Abstract
Temporal summation of “second pain” (TSSP) is considered to be the result of C-fiber-evoked responses of dorsal horn neurons, termed ‘windup’. This phenomenon is dependent on stimulus frequency (≥ 0.33 Hz) and relevant for central sensitization and chronic pain. Previous brain imaging studies have only been used to characterize neural correlates of second pain but not its temporal summation. We utilized functional magnetic resonance imaging (fMRI) in healthy volunteers to measure brain responses associated with TSSP. Region of interest analysis was used to assess TSSP related brain activation. Eleven pain-free normal subjects underwent fMRI scanning during repetitive heat pulses to the right foot at 0.33 Hz and 0.17 Hz. Stimulus intensities were adjusted to each individual’s heat sensitivity to achieve comparable TSSP ratings of moderate pain in all subjects. As predicted, experimental pain ratings showed robust TSSP during 0.33 Hz but not 0.17 Hz stimuli. fMRI statistical maps identified several brain regions with stimulus and frequency dependent activation consistent with TSSP, including contralateral thalamus (THAL), S1, bilateral S2, anterior and posterior insula (INS), mid-anterior cingulate cortex (ACC), and supplemental motor areas (SMA). TSSP ratings were significantly correlated with brain activation in somatosensory areas (THAL, S1, left S2), anterior INS, and ACC. These results show that neural responses related to TSSP are evoked in somatosensory processing areas (THAL, S2), as well as in multiple areas that serve other functions related to pain, such as cognition (ACC, PFC), affect (INS, ACC, PAG), pre-motor activity (SMA, cerebellum), and pain modulation (rostral ACC).
Keywords: Temporal summation, Second pain, Windup, fMRI
1. Introduction
Tonic impulse input over C-fibers and consequent temporal summation (TS) of dorsal horn (DH) neuronal responses, termed ‘windup’, can induce and maintain central hyperalgesic states that accompany persistent pain conditions (Dubner, 1991; Gracely et al., 1992; Staud et al., 2001). TS of “second pain” (TSSP) is considered to reflect ‘windup’ in DH-neurons and represents a psychophysical model for the study of hyperalgesic mechanisms (Herrero et al., 2000; Sarlani and Greenspan, 2005). Although spinal ‘windup’ is well established, little is known about the supraspinal representation of this clinically relevant pain mechanism.
A brief nociceptive stimulus, like a heat tap or electrical stimulation of A and C axons, can evoke two distinct pain sensations called, ‘first’ and ‘second’ pain (Price, 1972; Price et al., 1977; Vierck et al., 1997). First pain is an immediate sensation, whereas second pain occurs up to two seconds later and can be a dull, throbbing, or burning sensation. Second pain also often lingers beyond the brief stimulus that evokes it. Repeated activation of C-fibers results in ‘windup’ of DH-neurons and corresponding TSSP (Mendell and Wall, 1965; Price et al., 1978a; Price, 1999). In both cases, the magnitude and duration of the responses increase at stimulus frequencies of ≥ 0.33 Hz and this summation can be attenuated by N-methyl-D-aspartate receptor antagonists in a dose-dependent manner (Dickenson and Sullivan, 1987; Dickenson and Sullivan, 1990; Thompson and Woolf, 1990; Woolf and Thompson, 1991; Price et al., 1994; Arendt-Nielsen et al., 1995; Staud et al., 2005). The critical frequency for ‘windup’ mimics the natural frequency of peripheral C-nociceptors (0.3–1.0 Hz) when stimulus intensities are minimally painful (Torebjork and Hallin, 1974).
Despite strong parallels between ‘windup’ and TSSP, little is known about their neural representations and mechanisms within the human brain (Ploner et al., 2002; Qiu et al., 2005). It has been implicitly assumed that spinal ‘windup’ is reflected supra-spinally in brain structures known to receive synaptic input from dorsal horn neurons. However, since there are multiple types of spinal neurons, ascending pathways, and brain structures responding to C-fiber stimulation, the number and locations of brain sites showing supraspinal ‘windup’ are uncertain (Casey and Bushnell, 2000; Price, 2000; Hobson et al., 2005). Therefore, we used fMRI to examine the brain representation of TSSP in normal pain-free subjects. We sought to determine which brain sites show TSSP utilizing different rates of stimulation. We hypothesized that multiple brain sites would be involved in coding of TSSP and that activation of somatosensory processing regions including thalamus (THAL), insula (INS), and cingulate cortex would reveal close associations with pain ratings. We also sought to determine whether areas involved in cognition and affect [anterior cingulate cortex (ACC), prefrontal cortex (PFC), supplemental motor area (SMA), cerebellum], and pain-modulation [rostral-ACC and midbrain, periaqueductal grey (PAG)] are also activated during TSSP. Our experimental design relied on repetitive heat pulses at ≥ 0.33 Hz, a frequency known to result in TSSP. These stimulus-response characteristics represented the basis for our analysis of brain activation related to C-fiber pain and TSSP.
2. Materials and Methods
We recruited thirteen middle-aged healthy pain-free female subjects [mean age (SD): 42.9 (10.3) years] using advertisements posted throughout the University of Florida, Gainesville. We selected this age range because chronic pain conditions are most prevalent in middle and older age populations and to provide a relevant sample for future comparisons with pain patients. Subjects provided written informed consent to participate in the study. Two subjects were excluded from the analysis because of incomplete fMRI scans. All females who completed the study were right handed and Caucasian. The subjects were not allowed to take any medications, except vitamins and/or hormonal contraceptives. All pre-menopausal subjects were tested during the luteal phase of their menstrual cycles as determined by menstrual history. The University of Florida Institutional Review Board approved the procedures and protocols for this study.
2.1 Experimental Design
The purpose of this study was to image pain related brain activity associated with C-fiber activation and TSSP. For this purpose we applied 47 to 51°C heat pulses (see below) to the plantar surface of the foot. Whereas normal subjects usually rate such heat pulses as painless or only minimally painful when given as single stimuli (e.g., Figure 1), moderate pain and TSSP are reported during repetitive pulses at frequencies of ≥ 0.33 Hz but not at 0.17 Hz (Staud et al., 2001). The pain evoked by repetitive stimuli displays latencies consistent with C-fiber transmission (Staud et al., 2001). Because glabrous foot areas are most distant from the spinal cord and brain, delayed pain sensations (2nd pain) are well separated from early sensations such as 1st pain and can be easily detected by study subjects. Although heat pulses applied to the glabrous skin of the foot can evoke brief latency sensations of weak pain or warmth, they do so only during the first two pulses of a heat pulse train (Price et al., 1977; Price et al., 1994; Vierck et al., 1997). Thus, pain ratings and measures of neural activity in proximity to the last pulse of a six pulse stimulus train (as used in our study) almost exclusively result from C-fiber input and TSSP.
Figure 1.
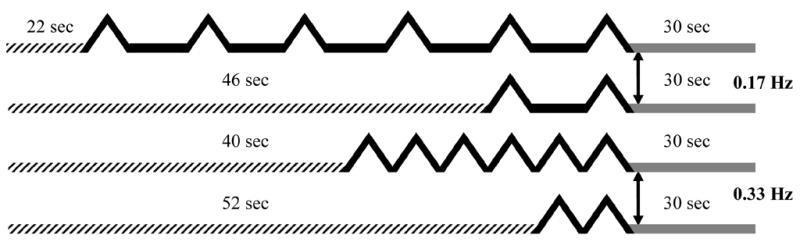
Repetitive thermal stimuli at 0.33 Hz and 0.17 Hz were used to image TSSP-related brain activation. Previous psychophysical studies have shown that stimulus frequencies of ≥ 0.33 Hz resulted in robust TSSP whereas frequencies of < 0.33 Hz were only minimally effective. During scanning runs, two and six heat stimuli at 0.33 Hz and 0.17 Hz were applied to the right plantar surface, respectively. Each run lasted for 88 sec and was comprised of discarded acquisitions/baseline, repetitive thermal stimuli at 0.17 Hz or 0.33 Hz, and a 30 sec follow-up period. Although the four different trains used during scanning runs varied in the timing of the first stimulus, the last stimulus of each train always occurred at the same time point of a run. Duration of discarded acquisitions/baselines is indicated by hatched lines; stimuli at 0.33 Hz or 0.17 Hz are shown by solid black lines; the 30 sec follow-up periods are represented by solid grey lines.
2.2 Ratings
2.2.1 Ratings of Experimental Pain
A standardized numerical pain scale (NPS) was utilized for rating the magnitude of painful sensations produced by thermal stimulation as described previously (Vierck et al., 1997; Staud et al., 2006). The scale ranged from 0 to 100, in increments of 5, with verbal descriptors at intervals of 10: 0 = no sensation, 10 = warm, 20 = a barely painful sensation (i.e. pain threshold), 30 = very weak pain, 40 = weak pain, 50 = moderate pain, 60 = slightly strong pain, 70 = strong pain, 80 = very strong pain, 90 = nearly intolerable pain, and 100 = intolerable pain. Previous experience with the scale has shown that increments of 5 provide appropriate resolution for discriminable levels of late sensation intensity from threshold to nearly intolerable levels (Vierck et al., 1997; Staud et al., 2001). This numerical scale has been found to be particularly advantageous for pain ratings during series of repetitive stimuli (Vierck et al., 1997).
2.2.2 Ratings of Somatic Pain and Anxiety
Numerical scales (0 – 100) were used for ratings of somatic pain and anxiety during the experimental protocol. Although the subjects were required to be pain free at enrollment somatic pain ratings were obtained before and during the scanning session to capture possible new onset pains like back pain, headache, etc. The scales were anchored on the left with “no pain/anxiety at all” and on the right with “the most intense pain/anxiety imaginable”(Vierck et al., 1997). Ratings of somatic pain and anxiety were obtained before and after each scanning run.
2.3 Thermal probe
An MR-compatible Peltier thermode with a 3 × 3 cm (9 cm2) contact surface (TSA-2001, Medoc Advanced Medical Systems, Ramat Yishai, Israel) was used for the thermal stimuli during the experiments. For TSSP testing the probe was firmly attached to the plantar surface of each subject’s right foot by Velcro® straps. The right foot was used for TSSP testing in all subjects.
2.3.1 Heat Pulses
During the fMRI scanning session, each subject received four different types of heat pulse trains (Figure 2). Two and six repetitive heat pulses at 0.17 and 0.33 Hz were used to test the dependence of TSSP on stimulus number and frequency, respectively, i.e. TSSP was expected to increase dependent on number and frequency of the stimuli. All heat pulse trains were counterbalanced to control for order effects. The interval between stimulus trains was always 2 min. For each heat pulse, the temperature of the thermal probe increased from baseline to peak temperature by 10 °C/sec, before returning to baseline at a rate of 10 °C/sec. The duration of each heat pulse was always 3 sec (1.5 sec rise time; 1.5 sec return time). The probe temperature was adjusted to each individual’s heat pain sensitivity which was determined in psychophysical experiments prior to fMRI scanning (see 2.3.2). During these experiments the probe temperature was determined that achieved maximal thermal TSSP ratings of 45 ± 10 NPS units after six heat pulses at 0.33 Hz.
Figure 2.
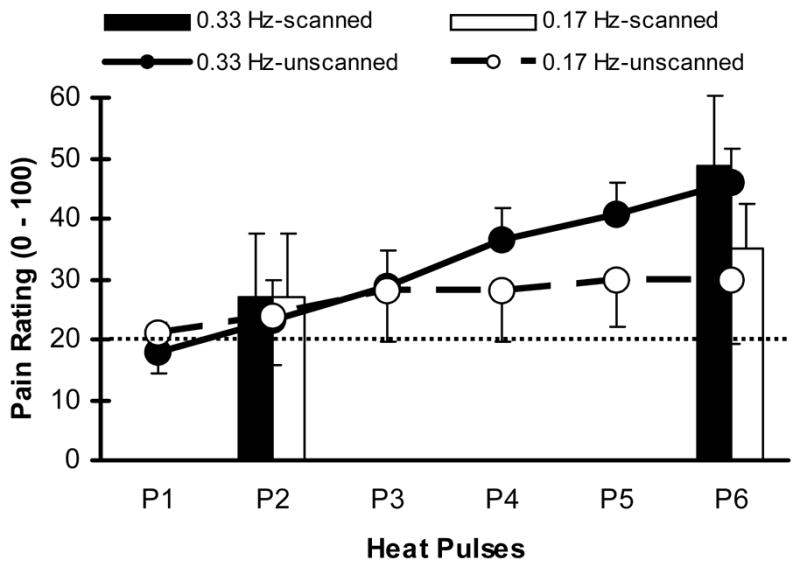
Mean (SD) pain ratings of study subjects during two and six pulse trains at stimulus frequencies of 0.33 Hz and 0.17 Hz. All subjects were asked to remember the number ratings of the pain intensity related to the last heat pulse in a series until the end of each scanning run. Solid bars (0.33 Hz) and hatched bars (0.17 Hz) show these memorized pain ratings of 2-pulse and 6-pulse scanning runs, respectively. Solid (0.33 Hz heat pulses) and dotted lines (0.17 Hz heat pulses) depict mean pain ratings of each of six heat pulses during separate unscanned trials. Pain ratings of the 2nd or 6th heat pulse obtained during scanned and unscanned trials were not significantly different at either stimulus frequency (all p > .05). A rating of 20 represents pain threshold (dotted line).
2.3.2 Adjustment of Thermal Stimuli to Each Subject’s Pain Sensitivity
Pain sensations from heat stimuli vary as a function of each subject’s peripheral and/or central sensitivity, which influences the rate of TSSP and its decay. Because this variability is likely to affect group comparisons of TSSP, we needed to provide measures of TSSP sensitivity across all subjects. Thus, to measure TSSP sensitivity, we determined the unique stimulus temperature for each subject outside the scanner that resulted in final heat pulse ratings of 45 ± 10 NPS units of the last (6th) stimulus at 0.33 Hz. Stimulus intensities resulting in moderate heat pain ratings (45 ± 10 NPS units) were chosen because they were unlikely to significantly alter peripheral and central pain sensitivity of study subjects during repeated trials. Testing was always started at 47°C (peak pulse temperature), and the temperature was subsequently raised until subjects achieved NPS ratings of 45 ± 10 (NPSmax) after 6 pulses. This stimulus intensity was used for subsequent TSSP testing in the MR scanner.
Due to technical constraints of the thermal stimulator used for the TSSP experiments, the maximal rise and fall of probe temperatures were limited to 8°C/sec. Thus, necessary adjustments of peak temperatures always required similar changes in baseline temperatures for TSSP heat stimuli. Although somewhat unusual, this heat pulse design was necessary to elicit similar magnitudes of TSSP in all subjects.
2.3.3 TSSP Training Sessions
All subjects were trained to attend to and rate late sensations evoked by repetitive thermal stimulation of the plantar surface of the right foot during at least two separate training sessions. Details of similar TSSP procedures have been previously reported in detail (Staud et al., 2001; Staud et al., 2004). Prior to the testing sessions, a technician who was unfamiliar with the hypotheses of the experiment instructed each subject to provide ratings of the intensity of any delayed pain sensations produced by repeated heat pulses to the plantar surface. Study subjects were told that they may or may not feel sensations of heat pain during each pulse and that a late sensation of heat or heat pain would likely be perceived 1 to 2 sec after each contact. They were also asked to pay attention to and provide numerical ratings of the magnitude of the late sensation, which could increase or decrease with stimulus repetition. A numerical pain scale with verbal anchors (NPS) was utilized for rating the magnitude of late sensations produced by thermal stimulations (Vierck et al., 1997). Before each fMRI session, the subjects practiced rating of late thermal sensations outside the scanner to familiarize themselves with the NPS.
2.3.4 Pain Ratings of Thermal Stimuli Before and During fMRI Scans
After resting comfortably on the scanner table the subjects were moved into the MR scanner to acclimatize themselves to the scanning environment. Subsequently, two six pulse trains of thermal stimuli at 0.33 Hz and 0.17 Hz were applied to the right foot without image acquisition. In contrast to subsequent scanning runs, the subjects were instructed to rate the pain intensity after each heat pulse using the NPS. This procedure served as an experimental check for the subsequent fMRI experimental manipulations in which the subjects reported the remembered heat pain ratings of the last heat pulse of a train after each scanning run. Specifically, the subjects were instructed before each scanning run to pay attention to the pain intensity of each stimulus and remember the number rating of the last stimulus of each heat pulse train which always occurred 30 sec before the end of each run (see Figure 2). After the scanner stopped the subjects were immediately asked to provide the remembered number rating of the last stimulus.
2.3.5 Estimation of the Conduction Velocity of Impulses Evoking Delayed Pain
Estimates of the conduction velocity of impulses evoking delayed pain were calculated after measuring the time delay from the beginning of the thermal pulse to the beginning of the delayed pain sensation, as described before (Staud et al., 2006). An electronic timer was activated at the beginning of the stimulation sequence. Thereafter, the subjects pressed a computer mouse button every time they felt the onset of delayed pain sensations during six 0.33 Hz heat pulses that elicited TSSP. A computer program automatically calculated the time delay between the stimuli and timed pain sensations. The means of at least four timed delays was used to calculate the conduction velocity of impulses giving rise to delayed pain. Calculations of C-fiber conduction velocity were based on the conduction distance between thenar foot eminences and the T12 spinous process, the central component of reaction time (including conduction within the spinal cord), and the latency to delayed pain (Price et al., 1977). Mean conduction velocity was computed as:
Reaction times to simple sensory stimuli, including somatosensory stimuli, range from about 0.1 to 0.2 sec (Robinson, 1934; Woodworth and Schlosberg, 1971). Thus the estimate of 0.15 sec central reaction time used for conduction velocity calculations is within the expected range of middle aged healthy populations. Given the very long peripheral conduction distance from the sole of the foot to the lumbosacral spinal cord and the slow conduction velocity of C-fibers, peripheral conduction time makes by far the largest proportion of the delay to second pain. Conduction velocities of less than 2 m/sec are considered to reflect impulse transmission by C-fibers.
2.4 Questionnaires
All subjects were asked to complete Beck’s Depression Inventory (BDI) (Beck and Beamesderfer, 1974) and the Spielberger State/Trait Anxiety Questionnaires (Spielberger et al., 1983). The BDI is a self-administered 21 item self-report rating inventory measuring characteristic attitudes and symptoms of depression. Scores can range from 0 – 63. A score of 19 and higher is indicative of clinical depression. Spielberger’s State/Trait Anxiety Inventory consists of 20 items each that ask how a person feels now, and reflects situational factors that may influence anxiety levels. Scores range from 20 to 80 and the higher the score the greater the level of anxiety. These questionnaires were only used to characterize the study subjects.
2.5 fMRI Scanning
2.5.1 fMRI Mock Scanning
In order to familiarize the subjects with the scanning environment, all participants underwent thermal TSSP trials in a mock scanner that simulated the conditions of the Siemens Allegra Head Scanner. Recorded scanner noise was played during the training sessions at realistic intensity levels. All subjects underwent at least two training sessions inside the mock scanner to reduce environment related anxiety and to familiarize themselves with the stimulation protocols.
2.5.2 TSSP Scanning Design
During the scanning session the subjects were comfortably placed on the scanning table in the supine position with their knees supported by a wedge cushion. Their head positions were fixated with foam cushions to prevent excessive motion. Ear phones were used for noise protection as well as communication with the subjects. All subjects underwent 32 scanning runs. Functional scanning runs comprised eight two-pulse trains and eight six-pulse trains at stimulus frequencies of 0.33 Hz and eight two-pulse trains and eight six-pulse trains at stimulus frequencies of 0.17 Hz (Figure 2). All heat pulse trains were applied to the subject’s right foot in counterbalanced order. At the end of each stimulus train brain scanning was continued for 30 sec to capture delayed pain related brain activity. The duration of each run was always 88 sec regardless of the number or frequency of the applied heat stimuli. Due to the two different frequencies used for testing, two and six pulse trains started either at 16 sec and 32 sec (0.17 Hz), or 60 sec and 76 sec (0.33 Hz), respectively, after the beginning of the scanning run (Figure 2). Therefore the last stimulus of every heat pulse train and similarly pain ratings occurred at exactly the same time point across all runs, regardless of the number and frequency of the applied heat stimuli. Using the NPS, the subjects were instructed to rate and then retain the pain rating of the last stimulus of each train in memory until the end of a run. After each run, the subjects were immediately asked to recall the number rating of the last stimulus. Before and after each run the subjects also rated clinical pain and anxiety using the numerical scale (0–100).
2.5.3 Image Acquisition
MRI data were acquired on a research-dedicated head scanner (Siemens Allegra, 3.0 Tesla) using a standard head RF coil. Functional images were acquired in 32 axial slices oriented parallel to the anterior commissure-posterior commissure (AC–PC) line using a T2*-weighted gradient echo planar imaging (EPI) sequence [Repetition time/echo time (TR/TE = 2000 ms/30 ms), flip angle (FA) = 90°, field of view (FOV) = 24cm, matrix = 64 × 64; voxel size = 3.75 mm × 3.75 mm × 3.8 mm; slice gap 0.4 mm]. Each functional run lasted for 88 sec, resulting in 44 volumes. The first two volumes were discarded at the scanner and two additional volumes were discarded during preprocessing to reduce T1-saturation effects, leaving a total of 40 volumes per functional run. Prior to functional scanning, high-resolution 3D anatomical images were acquired using a T1-weighted MP-RAGE protocol (128 1-mm axial slices; TR = 2000ms, TE = 4.13ms, FA = 8°, matrix = 256 × 256mm, FOV = 24cm) to enable transformation and localization of functional data into Talairach space (Talairach & Tournoux, 1988).
2.5.4 Image Analysis
2.5.4.1 Functional MRI Data Reduction
Data analysis was performed on a Xeon dual processor 3.4 GHz PC. fMRI data were preprocessed and analyzed using BrainVoyager QX 1.6 software (Brain Innovation, Maastricht, the Netherlands; http://www.brainvoyager.com). Image pre-processing consisted of rigid-body 3D motion correction using trilinear interpolation, slice-scan time correction with sinc interpolation, spatial smoothing with a 4-mm full-width at half maximum (FWHM) Gaussian kernel, voxel-wise linear detrending, and high-pass temporal filtering to remove nonlinear drifts at 3 Hz. Functional data were co-registered to a 3D anatomic volume and transformed into standard space using the standard 8-parameter landmark method of Talairach and Tournoux (Talairach and Tournoux, 1988). During Talairach transformation, functional voxels were interpolated to a resolution of 1mm.
2.5.4.2 Random Effects Analysis
The functional data were analyzed via an event-related design (with events specified in terms of seconds), using each thermal pulse as a predictor. This afforded the ability to estimate and control for variance associated with each thermal pulse. Using a typical hemodynamic response function [HRF; (Boynton et al., 1996)] with a delta of 2.5 and a tau of 1.25, a lag of 3 sec was added to the HRF in order and to ensure that the identified voxel time-courses corresponded to “second-pain” phenomena. This lag time was a-priori chosen to account for the estimated delay of C-fiber conducted second pain sensation originating from the foot. Given the very long peripheral conduction distance from the sole of the foot to the spinal cord and the slow conduction velocity of C fibers (< 2m/sec), 3 sec represents a conservative estimate of the delay of heat pulse related brain activation.
As the intent of this study was to characterize the net change in blood oxygen level dependent (BOLD) response to different stimulus-trains, we have chosen to use the data to show activation changes in terms of overall-magnitude (i.e., last minus first) across the four conditions. Thus, for each experimental condition a random-effects General Linear Model (RFX-GLM) was used to identify brain activity associated with TSSP. Linear contrasts were utilized to estimate the BOLD activation between the first and last thermal pulse. Linear contrasts were also used to determine the difference in BOLD responses for the last thermal pulse at each stimulus frequency (i.e., last pulse of the 6-pulse train to the last pulse of the 3-pulse train at 0.17 and 0.33 Hz respectively).
2.5.4.3 Determination of Regions of Interest
A more thorough evaluation of condition-related effects was done by examining changes in BOLD activation within specific a-priori determined regions of interest (ROI) including THAL, S1, S2, post INS, ant INS, right ACC, mid ACC, IFG, SMA, and PAG. In order to increase both construct and contrast specificity for the ROI analyses, the geographical extents of the ROIs were based on linear contrasts (first versus last pulse) of the most painful condition (i.e., 0.33 Hz 6-pulse train). Each ROI met the following criteria: voxels survived second-level threshold of p <.005, contained at least 50 contiguous voxels, and the center-of-gravity for the voxel cluster was within the targeted brain region.
These ROIs were then used as masks which served several functions. Statistically, they reduced the number of voxel-wise comparisons being done thus increasing the sensitivity in detecting changes in BOLD activation. Random Effects ROI-GLMs were then used to test differences in BOLD associated with the last pulse across conditions the same presentation rate, i.e. 0.17 Hz - 2nd pulses vs. 6th pulses and 0.33 Hz 2nd pulses vs. 6th pulses.
3. Results
3.1 Somatic Pain Ratings and Questionnaire Data
The subjects reported no somatic pain before and during the fMRI scans. Their mean (SD) Beck’s Depression Inventory score was 2.6 (3.9) (range 0 – 10) and their Spielberger State/Trait Anxiety scores were 29.7 (9.1) and 32.1 (3.5), respectively. These results suggest that our fMRI analysis was not confounded by incidental somatic pain or high levels of anxiety and depression.
3.2 Experimental Pain Ratings
The mean (SD) temperature of adjusted heat pulses applied to the right foot of the study subjects increased from a baseline of 38.4 (1.4) °C to a peak temperature of 46.4 (1.1) °C. The mean (SD) pain intensity of the first heat pulse during all conditions was rated at 19.4 (8.4) NPS units. The mean (SD) pain intensity of 0.33 Hz 1st and 6th pulses applied inside the scanner but before image acquisition was rated by the subjects as 24.5 (7.9) NPS units and 45.3 (12.1) NPS units, respectively. A dependent t-test showed that the pain ratings of the 6th pulse were significantly higher than the ratings of the 2nd pulse (t(5) = 5.1; p = .004), consistent with temporal pain summation. During scanning runs the number ratings of the last stimulus of each pulse train were kept in memory by the subjects. The mean (SD) of the remembered pain ratings obtained after scanning runs of two (short trains) and six (long trains) heat pulses was 27.2 (10.2) NPS units and 48.9 (11.4) NPS units for 0.33 Hz stimuli and 26.2 (9.0) NPS units and 34.3 (5.8) NPS units for 0.17 Hz stimuli, respectively. A dependent t-test showed that the difference scores of the last pain ratings of the short and long train during 0.33 Hz trains were significantly higher than during 0.17 Hz. trains (t(10) = 3.5; p = .01).
3.2.1 TSSP Ratings Obtained Inside the Scanner Before and During Runs
To avoid confounds of pain-related brain activation with rating tasks during the scanning runs, remembered pain ratings were always obtained immediately after the scanning run and only for the last pulse of stimulus trains at either 0.33 Hz or 0.17 Hz. To asses possible memory influences associated with remembered ratings, pain ratings of each pulse during stimulus trains at either 0.33 Hz or 0.17 Hz were obtained before scanning runs, while the subjects were inside the scanner. These ratings were similar to those recorded during scanning runs at either frequency (see Figure 1). In addition, the difference scores (SD) of pain ratings obtained before and during scanning runs at 0.33 Hz (21.7 (17.5) and 20.8 (10.2), respectively) and at 0.17 Hz (8.1 (7.4) and 9.8 (6.7), respectively) were not significantly different (p > .05) indicating that real time pain ratings and remembered ratings were similar.
3.3 Estimated Conduction Velocity of Delayed Pain Sensations
Six of the eleven subjects participated in this experiment. Reaction time measurements were made from the beginning of each heat pulse to the start of delayed pain sensations. The mean (SD) delay associated with late pain was 2.0 (0.5) sec (after subtracting 0.15 sec for the central component of reaction time) and the average distance between the thenar foot eminences and the T12 spinous process of the study subjects was 1.45 (8.3) m. Thus the mean (SD) estimated transmission velocity of impulses associated with late pain was 0.73 (0.43) m/sec, well within the range of C-fiber conduction velocity (Carpenter, 2002) and similar to recently published estimates (Granovsky et al., 2005).
3.4 Region of Interest Analysis
Random Effects ROI-GLMs were used to test differences in BOLD (associated with the last pulse) between conditions of identical frequency in 16 brain regions (Table 1). These regions were contained within the four general ones hypothesized to show increased activity during the experimental conditions. Consistent with the psychophysical data, there was significantly greater activation seen during the 6-pulse condition at 0.33 Hz than at 0.17 Hz (Figures 3–4). These differences occurred for all hypothesized regions (left post THAL, right post THAL, left S1, left S2, right S2, anterior and posterior INS, right ACC). During the 0.33 Hz pulses, S1 became activated not only at contralateral but also ipsilateral sides, consistent with previously published data of pain related brain activations (see Figure 3, Panel B) (Casey and Bushnell, 2000). Contrasts between 6-pulse and 2-pulse trains at 0.33 Hz showed significantly more activation in pain-related brain areas after the 6th pulse than after the 2nd pulse in all ROIs (Table 2). Similar contrasts at 0.17 Hz did not indicate greater activation within these pain related areas, except for the right S2, right post-INS, and ACC (Table 3). Thus, stimulus frequency (0.33 Hz > 0.17 Hz) and number of stimuli (6 > 2 stimuli) were important determinants of ROI activation, consistent with the psychophysical results.
Table 1. Regions of Interest (ROI): Locations and Volumes.
| Coordinates* |
||||
|---|---|---|---|---|
| ROI Volume (μL) | x | y | z | |
| Thal. post. R (VPL, MD, Pulvinar) | 78 | 7 | −19 | 8 |
| Thal. post. L (VPL, MD, Pulvinar) | 752 | −9 | −19 | 8 |
| S1/Post. Parietal Cortex L (BA 5/7) | 3272 | −13 | −44 | 57 |
| S1/Post. Parietal Cortex R (BA 5/7) | 2383 | 17 | −43 | 59 |
| S2 R (BA 40) | 2490 | 54 | −23 | 26 |
| S2 L (BA 40) | 526 | −52 | −20 | 21 |
| Ant. Insula R | 290 | 44 | 26 | 5 |
| Post. Insula R | 939 | 45 | −12 | 14 |
| Post. Insula L | 534 | −36 | −26 | 17 |
| Ant. Cingulate Cortex R (BA 24) | 505 | 8.4 | −3 | 39 |
| Dorsal Ant. Cing. Cortex L (BA 24B) | 1015 | −4 | −6 | 41 |
| Dorsal Ant. Cing. Cortex R (BA 24B) | 1335 | 21 | 42 | 35 |
| Rostral Ant. Cing. Cortex bil. | 110 | −0 | 13 | 29 |
| Ant. Cing. Cortex (BA 24) L A1 | 227 | −4 | 22 | 20 |
| Suppl. Motor Area L | 2155 | −5 | −9 | 67 |
| Mid-Cerebellum R | 135 | 3 | −61 | −19 |
Coordinates of voxels at center of mass for significant cluster. R = right; L = left; VPL = ventro posterior nucleus; MD = medio-dorsal nucleus; BA = Brodmann area
Figure 3.
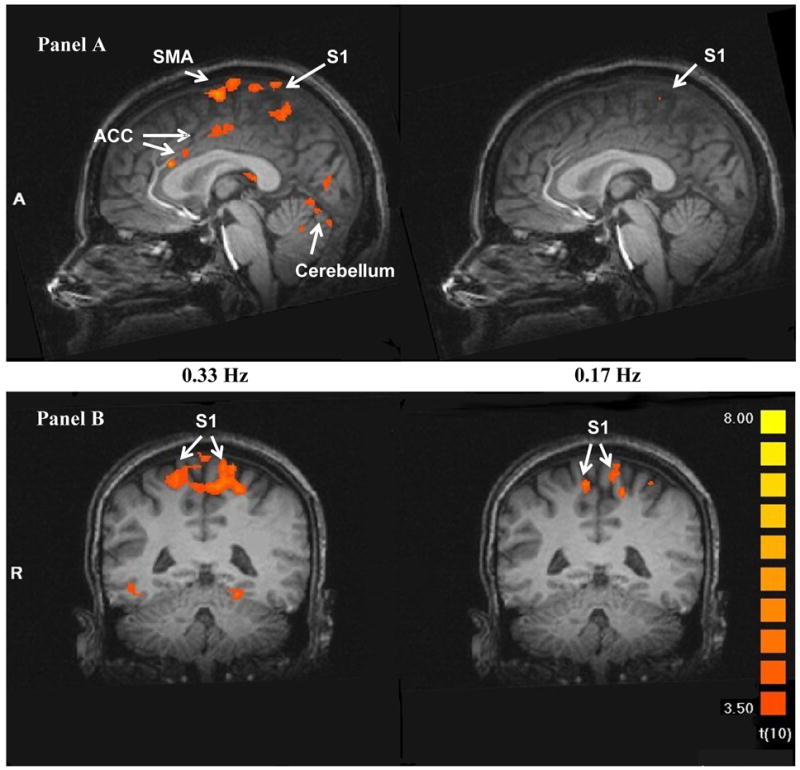
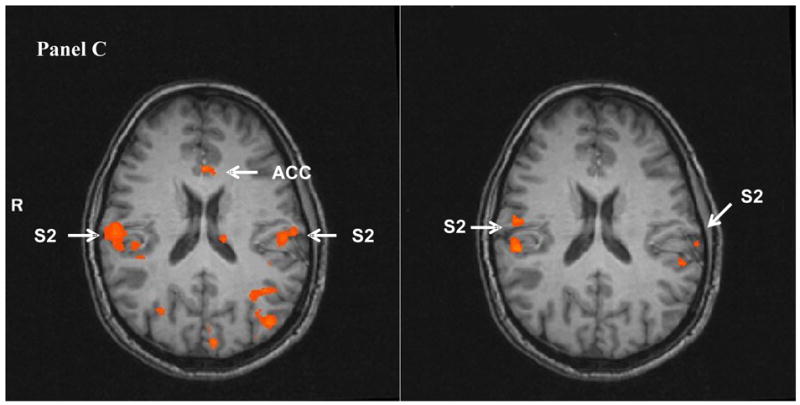
Heat pulse related brain activity during TSSP stimuli at 0.33 Hz (left) and 0.17 Hz (right) in sagittal (Panel A) and transverse (Panel B and C) sections. The fMRI images depict the difference in brain activation between the last stimulus of short (two stimuli) and long trains (six stimuli) at 0.33 Hz (left) and 0.17 Hz (right), respectively. The brain activity in all a-priori selected ROIs was significantly greater after six compared to two heat pulses at 0.33 Hz (left), including S1, S2, PFC, SMA, THAL, ACC, and INS, (p < .004). Similar comparisons at 0.17 Hz (right), however, produced only increased brain activity in M1, S2, and posterior-INS.
(THAL: thalamus; S: somato-sensory cortex; ACC: anterior cingulate cortex; PCC: posterior cingulate cortex; PFC: pre-frontal cortex; pINS: posterior insula; SMA: supplemental motor area).
Figure 4.
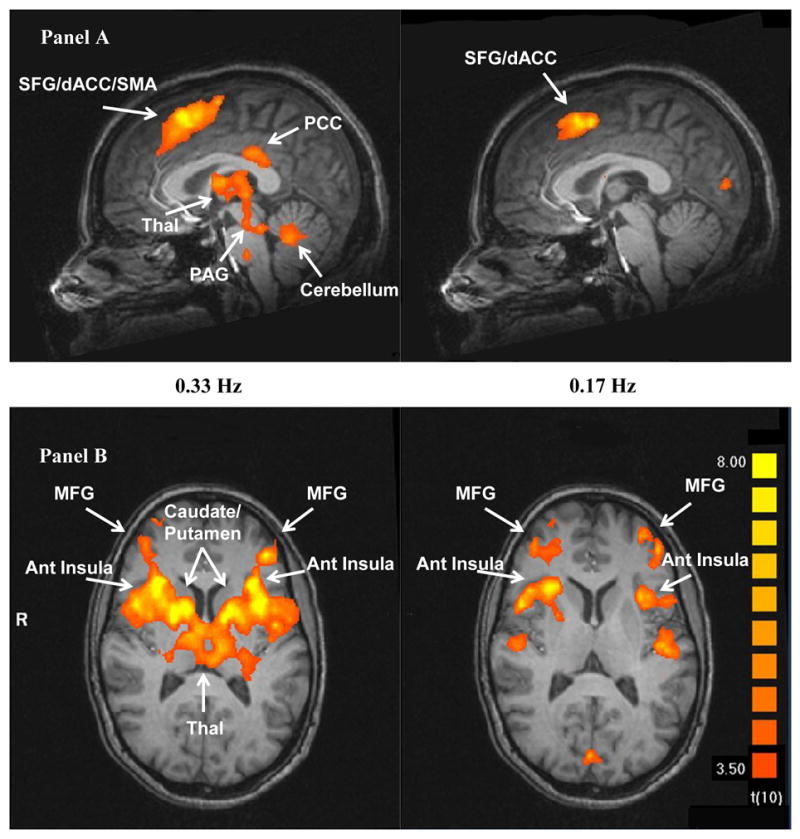
Pain related brain activity of the last of six heat pulses at 0.33 Hz and 0.17 Hz in sagittal (Panel A) and transverse (Panel B) sections, respectively. In contrast to Fig. 3 which illustrates the relationship between brain activity and the number of heat pulses, theses fMRI images depict the association of brain activity with TSSP pain ratings. Significantly increased brain activation was detected in PFC, SMA, ACC, THAL, ACC, and INS (p < .005).
Table 2. Contrasts of Brain Activity at 0.33 Hz TSSP (6th Heat Pulse versus 2nd Heat Pulse).
| T value | p | |
|---|---|---|
| Thalamus dorsal post. R | 4.12 | < .003 |
| Thalamus dorsal post. L | 6.20 | < .001 |
| S1/Post. Parietal Cortex L (BA 5/7) | 5.90 | < .001 |
| S1/Post. Parietal Cortex R (BA 5/7) | 5.95 | < .001 |
| S2 R (BA 40) | 5.13 | < .001 |
| S2 L (BA 40) | 5.49 | < .001 |
| Ant. Insula R | 4.60 | < .001 |
| Post. Insula R | 6.41 | < .001 |
| Post. Insula L | 5.27 | < .001 |
| Ant. Cingulate Cortex R (BA 24) | 5.62 | < .001 |
| Dorsal Ant. Cing. Cortex L (BA 24B) | 5.01 | < .001 |
| Dorsal Ant. Cing. Cortex R (BA 24B) | 5.73 | < .001 |
| Rostral Ant. Cing. Cortex bil. | 4.81 | < .001 |
| Ant. Cing. Cortex (BA 24) L A1 | 6.19 | < .001 |
| Suppl. Motor Area L | 7.18 | < .001 |
| Mid-Cerebellum R | 5.07 | < .001 |
R = right; L = left
Table 3. Contrasts of Brain Activity at 0.17 Hz TSSP (6th Heat Pulse versus 2nd Heat Pulse).
| T value | p | |
|---|---|---|
| Thalamus dorsal post. R | 0.04 | > .05 |
| Thalamus dorsal post. L | −0.65 | > .05 |
| S1/Post. Parietal Cortex L (BA 5/7) | 3.42 | < .007 |
| S1/Post. Parietal Cortex R (BA 5/7) | 2.95 | < .02 |
| S2 R (BA 40) | 4.36 | < .002 |
| S2 L (BA 40) | 2.48 | < .04 |
| Ant. Insula R | 0.46 | > .05 |
| Post. Insula R | 6.77 | < .001 |
| Post. Insula L | 2.17 | < .03 |
| Ant. Cingulate Cortex R (BA 24) | 1.77 | > .05 |
| Dorsal Ant. Cing. Cortex L (BA 24B) | 0.90 | > .05 |
| Dorsal Ant. Cing. Cortex R (BA 24B) | 1.77 | > .05 |
| Rostral Ant. Cing. Cortex bil. | 0.17 | > .05 |
| Ant. Cing. Cortex (BA 24) L A1 | 1.87 | > .05 |
| Suppl. Motor Area L | 0.01 | > .05 |
| Mid-Cerebellum R | 0.07 | > .05 |
R = right; L = left
3.5 Associations of Brain Activity with Experimental Pain Ratings
A second analysis was performed to identify brain areas associated with the cognitive-evaluative aspects of pain ratings, similar to (Kong et al., 2005). Experimental pain ratings were used as predictors of ROI activation. As shown in Figure 4, robust correlations existed between experimental pain ratings and brain activity. This analysis showed significant relationships within ROIs of most of the hypothesized regions (PAG, THAL, caudate, putamen, ant INS, ACC, PCC, SMA, left frontal gyrus, and left prefrontal gyrus). In addition, stronger relationships of pain ratings with brain activity were observed for 0.33 Hz as compared to 0.17 Hz stimulus trains (Figure 4).
3.6 BOLD Activity Time Course During TSSP Trials
BOLD activity increased during TSSP trials with 6 heat pulses at 0.33 Hz and remained elevated for more than 40 sec in all ROIs after the last heat stimulus (Figure 5). In contrast 6-pulse trials at 0.17 Hz resulted in the expected increase and return of BOLD activity to baseline after approximately 10 sec (Figure 5). Thus the observed brain activity during TSSP trials is unlikely to represent a BOLD artifact. In addition, the observed BOLD activity during 0.33 Hz pulses was reminiscent of the prolonged pain sensations intrinsic to TSSP as described in previous psychophysical experiments (Vierck et al., 1997; Staud et al., 2001).
Figure 5.
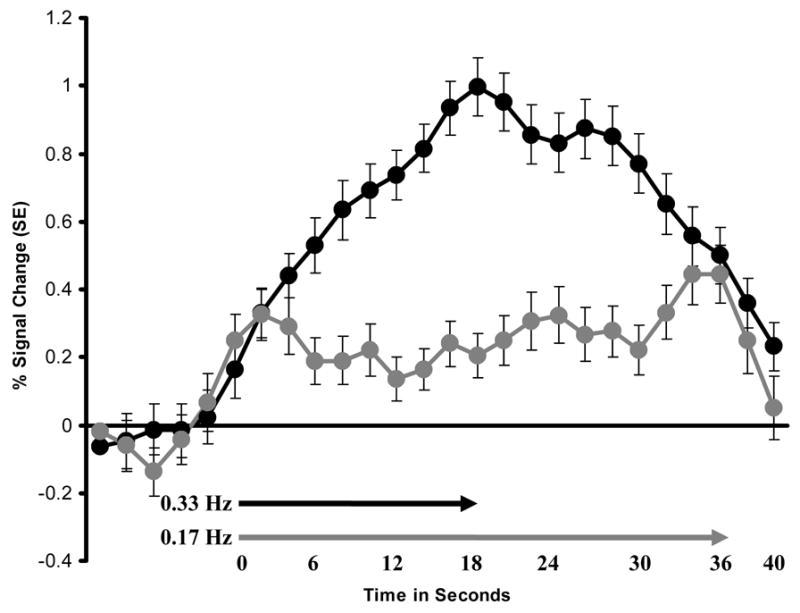
Representative sample of BOLD signal time courses related to six TSSP stimuli at 0.33 Hz (black circles) and 0.17 Hz (grey diamonds). ROI: right posterior-THAL. Arrows indicate the duration (in seconds) of six heat stimuli at 0.33 Hz (black arrow) and 0.17 Hz (grey arrow). Residual brain activation after 0.33 Hz 6-pulse trains took more than twice as long to resolve as expected. In contrast residual brain activity after 0.17 Hz 6-pulse trains resolved within the expected time period. Similar BOLD time courses were observed at all ROIs. TSSP: temporal summation of second pain; THAL: thalamus; BOLD: oxygen level dependent; SE: Standard error.
Thus four lines of evidence suggest that the observed activation of cortical and subcortical areas of normal subjects was related to TSSP, including the BOLD activity time course and the highly significant association of ROI BOLD responses with a) pulse frequency (0.33 Hz > 0.17 Hz); b) stimulus number (6th pulse > 2nd pulse); and c) experimental pain ratings.
4. Discussion
Using fMRI we identified the neural responses associated with TS of C-fiber evoked pain in multiple brain areas during repeated heat-pulses to the glabrous surface of the foot. Statistical parametric mapping of TSSP-related BOLD activity identified multiple brain areas known to receive input from ascending spinal pathways and sites involved in pain-related somatic sensation, affect, motor function, and pain modulation. These results are the first to characterize TSSP within the human brain.
4.1 Neural Responses Associated with TSSP
We applied trains of identical heat pulses to evoke C-fiber pain summation. It is important to recognize that single heat pulses used in our experiments evoke only weak ‘first pain’ or warmth, whereas repetitive heat-pulses (6-pulse trains at 0.33 Hz) result in moderate pain (approx. 50 VAS units) and TSSP (Price et al., 1978b; Vierck et al., 1997). Thus our experimental design differs considerably from other pain imaging studies that varied the perceived pain intensity by modulating the strength but not the number of stimuli as in our study. Moreover, previous experiments have shown that TSSP relies mostly on C-fiber input after the first two heat-pulses of a stimulus train because of rapid attenuation of A-delta fiber pain input (Price et al., 1978b; Vierck et al., 1997). Therefore, the heat-pulse trains used in our experiments were particularly useful to examine C-fiber pain and its summation. The same temporal parameters that evoke TSSP were used to identify neural responses in multiple brain areas. Thus the magnitudes of psychophysical ratings and neural responses depended on number and frequency of stimuli, i.e. responses were much greater after the sixth pulse of a 0.33 Hz train in comparison to the sixth pulse of a 0.17 Hz train (Figures 2–4). As hypothesized, TSSP-related brain activations occurred in somatosensory (THAL, S1, S2, ant-INS and post-INS), cognitive–evaluative/affective (ACC, PFC), and pain-modulating regions (rostral ACC).
Since similar magnitudes of summation occur for both spinothalamic-tract neurons and TSSP, spinal ‘windup’ is likely to make the largest contribution to TSSP (Price et al., 1978b; Price et al., 1979). It is possible that some additional TS occurs at supraspinal levels. It is also possible that different brain regions show magnitudes of TS that are above or below activation predicted by increases in perception of pain intensity. TS in some brain areas may be relevant to functions other than pain-intensity coding.
Additional, though small contributions may also result from non-neural carryover of BOLD during multiple stimuli, similar to that found in previous studies (Huettel et al., 2004). We think, however, that TS of BOLD responses in our study is the result of neuronal activation (see 4.3). Mostly, because non-neural components of BOLD activity decrease within approximately 10–15 seconds after a stimulus (Huettel et al., 2004), whereas TSSP related BOLD responses remained well above baseline levels for more than 40 seconds after moderately painful TSSP stimuli (Figure 5). In addition, a recent fMRI study demonstrated strong associations between BOLD activity and action potential activity within the same cortical area when repetitive acoustic stimuli were used (Mukamel et al., 2005).
4.2 Encoding of TSSP in Spino-Thalamo-Cortical Pathways to S1
Previous work using intrinsic optical density imaging of the somatosensory cortex has shown TS of heat-tap evoked neural responses in areas 3a and 1 of S1 in anesthetized squirrel monkeys (Tommerdahl et al., 1996; Tommerdahl et al., 1998; Whitsel et al., 2000). These results are limited because only one brain region was studied in these monkeys. A human study using magneto-encephalography showed weak activity in S1 related to second pain (evoked by C-fiber input) (Ploner et al., 2002). This weak S1-response may reflect the use of single stimuli. As pointed out above, moderate to intense levels of second pain can only be induced by repetitive stimulation and TS. The present study extends the results of these two studies in showing brain activation related to TSSP at all levels of somatosensory processing including THAL, S1, S2, PPC, and post-INS.
The demonstration of TS of C-fiber evoked responses within the spino-thalamo-cortical pathway to S1 further supports the role of these pathways in the encoding of TSSP and pain in general. Wide-dynamic-range (WDR) and nociceptive-specific neurons (NS) at all levels of these pathways have response characteristics that reflect refined sensory discrimination of nociceptive stimulation (Chudler et al., 1986; Maixner et al., 1986; Kenshalo, Jr. et al., 1988; Chudler et al., 1990; Chudler and Dong, 1995). Several studies have shown that WDR (Price et al., 1978b; Price et al., 1979; Chung et al., 1979) and NS spinothalamic tract neurons (Price et al., 1978b; Price et al., 1979; Chung et al., 1979; Craig and Andrew, 2002) display TS in response to repeated input from C-nociceptors, including heat-pulses like those used in the present study. Because spinothalamic tract neurons display robust ‘windup’, it is highly likely that ‘windup’ would be reflected in the target areas to which these neurons project. This prediction is consistent with results of our present study.
4.3 TSSP-Evoked Activity and the Pain Matrix
Our experiments showed that neural activity reflective of TS was not confined to somato-sensory pathways, including bilateral S1, but extended to brain regions involved in behavioral responses associated with pain. These regions included brain areas involved in pain-affect (ACC, INS, PFC, SMA), pain-modulation (rACC), and motor-preparation (SMA, cerebellum, caudate). TS of C-fiber evoked neural responses appear to be relevant to most or all functions associated with pain. Since this type of pain-summation is integrally related to relevant mechanisms of central sensitization (Woolf, 1996), one would expect similar distributions of activity in conditions of central sensitization and hyperalgesia. Future studies comparing brain activation by TSSP in chronic pain patients and normal controls will be necessary to answer this question.
4.4 Brain Activity Related to Pain Ratings
Our second analysis included a GLM-regression of brain activity on pain ratings within a single condition (e.g., 6-pulse train at 0.33 Hz). The variability in pain ratings across several trials of a single condition may partly result from factors other than the encoding of pain sensation and resultant affect. Kong et al (2005) recently characterized a brain network that reflects the cognitive evaluation required for mapping the nociceptive experience into semantic constructs such as words or a numbers. Our regression analysis shows a network of brain areas that is significantly correlated with pain ratings, including bilateral anterior-to-mid-INS cortex, dorsal-ACC (BA 24, 32), SMA, bilateral lateral-PFC, and caudate/putamen. These areas overlap with those described by Kong et al. (2005) to be involved in cognitive evaluation of pain and only partially overlap with areas likely to be involved in the encoding of pain. This partial overlap can be appreciated by comparing the results of contrast analyses (Figure 3) with pain-rating regression analyses (Figure 4). For example, whereas the contrast analysis across conditions (Figure 3) shows ventral (BA 24a and 24 b) as well as dorsal-ACC activation (BA 24b), the pain-rating regression analysis (Figure 4) depicts significant associations only in the dorsal-ACC (BA 24 and 32). Both sets of analyses identify significant regions in anterior-to-mid-INS and S2-cortex (Figures 3–4). Thus, similar to Kong et al. (2005), our results provide evidence for a distinction between brain activity involved in the encoding of pain dimensions (i.e., the sensation and feeling of pain) and brain activity involved in evaluative and other components not directly related to the subjective experience of pain itself (e.g., representing the pain dimensions by a word or number). Thus the combination of contrast analyses and pain-rating regressions bolster confidence that our study was able to characterize the brain regions that encode TSSP.
4.5 Limitations
Most but not all brain areas activated during TSSP-encoding were also detected during pain ratings. The best examples are S1 and PPC which showed robust activation during TSSP-encoding, yet were not identified in the pain rating regression analysis. These negative results, however, should not be interpreted that these brain areas are irrelevant for cognitive evaluations of pain. Evidence that S1 is involved in cognitive evaluations of pain was provided by a recent fMRI study (Moulton et al., 2005), which showed S1-activation during painful stimuli as well as several seconds later when pain ratings were made. Therefore the absent association of S1 with pain ratings in our study is puzzling but may be the result of our study design. To reduce pain rating variability we equated peak pain intensity across subjects in the 0.33 Hz 6-pulse trains. This procedure may not only have reduced variability in pain ratings but also variability in S1-activation. The observed activation in other hypothesized brain areas (e.g. dorsal-ACC) may have occurred as a result of increased variability secondary to cognitive evaluations and the production of pain ratings.
5. Conclusions
Our study provides evidence that brain activations associated with second pain and TSSP occur within widely distributed brain areas involved in somato-sensory processing, pain-related affect, pain modulation, and pain-related pre-motor activity. Furthermore, because spinal correlates of TSSP (‘windup’) reflect early mechanisms of central sensitization, this widespread pattern of activation may also occur in hyperalgesic states that are so characteristic for persistent pain conditions.
Table 4. Brain Regions of Interest (ROI) Related to Variability in Pain Ratings During TSSP.
| Brain regions | ROI Volume (μL) | Coordinates | ||
|---|---|---|---|---|
| x | y | z | ||
| Superior Frontal Gyrus L (SMA, ACC, Motor Cortex, BA 4,6, 24, 32) | 9460 | 0 | 13 | 50 |
| Middle Frontal Gyrus L Lateral PFC, BA 46 | 3068 | −40 | 37 | 15 |
| Middle Frontal Gyrus R Lateral PFC, BA 10 | 5262 | 35 | 42 | 19 |
| Anterior Insula L BA 13 | 5281 | −35 | 14 | 10 |
| Anterior Insula R BA 13 | 7864 | 31 | 13 | 11 |
| Caudate/Putamen/Lentiform Nucleus L | 6263 | −17 | 0 | 12 |
| Caudate/Putamen/Lentiform Nucleus R | 7780 | 18 | 4 | 11 |
| Thalamus, Medial Dorsal Nucleus L | 7708 | −12 | −18 | 12 |
| Thalamus, Medial Dorsal Nucleus R | 7100 | 10 | −16 | 12 |
| Midline Periaqueductal Grey | 1938 | −3 | −29 | −15 |
| Cerebellum | 3394 | −4 | −53 | −24 |
| Posterior Cingulate, BA 23 | 1316 | 1 | −32 | 29 |
| Inferior Parietal Lobule, S2, BA 40 | 647 | −53 | −32 | 26 |
all p ≤ 0.005
Acknowledgments
Supported by NIH grant NS-38767 and the American Fibromyalgia Syndrome Association (AFSA). The expert technical assistance of Myriam M. Lopez and Xeve S. Silver is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendt-Nielsen L, Petersen-Felix S, Fischer M, Bak P, Bjerring P, Zbinden AM. The effect of N-methyl-D-aspartate antagonist (ketamine) on single and repeated nociceptive stimuli: a placebo-controlled experimental human study. Anesth Analg. 1995;81:63–68. doi: 10.1097/00000539-199507000-00013. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. In: Pichot P, editor. Psychological measurements in psychopharmacology. Basel; Karger: 1974. pp. 151–169. [Google Scholar]
- Carpenter R. Neurophysiology. London: Hodder Arnold; 2002. pp. 1–320. [Google Scholar]
- Casey KL, Bushnell MC. Progress in Pain Research and Management. Vol. 18. Seattle: IASP Press; 2000. Pain Imaging. [Google Scholar]
- Chudler EH, Dong WK, Kawakami Y. Cortical nociceptive responses and behavioral correlates in the monkey. Brain Res. 1986;397:47–60. doi: 10.1016/0006-8993(86)91368-5. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Anton F, Dubner R, Kenshalo DR., Jr Responses of nociceptive SI neurons in monkeys and pain sensation in humans elicited by noxious thermal stimulation: effect of interstimulus interval. J Neurophysiol. 1990;63:559–569. doi: 10.1152/jn.1990.63.3.559. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60:3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- Chung JM, Kenshalo DR, Jr, Gerhart KD, Willis WD. Excitation of primate spinothalamic neurons by cutaneous C-fiber volleys. J Neurophysiol. 1979;42:1354–1369. doi: 10.1152/jn.1979.42.5.1354. [DOI] [PubMed] [Google Scholar]
- Craig AD, Andrew D. Responses of spinothalamic lamina I neurons to repeated brief contact heat stimulation in the cat. J Neurophysiol. 2002;87:1902–1914. doi: 10.1152/jn.00578.2001. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Differential effects of excitatory amino acid antagonists on dorsal horn nociceptive neurones in the rat. Brain Res. 1990;506:31–39. doi: 10.1016/0006-8993(90)91195-m. [DOI] [PubMed] [Google Scholar]
- Dubner R. Neuronal plasticity and pain following peripheral tissue inflammation or nerve injury. In: Bond M, Charlton E, Woolf CJ, editors. Proceedings of Vth World Congress on Pain. Pain Research and Clinical Management. Amsterdam: Elsevier; 1991. pp. 263–276. [Google Scholar]
- Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- Granovsky Y, Matre D, Sokolik A, Lorenz J, Casey KL. Thermoreceptive innervation of human glabrous and hairy skin: a contact heat evoked potential analysis. Pain. 2005;115:238–247. doi: 10.1016/j.pain.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Laird JMA, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hobson AR, Furlong PL, Worthen SF, Hillebrand A, Barnes GR, Singh KD, Aziz Q. Real-time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterology. 2005;128:610–619. doi: 10.1053/j.gastro.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates, Inc.; 2004. [Google Scholar]
- Kenshalo DR, Jr, Chudler EH, Anton F, Dubner R. SI nociceptive neurons participate in the encoding process by which monkeys perceive the intensity of noxious thermal stimulation. Brain Res. 1988;454:378–382. doi: 10.1016/0006-8993(88)90841-4. [DOI] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Hum Brain Mapp. 2005. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Dubner R, Bushnell MC, Kenshalo DR, Jr, Oliveras JL. Wide-dynamic-range dorsal horn neurons participate in the encoding process by which monkeys perceive the intensity of noxious heat stimuli. Brain Res. 1986;374:385–388. doi: 10.1016/0006-8993(86)90435-x. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional Intensive and Temporal Patterns of Functional MRI Activation Distinguishing Noxious and Innocuous Contact Heat. J Neurophysiol. 2005;93:2183–2193. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling Between Neuronal Firing, Field Potentials, and fMRI in Human Auditory Cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Ploner M, Gross J, Timmermann L, Schnitzler A. Cortical representation of first and second pain sensation in humans. PNAS. 2002;99:12444–12448. doi: 10.1073/pnas.182272899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp Neurol. 1972;37:371–387. doi: 10.1016/0014-4886(72)90081-7. [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Price DD, Hayes RL, Ruda M, Dubner R. Neural representation of cutaneous aftersensations by spinothalamic tract neurons. Fed Proc. 1978a;37:2237–2239. [PubMed] [Google Scholar]
- Price DD, Hayes RL, Ruda M, Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol. 1978b;41:933–947. doi: 10.1152/jn.1978.41.4.933. [DOI] [PubMed] [Google Scholar]
- Price DD, Hayashi H, Dubner R, Ruda MA. Functional relationships between neurons of marginal and substantia gelatinosa layers of primate dorsal horn. J Neurophysiol. 1979;42:1590–1608. doi: 10.1152/jn.1979.42.6.1590. [DOI] [PubMed] [Google Scholar]
- Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological mechanisms of pain and analgesia: Progress in pain research and management. Seattle: IASP Press; 1999. pp. 1–248. [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Noguchi Y, Honda M, Nakata H, Tamura Y, Tanaka S, Sadato N, Wang X, Inui K, Kakigi R. Brain Processing of the Signals Ascending Through Unmyelinated C Fibers in Humans: An Event-Related Functional Magnetic Resonance Imaging Study. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj071. in press. [DOI] [PubMed] [Google Scholar]
- Robinson ES. Work on the integrated organism. In: Murchinson C, editor. A Handbook of General Experimental Psychology. Worcester, MA: Clark University Press; 1934. pp. 571–650. [Google Scholar]
- Sarlani E, Greenspan JD. Why look in the brain for answers to temporomandibular disorder pain? Cells Tissues Organs. 2005;180:69–75. doi: 10.1159/000086200. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (STAI) (Self Evaluation Questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–696. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Robinson ME, Price DD. Effects of the NDMA receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal controls. J Pain. 2005;6:323–332. doi: 10.1016/j.jpain.2005.01.357. [DOI] [PubMed] [Google Scholar]
- Staud R, Price DD, Fillingim RB. Advanced continuous-contact heat pulse design for efficient temporal summation of second pain (wind-up) J Pain. 2006;7:575–582. doi: 10.1016/j.jpain.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thompson SW, Woolf CJ. Primary afferent-evoked prolonged potentials in the spinal cord and their central summation: role of the NMDA receptor. In: Bond MR, Woolf CJ, editors. Proceedings of the VI. World Congress of Pain. Amsterdam: Elsevier; 1990. pp. 291–298. [Google Scholar]
- Tommerdahl M, Delemos KA, Vierck CJ, Favorov OV, Whitsel BL. Anterior parietal cortical response to tactile and skin-heating stimuli applied to the same skin site. J Neurophysiol. 1996;75:2662–2670. doi: 10.1152/jn.1996.75.6.2662. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Delemos KA, Favorov OV, Metz CB, Vierck CJ, Whitsel BL. Response of anterior parietal cortex to different modes of same-site skin stimulation. J Neurophysiol. 1998;80:3272–3283. doi: 10.1152/jn.1998.80.6.3272. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Hallin RG. Identification of afferent C units in intact human skin nerves. Brain Res. 1974;67:387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- Whitsel BL, Tommerdahl M, Kohn A, Vierck CJ, Favorov O. The S1 response to noxious skin heating by optical intrinsic signal imaging. In: Casey KL, Bushnell MC, editors. Pain Imaging, Progress in Pain Research and Managment. Vol. 18. Seattle: IASP Press; 2000. pp. 47–93. [Google Scholar]
- Woodworth RS, Schlosberg H. Woodworth & Schlosberg’s experimental psychology. New York: Holt, Rinehart and Winston; 1971. pp. 1–1296. [Google Scholar]
- Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]


