula: anterior/posterior insula; SI: secondary somatosensory area; SMG: supramarginal gyrus; SPL: superior parietal lobule; VMPFC: ventromedial prefrontal cortex. Z-score: most significant voxel in cluster; %Δ: percent signal change expressed as mean ± standard deviation in cluster)
| STIM × TIME Interaction:
Carpal Tunnel Syndrome:(CTSbase.acup – CTSbase.sham) - (CTSpost.acup – CTSpost.sham) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region (Brodmann Area) | Talairach | CTSbase %Δ Acup (μ±σ) | CTSbase %Δ Sham (μ±σ) | CTSpost %Δ Acup (μ±σ) | CTSpost %Δ Sham (μ±σ) | ||||
| Side | X (mm) | Y (mm) | Z (mm) | Z-score | |||||
| amACC (24) | R | 7 | 28 | 23 | −3.27 | −0.21±0.02 | 0.38±0.03 | 0.22±0.04 | −0.03±0.06 |
| Amygdala | L | −22 | −8 | −11 | −3.79 | −0.45±0.09 | 0.20±0.11 | −0.03±0.05 | −0.11±0.05 |
| VMPFC | R | 12 | 48 | −11 | −3.37 | −0.46±0.05 | 0.19±0.12 | −0.08±0.05 | −0.28±0.07 |
| LatHypothA | L | −8 | −8 | −7 | 3.37 | 0.29±0.12 | −0.12±0.05 | −0.06±0.07 | 0.16±0.03 |
| aInsula | R | 37 | 2 | 3 | −3.54 | 0.22±0.02 | 0.22±0.01 | 0.21±0.01 | 0.00±0.03 |
| a/pInsula | L | −37 | −3 | 7 | −3.95 | −0.06±0.05 | 0.32±0.10 | 0.23±0.11 | 0.12±0.06 |
| SI (3b/1) | L | −21 | −28 | 55 | −4.37 | −0.21±0.05 | 0.10±0.06 | 0.10±0.03 | −0.16±0.04 |
| Healthy Controls: (HCbase.acup – HCbase.sham) - (HCpost.acup – HCpost.sham) | |||||||||
| Region (Brodmann Area) | Talairach | HCbase %Δ Acup (μ±σ) | HCbase %Δ Sham (μ±σ) | HCpost %Δ Acup (μ±σ) | CTSpost %Δ Sham (μ±σ) | ||||
| Side | X (mm) | Y (mm) | Z (mm) | Z-score | |||||
|
| |||||||||
| cuneus | L | −16 | −74 | 28 | −3.66 | −0.04±0.01 | −0.02±0.02 | 0.13±0.01 | −0.20±0.02 |
| SMG (40) | R | 59 | −36 | 37 | 3.96 | 0.32±0.09 | 0.16±0.03 | −0.06±0.02 | 0.46±0.06 |
| SPL (7) | R | 29 | −50 | 60 | −3.55 | 0.06±0.10 | 0.30±0.10 | 0.05±0.05 | −0.29±0.02 |
| VMPFC | L | −1 | 43 | −7 | 2.85 | −0.30±0.03 | −0.64±0.10 | −0.44±0.10 | 0.01±0.05 |
Figure 4.
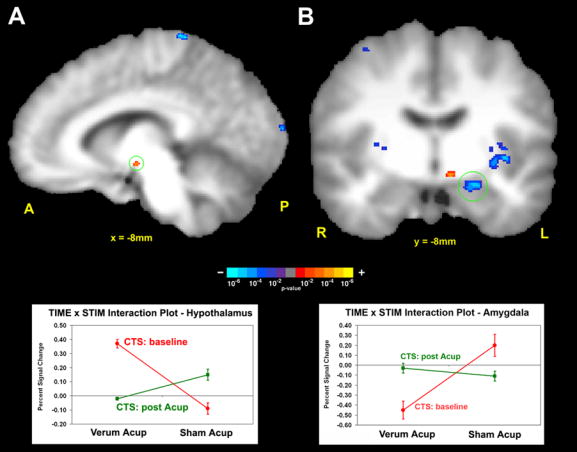
Longitudinal fMRI analysis of CTS patients at baseline and after 5 weeks of acupuncture treatment. The ANOVA interaction tests: (CTSbase.acup – CTSbase.sham) – (CTSpost.acup – CTSpost.sham). A positive interaction was found in the hypothalamus, while a negative interaction was found in the amygdala. Interaction plots demonstrated that the greatest %-signal change in the interaction was by the subgroup: CTS patients at baseline with verum acupuncture stimulation.
Functional connectivity for CTS patients at baseline was explored by comparing fMRI signal response to verum acupuncture in brain regions noted in Table 1, the baseline cross-sectional ANOVA interaction. Of the 13 brain regions modulated by this interaction, only one correlation was found to be significant. A positive correlation (r=0.89, p<0.05, corrected) was found between signal change in the amygdala and LHA (Figure 5a). Specifically, those patients that responded to verum acupuncture with less deactivation in the amygdala, had greater activation in the LHA, and vice versa (Figure 5b).
Figure 5.
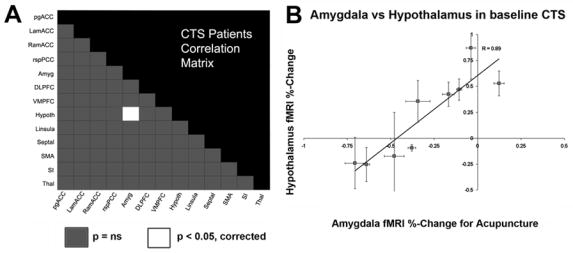
Functional connectivity was explored by calculating the percent signal change correlation matrix for CTS patients at baseline responding to verum acupuncture. Potential brain regions (13) were drawn from those demonstrating a significant cross-sectional ANOVA interaction (table 1). (a) The only two regions which demonstrated a significant correlation were the amygdala and hypothalamus. (b) The less deactivation in the amygdala, the more activation in the hypothalamus for CTS patients at baseline in response to acupuncture.
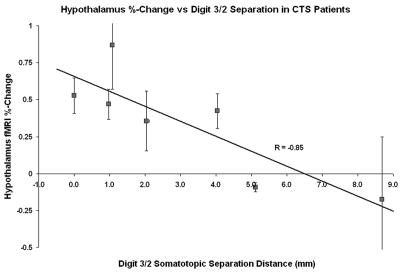
Furthermore, we found that baseline CTS patients’ fMRI signal response in the LHA in response to verum acupuncture was significantly correlated (r=−0.85, p<0.05, Figure 6) with the separation of somatotopic representations of digits 2 and 3 in contralateral SI. This separation distance was found to be a marker of maladaptive cortical plasticity in CTS patients in a previous publication (Napadow et al. 2006a). No other neuroimaging or clinical biomarker correlated with patient limbic response to verum acupuncture. However, a negative trend was found between the fMRI signal response in the hypothalamus and the intensity of sharp pain elicited by verum acupuncture stimulation (r = −0.66, p = 0.055) - i.e. the more sharp pain, the less fMRI signal increase in the LHA. While some authors have suggested that acupuncture works as stress-induced analgesia (Bragin et al. 1983), this result suggests that activation of the LHA in CTS patients at the group level was not likely due to a sharp pain/stress response.
Figure 6.
The magnitude of hypothalamic activation in response to verum acupuncture for CTS patients at baseline was found to correlate negatively with their digit 2/digit 3 somatotopic separation in contralateral SI. The worse a patient’s central maladaptive neuroplasticity, the more they responded to acupuncture with hypothalamic activation.
A statistical analysis found no difference between CTS patients and HC in regard to the prevalence of different sensations elicited by verum acupuncture stimulation. There were also no statistically significant differences in sensations elicited at baseline versus after 5 weeks of acupuncture for CTS patients. Moreover, there were no statistically significant differences in reported anxiety during verum acupuncture (CTS baseline: 4/15 runs; CTS postAcup: 1/11; HC baseline: 3/16; HC re-test: 1/11) or sham acupuncture (CTS baseline: 1/20; CTS postAcup: 0/15; HC baseline: 1/14; HC re-test: 0/11). The mean intensity (across subjects) of the maximum deqi sensation was not significantly different between any subgroups (CTSbase.verum: 3.6±2.1; CTSbase.sham: 2.9±1.6; CTSpost.verum: 3.7±1.6; CTSpost.sham: 2.7±1.0; HCbase.verum: 4.1±2.0; HCbase.sham: 3.3±2.0; HCpost.verum: 4.7±2.2; HCpost.sham: 3.2±2.0; mean±SD). The mean intensity of sharp pain (when experienced) in response to verum acupuncture at LI-4 was in the mild to low moderate range for both groups at baseline (CTS baseline: 3.2±0.8; HC: 3.4±0.7, mean±SEM), as well as for both groups after clinical acupuncture treatment (CTS postAcup: 4.0±1.6) or 5 weeks re-test (HC: 2.7±1.0).
Differences did exist between verum (VA) and sham (SA) acupuncture in regard to the types of sensations elicited. Specifically, the prevalence of aching (VA: 75.0% of runs, SA: 21.4%, p=0.003), soreness (VA: 62.5%, SA: 14.3%, p=0.007), dull pain (VA: 75.0%, SA: 14.3%, p<0.001), sharp pain (VA: 68.8%, SA: 14.3%, p=0.003), and spreading (VA: 50.0%, SA: 14.3%, p=0.038) were greater during VA for HC subjects. For re-tested HC subjects, the prevalence of aching (VA: 90.9%, SA: 9.1%, p<0.001), soreness (VA: 81.8%, SA: 18.2%, p=0.003), sharp pain (VA: 63.6%, SA: 18.2%, p<0.030), and spreading (VA: 63.6%, SA: 9.1%, p=0.008) was greater for VA. For baseline CTS patients, the prevalence of aching (VA: 80.0%, SA: 15.0%, p<0.001), fullness (VA: 33.3%, SA: 5.0%, p=0.028), dull pain (VA: 66.7%, SA: 0.0%, p<0.001), sharp pain (VA: 60.0%, SA: 15.0%, p=0.006), and spreading (VA: 53.3%, SA: 20.0%, p=0.040) was greater during VA compared to SA. For CTS patients after acupuncture therapy, the prevalence of aching (VA: 72.7%, SA: 6.7%, p<0.001), soreness (VA: 54.5%, SA: 0.0%, p<0.001), dull pain (VA: 72.7%, SA: 6.7%, p<0.001), and spreading (VA: 45.5%, SA: 6.7%, p=0.020) was greater for VA. Thus, for the most part, VA and SA differed consistently for the different sub-groups - CTS baseline, CTS post-acupuncture, and HC test-retest. In general, the fact that some sensations were more common for VA compared to SA should not be surprising. Several studies have noted that non-insertive forms of sham acupuncture (e.g. placebo-needles) are better at mimicking verum needle insertion, rather than needle manipulation (Tsukayama et al. 2006). This observation applies to our protocol design as well, since our acupuncture stimulation is best characterized as needle manipulation and identical sensations for VA and SA would not be expected. In sum, our results suggest there was little difference in psychophysical response between different sub-groups and thus, the significant imaging findings were not the result of differences in the sensations elicited.
Discussion
Brain response to acupuncture stimulation may underlie the efficacy of this age-old healing modality. In this study we used neuroimaging to explore cross-sectional and longitudinal differences in acupuncture processing between CTS patients (before versus after clinical acupuncture treatment) and HC, controlling for superficial somatosensory/cognitive elements of stimulation. Our results demonstrated that baseline CTS patients respond to acupuncture with more pronounced modulation in a network of limbic brain regions including the amygdala and hypothalamus. Furthermore, brain processing of acupuncture stimulation may change after a course of clinical acupuncture treatment.
Amygdala Response to Acupuncture
The amygdala is a critical component of the limbic system which can translate somatosensory stimuli (e.g. acupuncture) into affective states. The amygdala is important in processing emotions, especially fear and defensive behavior (Zald 2003). Neuroimaging studies have demonstrated amygdala activation in response to acute pain (Bingel et al. 2002; Bornhovd et al. 2002). Electrophysiological studies in rats have corroborated these findings (Bernard et al. 1992), and have also provided evidence of sensitization and hyperactivation in the amygdala following induction of an inflammatory chronic pain state (Neugebauer and Li 2003). Such neuroplastic change is thought to result from long term potentiation (LTP) which induces synaptic strengthening for connections within the amygdala as well as between the amygdala and other limbic regions (Chapman et al. 1990; Neugebauer et al. 2004).
In our data, the amygdala was found to be deactivated in CTS patients in response to verum acupuncture. As chronic pain has been associated with high background or baseline activity in the amygdala (Neugebauer and Li 2003), we propose that in some patients, acupuncture may function to ameliorate the affective component of chronic pain by deactivating a sensitized, hyperactive amygdala. A short term deactivation within the amygdala may initiate a progressive normalization of activity via neuroplasticity, leading to long term physiologically and clinically relevant response.
Interestingly, while experimental pain typically produces signal increase in the amygdala, several neuroimaging studies have also noted signal decrease in this limbic region (Derbyshire et al. 1997; Becerra et al. 1999; Petrovic et al. 1999; Becerra et al. 2001; Petrovic et al. 2004). It has been theorized that down-regulating limbic activity, particularly in the amygdala, is part of a cognitive coping mechanism for pain suppression (Hsieh et al. 1999; Petrovic and Ingvar 2002). Typically, this occurs when the subject is unable to make a behavioral response to avoid an aversive context (e.g. acupuncture or pain experiments inside an MRI scanner). In some of our patients, subjective expectation of pain from acupuncture may have evoked cognitive coping mechanisms manifest by amygdala deactivation. This may have been particularly amplified for chronic pain patients, naïve to acupuncture at baseline fMRI testing. As therapeutic acupuncture rarely produces analgesia from a single exposure, rather working through accumulative, regularly spaced treatments (Carlsson 2002), a learned cognitive coping strategy may become more entrained with repeated exposure to acupuncture, leading to a longer term strategy for pain suppression.
Hypothalamus Response to Acupuncture
We also found that acupuncture is associated with activity in LHA for CTS patients. Somatosensory input to the hypothalamus is carried by both direct (unmodulated, monosynaptic) and indirect (modulated by cortical and sub-cortical regions, polysynaptic) pathways (Bernard et al. 1996; Burstein 1996). Limbic input to the LHA from the amygdala is transmitted by the ventral amygdalofugal pathway (Parent 1996), thereby translating affect and emotion into autonomic response. Interestingly, our data suggested functional connectivity between the amygdala and LHA for baseline CTS patients (see Figure 5b). In addition, the greater the maladaptive plasticity in contralateral SI (as measured by digit 2/digit 3 representation blurring), the greater the hypothalamic signal increase in response to acupuncture (see Figure 6). Hence, patients with central manifestations of their peripheral CTS lesion may preferentially respond to acupuncture stimulation with hypothalamic activity.
The LHA is capable of producing anti-nociception through efferents to modulatory serotonergic neurons in the rostroventral medulla (RVM) acting on the spinal dorsal horn (Holden et al. 2005). The hypothalamus is also an important component of the endogenous opioidergic pain control system, which functions through connections from the hypothalamus to periaqueductal gray to RVM, and has been implicated as a potential mechanism of acupuncture action (Pomeranz and Chiu 1976; Takeshige et al. 1992; Han 2004). However, this system is associated more with arcuate nucleus than LHA activity.
The hypothalamus is also an important component of the central autonomic network. Through the cholinergic anti-inflammatory pathway, hypothalamic (autonomic) activity can inhibit inflammation (Tracey 2002). Acupuncture may be able to down-regulate inflammatory response by tilting sympathovagal balance towards vagal enhancement. In fact, acupuncture has been noted to affect sympathovagal balance in human studies assessing heart rate and heart rate variability (Nishijo et al. 1997; Haker et al. 2000). However, CTS is more typically characterized by non-inflammatory fibrosis and it remains to be seen if a general anti-inflammatory effect could impact CTS. Even so, peripheral autonomic pathology has been noted in CTS via thermography (Ming et al. 2005; Orlin et al. 2005) and sympathetic skin response (SSR) (Kiylioglu et al. 2005). As the hypothalamus is one of the principal SSR effector regions in the brain (Vetrugno et al. 2003), acupuncture may modulate sympathetic response local to the CTS lesion via central modulation of hypothalamic activity.
Other studies have found either fMRI signal increase (Wu et al. 1999) or signal decrease (Hui et al. 2000) in the hypothalamus in response to LI-4 manual acupuncture stimulation in healthy adults. Our results demonstrated that the significant positive interaction in the LHA was formed by a signal increase for CTS patients and signal decrease for HC – more consistent with Hui et al. (i.e. signal decrease for healthy adults). The hypothalamic signal increase seen by Wu et al. may have been due to the type of needle stimulation (combined twisting and lifting-thrusting) which produced more intense psychophysical response (mean of 6 on a 0–10 scale) compared to our study (HC: 4.1; CTS: 3.6).
Ultimately, acupuncture most likely affects CTS pathology and symptomatology through both peripheral and central mechanisms. As CTS is due to ischemic neuropathology, the ability of acupuncture to induce increased blood flow both superficially and deep to the surface (Sandberg et al. 2003) may improve microcirculation to the impacted median nerve within the carpal tunnel. As part of our therapeutic acupuncture protocol, CTS patients received electroacupuncture at acupoint PC-7, which is centered over the median nerve at the wrist crease. It has been suggested that the vasodilatory effect of acupuncture is due to the release of CGRP and other vasodilatory neuropeptides (Sato et al. 2000). Future studies should assess peripheral effects of acupuncture concurrently with central effects.
Processing of Sham Acupuncture
Deactivations in response to verum acupuncture in baseline CTS patients were in stark contrast to the greater degree of activation in response to a sham acupuncture control for CTS patients at baseline compared to HC. More pronounced activity in primary sensorimotor regions corroborated our previous data for sensorimotor cortical hyperactivation in CTS patients in response to non-noxious stimulation of affected digits (Napadow et al. 2006a). Additionally, our data demonstrated that when the stimulus was a context-laden sham acupuncture intervention, cognitive (DLPFC) and affective (rACC, VMPFC) brain regions were also activated more for CTS patients compared to HC. Interestingly, these regions have also been implicated in executive control of placebo analgesia (Zubieta et al. 2005; Kong et al. 2006).
Methodological Issues and Limitations
To explore the specificity of acupuncture limbic modulation in a longitudinal CTS analysis, a similar test-retest analysis was performed in HC (see Table 3). In contrast to the limbic modulations, the HC test-rested analysis yielded significant interactions in mostly higher associative and cognitive cortical regions, perhaps corresponding to a learning effect inherent in a test-retest experiment. The one exception was the VMPFC, implicated as a para-limbic region processing affective states. However, this region is also part of the task-nonspecific default network (Gusnard et al. 2001), and fMRI signal decreases were found in 3 of the 4 HC sub-groups (Table 3). Hence, this particular interaction may not be specifically related to differences in acupuncture processing but simply learned differences in task salience and attention (McKiernan et al. 2003).
Multiple controls were used in this fMRI study. When fMRI results are presented by an interaction which is composed of a difference of differences (not uncommon in neuroimaging studies), it is important to appreciate which subgroups were the prime delimiters of the significant interaction. We chose to report this information through interaction plots in our figures and as separate data columns in our tables. This approach allowed us to focus our discussion on brain regions where interaction effects were mainly due to verum acupuncture processing in CTS patients – i.e. the amygdala and LHA.
In conclusion, we have presented evidence that acupuncture processing in the brains of CTS patients differs from that of HC. Patients with CTS respond to acupuncture with more pronounced fMRI signal decrease in the amygdala and signal increase in the LHA. The functional connectivity found between the amygdala and hypothalamus suggests that a coordinated limbic response to acupuncture stimuli may underlie the efficacy of this controversial healing modality.
Table 4.
Psychophysics analysis for different sub-groups. A chi-square test was performed to evaluate the difference in proportion of total runs where each sensation was experienced. The table shows the Pearson Chi-Square 2-sided p-value and percentage of runs for individual subgroups. n.s.: non-significant.
| Ache | Sore | Press | Heavy | Full | Warm | Cool | Numb | Tingle | Pain (dull) | Pain (sharp) | Spread | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTSbase.verum vs. HCbase.verum | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| CTSbase.verum vs. CTSpost.verum | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| HCbase.verum vs. HCpost.verum | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| CTSbase.verum vs. CTSbase.sham | 0.001 | n.s. | n.s. | n.s. | 0.028 | n.s. | n.s. | n.s. | n.s. | 0.001 | 0.006 | 0.040 |
| Verum | 80.0% | 33.3% | 66.7% | 60.0% | 53.3% | |||||||
| Sham | 15.0% | 5.0% | 0.0% | 15.0% | 20.0% | |||||||
| HCbase.verum vs. HCbase.sham | 0.003 | 0.007 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.001 | 0.003 | 0.038 |
| Verum | 75.0% | 62.5% | 75.0% | 68.8% | 50.0% | |||||||
| Sham | 21.4% | 14.3% | 14.3% | 14.3% | 14.3% | |||||||
| CTSpost.verum vs. CTSpost.sham | 0.001 | 0.001 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.001 | n.s. | 0.020 |
| Verum | 72.7% | 54.5% | 72.7% | 45.5% | ||||||||
| Sham | 6.7% | 0.0% | 6.7% | 6.7% | ||||||||
| HCpost.verum vs. HCpost.sham | 0.001 | 0.003 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.030 | 0.008 |
| Verum | 90.9% | 81.8% | 63.6% | 63.6% | ||||||||
| Sham | 9.1% | 18.2% | 18.2% | 9.1% |
Acknowledgments
This research was supported by grants from the NIH: NCCAM (K01-AT002166-01, P01-AT002048-02), NCRR (P41RR14075), and the Mental Illness and Neuroscience Discovery (MIND) Institute, as well as the Department of Physical Medicine and Rehabilitation Mini-grant Program, Harvard Medical School (#R02034). We would also like to thank Richard Harris and Bruce Rosen for constructive criticism of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audette J, Ryan A, Liu J, Li M, Kettner NW, Kwong KK, Hui KK, Napadow V. Acupuncture for Chronic Carpal Tunnel Syndrome: A pilot prospective controlled outcome study. American Journal of Physical Medicine & Rehabilitation. 2006 in press. [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32(5):927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41(5):1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68(2):551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99(1–2):313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125(Pt 6):1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Bragin EO, Vasilenko GF, Durinjan RA. The study of the central grey matter in mechanisms of different kinds of analgesia: effects of lesions. Pain. 1983;16(1):33–40. doi: 10.1016/0304-3959(83)90083-0. [DOI] [PubMed] [Google Scholar]
- Burstein R. Somatosensory and visceral input to the hypothalamus and limbic system. Prog Brain Res. 1996;107:257–267. doi: 10.1016/s0079-6123(08)61869-5. [DOI] [PubMed] [Google Scholar]
- Carlsson C. Acupuncture mechanisms for clinically relevant long-term effects--reconsideration and a hypothesis. Acupunct Med. 2002;20(2–3):82–99. doi: 10.1136/aim.20.2-3.82. [DOI] [PubMed] [Google Scholar]
- Chapman PF, Kairiss EW, Keenan CL, Brown TH. Long-term synaptic potentiation in the amygdala. Synapse. 1990;6(3):271–278. doi: 10.1002/syn.890060306. [DOI] [PubMed] [Google Scholar]
- Chiang C, Chang C, Chu H, Yang l. Peripheral afferent pathway for acupuncture analgesia. Science Sinica. 1973;16:210–217. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73(3):431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haker E, Egekvist H, Bjerring P. Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects. J Auton Nerv Syst. 2000;79(1):52–59. doi: 10.1016/s0165-1838(99)00090-9. [DOI] [PubMed] [Google Scholar]
- Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1–3):258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Holden JE, Farah EN, Jeong Y. Stimulation of the lateral hypothalamus produces antinociception mediated by 5-HT1A, 5-HT1B and 5-HT3 receptors in the rat spinal cord dorsal horn. Neuroscience. 2005;135(4):1255–1268. doi: 10.1016/j.neuroscience.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Stone-Elander S, Ingvar M. Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neurosci Lett. 1999;262(1):61–64. doi: 10.1016/s0304-3940(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Tu CH, Chen FP, Chen MC, Yeh TC, Cheng HC, Wu YT, Liu RS, Ho LT. Activation of the hypothalamus characterizes the acupuncture stimulation at the analgesic point in human: a positron emission tomography study. Neurosci Lett. 2001;307(2):105–108. doi: 10.1016/s0304-3940(01)01952-8. [DOI] [PubMed] [Google Scholar]
- Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp. 2000;9(1):13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage. 2005;27(3):479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Kaptchuk T. The Web That Has No Weaver: Understanding Chinese Medicine. Chicago: Contemporary Books; 2000. [Google Scholar]
- Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136(5):374–383. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- Keir PJ, Rempel DM. Pathomechanics of peripheral nerve loading. Evidence in carpal tunnel syndrome. J Hand Ther. 2005;18(2):259–269. doi: 10.1197/j.jht.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Kiylioglu N, Akyol A, Guney E, Bicerol B, Ozkul A, Erturk A. Sympathetic skin response in idiopathic and diabetic carpal tunnel syndrome. Clin Neurol Neurosurg. 2005;108(1):1–7. doi: 10.1016/j.clineuro.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Ming Z, Zaproudina N, Siivola J, Nousiainen U, Pietikainen S. Sympathetic pathology evidenced by hand thermal anomalies in carpal tunnel syndrome. Pathophysiology. 2005;12(2):137–141. doi: 10.1016/j.pathophys.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Napadow V, Kettner N, Ryan A, Kwong KK, Audette J, Hui KK. Somatosensory cortical plasticity in carpal tunnel syndrome--a cross-sectional fMRI evaluation. Neuroimage. 2006a;31(2):520–530. doi: 10.1016/j.neuroimage.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Napadow V, Liu J, Kaptchuk TJ. A systematic study of acupuncture practice: acupoint usage in an outpatient setting in Beijing, China. Complement Ther Med. 2004;12(4):209–216. doi: 10.1016/j.ctim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Napadow V, Liu J, Li M, Kettner N, Ryan A, Kwong KK, Hui KKS, Audette J. Somatosensory Cortical Plasticity in Carpal Tunnel Syndrome Treated by Acupuncture. Human Brain Mapping. 2006b doi: 10.1002/hbm.20261. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005;24(3):193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol. 2003;89(2):716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10(3):221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Nishijo K, Mori H, Yosikawa K, Yazawa K. Decreased heart rate by acupuncture stimulation in humans via facilitation of cardiac vagal activity and suppression of cardiac sympathetic nerve. Neurosci Lett. 1997;227(3):165–168. doi: 10.1016/s0304-3940(97)00337-6. [DOI] [PubMed] [Google Scholar]
- Orlin JR, Stranden E, Slagsvold CE. Effects of mechanical irritation on the autonomic part of the median nerve. Eur J Neurol. 2005;12(2):144–149. doi: 10.1111/j.1468-1331.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- Papanicolaou GD, McCabe SJ, Firrell J. The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg [Am] 2001;26(3):460–466. doi: 10.1053/jhsu.2001.24972. [DOI] [PubMed] [Google Scholar]
- Parent A. Carpenter’s Human Neuroanatomy. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- Pariente J, White P, Frackowiak RS, Lewith G. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. Neuroimage. 2005;25(4):1161–1167. doi: 10.1016/j.neuroimage.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci. 2004;16(7):1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95(1–2):1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83(3):459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: endorphin implicated. Life Sci. 1976;19(11):1757–1762. doi: 10.1016/0024-3205(76)90084-9. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Lundeberg T, Lindberg LG, Gerdle B. Effects of acupuncture on skin and muscle blood flow in healthy subjects. Eur J Appl Physiol. 2003;90(1–2):114–119. doi: 10.1007/s00421-003-0825-3. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y, Shimura M, Uchida S. Calcitonin gene-related peptide produces skeletal muscle vasodilation following antidromic stimulation of unmyelinated afferents in the dorsal root in rats. Neurosci Lett. 2000;283(2):137–140. doi: 10.1016/s0304-3940(00)00932-0. [DOI] [PubMed] [Google Scholar]
- Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Sud V, Freeland AE. Biochemistry of carpal tunnel syndrome. Microsurgery. 2005;25(1):44–46. doi: 10.1002/micr.20071. [DOI] [PubMed] [Google Scholar]
- Takeshige C, Sato T, Mera T, Hisamitsu T, Fang J. Descending pain inhibitory system involved in acupuncture analgesia. Brain Res Bull. 1992;29(5):617–634. doi: 10.1016/0361-9230(92)90131-g. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tsukayama H, Yamashita H, Kimura T, Otsuki K. Factors that influence the applicability of sham needle in acupuncture trials: two randomized, single-blind, crossover trials with acupuncture-experienced subjects. Clin J Pain. 2006;22(4):346–349. doi: 10.1097/01.ajp.0000176359.94644.mL. [DOI] [PubMed] [Google Scholar]
- Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13(4):256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- Wu MT, Hsieh JC, Xiong J, Yang CF, Pan HB, Chen YC, Tsai G, Rosen BR, Kwong KK. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain--preliminary experience. Radiology. 1999;212(1):133–141. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhu L. New Acupuncture (Xian Zhen-jiu Xue) Beijing: People’s Press; 1954. [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]