Abstract
Topically applied morphine is routinely used to alleviate pain in cutaneous wounds such as burns and pressure sores. Evidence suggests the topical administration of exogenous opioid drugs may impair wound closure. This study examined the effects of topical morphine on a standardized model of cutaneous wound healing in the rat. Full-thickness 4mm diameter circular skin flaps were excised from the intrascapular region of male Sprague-Dawley rats. IntraSite™® Gel infused with either morphine-sulfate, neurokinin-1 (NK-1) or neurokinin-2 (NK-2) receptor antagonists, substance P (SP), neurokinin A (NKA), SP + morphine-sulfate, or NKA + morphine-sulfate was applied to the wound twice daily. Results demonstrated a significant overall delay in the time course of wound contraction in morphine-treated animals when compared with gel-only treated controls. The delay in wound contraction seen in morphine-treated animals increased in a concentration-dependent manner. Topical application of NK-1 or NK-2 receptor antagonists mimicked the effects of morphine in delaying wound closure, suggesting topical opioids impair wound closure via the inhibition of SP and NKA release peripherally into the healing wound. Additionally, no significant delays in closure were seen in rats receiving morphine combined with SP or NKA, demonstrating the ability of each neuropeptide to attenuate the effects of morphine in delaying wound closure and restore normal wound closure rates. The combination of SP or NKA and morphine-sulfate for wound therapy may provide local analgesia while maintaining normal closure rates.
Keywords: Substance P, neurokinin A, neurokinin-1 receptor, neurokinin-2 receptor, opioid, primary afferent neuron
1. Introduction
Several prominent medical factors contribute to the incidence of cutaneous wounds, including diabetes mellitus, obesity, and aging. Cutaneous wounds can be extremely painful. Opioid drugs, such as morphine, are highly effective and widely used analgesic agents in the treatment of these wounds. However, the effectiveness of pain management for wound patients can be problematic given that side-effects associated with systemic use of opioids can create severe complications in their current medical conditions. Analgesia during topical opioid administration can be obtained via the activation of opioid receptors located on afferent sensory nerve terminals in peripheral tissues [1, 2]. The mode of action is believed to be local rather than systemic, avoiding negative effects seen upon stimulation of opioid receptors located within the central nervous system. Thus, the topical application of opioids has emerged as a successful strategy for reducing the pain associated with cutaneous wounds [3].
Evidence suggests that sensory neuropeptides play an important role in wound repair, as healing is enhanced by their exogenous application [4–6] and impaired by their depletion [7–10]. Tachykinin neuropeptides, such as SP and NKA, serve as a link between the immune and nervous system [11, 12]. In the periphery, neuropeptides are located in both noradrenergic and cholinergic autonomic nerve fibers as well as in free nerve endings of afferent sensory nerves [13, 14]. Stimulation of these nerve fibers as a result of injury or inflammation causes the release of neuropeptides stored within the peripheral terminals, which facilitates wound healing by regulating blood flow and modulating the migration and function of immunocompetent, inflammatory, and parenchymal cells.
The biological actions of SP and NKA are mediated via the membrane-bound G protein-coupled receptors, NK-1 and NK-2 respectively. These receptors are expressed by several non-neuronal cell types within peripheral tissues, including smooth muscle cells, acinar cells, endothelial cells, fibroblasts, keratinocytes, and various circulating immune cells and inflammation-activated immune cells [15–20]. Activation of neurokinin receptors results in vasodilation, increased vascular permeability, magnification of the inflammatory response, as well as the recruitment, proliferation, and activation of various immune and parenchymal cells essential for normal wound healing [7, 12, 21–28].
Although topical opioids offer a promising new therapeutic strategy for alleviating the pain associated with cutaneous wounds, they may also adversely effect wound healing, limiting the usefulness of this approach. Opioids inhibit action potential generation within neurons and, consequently, suppress the release of pro-inflammatory neuropeptides from sensory nerve terminals [29, 30], disrupting the connection between the nervous and immune systems. Therefore, this study addressed the effects topical morphine application and neuropeptide replacement have on cutaneous wound closure.
2. Materials & Methods
2.1 Animals and Experimental Design
A model of cutaneous wound healing was utilized to determine wound closure rates in rats. Ninety male Sprague-Dawley rats (Harlan, Indianapolis, IN) at approximately 8 weeks of age (200–220 grams body weight) were randomly assigned to one of fifteen treatment groups. Rats were then anesthetized by intraperitoneal administration of 65 mg/kg ketamine HCl and 5.5 mg/kg xylazine HCl and the mid-periscapular region shaved. A 4-mm diameter (12.6 mm2) full-thickness circular skin flap was excised from the midline just below the scapulae using a skin biopsy punch to a depth just above the panniculus carnosus muscle [31]. All rats were subsequently housed individually to prevent cage mates from grooming or otherwise perturbing the wound. Animal facilities were temperature- and humidity-controlled with a 12-h dark–light cycle and food and water ad libitum. All surgical procedures and animal handling were performed in accordance with National Institutes of Health laboratory care standards and approved by the University of Kansas Medical Center Animal Care and Use Committee.
2.2 Drug Preparation and Gel Administration
Morphine sulfate (25 mg/ml) (Abbott Laboratories, Inc., North Chicago, IL) was infused into IntraSite™® Gel (amorphous hydrogel; Smith+Nephew, England) at concentrations of 0.5 mM, 1.5 mM, 5 mM, and 15 mM. The peptides, SP and NKA (Sigma-Aldrich, St. Louis, MO) and NK-1 and NK-2 receptor antagonists, RP 67580 and GR 159897 (Tocris, Ellisville, Missouri) were solubilized in 0.9% saline to a concentration of 10 mM and then infused into IntraSite™® Gel to a final concentration of 1 mM for SP, NKA, and RP 67580 and 3 mM for GR 159897. Initial studies using 1.5 mM morphine modeled the 0.1% morphine-infused gel used clinically [32]; antagonist and peptide doses were selected to provide similar ratios between concentration and drug affinity at the specific receptor (roughly 1 × 106).
Peptidase inhibitor-infused gel was prepared by combining 0.17 mg/ml bacitracin, 0.02 mg/ml leupeptin, 0.02 mg/ml chymostatin and 0.85 mg/ml BSA in IntraSite™® Gel. Gel and drug were combined in 3 cc syringes by repeated passage through a Luer-lock stopcock. Drug solutions were made the day of or the day prior to use. Control animals received IntraSite™® Gel alone treatments. Saline was added to control gel to match the consistency of the drug-infused gels. Beginning one hour post-surgery, 150μL IntraSite™® Gel alone or IntraSite™® Gel infused with a drug was applied twice daily (7:00 a.m. and 5:00 p.m.) for 10–14 days (control, n=25; 5 mM morphine sulfate, n=11; 1 mM SP, 1 mM NKA, 5 mM morphine sulfate + 1 mM SP, 5 mM morphine sulfate + 1 mM NKA, n=5 each; RP67580, n=8; 0.5 mM and 1.5 mM morphine sulfate, n=7 each; GR159897, 15 mM morphine sulfate, n=6 each; peptidase inhibitor, n=4).
2.3 Wound Imaging and Data Analysis
Each morning prior to treatment, wound images were captured using a hand-held digital camera. A bar attached to the camera provided a fixed focal distance target for wound imaging. A size standard with known surface area was fastened to the target bar and included in each image. Wound area was determined daily using a computerized planimetric program (Scion Image, Fredrick, MD). The area occupied by the wound was defined by the boundary created by the granulation tissue or scab/intact tissue interface. Wound area data generated by Scion Image were converted from pixels to area units of mm2 by comparison to the known area of the fixed size standard and are reported as area (mm2) mean ± SEM. Statistical analyses were performed using InStat (GraphPad Software, San Diego, CA). Data were analyzed using a one-way ANOVA with Tukey’s post hoc or unpaired t-test. Differences between means were considered significant when p ≤ 0.05.
3. Results
3.1 Effects of topical administration of morphine sulfate on cutaneous wound closure rates
The impact of increasing concentrations of topical morphine sulfate application on cutaneous wound closure rates in rats was assessed using a standardized model of cutaneous wound healing. Animals receiving topical morphine sulfate treatment demonstrated a significant delay in wound closure rates when compared to gel-only treated controls. In animals receiving 0.5 mM morphine sulfate applications, wound area was significantly larger on days 1 and 6 post-wounding (Figure 1). The wound area of animals receiving 1.5 mM morphine sulfate treatments was significantly larger on wound days 1–7 (Figure 1). Topical application of 5 mM morphine sulfate significantly increased wound area on wound days 1–9, whereas 15 mM morphine sulfate treatment produced significantly larger wounds on days 1–10 post-wounding (Figure 1). In addition, the delay in wound contraction observed in morphine-treated animals increased in a concentration-dependent manner. Total wound area over the complete time course of animals receiving 0.5, 1.5, 5, and 15 mM morphine sulfate treatments was significantly larger (approximately 6, 16, 26, and 33% respectively) than gel-only treated control rats. In addition, the total wound area of animals treated with 1.5 mM morphine-sulfate was significantly larger than animals in the 0.5 mM morphine sulfate-treated group, and the total wound area of animals treated with 5 mM morphine sulfate was significantly larger than animals in the 1.5 mM morphine sulfate-treated group.
Fig. 1. Time course of wound closure for rats receiving increasing concentrations of morphine sulfate-infused gel treatments.
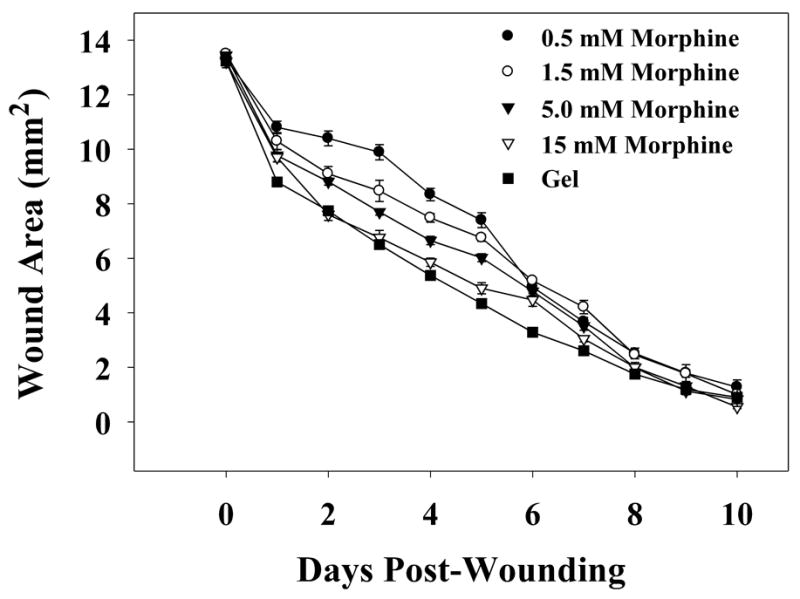
IntraSite™® gel (150 μl) was applied to the wound twice daily through wound day 10. Wound size is presented as area (mm2) mean ± SEM and was determined by analysis of digital images. Note that IntraSite™® gel infused with 0.5, 1.5, 5, or 15 mM morphine sulfate significantly delayed wound closure compared to gel-only treatment. Additionally, the delay in wound closure seen in gel + morphine treated animals increased in a concentration-dependent manner (n=6 to 7; p ≤ 0.05; ANOVA, Tukey’s post-hoc test).
3.2 Effects of topical application of selective, non-peptide neurokinin-1 and neurokinin-2 receptor antagonists on cutaneous wound closure rates
Selective, non-peptide NK-1 and NK-2 receptor antagonists were utilized to determine the effects their topical administration have on cutaneous wound closure rates in rats. A standardized model of cutaneous wound healing was used to evaluate the wounds. Animals receiving topical NK-1 or NK-2 receptor antagonists demonstrated a significant delay in wound closure rates when compared to gel-only treated controls. Wound area of animals treated with gel infused with 1 mM RP 67580, a selective NK-1 receptor antagonist, was significantly larger on days 2, 3, 4, 5, 6, and 8 post-wounding when compared to gel-only treated control animals (Figure 2A). A 25% increase in the total wound area over the complete time course of animals receiving the NK-1 receptor antagonist was seen when compared to controls. Similar results were observed in the wounds of animals receiving topical treatment with 3mM of the selective, non-peptide NK-2 receptor antagonist GR 159897. A significant increase in the area of the wounds was seen on wound days 1–8 (Figure 2B) with a 19% increase in the total wound area.
Fig. 2. Wound closure time course for rats receiving IntraSite™® gel infused with the selective, nonpeptide NK-1 or NK-2 receptor antagonist, RP 67580 or GR 159897.
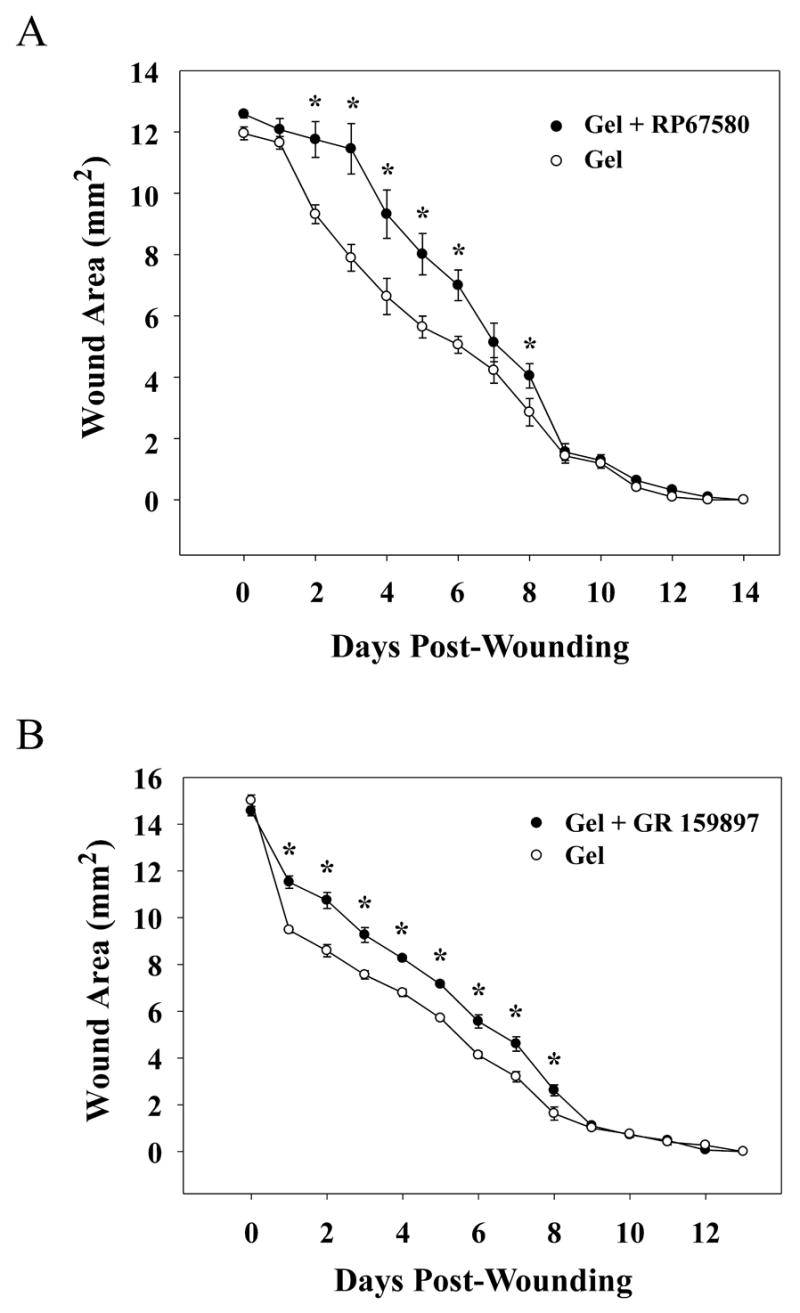
Data are presented as area (mm2) mean ± SEM and were determined by analysis of digital images. (A) Rats received applications of IntraSite™® gel (150 μl) to the wound twice daily through wound day 14. IntraSite™® gel infused with 1 mM RP 67580 (n=8) significantly delayed wound closure compared to gel-only controls (n=8). Gel + RP 67580 treated rats had significantly larger wound areas when compared to gel-only controls on wound days 2, 3, 4, 5, 6, and 8. (B) IntraSite™® gel (150 μl) was applied topically to the wound twice daily through wound day 13. Treatment with 3 mM GR 159897 (n=6) significantly delayed wound closure compared to gel-only controls (n=6) with significant increases in wound area compared to control on days 1–8 post-wounding (*p ≤ 0.05; ANOVA, Tukey’s post-hoc test).
3.3 Effects of neuropeptide replacement in morphine sulfate-infused gel on cutaneous wound closure rates
A standardized model of cutaneous wound healing was used to determine the effects of the addition of SP or NKA into morphine sulfate-infused gel applications on wound closure rates in rats. As previously demonstrated, 5 mM morphine sulfate significantly increased the area of healing wounds. In this experiment, significant increases in wound area of morphine sulfate treated rats were seen on days 1, 2, 3, 5, 6 and 8 post-wounding (Figure 3A & B). A 17% increase in the total wound area was seen for animals in this treatment group. In addition, topical application of 1 mM SP significantly decreased the wound area on wound days 1, 2, 6, and 8 (Figure 3A), with an 11% decrease in the total wound area over the entire time course demonstrating acceleration in wound closure. However, a significant difference was not seen between topical treatment of 1 mM NKA and control (Figure 3B). Wounds treated with a combination of either 1 mM SP or 1 mM NKA and 5 mM morphine sulfate did not exhibit significant changes in wound area when compared to gel-only treated controls (Figure 3A & B). Furthermore, no noticeable erythema or pain-related behaviors were observed in rats receiving topical application of either peptide.
Fig. 3. Wound closure time course for rats receiving IntraSite™® gel treatments infused with morphine and/or neuropeptides.
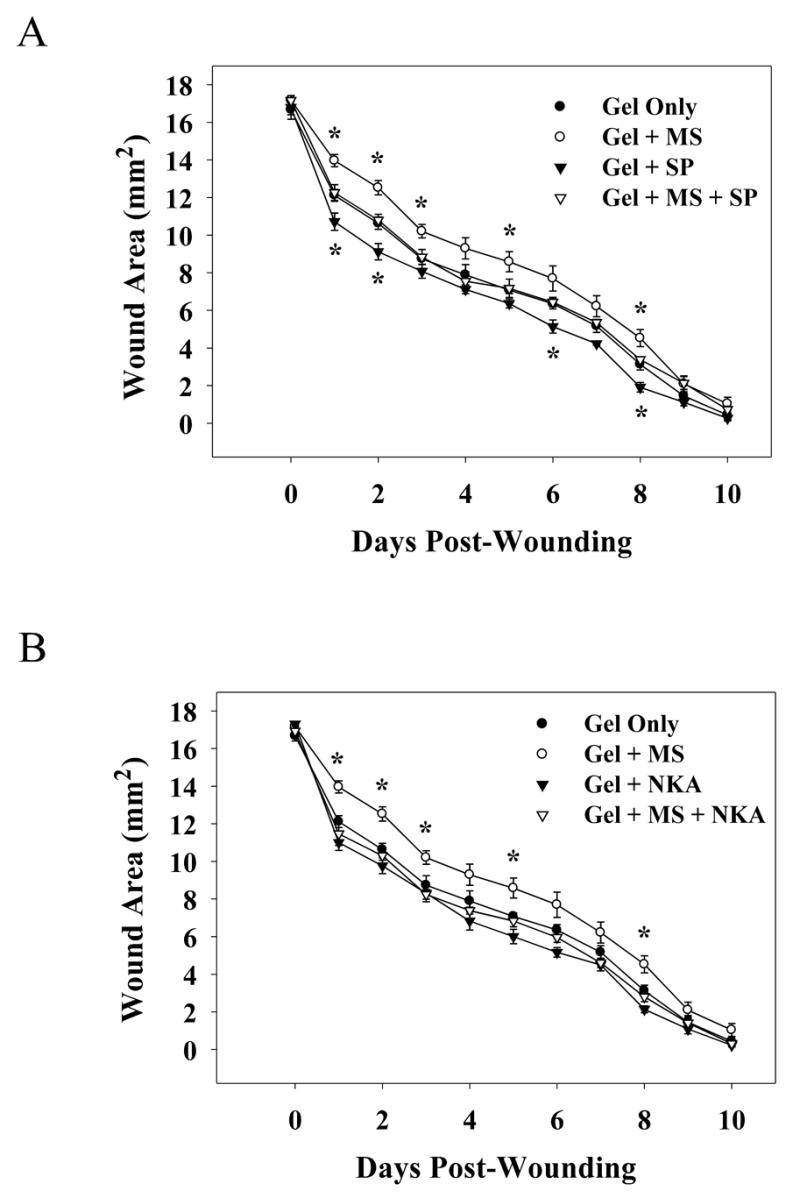
Rats were treated with IntraSite™® gel (150 μl) twice daily through wound day 10. Wound size is presented as area (mm2) mean ± SEM and was determined by analysis of digital images. IntraSite™® gel infused with 5 mM morphine sulfate significantly delayed wound closure compared to gel-only treated controls. Wound area was significantly larger in the morphine treated rats when compared to the gel-only treated rats on wound days 1,2,3,5, and 8. (A) Rats were treated with 5 mM morphine sulfate, 1 mM SP, or 5 mM morphine sulfate + 1 mM SP. IntraSite™® gel infused with SP significantly increased wound closure compared to gel-only treatment. In animals treated with SP wound area was significantly smaller than wounds of rats receiving gel-only on wound days 1, 2, 6, and 8. Note that a significant difference was not seen in rats receiving morphine + SP treatment when compared to controls. (B) IntraSite™® gel treatments were infused with 5 mM morphine sulfate, 1 mM NKA, or 5 mM morphine sulfate + 1 mM NKA. Note a significant difference was not seen in either NKA or morphine + NKA treated groups when compared to controls (*p ≤ 0.05; ANOVA, Tukey’s post-hoc test; n=5).
3.4 Effects of peptidase inhibitors on wound closure rates
Peptidase inhibitors were utilized to determine the effects of local peptidases and the stability of peptides within the healing wound. Rats were treated with peptidase inhibitor-infused gel on days 0 – 5 post-wounding and the area of the wounds quantified. The closure of wounds treated with peptidase inhibitors did not differ significantly from control wounds (data not shown).
4. Discussion
Topical morphine is currently being used clinically to provide analgesia to patients with painful cutaneous wounds [3, 32]. However, this therapeutic strategy may negatively impact wound healing. Unmyelinated, capsaicin-sensitive primary afferent neurons are primarily responsible for the peripheral analgesic effects of topical morphine application [33, 34]. Opioids provide analgesia in part by inhibiting the generation of action potentials within these neurons, which subsequently results in blocking the retrograde release of important pro-inflammatory neuropeptides, such as SP and NKA, into peripheral tissues [29, 35, 36]. This study investigated the effects topical morphine application and neuropeptide replacement have on the closure rate of cutaneous wounds.
A standardized model of cutaneous wound healing was utilized to examine the impact increasing concentrations of topical morphine-sulfate application have on cutaneous wound closure rates in rats. This model of wound healing provides sensitivity sufficient to detect age-related variations in wound closure, as well as the negative impact of partial sensory denervation on wound healing [10, 31]. The results of this study demonstrate that the rate of closure is significantly slower in animals receiving topical morphine treatment when compared to control animals (Figure 1). This delay occurs in a concentration-dependent manner consistent with an opioid receptor-mediated effect.
If morphine is delaying wound closure rates by inhibiting the release of neuropeptides from primary afferent neurons, then functional blockade of peripheral neuropeptide receptors should mimic the effect seen with morphine application. When cutaneous wounds were treated with a selective, non-peptide NK-1 or NK-2 receptor antagonist, RP 67580 or GR 159897 respectively, wound closure rates were significantly delayed when compared to control (Figure 2A & B). This observation suggests that topical morphine application slows wound closure by inhibiting the release and peripheral action of SP and NKA at NK-1 and NK-2 receptors and confirms the importance of SP/NK-1 receptor and NKA/NK-2 receptor interactions during wound healing. Both RP 67580 and GR 159897 are high-affinity antagonists for the NK-1 or NK-2 receptor, respectively, and exhibit at least 3,000-fold selectivity between these receptors. However, the degree of penetration of drug from the gel into the peri-wound skin and the actual concentration of antagonist drug at the receptor in these studies are unknown. The similarity of magnitude of delay in wound closure evoked by the NK-1 and NK-2 receptor antagonists does not reveal whether one of the two receptors is more important, mechanistically, in promoting wound closure. Extensive concentration-response relationships would need to be established to determine the relative potency of NK-1 and NK-2 receptor agents in modifying cutaneous wound closure.
Neuropeptides mediate early components of the cutaneous neurogenic inflammatory response. Previous studies have demonstrated that denervation with the neurotoxin capsaicin results in diminished wound healing [8, 10]. The primary reason for delayed wound healing in the absence of neuropeptides appears to be due to a lag phase between injury and the initiation of wound contraction [7]. Similar to morphine treatment, the delay in closure seen with selective receptor antagonists is most evident within the first few days of the time course, suggesting an essential neurokinin receptor-mediated neuromodulation by SP and NKA early in the time course of wound healing.
Morphine slows wound closure by blocking the release of neuropeptides into the healing wound. Replacement of the neuropeptides should be able to attenuate the deleterious effects of morphine. Therefore, the ability of SP or NKA to restore normal wound closure in morphine-treated animals was determined by addition of the neuropeptides in morphine-infused gel. In this study, twice-daily topical administration of morphine significantly delayed wound closure, while treatment of wounds with SP accelerated wound closure (Figure 3A). The delay in wound closure seen with morphine treatment was fully reversed by the addition of either SP or NKA into morphine-infused gel, demonstrating the neuropeptides’ capacity to restore normal wound closure rates. (Figure 3A & B). While peri-wound pain thresholds were not directly quantified, treatment of the wounds with either SP or NKA did not produce any overt algesic effects (e.g., enhanced wound-directed biting or scratching behaviors). The results demonstrate the potential use of the neuropeptides in reversing the detrimental effect of topical morphine therapy in wound healing.
Most cells expressing neuropeptide receptors also express neuropeptide-degrading enzymes such as neutral endopeptidase (NEP), potentially providing a feedback mechanism to effectively control the bioavailability of neuropeptides and regulate their inflammatory effects [37]. Studies have shown that chronic, non-healing diabetic ulcers have increased NEP localization and activity [38]. Therefore, the role of local peptidases on normal wound closure rates was determined. Wounds receiving twice-daily application of gel infused with a cocktail of peptidase inhibitors were not significantly different in size when compared to control wounds. This result suggests that enzymatic degradation of neuropeptides within the wound does not impact normal healing rates. In addition, treatment with either SP- or NKA-infused gel significantly increased wound closure rates, suggesting that the peptides are stable in the hydrogel.
These data suggest that topical morphine alters wound healing by activating opioid receptors on primary afferent neurons, thereby inhibiting the release of neuropeptides such as SP and NKA. Although morphine-treated wounds do ultimately close at times similar to controls, the area of these wounds is significantly larger during earlier days of the time course. Wound healing is a dynamic process consisting of multiple overlapping phases, which rely heavily on the orchestrated movement of various inflammatory and parenchymal cells into the wound. Data from this study suggest morphine treatment may be disrupting cellular processes which occur early in wound healing. In addition, interruption in the normal progression of healing by topical morphine administration may result in long-term detrimental alterations in the cellular architecture of healed skin.
Acknowledgments
This study was supported in part by DA12505 (KMcC) and a Basic Science Research Pilot Grant from the Lied foundation/KUMC Research Institute (KMcC). The authors would like to thank Michelle Winter for her expert technical assistance.
Abbreviations
- NK-1
neurokinin-1
- NK-2
neurokinin-2
- SP
substance P
- NKA
neurokinin A
- NEP
neutral endopeptidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith TW, Buchan P, Parsons DN, Wilkinson S. Peripheral antinociceptive effects of N-methyl morphine. Life Sci. 1982;31:1205–8. doi: 10.1016/0024-3205(82)90343-5. [DOI] [PubMed] [Google Scholar]
- 2.Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–75. [PubMed] [Google Scholar]
- 3.Long TD, Cathers TA, Twillman R, O’Donnell T, Garrigues N, Jones T. Morphine-Infused silver sulfadiazine (MISS) cream for burn analgesia: a pilot study. J Burn Care Rehabil. 2001;22:118–23. doi: 10.1097/00004630-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Delgado AV, McManus AT, Chambers JP. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp Biol Med (Maywood) 2005;230:271–80. doi: 10.1177/153537020523000407. [DOI] [PubMed] [Google Scholar]
- 5.Engin C. Effects of calcitonin gene-related peptide on wound contraction in denervated and normal rat skin: a preliminary report. Plast Reconstr Surg. 1998;101:1887–90. doi: 10.1097/00006534-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Kjartansson J, Dalsgaard CJ. Calcitonin gene-related peptide increases survival of a musculocutaneous critical flap in the rat. Eur J Pharmacol. 1987;142:355–8. doi: 10.1016/0014-2999(87)90073-2. [DOI] [PubMed] [Google Scholar]
- 7.Khalil Z, Helme R. Sensory peptides as neuromodulators of wound healing in aged rats. J Gerontol A Biol Sci Med Sci. 1996;51:B354–61. doi: 10.1093/gerona/51a.5.b354. [DOI] [PubMed] [Google Scholar]
- 8.Kjartansson J, Dalsgaard CJ, Jonsson CE. Decreased survival of experimental critical flaps in rats after sensory denervation with capsaicin. Plast Reconstr Surg. 1987;79:218–21. doi: 10.1097/00006534-198702000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Peskar BM, Lambrecht N, Stroff T, Respondek M, Muller KM. Functional ablation of sensory neurons impairs healing of acute gastric mucosal damage in rats. Dig Dis Sci. 1995;40:2460–4. doi: 10.1007/BF02063255. [DOI] [PubMed] [Google Scholar]
- 10.Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res. 2002;307:281–91. doi: 10.1007/s00441-001-0477-8. [DOI] [PubMed] [Google Scholar]
- 11.Felten DL, Felten SY, Bellinger DL, Lorton D. Noradrenergic and peptidergic innervation of secondary lymphoid organs: role in experimental rheumatoid arthritis. Eur J Clin Invest. 1992;22 (Suppl 1):37–41. [PubMed] [Google Scholar]
- 12.Payan DG. Neuropeptides and inflammation: the role of substance P. Annu Rev Med. 1989;40:341–52. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- 13.Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, et al. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987;100:225–60. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 14.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–68. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 15.Ansel JC, Kaynard AH, Armstrong CA, Olerud J, Bunnett N, Payan D. Skin-nervous system interactions. J Invest Dermatol. 1996;106:198–204. doi: 10.1111/1523-1747.ep12330326. [DOI] [PubMed] [Google Scholar]
- 16.Bowden JJ, Baluk P, Lefevre PM, Vigna SR, McDonald DM. Substance P (NK1) receptor immunoreactivity on endothelial cells of the rat tracheal mucosa. Am J Physiol. 1996;270:L404–14. doi: 10.1152/ajplung.1996.270.3.L404. [DOI] [PubMed] [Google Scholar]
- 17.Krause JE, Takeda Y, Hershey AD. Structure, functions, and mechanisms of substance P receptor action. J Invest Dermatol. 1992;98:2S–7S. doi: 10.1111/1523-1747.ep12462082. [DOI] [PubMed] [Google Scholar]
- 18.Haley KJ, Sunday ME, Osathanondh R, Du J, Vathanaprida C, Karpitsky VV, et al. Developmental expression of neurokinin A and functional neurokinin-2 receptors in lung. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1348–58. doi: 10.1152/ajplung.2001.280.6.L1348. [DOI] [PubMed] [Google Scholar]
- 19.Song IS, Bunnett NW, Olerud JE, Harten B, Steinhoff M, Brown JR, et al. Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R) Exp Dermatol. 2000;9:42–52. doi: 10.1034/j.1600-0625.2000.009001042.x. [DOI] [PubMed] [Google Scholar]
- 20.Renzi D, Pellegrini B, Tonelli F, Surrenti C, Calabro A. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol. 2000;157:1511–22. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carolan EJ, Casale TB. Effects of neuropeptides on neutrophil migration through noncellular and endothelial barriers. J Allergy Clin Immunol. 1993;92:589–98. doi: 10.1016/0091-6749(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg JM, Saria A, Brodin E, Rosell S, Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci U S A. 1983;80:1120–4. doi: 10.1073/pnas.80.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson J, von Euler AM, Dalsgaard CJ. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985;315:61–3. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- 24.Perretti M, Ahluwalia A, Flower RJ, Manzini S. Endogenous tachykinins play a role in IL-1-induced neutrophil accumulation: involvement of NK-1 receptors. Immunology. 1993;80:73–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Roch-Arveiller M, Regoli D, Chanaud B, Lenoir M, Muntaner O, Stralzko S, et al. Tachykinins: effects on motility and metabolism of rat polymorphonuclear leucocytes. Pharmacology. 1986;33:266–73. doi: 10.1159/000138225. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Danno K, Ikai K, Imamura S. Effects of substance P and substance K on the growth of cultured keratinocytes. J Invest Dermatol. 1988;90:399–401. doi: 10.1111/1523-1747.ep12456487. [DOI] [PubMed] [Google Scholar]
- 27.Thureson-Klein A, Hedqvist P, Ohlen A, Raud J, Lindbom L. Leukotriene B4, platelet-activating factor and substance P as mediators of acute inflammation. Pathol Immunopathol Res. 1987;6:190–206. doi: 10.1159/000157045. [DOI] [PubMed] [Google Scholar]
- 28.Ziche M, Morbidelli L, Pacini M, Dolara P, Maggi CA. NK1-receptors mediate the proliferative response of human fibroblasts to tachykinins. Br J Pharmacol. 1990;100:11–4. doi: 10.1111/j.1476-5381.1990.tb12043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroeder JE, Fischbach PS, Zheng D, McCleskey EW. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991;6:13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- 30.Werz MA, MacDonald RL. Opioid peptides selective for mu- and delta-opiate receptors reduce calcium-dependent action potential duration by increasing potassium conductance. Neurosci Lett. 1983;42:173–8. doi: 10.1016/0304-3940(83)90402-0. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Warn JD, Fan Q, Smith PG. Relationships between nerves and myofibroblasts during cutaneous wound healing in the developing rat. Cell Tissue Res. 1999;297:423–33. doi: 10.1007/s004410051369. [DOI] [PubMed] [Google Scholar]
- 32.Twillman RK, Long TD, Cathers TA, Mueller DW. Treatment of painful skin ulcers with topical opioids. J Pain Symptom Manage. 1999;17:288–92. doi: 10.1016/s0885-3924(98)00140-7. [DOI] [PubMed] [Google Scholar]
- 33.Bartho L, Stein C, Herz A. Involvement of capsaicin-sensitive neurones in hyperalgesia and enhanced opioid antinociception in inflammation. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:666–70. doi: 10.1007/BF00175710. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Zhang Q, Stein C, Schafer M. Contribution of opioid receptors on primary afferent versus sympathetic neurons to peripheral opioid analgesia. J Pharmacol Exp Ther. 1998;286:1000–6. [PubMed] [Google Scholar]
- 35.Brodin E, Gazelius B, Panopoulos P, Olgart L. Morphine inhibits substance P release from peripheral sensory nerve endings. Acta Physiol Scand. 1983;117:567–70. doi: 10.1111/j.1748-1716.1983.tb07228.x. [DOI] [PubMed] [Google Scholar]
- 36.Werz MA, Macdonald RL. Opioid peptides with differential affinity for mu and delta receptors decrease sensory neuron calcium-dependent action potentials. J Pharmacol Exp Ther. 1983;227:394–402. [PubMed] [Google Scholar]
- 37.Damas J, Bourdon V, Liegeois JF, Simmons WH. Influence of several peptidase inhibitors on the pro-inflammatory effects of substance P, capsaicin and collagenase. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:662–9. doi: 10.1007/BF00170843. [DOI] [PubMed] [Google Scholar]
- 38.Spenny ML, Muangman P, Sullivan SR, Bunnett NW, Ansel JC, Olerud JE, et al. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen. 2002;10:295–301. doi: 10.1046/j.1524-475x.2002.10504.x. [DOI] [PubMed] [Google Scholar]


