Abstract
CpG oligodeoxynucleotides are potent immunostimulants. In this study, CPG 7909 was formulated with the recombinant Plasmodium falciparum protein AMA1-C1 adsorbed to Alhydrogel (aluminum hydroxide) and used to immunize mice. Mice receiving free CPG 7909 in a separate same site injection to the AMA1-C1/Alhydrogel had the same antibody responses as mice receiving AMA1-C1/Alhydrogel alone. For mice immunized with CPG 7909 bound to the AMA1-C1/Alhydrogel formulation, there was a bell shaped CPG 7909 dose response curve with the highest antibody response co-incident with the concentration of CPG 7909 that saturated binding to the Alhydrogel. At a higher CPG 7909 dose where 74% was unbound, there was no enhancement of response over AMA1-C1/Alhydrogel alone. Our results suggest that the adjuvant effects of CpGs are optimal when adsorbed to Alhydrogel and highlight the need for careful characterization of the vaccine formulation.
1. Introduction
CpGs are oligodeoxynucleotides (ODN) containing unmethylated CpG dinucleotide motifs that possess immunostimulatory properties and are potentially useful as adjuvants [1]. In the first study to describe their action, CpG motifs in bacterial DNA and synthetic ODN were found to enhance B-cell activation in mice [2]. Subsequent studies showed that, in mammals, the immune enhancement is mediated by binding of the CpG ODN to Toll-like receptor 9 (TLR9) found on B cells and, depending on the species, a variety of antigen presenting cells. The interaction of TLR9 with CpG motifs initiates a cascade of events resulting in the activation of B cells and secretion of T helper (Th)1-type cytokines and chemokines [3]. In animal studies, CpG immunostimulation was more efficient if the CpG ODN is chemically coupled to the antigen [4] suggesting that simultaneous activation of a cell by both antigen and CpG is required for optimal effect.
One CpG ODN, designated ODN 2006, is a 24-mer that contains three CpG motifs (5′-GTCGTT-3′) and has been selected for human use although it stimulates immune responses in a wide range of animals including primates [5], mice, rats and guinea pigs [6]. It is named CPG 7909 or VaxImmune™ and is produced by Coley Pharmaceutical Group. This ODN contains a phosphorothioate backbone, making it resistant to nuclease attack and increasing its in vivo half-life.
In the first Phase I/II vaccine trial of CPG 7909, it was added to a non-adjuvanted influenza vaccine [7]. In this trial CPG 7909 did not significantly enhance antibody production. However, in a Phase I trial of CPG 7909 with an alum-based hepatitis B vaccine, Engerix-B®, in healthy Canadian adult volunteers, the vaccine gave substantially higher antibody responses compared with Engerix-B® alone [8]. The addition of CPG 7909 significantly increased antigen-specific antibody titers and enhanced the avidity maturation of IgG1 to hepatitis B surface antigen [9]. In a second Phase 1 trial with Engerix-B®, CPG 7909 was able to stimulate antibody response in immuno-compromised HIV infected recipients [10]. CPG 7909 is currently being tested in human Phase 1 vaccine trials with several other vaccine candidates, including the malaria antigens Merozoite Surface Protein 1 (MSP142) [11] and Apical Membrane Antigen 1 (AMA1) [12], both of which are adsorbed to aluminum hydroxide (Alhydrogel).
In this report, we compare the enhancement of antibody response to alum-based malaria vaccine candidates by CPG 7909 and show that the binding of CPG 7909 to the alum is critical.
2. Materials and Methods
2.1. CPG 7909
CPG 7909 (Coley Pharmaceutical Group, Wellesley, MA) has a phosphorothioate backbone and the sequence 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′. Clinical lot 207-03-002, a gift under Clinical Trials Agreement from Coley, was supplied as 10 mg/ml in 6 mM monobasic sodium phosphate, 94 mM dibasic sodium phosphate, 154 mM sodium chloride.
2.2. Vaccine formulations
AMA1-C1 is an equal mixture (by mass) of two recombinant allelic forms of P. falciparum apical membrane antigen 1 (FVO and 3D7 clones) expressed in Pichia pastoris. Synthetic gene design, recombinant protein expression, and purification have been described [13, 14]. All vaccines were formulated to contain 30 ng of AMA1-C1 and 80 μg of Alhydrogel (1.6 mg/ml Al2O3) per 50 μL dose. Various amounts of CPG 7909 were added (Table 1). After mixing, formulations were immediately placed on ice at 0°C until injected. Vaccines containing CpG were used within 3 h of formulation. In previous studies, we showed that 30 ng AMA1-C1/Alhydrogel in female BALB/c mice generated responses that lie on the steep part of the dose-response curve [15].
Table 1.
AMA1-C1/Alhydrogel and CpG formulations, doses, and percent CpG bound
| Groupc | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| 1st injectiona | PBS | 0.1 μg
CpG |
0.3 μg
CpG |
1 μg
CpG |
3 μg
CpG |
12.5 μg
CpG |
50 μg
CpG |
||||||
| 2nd injectionb | AMA1-C1/Alhydrogel | AMA1-C1/Alhydrogel/0.1μg CpG | AMA1-C1/Alhydrogel/0.3 μg CpG | AMA1-C1/Alhydrogel/1 μg CpG | AMA1-C1/Alhydrogel/3 μg CpG | AMA1-C1/Alhydrogel/12.5 μg CpG | AMA1-C1/Alhydrogel/50 μg CpG | AMA1-C1/Alhydrogel | |||||
| CpG bound | - | 100 % | 26%d | 0 % | |||||||||
1st injections contained 20 μl of various amounts of CpG or PBS.
2nd injections contained 30 ng AMA1-C1, 80 μg Alhydrogel, and various amounts of CpG or PBS in 50 μl doses and were administered 30 min after the 1st injection in the same site.
10 female BALB/c mice were used in each group.
26% of the CpG was bound in the day 0 formulation, and 35% was bound in the day 29 formulation.
2.3. CPG 7909 analysis by spectrophotometry
To calculate the amount of CpG not bound to Alhydrogel, the vaccine formulations were centrifuged to pellet the Alhydrogel, and the CpG concentration in the supernatant was quantified by measuring absorbance at 260 nm (A260) with an Ultrospec 3300 Pro UV/Visible Spectrophotometer (Amersham Pharmacia Biotech, Piscataway, NJ). Samples were diluted 1:25 in saline and the readings compared with those of a 40 μg/ml reference solution of CpG.
2.4. Comparative immunogenicity of vaccines containing bound or unbound CPG 7909
All animal care and handling was performed in accordance with National Institutes of Health guidelines and with Animal Care and Use Committee-approved protocols. On day 0, groups of 10 female BALB/c mice (Taconic, Germantown, NY) were immunized intramuscularly in the anterior tibialis with 0.02 ml of CPG 7909 or 1× PBS (see Table I). A second immunization was administered 30 min later in the same site, targeting both injections to the same draining lymph nodes. On day 29, each mouse received the same injections it had on day 0 (Table I). Thus, some mice received CpG that was completely or partially bound to Alhydrogel, while others received CpG that was completely unbound (assured by a separate injection). Each of the six formulations containing CpG (Groups 2-7, Table I) was analyzed by spectrophotometry to assess CpG binding. Sera were collected on days 29 and 43 and analyzed for total antigen-specific IgG by ELISA as previously described [16]. Briefly, ELISA plates were coated with 1 μg/ml of either AMA1-FVO or AMA1-3D7 overnight at 4°C, blocked at room temperature and the mouse sera added. Diluted sera were compared with standard curves of reference antisera on each ELISA plate, and antibody units for each mouse were defined as the reciprocal dilution that gave an optical density (OD) of 1.0 at 405 nm in this standardized, qualified ELISA.
IgG subclass determination was performed by ELISA. Briefly, ELISA plates were coated with 1 μg/ml AMA1-FVO overnight at 4°C, blocked at room temperature, and the mouse sera added. These were normalized by adding the dilution of each serum that gave an optical density of 3 in the standard, antigen-specific total IgG ELISA. The secondary goat anti-mouse IgG-specific subclass (IgG1, IgG2a, IgG2b, and IgG3) alkaline phosphatase conjugates (Southern Biotechnology Associates, Inc., Birmingham, AL) were normalized by adding the dilution of each that gave an OD of 2 in a direct ELISA on specific IgG subclass-coated plates. In this manner, the level of each IgG subclass in a serum sample was compared, and animals were compared to each other.
Statistically significant differences between groups were determined by the Mann-Whitney U test for two groups or by Kruskal-Wallis One-Way ANOVA for multiple groups, with p values < 0.05 considered significant. Dose-response relationships were determined by Spearman rank correlation, with SRC values > 0 and p values < 0.05 considered significant.
3. Results
3.1. Antibody response with unbound CPG 7909
In mice receiving increasing quantities of unbound CPG 7909 followed by AMA1-C1/Alhydrogel in separate injections in the same site, no CPG 7909 dose-response relationship was evident (day 43 sera, SRC -0.1921, p=0.0569) (Fig. 1A). Moreover, antibody responses in these groups were similar to the group that received AMA1-C1/Alhydrogel alone (Kruskal-Wallis One-Way ANOVA, p=0.1581).
Fig. 1.
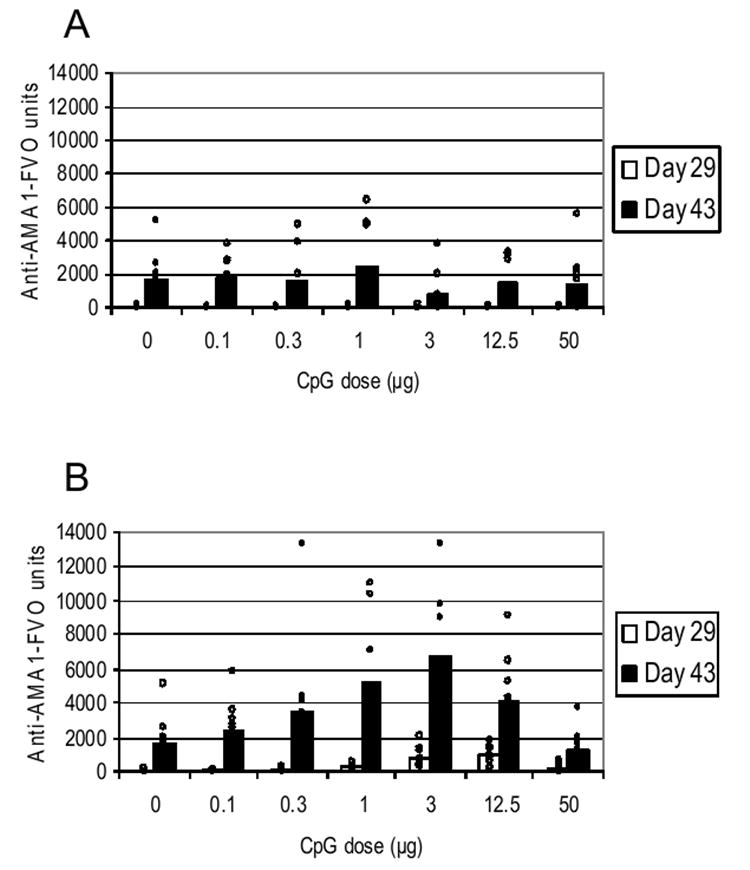
Antibody responses in mice to AMA1-C1/Alhydrogel vaccines containing 30 ng AMA1-C1 and various amounts of unbound (A) or bound (B) CPG 7909 per dose. The formulation containing 50 μg of partially bound CpG (B) consisted of 26% and 35% bound CpG for the day 0 and 28 immunizations, respectively (determined by spectrophotometry). Arithmetic mean antibody in the groups of 9-10 mice were determined (bars). Individual mice are indicated as small circles.
3.2. Antibody response with CPG 7909 bound to the AMA1-C1/Alhydrogel
By spectroscopy, there was no detectable unbound CPG 7909 in formulations containing 0.1 to 12.5 μg CpG. The formulation containing 50 μg of CpG had approximately 25% (12.5 μg) of the CpG bound.
Unlike the mice receiving free CPG 7909, mice receiving the CPG 7909 bound to alum had a significant CPG 7909 dose-response relationship as determined by serum antibody ELISA on AMA1-FVO-coated plates in both day 29 sera (SRC 0.8454, p<0.0001) and day 43 sera (SRC 0.5222, p<0.0001) (Fig. 1B). In day 29 sera (post-primary immunization), the highest average antibody response occurred in mice that received 12.5 μg of 100% bound CpG per dose (although this was not statistically different from those receiving 3 μg, Mann-Whitney U test, p=0.5787). In day 43 sera (2 weeks post-secondary), however, the maximal average response shifted to 3 μg of bound CpG, and the dose-response plateaued with no significant difference between the 1, 3, and 12.5 μg bound CpG groups (Kruskal-Wallis One-Way ANOVA, p=0.2366).
After either 1 or 2 immunizations, the highest dose of CpG (50 μg), which was partially bound to Alhydrogel, gave rise to significantly less antibody compared with the 12.5 μg dose group (Mann-Whitney U tests, day 29 p=0.0021; day 43 p=0.0015). After 2 doses of 50 μg of partially bound CpG, antibody responses were no greater than those with Alhydrogel alone (Mann-Whitney U test, p=0.5288).
IgG isotyping was performed to determine the levels of IgG1 (indicative of a Th2-type response and expected from aluminum hydroxide) and IgG2a, IgG2b, and IgG3 (indicative of a Th1-type response and expected from CpG ODN) in selected groups of post-secondary immune sera. The average ODs of the IgG subclasses for each group are shown in Fig. 2. IgG1 levels are highest in each group. In mice receiving vaccine with bound or partially bound CpG, levels of IgG2a and 2b increased over those receiving no CpG. The group receiving 3 μg of bound CpG developed the highest total antigen-specific IgG response (Fig. 2), but surprisingly, the 12.5 μg bound CpG group produced the highest levels of IgG2a and 2b.
Fig. 2.
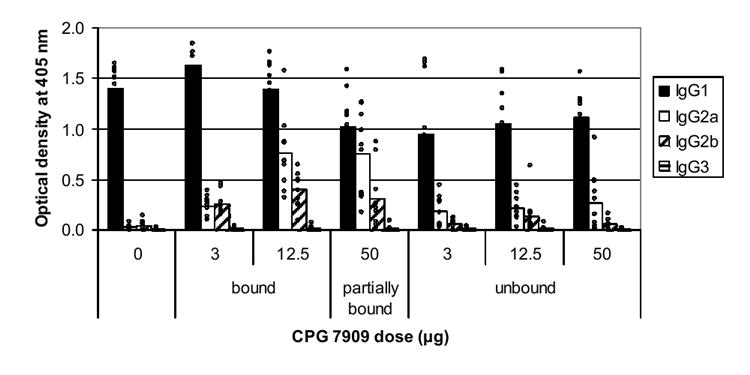
IgG subclass determination in sera from selected groups of mice. AMA1-C1/Alhydrogel vaccines contained various amounts of unbound, partially bound or bound CPG 7909. Anti-AMA1-FVO-specific IgG1, IgG2a, IgG2b, and IgG3 levels were measured by ELISA using normalized sera and secondary antibody conjugates. Bars represent the arithmetic means of optical density readings of day 43 sera from groups of 9-10 mice with values for individual mice indicated with small circles.
Although mice that received either unbound CPG 7909 or the partially bound CPG 7909 in the 50 μg group made the same total amount of antibody as mice that received no CPG 7909, there was still some switch towards Th1-type antibodies although the Th1 responses were generally less than those seen with completely or partially bound CpG (Fig.2).
4. Discussion
For the AMA1-C1/Alhydrogel formulations tested by intramuscular injection in mice, no enhancement of total antigen specific IgG response was seen unless the CpG ODN was bound to the alum. Four weeks after a single injection, the peak antibody response was co-incident with the maximum dose of CpG ODN (12.5 μg) that was bound to the alum. Two weeks following a second immunization, the peak response was much broader: CPG 7909 doses between 3 and 12.5 μg gave similar responses. The decreased response at higher CPG 7909 doses was striking. At 50 μg, even though the vaccine contained the same 12.5 μg of bound CpG, there was no increase in total antigen specific IgG relative to the vaccine with no CpG. Apparently the 37.5 μg of free CPG 7909 was deleterious.
As expected from the known Th1 bias in the response to CpG containing adjuvants, mice receiving AMA1-C1/Alhydrogel with bound CPG 7909 not only had an increased total antigen specific IgG response, but also a marked increase in IgG2a and IgG2b, indicative of a Th1-type response compared to the animal that did not receive any CPG. In mice receiving unbound CpG or a 50 μg dose containing both bound and unbound CpG, there was a significant increase in IgG2 production even though there was no detectible increase in total antigen specific IgG. These results suggest that the free CpG had some impact on the nature of the immune response, although it was diminished compared to the bound CpG.
These studies were designed only to look for the impact of CPG 7909 on antibody levels and not for the ability of the antibody to kill parasites since other studies conducted with recombinant AMA1, in both mice and humans, have shown that the ability of the resulting anti-AMA1 antibodies to inhibit the grown of parasites in vitro depends only on the total anti-AMA1 antibody and not on the relative levels of different subclasses (Mullen et al, unpublished). However, for other vaccines, the possibility exists that not only will binding of CpG to alum affect total antibody, but it may additionally impact on the ability of the resulting antibodies to kill their target.
Importantly these results highlight the need for a physical association of the CpG and antigens for optimal effect. In humans, CPG 7909 has substantially boosted antibody response with Hepatitis B Surface Antigen [17, 18] and with AMA1 (Mullen et al, unpublished) and MSP142 (Martin et al, unpublished) when these antigens were formulated with alum, but not with the un-adjuvanted influenza vaccine [19]. The mouse data presented in this paper are consistent with these human studies, and both are consistent with studies that show a substantial enhancement of antibody production with CpG covalently linked to the antigen [20].
It is unclear if the deleterious impact of free CpG on the immunostimulation of bound CpG seen in this study will also be a problem in human vaccines since at the doses used, the amount of free CpG in the mice was several orders higher than could be achieved in humans when used at the recommended dose of approximately 500 μg. Nevertheless, these data show that for alum based vaccines, since the effective CpG dose may be related to the bound concentration, it will be important to carefully optimize and characterize the binding of CpG to the alum in order to optimize the immunogenicity and to be able to fully interpret the resulting immune responses.
Acknowledgments
We are grateful to Cheryl Kothe and Brian Keegan for providing excellent animal care, handling and manipulations and to Heather L. Davis, Coley Pharmaceutical Group, for providing clinical grade CPG 7909.
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201–16. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 2.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 3.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 4.Tighe H, Takabayashi K, Schwartz D, et al. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30(7):1939–47. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann G, Weeratna RD, Ballas ZK, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164(3):1617–24. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 6.Mullen GE, Giersing BK, Ajose-Popoola O, et al. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24(14):2497–505. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004;22(2324):3136–43. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 8.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 9.Siegrist CA, Pihlgren M, Tougne C, et al. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine. 2004;23(5):615–22. doi: 10.1016/j.vaccine.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19(14):1473–9. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Kennedy MC, Long CA, Saul AJ, Miller LH, Stowers AW. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect Immun. 2003;71(12):6766–74. doi: 10.1128/IAI.71.12.6766-6774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malkin EM, Diemert DJ, McArthur JH, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73(6):3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malkin EM, Diemert DJ, McArthur JH, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73(6):3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy MC, Wang J, Zhang Y, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70(12):6948–60. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullen GE, Giersing BK, Ajose-Popoola O, et al. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24(14):2497–505. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Miles AP, McClellan HA, Rausch KM, et al. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine. 2005;23(19):2530–9. doi: 10.1016/j.vaccine.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 18.Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19(14):1473–9. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 19.Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004;22(2324):3136–43. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 20.Tighe H, Takabayashi K, Schwartz D, et al. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30(7):1939–47. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]


