Abstract
Stimulant-dependent individuals (SDI) have abnormal brain metabolism and structural changes involving dopaminergic target areas important for the processing of time. These individuals are also more impulsive and impaired in working memory and attention. The current study tested whether SDI show altered temporal processing in relation to impulsivity or impaired prefrontal cortex functioning. We employed a series of timing tasks aimed to examine time processing from the milliseconds to multiple seconds range and assessed cognitive function in 15 male SDI and 15 stimulant-naïve individuals. A mediation analysis determined the degree to which impulsivity or executive dysfunctions contributed to group differences in time processing. SDI showed several abnormal time processing characteristics. SDI needed larger time differences for effective duration discrimination, particularly for intervals of around 1 sec. SDI also accelerated finger tapping during a continuation period after a 1 Hz pacing stimulus was removed. In addition, SDI overestimated the duration of a relatively long time interval, an effect which was attributable to higher impulsivity. Taken together, these data show for the first time that SDI exhibit altered time processing in several domains, one which can be explained by increased impulsivity. Altered time processing in SDI could explain why SDI have difficulty delaying gratification.
Keywords: Methamphetamine, Cocaine, Dopamine, Temporal processing, Working memory, Impulsivity
1. Introduction
Cocaine and amphetamine-like drugs are psychostimulants which alter the dopaminergic system, e.g. by blocking the dopamine transporter (White and Kalivas, 1998) or releasing of dopamine via reverse transport (Sulzer et al., 2005). Methamphetamine (METH), a compound structurally similar to amphetamine also affects the dopamine system, e.g. it leads to downregulation of striatal D2 dopamine receptors (Chang and Haning, 2006) and dopamine transporters in the striatum (McCann et al., 1998), orbitofrontal cortex, and dorsolateral prefrontal cortex (Sekine et al., 2003). Although cocaine and amphetamines have different pharmacodynamic properties, both substances reduce the availability of D2 receptors, which is thought to alter the individual's ability to perform reward-related behaviors (Volkow et al., 2001a).
The dopamine system and its target neural substrates, e.g. the striatum and the prefrontal cortex, are an important neural system for the modulation of time perception and the timing of motor acts. Patients with structural damage such as focal brain lesions to the frontal lobes (Kagerer et al., 2002; Nichelli et al., 1995; von Steinbüchel et al., 1999) or traumatic brain injury predominantly affecting frontal areas (Pouthas and Perbal, 2004) can show substantial impairments in the estimation of temporal intervals. Patients with Parkinson's disease, who have decreased dopaminergic function in the basal ganglia, show deficits in motor timing as well as in duration discrimination (Hellström et al., 1997; O'Boyle et al., 1996). Neuroimaging studies have shown that temporal processing is associated with activation in right prefrontal regions (Rao et al., 2001; Rubia et al., 1998; Rubia and Smith, 2004). Other neuroimaging studies of timing have implicated a fronto-striatal network as the neural basis of the internal clock (Cuoll et al., 2004; Hinton and Meck, 2004; Nenadic et al., 2003). Pharmacological studies on animals and humans support the general hypothesis that fronto-striatal circuits are critical for temporal processing. Dopamine antagonists (like haloperidol) that affect the meso-striatal dopamine system slow down the clock rate in healthy subjects (Rammsayer, 1989; 1999) whereas timing behavior in animals and humans under the acute influence of METH can be interpreted as the result of speeding up the clock rate (Buhusi and Meck, 2002; Cevik, 2003; Mohs et al., 1980).
Functional neuroimaging and neuropsychological studies show that chronic stimulant users have significant brain activation changes in frontostriatal regions that are associated with impairments in attention, working memory, and decision making (Fein et al., 2002: McKetin and Mattick, 1997; Nordahl et al., 2003; O'Mally et al., 1992; Paulus et al. 2003; Salo et al., 2005). Stimulant-using individuals and stimulant-dependent individuals (SDI) show fundamental cognitive deficits and increased impulsivity when making decisions (Leland and Paulus, 2005; Fillmore and Rush, 2002; Paulus et al., 2002). Similar to other drug-dependent individuals, SDI show less self-control in decision making tasks as they discount future rewards more strongly than control subjects, that is, they tend to prefer smaller and sooner over larger but later rewards (Hoffmann et al., 2006; Kirby and Petry, 2004). Overall, the prefrontal cortex is strongly involved in executive functions such as attention regulation, the control of behavior and thoughts, and planning for the future (Arnsten and Li, 2005). These complex cognitive functions are intimately related to impulse control and are impaired in substance abusing individuals (Cardinal et al., 2004; Evenden, 1999; Kirby and Petry, 2004; Monterosso et al., 2006).
Although several cognitive dysfunctions have been reported in stimulant dependent individuals, it is not known whether these individuals have fundamental problems with temporal processing. An answer to this question would be important because many higher order cognitive functions dependent on intact temporal processing (von Steinbüchel and Pöppel, 1993). Thus, this is the first study to assess temporal processing in patients with a dependence on substances that have a neurotoxic effect on the dopamine system in fronto-striatal areas of the brain. We hypothesized that SDI would be affected in the accuracy and precision of duration perception and the timing of their behavior. Accuracy refers to the mean error in performance resulting from the difference between the physical duration and the subjective estimate of duration. Precision refers to the prevention of constant errors over trials, assessed by the variance in performance. We were especially interested in whether we would find specific effects of chronic stimulant use depending on the time intervals involved. Different temporal processing mechanisms seem to be involved for different time scales (Buhusi and Meck, 2005; Mauk and Buonomano, 2004; Wittmann, 1999). Temporal integration windows of around 250 to 500 ms (Rammsayer, 1999; Wittmann et al., 2001), of around 1 second (Madison, 2001) and for intervals up to 2 to 3 seconds (Fraisse, 1984; Pöppel, 1997; Wittmann et al., 2007) have been postulated. To probe temporal processing at a variety of time scales, from the milliseconds range to the seconds range, a set of tasks was employed assessing duration discrimination, temporal reproduction, time estimation, and paced finger tapping.
2. Materials and methods
2.1 Subjects
This study was approved by the University of California San Diego (UCSD) Institutional Review Board and all subjects provided written informed consent to participate. The SDI group consisted of 15 men meeting criteria for methamphetamine and/or cocaine dependence as determined by interview for DSM-IV diagnoses (SSAGA; Buchholz et al., 1994) and consensus of a team of experienced clinicians. The mean age of the SDI group was 42.3 ± 8.5 (range: 22-52 years), with a mean of 12.9 ± 1.24 years of education (range: 10-15). The SDI met criteria for current dependence when they voluntarily entered the 28-day inpatient Alcohol and Drug Treatment Program (ADTP) at the San Diego Veterans Affairs Medical Center. At time of testing, these subjects had been abstinent from stimulants for an average of 27.7 ± 5.6 days (range: 20-40). They were dependent on methamphetamine or cocaine for an average of 12.2 ± 8.5 years (range: 6 months-27 years) and had an estimated mean of 5373 ± 5858 lifetime uses of stimulants (range: 152-23036). Of the 15 stimulant-dependent subjects, 2 consumed cannabis but were not dependent, 2 fulfilled criteria for current alcohol dependence, 2 patients had past but not current alcohol dependence, and 11 were smokers.
15 normal comparison men formed the control group, aged 40.9 ± 8.8 years (range: 21-54) with 15.2 ± 1.25 years of education (range: 13-17). They were recruited via advertisement in local newspapers and the internet. The two groups did not differ in age (t(28) = −0.42, ns), but stimulant-dependent subjects were less educated than normal comparison subjects (t(28) = 4.9, p < .001). Of the 15 control subjects, none fulfilled criteria for past or current drug dependence and none were smokers.
Exclusion criteria for both stimulant-dependent and normal comparison subjects were: medication that affects the central nervous system; serious neurological or medical condition; history of serious head injury or unconscious periods lasting more than 30 min; current major depressive, bipolar, schizophrenic, posttraumatic stress, panic, or obsessive-compulsive disorder; lifetime history of antisocial personality disorder; acute signs of withdrawal as indicated by the presence of at least two DSM-IV withdrawal signs. In addition, urine toxicology was obtained for all subjects before testing and revealed no evidence of recent cannabis, amphetamine, sedative hypnotic, cocaine, or PCP use.
2.3.Temporal processing measures
2.3.1 Duration Discrimination
Auditory duration comparisons were conducted in two separate conditions using short (100 ms) and long (1000 ms) standard stimuli. The stimuli consisted of white noise presented via headphones at 80 dB SPL. The duration of the comparison stimulus ranged between 101 ms and 400 ms for the short condition and between 1005 ms and 2000 ms for the long condition. Subjects had to indicate which of the two presented stimuli was longer: the first or the second one. The duration of the stimuli varied according to the adaptive maximum-likelihood based algorithm YAAP (Treutwein, 1997) which takes into account the subjects' responses for the determination of the next duration of the comparison stimulus. Standard and comparison intervals were separated by a pause interval of 500 ms and their sequence was randomized. Stimulus presentation was stopped when a certain criterion for the estimation of the difference threshold was reached. Based on a logistic psychometric function the tracking procedure estimates a threshold corresponding to 75% correct duration discrimination. Stimulus presentation is terminated when the location of the true threshold lies with a probability of 95% within a confidence interval of ± 10 ms around the estimated threshold.
2.3.2 Temporal Reproduction
Subjects were instructed to reproduce the duration of standard tones that were presented at 70 dB SPL via headphones. Five different standard tone durations of 1000 ms, 2000 ms, 3000 ms, 4000 ms, and 5000 ms were used. Each was presented six times in random order, resulting in 30 trials per subject. Each trial consisted of two tones, starting with a 300 Hz tone presented for one of the standard durations. After that standard tone ended, there was a fixed pause of 1000 ms before onset of a 600 Hz tone. Subjects were instructed to reproduce the duration of the standard tone by pressing a key to switch off the second tone when they believed the same duration had elapsed. Mean duration of reproduced intervals and the coefficient of variation (CV) were calculated over the six trials per standard interval. Subjects were instructed not to count. It was stressed that the investigator was not interested in the subjects' counting abilities, but the subjective estimates of duration. Nevertheless, to further discourage counting, a non-temporal secondary task was employed. Before the presentation of the standard tone, two symbols were presented, which the subjects were told to memorize. After reproduction of the interval, a single symbol appeared on the screen and the subjects had to decide whether or not it was one of the former two. Stimulus presentation and response registration were controlled using a program created with the WinVis toolbox (Neurometrics Institute) for MATLAB (Version 5.3, MathWorks Inc.).
2.3.3 Paced Motor Timing
Through headphones subjects heard a regular sequence of 20 tones (500 Hz, duration: 50 ms) that had to be synchronized precisely by tapping the index finger repeatedly on a key (sensorimotor synchronization). Each sequence used one of three different inter-tone intervals for the pacer signal: 1000 ms, 2000 ms, and 4000 ms. In a separate condition (continuation tapping), subjects again synchronized their tapping to a sequence of tones, but after 10 tones the beat stopped and subjects were asked to continue tapping at the same tempo without the pacer signal. This continuation tapping was performed for another 40, 20, or 10 taps depending on the inter-tone interval for that trial (1000 ms, 2000 ms, or 4000 ms, respectively). A software program (Mates, 1990) controlled stimulus presentation and the registration of the inter-tap intervals (as a measure of tapping speed), as well as recording the asynchrony between the tone onset and the tap onset (as measure of accuracy in sensorimotor synchronization). Only when the asynchrony between tone and tap did not exceed a positive value of 120 ms, that is, when the tap followed the tone by no more than that amount of time, was the value taken as a synchronization trial (see below). Taps following a tone by 120 ms or more are most likely the result of motor commands initiated in response to, rather than in synchrony with, the tone and were operationally defined as reactions (see Wittmann et al., 2007). The number of such reactions per trial was calculated as a further measure of synchronization ability.
2.3.4 Time estimation
In a prospective time estimation task, subjects were instructed to estimate a temporal interval from an indicated moment to the offset of the interval as signaled by the bell of an alarm clock. The interval to be estimated lasted 53 seconds. We used the following instruction: “In a moment I am going to say ‘start’ and then after a while the alarm of the clock will ring. When it rings, please indicate how much time you think has gone by in seconds. Please try not to count in your head, but just estimate how much time you feel has gone by.” After the end of the interval subjects marked the estimated duration on a visual scale representing a time arrow covering a range from 0 to 3 minutes with bars indicating 1-seconds steps. With this procedure we wanted to reduce effects of whole number bias that can appear in verbal accounts of time intervals.
2.4. Measures of prefrontal cortical function and impulsivity
2.4.1 Attention and working memory
Computerized tasks were selected from the English version of the Test for Attentional Performance (TAP) battery (Zimmermann and Fimm, 1997), during which subjects react to a series of visual and auditory stimuli. In the phasic alertness task the difference in reaction time between cued and uncued runs gives an index of the phasic alertness response, with shorter reactions on cued runs associated with greater phasic alertness. In the focused attention task, arrows pointing left or right are presented left or right of fixation. The subject responds as quickly as possible with the hand corresponding to the direction in which the arrow is pointing. When pointing direction is incongruent with presentation side, reaction times are slowed. This slowing is a measure of distraction and inversely related to focused attention. In the 2-back working memory task, a series of 1-digit numbers is presented and the subject responds whenever a number shown is the same as the one displayed two numbers back in the sequence. In addition to these computerized tasks, we employed the Digit Span test (forward and backward) for a further measure of short-term/working memory. Subjects are asked to repeat 2, 3, 4, etc. digits forward and then in subsequent trials they have to repeat presented digits backwards. The experimenter continues to the next step with an increased number of digits only if the subject succeeds with at least one of two rows of digits at a given step.
2.4.2 Impulsivity
The Barratt Impulsiveness Scale (BIS-11) (Barratt et al., 1999) consists of 30 items that can be grouped into 3 subscales: Non-planning impulsivity (“I plan tasks carefully”, “I change jobs”), motor impulsivity (“I do things without thinking”, “I buy things on impulse”), and attention/cognition impulsivity (“I concentrate easily”, “I get easily bored when solving thought problems”) (Patton et al 1995).
2.5. Statistical analyses
As an index of temporal performance accuracy, the ratio theta (θ), was used, where θ = subjective / objective duration (Block et al. 2000). Subjective duration refers to the produced, reproduced, or estimated duration (as appropriate to the task) and objective duration similarly refers to the presented or actual time interval. A 1:1 ratio (i.e. theta = 1) indicates perfect accuracy. Depending on the timing task, ratios of > 1 and < 1 signify a longer and shorter reproduction of tones, a longer and shorter production of tapping intervals, and a longer and shorter estimate of the 53 seconds duration, respectively. For the duration discrimination task the Weber fraction Δt / t was calculated as an accuracy index, where Δt is the difference between the base interval t (100 ms, 1000 ms) and the length of the comparison interval at which the duration discrimination threshold was estimated. Statistical analyses were carried out on the mean accuracy index in each condition.
Analyses of covariance (ANCOVA) with ‘group’ as the main factor and ‘education’ as the covariate were performed for each interval to assess performance differences between stimulant-dependent individuals and control subjects. Because of different underlying timing processes, we tested each of the intervals separately. Alpha levels were set to p < 0.05. Bonferroni correction was used to protect against Type I error when multiple tests (different durations) for a given measure were applied.
For variables where we had clear predictions of effect directionality based on results from comparable patient populations (patients with damage to or dysfunction of fronto-striatal areas of the brain) we used one-tailed tests; otherwise we used two-tailed tests. For most performance measures we used one-tailed tests as we assumed SDI to be impaired in their timing precision (larger coefficients of variation) and accuracy (larger discrimination thresholds, stronger under-reproductions of intervals, larger asynchrony in sensorimotor synchronization, larger overestimation of the 53-seconds interval). Only for finger tapping in the continuation mode did we use a two-tailed test since we had no a priori expectation that SDI tapping would be slower versus faster.
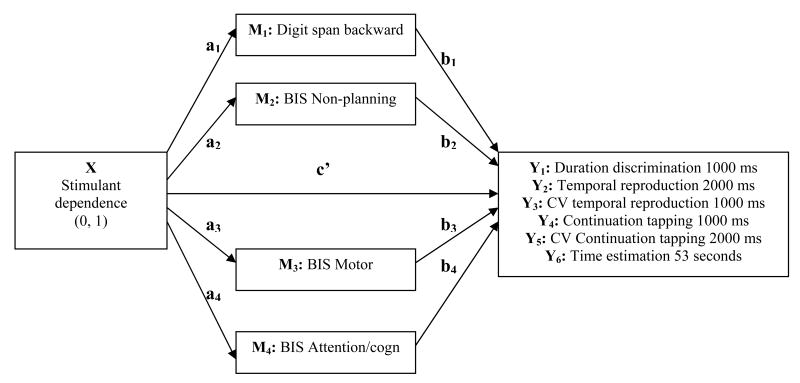
The effects that drug dependence have on the brain may not affect time perception directly (for example, by disturbing an internal clock) but rather indirectly via the influence of impaired attention, working memory and impulsivity – dimensions that are known to be influenced in SDI and also influence the experience of time. Therefore, we assessed the influence of possible indirect effects on time perception using mediator analyses. A variable may be said to function as a mediator to the extent that it accounts for the relation between the predictor (timing functions) and the criterion (group membership) (Preacher and Hayes, 2004). Specifically, a variable can be considered a mediator M if it carries the influence of a given independent variable X (in our case: stimulant dependence) to a given dependent variable Y (temporal processing). Based on effect analyses between the three components in question, X (drug dependence), Y (temporal processing), and M (prefrontal cortical function), we calculated whether X had a direct effect on Y or whether M mediated the effects of X on Y. In this model of multiple mediation effects (see Fig. 1), a, b, and c' represent the path coefficients for the effects of X → M, M → Y, and X → Y, respectively. The path coefficient c' represents the direct path, whereas a and b represent the indirect paths. The total effect c, that is, the initial effect of X on Y when mediators are not introduced in the model, is the sum of the indirect (a, b) and direct effect (c').
Figure 1. Model of multiple mediation effects.
The independent variable X (stimulant dependence) affects the dependent variable Y (temporal-processing measure) either directly (c') or indirectly (ab path) via the mediators M1 to M4.
We used a multiple mediator approach separately for each dependent variable Y since we had several possible factors that had to be accounted for in the model (Preacher and Hayes, submitted; SPSS macros available at: http://www.quantpsy.org). We included as mediators M1 through Mj the measures of prefrontal cortical function that differentiated SDI and controls. A bootstrapping method was part of the procedure to analyze indirect effects (n=5000, confidence intervals set at 95%). Bootstrapping methods offer a powerful method for obtaining confidence limits for specific indirect effects (MacKinnon et al., 2007). There are three possible outcomes of a mediation analysis. When the effect of X on Y reaches zero after the inclusion of M, complete mediation has occurred. When the effect of X on Y decreases by a certain amount but is still existent, partial mediation has occurred. In case that no decrease of the effect of X on Y is registered by including M into the model, no mediation at all has occurred (MacKinnon et al., 2007; Preacher & Hayes, 2004). The variable of education which was significantly lower in SDI than in control subjects was included as a covariate in the mediation models.
3. Results
3.1. Group differences
For the duration discrimination task, ANCOVAs revealed that stimulant users had a significantly higher Weber fraction (corresponding to a higher threshold) than controls at the 1000 ms interval duration [F(2,27) = 9.292, p < 0.0025], but the two groups did not differ in the 100 ms condition [F(2,27) = .361, p < 0.553] (see Table 1).
Table 1.
Performance differences in the temporal processing tasks between SDI and control subjects with education controlled for as a covariate.
| Temporal processing tasks | Stimulant-dependent individuals | Control subjects | F
df = 27 |
p |
|---|---|---|---|---|
| Mean | Mean | |||
| Duration discrimination | ||||
| Weber fraction Δt / t | ||||
| 100 ms | .440 | .315 | .361 | .553 |
| 1000 ms | .183 | .118 | 9.292 | .0025* |
| Temporal reproduction | ||||
| Mean reproduced interval [θ] | ||||
| 1000 ms | 1.016 | 1.076 | 2.440 | .075 |
| 2000 ms | .912 | 1.014 | 9.500 | .0025* |
| 3000 ms | .908 | .947 | .433 | .251 |
| 4000 ms | .827 | .916 | 1.311 | .131 |
| 5000 ms | .804 | .871 | .463 | .250 |
| CV reproduced interval | ||||
| 1000 ms | 21.6 | 11.3 | 10.531 | .0015* |
| 2000 ms | 15.9 | 8.6 | 2.364 | .075 |
| 3000 ms | 11.0 | 8.2 | 2.064 | .081 |
| 4000 ms | 11.3 | 7.4 | 1.186 | .143 |
| 5000 ms | 13.4 | 8.7 | 4.318 | .023 |
|
| ||||
| Sensorimotor Synchronization | ||||
| Mean Asynchrony [ms] | ||||
| 1000 ms | 2.60 | −34.0 | .159 | .346 |
| 2000 ms | −40.3 | −51.4 | 1.340 | .129 |
| 4000 ms | −169.8 | −144.8 | .275 | .303 |
| Missed synchronizations [%] | ||||
| 1000 ms | 17.4% | 9.5% | .065 | .400 |
| 2000 ms | 32.4% | 27.6% | .267 | .305 |
| 4000 ms | 60.6% | 46.3% | .144 | .303 |
|
| ||||
| Continuation Tapping | ||||
| Mean inter-tap interval [θ] | ||||
| 1000 ms | .938 | 1.036 | 5.497 | .0135* |
| 2000 ms | .960 | .991 | .309 | .291 |
| 4000 ms | .961 | 1.025 | .810 | .188 |
| CV inter-tap interval | ||||
| 1000 ms | 6.2 | 4.7 | 4.299 | .024 |
| 2000 ms | 7.6 | 4.9 | 7.605 | .005* |
| 4000 ms | 5.5 | 5.4 | .055 | .996 |
|
| ||||
| Time estimation (53 sec interval) | ||||
| Mean estimate [θ] | 1.513 | 1.122 | 3.984 | .0253* |
significant difference with initial alpha level of .05. Bonferroni-adjusted for multiple tests (duration discrimination: p < 0.025, temporal reproduction: p < 0.001, sensorimotor synchronization and continuation tapping: p < 0.0167, time estimation: p < 0.05)
In the temporal reproduction task a significant difference in timing accuracy between the groups was only revealed for the 2000 ms duration interval [F(2,27) = 9.500, p < 0.0025]. The difference in the θ value reflects the under-reproduction of that interval (θ = .912) in the group of stimulant-dependent individuals as compared to the slight over-reproduction in the controls (θ = 1.014). The analysis of the variability of temporal reproduction, an indicator of timing precision, revealed that SDI performed more variably relative to comparison subjects during the 1000 ms intervals [F(2,27) = 10.531, p < 0.0015].
Tempo matching did not differ between SDI and comparison subjects on the sensorimotor synchronization task, that is, neither the mean asynchrony between taps and tones differed between the SDI and the control group nor was there a group effect for the percentage of missed synchronizations. During the continuation phase of the continuation tapping task, when subjects were instructed to continue tapping at the same pace after the pacer signal stopped, SDI relative to comparison subjects tapped faster (θ = .938) during the continuation interval with a 1000 ms duration than controls (θ = 1.036) [F(2,27) = 5.497, p = 0.0135]. Examining the variability of tapping performance during the continuation phase revealed that SDI were more variable than comparison subjects in the 2000 ms interval [F(2,27) = 7.605, p = 0.005].
For the time estimation task (53 seconds), the ANCOVA revealed that SDI (90.8 sec, θ = 1.513) had significantly longer time estimates than the control subjects (67.0 sec, θ = 1.122) [F(2,27) = 3.984, p = 0.0253].
SDI did not differ from comparison subjects on phasic alertness, focused attention, or the 2-back working memory tasks (see Table 2). In the digit span forward task they also did not show group differences. However, in the digit span backward task SDI had significantly lower scores (7.93) than controls (10.13). SDI scored higher on all three BIS subscales non-planning impulsivity, motor impulsivity, and attention/cognitive impulsivity than control subjects.
Table 2.
Performance differences in the prefrontal cortex functioning tasks and the impulsivity scales between SDI and control subjects.
| Tasks of prefrontal cortex functioning and impulsivity scales | Stimulant-dependent individuals (SDI) | Control subjects | T
df = 27 |
p |
|---|---|---|---|---|
| Mean | Mean | |||
| Tasks for Assessing Attentional Performance | ||||
| Phasic alertness [index] | 0.031 | 0.019 | .456 | .150 |
| Focused attention [index] | 6.86 | 7.11 | .189 | .667 |
| 2-back working memory [N valid reactions] | 13.00 | 13.86 | 1.866 | .090 |
|
| ||||
| Digit span forward [points] | 7.93 | 10.13 | 1.995 | .085 |
| Digit span backward [points] | 5.67 | 9.13 | 4.377 | .023* |
|
| ||||
| Barratt Impulsivity Scale (BIS) | ||||
| Non-planning impulsivity | 28.4 | 20.7 | 13.933 | .0005* |
| Motor impulsivity | 28.6 | 20.2 | 10.646 | .002* |
| Attention/cognition impulsivity | 17.9 | 14.1 | 7.403 | .006* |
significant difference with initial alpha level of .05. Bonferroni-adjusted for multiple tests (Digit span: p < 0.025; BIS: p < 0.0167)
3.2. Mediation analyses
Group status (stimulant dependent versus stimulant-naïve) significantly affected the mediator variables (‘Effects of X on M’ in Table 3), which overall parallel the group differences found in the ANCOVAs. SDI scored lower on the digit span backward task and had higher impulsivity ratings (the BIS subscales Non-planning and Attention/cognition). In general, the mediation analyses revealed two types of results: the assessed psychological variables had no mediation effect on the timing tasks or a variable completely mediated the effects between X and Y (in one case).
Table 3.
Summary of mediation results with education controlled for as a covariate.
| Independent variable X | Mediating variable M1 - Mj | Dependent variable Y | Effect of X on M (a) | Effect of M on Y (b) | Indirect effect (ab) | Total indirect effect (ab) | Direct effect (c') | Total effect (c) | Degree of mediation |
|---|---|---|---|---|---|---|---|---|---|
| Stimulant dependent (1)
vs. non-dependent (0) subjects |
Digit span backward | Duration Discrimination Δt / t 1000 m | −2.45* | −.0048 | .0118 | .0058 | .091* | .097** | None |
| BIS Non-planning | 7.24*** | −.0018 | −.0133 | ||||||
| BIS Motor | .8225 | .0010 | −.0002 | ||||||
| BIS Attention/cognition | 7.37** | −.0003 | .0074 | ||||||
| Digit span backward | Reproduction interval θ 2000ms | −2.45* | −.0108 | .0265 | .0311 | −.195* | −.164** | None | |
| BIS Non-planning | 7.24*** | .0030 | .0214 | ||||||
| BIS Motor | .8225 | .0070 | .0057 | ||||||
| BIS Attention/cognition | 7.37** | −.0031 | −.0226 | ||||||
| Digit span backward | CV reproduction 1000ms | −2.45* | .1040 | −.2550 | −4.177 | 19.4** | 15.2** | None | |
| BIS Non-planning | 7.24*** | −1.371* | −9.926 | ||||||
| BIS Motor | .8225 | .2390 | .1966 | ||||||
| BIS Attention/cognition | 7.37** | .7883* | 5.808 | ||||||
| Digit span backward | Continuation tapping θ 1000ms | −2.45* | −.0037 | .0092 | .0539 | −.135* | −.081* | None | |
| BIS Non-planning | 7.24*** | .0034 | .0246 | ||||||
| BIS Motor | .8225 | .0008 | .0194 | ||||||
| BIS Attention/cognition | 7.37** | .0026 | .0007 | ||||||
| Digit span backward | Continuation tapping CV 2000ms | −2.45* | .0040 | −.0097 | .3742 | 3.107 | 3.48* | None | |
| BIS Non-planning | 7.24*** | .1047 | .7576 | ||||||
| BIS Motor | .8225 | −.1575 | −.1296 | ||||||
| BIS Attention/cognition | 7.37** | −.0331 | −.2441 | ||||||
| Digit span backward | Time estimation 53 sec θ | −2.45* | .0375 | −.0920 | .2015 | .3050 | .5065* | Complete | |
| BIS Non-planning | 7.24*** | .0559* | .4047* | ||||||
| BIS Motor | .8225 | −.0176 | −.0145 | ||||||
| BIS Attention/cognition | 7.37** | −.0131 | −.0966 |
significant coefficients: *p < 0.05, **p < 0.01, ***p < 0.001. Note that coefficients indicating effects are not standardized values. Significant effects correspond to t values ≥ 2.0.
Two BIS subscale scores affected time perception scores (Table 3, ‘Effect of M on Y’). The subscales of non-planning as well as attention/cognition impulsivity affect the variation in the 1000 ms reproduction task. However, these influences did not mediate the effects of stimulant dependence on the timing task (there is no significant indirect effect). A mediating effect of the BIS subscale of non-planning impulsivity was revealed in the 53-seconds time estimation task. Specifically, non-planning impulsivity mediated the significant indirect effect of drug dependence on time estimation and the direct effect between drug dependence and time estimation was not significant, which implied that the indirect effect completely explained the total effect. In other words, the more impulsive the SDI the longer they estimated the interval.
In contrast to the above mediation effect, all other total group effects on temporal processing were not mediated by any of the variables tested. The possible mediator variables did not account for differences between SDI and comparison subjects as found in the ANCOVAs and represented by the total effects.
4. Discussion
This investigation showed that stimulant-dependent subjects (SDI) show impairments in time perception and in sensorimotor timing. Moreover, these impairments are task dependent and possibly specific for the duration of the intervals processed. The mediator models put forth here tested the hypothesis that (neuro-) psychological dysfunctions might carry the effects as mediator variables between stimulant dependence status and temporal processing abilities. We found partial support for this hypothesis, specifically for the estimation of the 53-second interval which is mediated by one form of self-reported impulsivity (non-planning impulsiveness). In contrast, impaired timing in SDI for the processing of 1 and 2-second intervals over a variety of tasks could not be explained by the included mediator variables. Taken together, these findings support the hypothesis that SDI have basic time processing dysfunctions within a specific time range (around 1 to 2 seconds) that are not due to altered levels of impulsivity or other cognitive dysfunctions.
Methamphetamine and cocaine abusers show altered function in prefrontal and striatal dopaminergic circuits (Baxter et al., 1988; Chang et al., 2005; Sekine et al., 2003; Volkow et al., 2001a; 2001b). Dopaminergic systems in the striatum and prefrontal cortex are also thought to be involved in attention, working memory and impulse control (Arnsten and Li, 2005; Cardinal et al., 2004; Evenden, 1999; Paulus et al., 2002). As these brain systems have also been identified as guiding the estimation of time intervals and the timing of movement (Cuoll et al., 2004; Matell and Meck, 2004; Rubia and Smith, 2004), we expected to find temporal processing impairments in SDI. Although our cross-sectional design limits our ability to draw strong conclusions regarding the causal effect of stimulant dependence on time processing, the current evidence points to the same specific brain structures and neural systems known to be involved in time perception and to be affected in SDI. Therefore, we propose that temporal processing abnormalities in SDI result from dysfunctions in fronto-striatal areas of the brain, whether that dysfunction contributes to or is a result of stimulant dependence.
Just as stimulant dependence might lead to brain changes underlying altered time processing, the reverse it also possible. For instance, the estimation of the 53-second interval was influenced by subjects' impulsiveness. However, higher impulsiveness and an altered sense of time in these SDI may have been an antecedent to initial drug use, i.e. a temperamental pre-disposition encouraging our tested SDI to fall into the habit of drug taking. Only longitudinal studies will reveal whether an altered experience of time in combination with other factors predisposes an individual to drug abuse. As is often the case in other complex behavioral domains, stimulant use and altered time perception may interact, leading to a mutually reinforcing effect on brain function.
Based on our results, one can conclude that long-term exposure to methamphetamine or cocaine is associated with impairments of processing temporal intervals. The fact that the statistical analyses found significant direct group effects especially for the processing of intervals of 1000 and 2000 ms should be taken with caution as it may imply only that in our specific tasks these intervals were more sensitive to group differences. For example, at longer intervals subjects might have used a strategy that subdivides the interval by subtle rhythmic movements, an automatic behavior that is hard to control for. This structuring of the intervals could have guided the temporal responses and made them more accurate. At longer intervals these strategies become more effective, therefore revealing timing impairments only at shorter interval lengths. In addition, the fact that in the temporal reproduction task we found significant group differences only for the 2-second interval (and not at 1 second) urges caution with our interpretation. It is, however, worth considering the results within the conceptual framework of the psychology of time. Researchers have established the categorical distinction between perception of duration (for intervals up to 2 - 3 seconds) and estimation of duration (for longer intervals). Events lasting only a few seconds are processed in the present as a perceptual whole, whereas longer intervals must be estimated from memory (Fraisse, 1984; Pöppel, 1997). It is possible that the impairments found in the SDI are specific to temporal processing in this shorter time range.
According to Rammsayer (1999) a dissociation of effects as seen in our results on duration discrimination (impairment only for the processing of 1000 ms but not for 100 ms intervals) would favor the interpretation that timing processes per se are not disturbed in SDI. Intervals with a length of up to some hundreds of milliseconds are supposed to be processed based on brain mechanisms outside of motor and cognitive control. The higher duration difference thresholds of SDI at intervals around 1000 ms, in contrast, would be interpreted as based on disturbances of additional cognitive processes that come into play only at longer intervals. Based on prior studies using tapping tasks, there is evidence that a specific temporal processing function occurs in a time range between approximately 300 ms and one or two seconds (Madison, 2001; Mates et al., 1994; Wittmann et al., 2001). In fact, inferring from a recent synchronization tapping study employing a secondary attention task, Miyake et al. (2004) concluded that in a time range between 450 to 1500 ms automatic processing that is not strongly affected by attention, whereas attention and working memory affect intervals in the range between 1800 and 3600 ms. This empirical finding is in accordance with similar theoretical proposals suggesting that an automatic timing system for shorter intervals can measure time without attentional modulation whereas a cognitively controlled timing system for supra-seconds intervals draws upon cognitive circuits of the brain (Lewis and Miall, 2003). We show here that in timing performance with 1000 and 2000 ms intervals a variety of tasks are not mediated by attention or working-memory processes. First, timing disturbances occurred in SDI in the absence of impairments in most of the tasks of a battery of attention and working-memory tests. Second, an impairment of SDI in the backward digit span task did not mediate performance in time perception. Thus, it is possible that our group effects reveal a specific disturbance of a temporal processing mechanism that is active for durations of 1 to 2 seconds and which is not mediated by other cognitive processes.
In the 53-seconds time-estimation task SDI estimated the time interval to have lasted longer than did the control subjects. The fact that this group effect was mediated by greater impulsivity of SDI can be explained by models of prospective time perception. Subjects estimate the duration of a given interval as longer when the focus of attention is on the passage of time as opposed to a condition where the same interval is filled with activities that distract an observer from attending to time (Wittmann and Lehnhoff, 2005; Zakay and Block, 1996). An overestimation of time intervals is a sign of boredom or emotional distress that draws attention away from meaningful thoughts and actions and directs it to the passage of time (Danckert and Allman, 2005; Twenge et al., 2003). The higher impulsivity of SDI could lead to the subjective experience of being trapped in time during the time-estimation task. Then, SDI would focus their attention more on the passage of time and overestimate its duration. Although results are not unequivocal, a relationship between impulsivity and time perception also has been postulated for children with attention deficit hyperactivity (Barkley et al., 2001) and patients with orbitofrontal cortex lesions (Berlin et al., 2004). Our finding could have clinically relevant implications for the treatment of drug addicts with impulse control problems. It has been suggested that an altered sense of time could be one reason for impulsive individuals to discount the value of temporally delayed reinforcers more strongly and to persist in goal directed behavior that results in immediate or short-term gains at the expense of future or long-term interests (Barratt, 1983, Takahashi, 2006). For a person addicted to a drug, the benefit of resisting the temptation to use a drug, that is, to delay gratification, might lie subjectively too far in the future. Treatment programs could develop intervention strategies that manipulate the temporal delay of rewards or to cognitively restructure the perception of inter-temporal choices in order to shape more adaptive and health-promoting behavior (Monterosso and Ainslie, 2006).
In summary, we found SDI to exhibit unmediated deficits in sensorimotor timing and an overestimation of a 53-second interval that was mediated by an increase in impulsivity. Methamphetamine and cocaine abusers show abnormal metabolic activity of the dopaminergic system and have structural brain changes in fronto-striatal regions, both factors that have been identified as important for contributing to changes in the processing of time. We therefore conclude that SDI have impairments in sensorimotor timing and that longer time intervals are overestimated due to more impulsivity. Diagnostic and therapeutic tools in psychiatry and neurology are being developed that assess/treat altered time-perception in various patient populations (Monterosso and Ainslie, 2006; von Steinbüchel and Pöppel, 1993). Patients addicted to methamphetamine and cocaine could become beneficiaries of such an approach.
Acknowledgments
We would like to acknowledge the invaluable help of Heather Donovan and Mani Mortezaei. This work was supported by grants from NIDA (R01DA016663, R01DA018307) and from the Veterans Administration via a Merit Grant (MPP), and an NIH training grant (5T32MH18399). The Max Kade Foundation who supported M. Wittmann by a grant is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnsten FT, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Barkley R, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J Abnormal Child Psychol. 2001;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Barratt ES. The biological basis of impulsiveness: the significance of timing and rhythm disorders. Person Indiv Diff. 1983;4:387–391. [Google Scholar]
- Barratt ES, Stanford MS, Dowdy L, Liebman MJ, Kent TA. Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Res. 1999;86:163–173. doi: 10.1016/s0165-1781(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Barrio J, Rawson RA, Engel J, Guze BH, Selin C, Sumida R. Localization of neurochemical effects of cocaine and other stimulants in the human brain. J Clin Psychiatry. 1988;49:23–26. [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Block R, Hancock P, Zakay D. Sex differences in duration judgements: a meta-analytic review. Mem Cog. 2000;28:1333–1346. doi: 10.3758/bf03211834. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Cevik MÖ. Effects of methamphetamine on duration discrimination. Behav Neurosci. 2003;117:774–784. doi: 10.1037/0735-7044.117.4.774. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller E, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Haning W. Insights from recent positron emission tomographic studies of drug abuse and dependence. Curr Opin Psychiatry. 2006;19:246–252. doi: 10.1097/01.yco.0000218594.46431.2f. [DOI] [PubMed] [Google Scholar]
- Cuoll JT, Vidal F, Nazarin B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Danckert JA, Allman AA. Time flies when you're having fun: temporal estimation and the experience of boredom. Brain Cog. 2005;59:236–245. doi: 10.1016/j.bandc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Evenden J. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmcology. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fraisse P. Perception and estimation of time. Annu Rev Psychol. 1984;35:1–36. doi: 10.1146/annurev.ps.35.020184.000245. [DOI] [PubMed] [Google Scholar]
- Hellström A, Lang H, Portin R, Rinne J. Tone duration discrimination in Parkinson's disease. Neuropsychologia. 1997;35:737–740. doi: 10.1016/s0028-3932(96)00122-4. [DOI] [PubMed] [Google Scholar]
- Hinton S, Meck W. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Brain Res Cog Brain Res. 2004;21:171–182. doi: 10.1016/j.cogbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Kagerer F, Wittmann M, Szelag E, von Steinbüchel N. Cortical involvement in temporal reproduction: evidence for differential roles of the hemispheres. Neuropsychologia. 2002;40:357–366. doi: 10.1016/s0028-3932(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Leland DS, Paulus MP. Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug Alcohol Depend. 2005;78:83–90. doi: 10.1016/j.drugalcdep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Madison G. Variability in isochronous tapping: higher order dependencies as a function of intertap interval. J Exp Psychol Hum Percept Perform. 2001;27:411–422. doi: 10.1037//0096-1523.27.2.411. [DOI] [PubMed] [Google Scholar]
- Matell M, Meck W. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cog Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Mates J. A system of personal computer control programs for tapping experiments. Comp Meth Prog Biomed. 1990;33:43–48. doi: 10.1016/0169-2607(90)90022-2. [DOI] [PubMed] [Google Scholar]
- Mates J, Radil T, Müller U, Pöppel E. Temporal integration in sensorimotor synchronization. J Cog Neurosci. 1994;6:332–340. doi: 10.1162/jocn.1994.6.4.332. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neuroci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users. Drug Alcohol Depend. 1997;48:235–242. doi: 10.1016/s0376-8716(97)00132-4. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Ann Rev Psychol. 2007;58:17.1–17.22. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Onishi Y, Pöppel E. Two types of anticipation in synchronous tapping. Acta Neurobiol Exp. 2004;64:415–426. doi: 10.55782/ane-2004-1524. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Tinklenberg J, Roth WT, Kopell B. Sensitivity of some human cognitive functions to effects of methamphetamine and secobarbital. Drug Alcohol Depend. 1980;5:145–150. doi: 10.1016/0376-8716(80)90191-x. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. The behavioral economics of will in recovery from addiction. Drug Alcohol Depend. 2006 doi: 10.1016/j.drugalcdep.2006.09.004. in press. accessed on October 9, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2006 doi: 10.1002/hbm.20281. in press. accessed on August 30, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadic I, Gaser C, Volz HP, Rammsayer T, Hager F, Sauer H. Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp Brain Res. 2003;148:238–246. doi: 10.1007/s00221-002-1188-4. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Clark K, Hollnagel C, Grafman J. Duration processing after frontal lobe lesions. Ann N Y Acad Sci. 1995;769:183–190. doi: 10.1111/j.1749-6632.1995.tb38139.x. [DOI] [PubMed] [Google Scholar]
- O'Boyle DJ, Freeman JS, Cody FWJ. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson's disease. Brain. 1996;119:51–70. doi: 10.1093/brain/119.1.51. [DOI] [PubMed] [Google Scholar]
- O'Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18:131–144. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psych. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Pöppel E. A hierarchical model of temporal perception. Trends Cog Sci. 1997;1:56–61. doi: 10.1016/S1364-6613(97)01008-5. [DOI] [PubMed] [Google Scholar]
- Pouthas V, Perbal S. Time perception does not only depend on accurate clock mechanisms but also on unimpaired attention and memory processes. Acta Neurobiol Exp. 2004;64:367–385. doi: 10.55782/ane-2004-1520. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Meth Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in simple and multiple mediator models. submitted. http://www.geocities.com/quantpsy/ph2abs.htm. [DOI] [PubMed] [Google Scholar]
- Rammsayer T. Dopaminergic and serotoninergic influence on duration discrimination and vigilance. Pharmacopsychiatry. 1989;22 1:39–43. doi: 10.1055/s-2007-1014623. [DOI] [PubMed] [Google Scholar]
- Rammsayer T. Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psych. 1999;52B:273–286. doi: 10.1080/713932708. [DOI] [PubMed] [Google Scholar]
- Rao S, Mayer A, Harrington D. The evolution of brain activation during temporal processing. Nature Neurosci. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, Bullmore ET. Prefrontal involvement in “temporal bridging” and timing movement. Neuropsychologia. 1998;36:1283–1293. doi: 10.1016/s0028-3932(98)00038-4. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp. 2004;64:329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Moore C, Waters C, Natsuaki Y, Galloway GP, Kile S, Sullivan EV. A dissociation in attentional control: evidence from methamphetamine dependence. Biol Psychiatry. 2005;57:310–313. doi: 10.1016/j.biopsych.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Suzuki K, Tsukada H, Yoshikawa E, Futatsubashi M, Mori N. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortex with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160:1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Time-estimation error following Weber-Fechner law may explain subadditive time-discounting. Med Hypotheses. 2006;67:1372–1374. doi: 10.1016/j.mehy.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Treutwein B. YAAP: yet another adaptive procedure. Spatial Vision. 1997;11:129–134. [PubMed] [Google Scholar]
- Twenge JM, Catanese KR, Baumeister RF. Social exclusion and the deconstructed state: time perception, meaninglessness, lethargy, lack of emotion, and self-awareness. J Pers Soc Psychol. 2003;85:409–423. doi: 10.1037/0022-3514.85.3.409. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G, Fowler J, Ding Y, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Papas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G, Fowler J, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- von Steinbüchel N, Pöppel E. Domains of rehabilitation: a theoretical perspective. Behav Brain Res. 1993;56:1–10. doi: 10.1016/0166-4328(93)90017-k. [DOI] [PubMed] [Google Scholar]
- von Steinbüchel N, Wittmann M, Szelag E. Temporal constraints of perceiving, generating, and integrating information: clinical indications. Restor Neurol Neurosci. 1999;14:167–182. [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wittmann M. Time perception and temporal processing levels of the brain. Chronobiol Int. 1999;16:17–32. doi: 10.3109/07420529908998709. [DOI] [PubMed] [Google Scholar]
- Wittmann M, von Steinbüchel N, Szelag E. Hemispheric specialisation for self-paced motor sequences. Brain Res Cog Brain Res. 2001;10:341–344. doi: 10.1016/s0926-6410(00)00052-5. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Lehnhoff S. Age effects in the perception of time. Psychol Rep. 2005;97:921–935. doi: 10.2466/pr0.97.3.921-935. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Carter O, Hasler F, Cahn R, Grimberg U, Spring D, Hell D, Flohr H, Vollenweider FX. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. 2007;21:50–61. doi: 10.1177/0269881106065859. [DOI] [PubMed] [Google Scholar]
- Zakay D, Block R. The role of attention in time estimation processes. In: Pastor MA, Artieda J, editors. Time Internal Clocks and Movement. Elsevier; North-Holland: 1996. pp. 143–164. [Google Scholar]
- Zimmermann P, Fimm B. Test for Assessing Attentional Performance (TAP) Version 1.5. Psytest; Herzogenrath: 1997. [Google Scholar]