Abstract
The cytochrome bc1 complex (commonly called Complex III) is the central enzyme of respiratory and photosynthetic electron transfer chains. X-ray structures have revealed the bc1 complex to be a dimer, and show that the distance between low potential (bL) and high potential (bH) hemes, is similar to the distance between low potential hemes in different monomers. This suggests that electron transfer between monomers should occur at the level of the bL hemes. Here we show that although the rate constant for bL → bL electron transfer is substantial, it is slow compared to the forward rate from bL to bH, and the intermonomer transfer only occurs after equilibration within the first monomer. The effective rate of intermonomer transfer is about 2-orders of magnitude slower than the direct intermonomer electron transfer.
Keywords: Cyt bc1, Complex III, Monomer, Dimer, Electron transfer, Coenzyme Q-10, Ubiquinone, Superoxide
1. Introduction
1.1. The cytochrome bc1 complex in respiratory and photosynthetic electron transport
Cytochrome (cyt) bc complexes (Complex III in mitochondria) occupy the central position in the energy transducing membranes of mitochondria, bacteria and chloroplasts (the related cyt b6f complex), as well as many archaea, and the evolutionary homology of the bc complex among these groups is well established [1]. The functional core of all bc1 complexes comprises 3 catalytic subunits that contain all the redox active centers, with a variable number of additional, non-redox active polypeptides. There are four identifiable redox centers and at least two quinone-binding sites. Two b-type hemes have relatively low redox mid-point potentials (Em). Cyt c1 and the Rieske iron-sulfur protein (ISP) are the high potential components of this complex. The two known quinone binding sites are center “o” (or Qo site), where oxidation of ubiquinol provides reducing equivalents to the complex, and center "i" (hence Qi site), where ubiquinone is reduced (reviewed in [1–5]).
1.2. The bc1 complex as a functional dimer
Cyt bc1 complexes are organized as dimers both in detergent solution (see, e.g., [6,7]) and in crystals [8–13], and it is now generally accepted that the dimer is the functional form. Strong implication of a functional role for the dimer comes from three sources: (i) the ISP is oriented so that its functional globular domain is associated with one b-subunit, while the transmembrane helix is associated with the other b-subunit, across the symmetry axis; (ii) the two “i” sites share a common volume from which quinone and quinol exchange with the larger membrane pool; (iii) the proximity of the low potential bL hemes in each monomer strongly suggests the occurrence of intermonomer electron transfer (Fig. 1).
Figure 1.
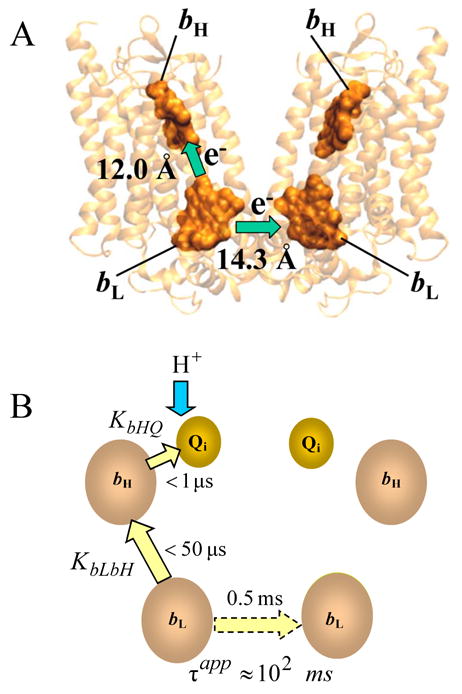
A - Cytochrome b dimer with 4 hemes. Distances are between the nearest edges of each porphyrin macrocycle. The figure was prepared in VMD [35] using file 2A06.pdb. B - Intermonomer electron transfer (shown by dashed arrow) occurs after equilibration within the first monomer via equilibrium constant KbLbH for electron transfer between hemes bL and bH, and KbHQ, for electron transfer from bH to Qi. This scheme shows only the case when, initially, there is no semiquinone at the Qi site. The value of 0.5 ms shown for the intrinsic monomer-monomer electron transfer is based on the assumption that λ =1 eV.
The X-ray structures show that various specific inhibitors bind to both monomers, in a seemingly symmetrical fashion [14], and the only direct evidence suggesting any non-equivalence of the two monomers comes from the co-crystal structure of Lange and Hunte [15], in which cyt c is bound to only one cyt c1 in each dimer. Nevertheless, there are numerous observations, especially involving inhibitor actions at substoichiometric titres, that have suggested functional asymmetry.
Several models for a functional role of the two monomers within a dimer have been suggested, beginning with the pioneering work of de Vries [16–19]. De Vries proposed a double Q cycle in which the two monomeric halves of the enzyme act cooperatively to complete the catalytic cycle [16]. Similarly, Gopta et al. [18] proposed a dimeric Q-cycle where the energetically unfavorable oxidation of the first ubiquinol molecule by one of the bc1 monomers is driven by the energetically favorable oxidation of the second ubiquinol by the other monomer. Nieboer and Berden [17] explained their titration of the steady-state activity of mitochondrial ubiquinol-cyt c oxidoreductase with combinations of antimycin, myxothiazol and DCCD (N, N '-dicyclohexylcarbodiimide) by assuming that the bc1 complex is a functional dimer, consisting of “electrically interacting protomers”. Gutierrez and Trumpower [19] observed that one molecule of stigmatellin per dimer completely inhibits bc1 complex activity (see, however, [20]). The results indicated an anticooperative interaction between monomer sites, and the authors proposed a half-of-the-sites reactivity for the bc1 complex. Schmitt and Trumpower [21] had earlier suggested a half-of-the-sites reactivity with respect to cyt c, consistent with the structural findings of Lange and Hunte [15] for the bc1-cyt c co-crystals.
Inhibitor titration curves have frequently been taken to indicate interactions or cooperativity within the bc1 complex (see, e.g., [17,22,23]). However, there are other possible sources of such titration behavior (see, e.g., [23–25]). Bechmann et al. [23] suggested that the S-shaped dependence of many apparently high affinity inhibitors of the bc1 complex was due to their fast movement between binding sites in the dimer and a large partition coefficient between lipid and water. They also considered fast electron transfer between monomers in a dimer, but regarded this as less probable.
1.3. “Cross-dimer” (monomer-to-monomer) electron transfer
The correlation between the rate of electron transfer and distance between the cofactors (see, e.g., [26]) strongly supports the likelihood of effective electron sharing between monomers, via the bL hemes, during bc1 complex turnover [27]. At the same time, the symmetry of the complex has confounded the measurement of this electron transfer. In general, two different approaches have been used to date to try to elicit evidence for it, but there is still no unequivocal demonstration.
The first method, which predates the structural knowledge, is the creation of heterogeneity in the dimer by binding specific inhibitors sub-stoichiometrically and measuring the electron-transfer characteristics in such inhibitor-induced “heterodimers” (see, e.g., [17,19,28,29]). The classic difficulty with this approach is the statistical creation of distinct populations of bc1 dimers with 0, 1 and 2 sites blocked (see, e.g., [17]). This creates the possibility for alternative explanations of any observed effect, especially in the case of steady-state rate measurements, where multiple turnovers of both Qi and Qo sites are needed. These include the well-known Kröger-Klingenberg effect [25] (a reflection of the two-substrate nature of the bc1 complex), the possibility of fast intermonomer inhibitor exchange [23], number heterogeneity in the distribution of bc1 complexes (and reaction centers) in natural vesicles [30,31], rate limitation in quinone delivery (especially detergent solubilized, isolated bc1 complex), and modified kinetics and thermodynamics of artificial quinones.
A second approach has been to try to augment or diminish the direct intermonomer electron transfer rate by modifying the protein medium between the bL hemes [32]. However, if what we know about electron transfer is even half right, this is doomed to failure – the hemes are sufficiently close that even putting a vacuum between them would be unlikely to modify the rate enough to make a noticeable difference.
2. Hypothesis
Rate-distance correlations based on established theory [33,34] predict a substantial bL-bL electron transfer rate, but the bL-bH electron transfer is expected to be ~2 orders of magnitude faster. We therefore suggest that net monomer-monomer electron transfer (MET) occurs only after pre-equilibration of the electron within one monomer (bL↔bH↔Qi). This results in an effective rate of transfer that is 2 orders of magnitude slower than the direct process, under uncoupled conditions. However, the bL↔bH equilibrium constant is sensitive to the membrane potential. Thus, as the membrane potential builds up, the population of reduced bL increases and the net rate of MET also increases. In effect, direct intermonomer electron transfer (without pre-equilibration of the electron within one monomer) is observed only under conditions when bL-bH electron transfer is impaired, and there is no significant electron transfer between monomers under uncoupled conditions, or in isolated bc1 complexes, when the reaction is activated by ubiquinone.
3. Discussion
3.1. Estimation of bL-bL and bL-bH electron transfer rates from rate-distance correlation
The rate of electron transfer between electron carriers in proteins decreases exponentially with distance (see, for example, [26,33,34,36–38]). The logarithm of the rate constant, k, of intraprotein electron transfer between two electron carriers with edge-to-edge distance r can be described by the following equation:
| (1) |
where r is in Angstroms, σ is a “length constant” commonly taken to be equal to 0.6 Å−1 [34], but which may have some dependence on the packing density of a protein [34,38]; ΔG0 is the standard reaction free energy in eV; λ is the reorganization energy in eV. In classical Marcus theory [33], the value of γ is given by F/(4RT·ln10) = 4.2 (at T = 298 K), where F is the Faraday constant, R is the gas constant, and T is the absolute temperature. Moser et al. [34] consider γ as an empirical term and obtained a value of 3.1 from best fits to large data collections, thereby possibly accommodating some quantum contributions. For the bc1 complex, all parameters, except the reorganization energy, are known for both bL-bL and bL-bH electron transfer. The edge-to-edge distance between low-potential hemes estimated from X-ray structural analysis of the bovine bc1 complex with resolution 2.1 Å is 14.3 Å, while the edge-to-edge distance between bL and bH in one monomer is 12.0 Å [14]. Following accepted practices, we refer to “edge-to-edge” distances, taken between the macrocycle pi-electron systems of the hemes [38,39]. The standard reaction free energy, ΔG0, is 0 for bL-bL electron transfer and approx. −0.1 eV for bL-bH electron transfer.
For λ=1 eV, equation 1 yields an intrinsic time of ~0.5 ms and ~5 μs for electron transfer between bL-bL and bL-bH, respectively. The two orders of magnitude difference between rates of bL-bH and bL-bL electron transfer leads to the predominant reduction of bH in the same monomer. The absolute values of these rates are very sensitive to uncertainties in the parameters, but the relative rates are much less so.
3.2. The ratio of bL-bH to bL-bL electron transfer rates is large and almost independent of the reorganization energy
Using Eq. 1 one can estimate the ratio of bL-bH to bL-bL electron transfer rates, kbLbH/kbLbL. While the precise values of the reorganization energy are unknown, the difference Δλ=λbLbH - λbLbL is expected to be small. For Δλ=0, Figure 2 shows that the ratio of bL-bL and bL-bH electron transfer rates is ~102 and is almost independent of the reorganization energy. The two orders of magnitude difference in the bL-bL and bL-bH electron transfer rate constants leads to the dominant reduction of heme bH first, i.e., the branching ratio, P = kbLbL/(kbLbL+kbLbH), is small (see below). Therefore, we can assume that the equilibrium between bL and bH within one monomer is established before any significant electron exchange between monomers takes place.
Figure 2.
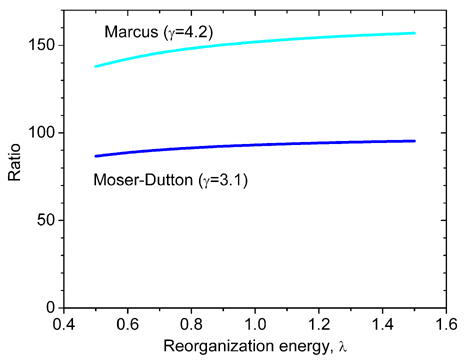
The dependence of the ratio of the intrinsic rate constants for bL-bL and bL-bH electron transfer on the reorganization energy, calculated from Eq. 1 for Marcus and Moser-Dutton treatments. The edge-to-edge distance between low-potential hemes was assumed to be 14.3 Å, while the edge-to-edge distance between bL and bH in the same monomer was taken as 12.0 Å [14]. The standard reaction free energy, ΔG0, is 0 for bL-bL electron transfer and ~ −0.1 eV for bL-bH electron transfer. It is assumed that λbLbH = λbLbL.
3.3. The apparent time of monomer-monomer electron transfer
It is straightforward to estimate the apparent rate of single electron transfer between monomers, kapp (assuming that there is no more than one electron in either monomer of the dimer). The net rate of electron transfer is determined by the population of bL- and the intrinsic bL - bL electron transfer rate. Since the forward rates within a monomer are clearly fast, the electron is initially in quasi-equilibrium within one monomer:
Here KbLbH is the equilibrium constant of electron transfer between bL and bH hemes; KbHQ, is the equilibrium constant of electron transfer from bH heme to Qi; kbLbL is the intrinsic rate constant for direct electron transfer between bL hemes (see also Fig. 1).
In this case the apparent rate constant of the MET can be approximated by the product of the intrinsic rate constant of the electron transfer between bL hemes and the quasi-equilibrium relative population of reduced bL in the monomer, kapp ≈ kbLbL{QibHbL−}. According to Scheme 1 the fraction of reduced bL is:
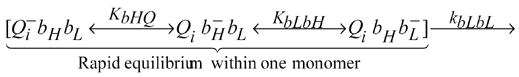
Scheme 1
| (1) |
The observed time for the “leaking” of electrons from the first to the second monomer is given by:
| (3) |
The equilibrium constant KbLbH for one-electron transfer from bL to bH, measured in the presence of myxothiazol and antimycin, is equal to ~20 in the absence of Δψ [40]. However, it is known that myxothiazol shifts the redox potential of bL by ~ 20 mV [41]. Thus, the value of 15–25 for the equilibrium constant KbLbH determined in the presence of myxothiazol [40] should correspond to a value of 30–50 in the absence of myxothiazol. In all further calculations we will assume that KbLbH=40 in the absence of myxothiazol.
The equilibrium constant between bH and quinone is ~3 (estimated from the difference in midpoint potentials of bH /bH− (~50 pH 7) and Q/Q− (~80 mV, pH 7)). If we take τbLbL ~0.5 ms, as estimated above for λ=1 eV, the apparent time of monomer-monomer electron transfer will be:
| (4) |
This estimate illustrates that the apparent time of monomer-monomer electron transfer could be substantially slower than the typical turnover time of the bc1 complex under uncoupled conditions (~1 ms when quinone pool is reduced, and ~ 10 ms when the quinone pool is oxidized). However, we should stress here that this estimate involves the absolute value of the rate constant kbLbL, which is based on the use of the distance-rate correlation. In contrast to the ratio of rates, it is significantly dependent on the value of the reorganization energy. As a result, the apparent time of MET also depends on this unknown value (Figure 3).
Figure 3.

Dependence of the apparent time of monomer-monomer electron transfer, τapp, on the reorganization energy, calculated from Eq. 3, with τbLbL calculated from Eq. 1. The equilibrium constant KbLbH for electron transfer from bL to bH is equal to 40. The equilibrium constant KbHQ, for electron transfer from bH heme to Qi is taken as 3; τbLbL =0.5 ms (see text); other parameters as for Fig. 2. Shaded area indicates the characteristic times of bc1 complex turnover.
If the Qi site of the initial monomer is already occupied by semiquinone, the new electron will form QH2, which will equilibrate rapidly with the pool. A similar description of the intramonomer equilibrium is valid, but includes unbinding of QH2. The overall equilibrium with the quinone pool will strongly deplete electrons from bL and the net rate of intermonomer transfer by this route will be negligible, and certainly not distinguishable from diffusion of QH2 between sites.
3.4. The transmembrane electric potential controls the monomer-monomer electron transfer
Due to the transmembrane orientation of the bL and bH hemes, the equilibrium constant of electron transfer between them, KbLbH, depends on the value of the transmembrane electric potential, Δψ:
| (5a) |
Here K0bLbH is the value of the equilibrium constant of electron transfer between bL and bH hemes at zero transmembrane potential, R is the gas constant, T is the absolute temperature, F is the Faraday constant, α is the fraction of Δψ applied between bH and bL. Because the center-to-center distance between bL and bH is about 20 Å, compared to a typical membrane thickness of 40Å, we have assumed a value of α=0.5. Structures of the bc1 complex indicate that electron transfer between bH heme and Qi has little transmembrane vectoriality. However, the reaction is clearly electrogenic [42–46] and this is now ascribed to electrogenic protonation of reduced Qi species [45,47]. Whatever the origin of the electrogenicity the equilibrium constant, KbHQ, is also dependent on Δψ, in much the same way as for bL-bH electron transfer:
| (5b) |
For the sake of illustration, we will assume that the two steps - bL-bH and bH-Qi – are equally electrogenic (α=0.5 for both equilibria), which is close to relative contributions suggested for the bc1 [43] and b6f [46] complexes. Combining equations 3 and 5, and assuming that RT/F = 25 mV, we have:
| (6) |
Equation 6 predicts that increasing Δψ, increases the population of reduced bL and accelerates the apparent electron transfer between monomers.
Figure 4 shows the dependence of the apparent time of electron transfer between monomers on the transmembrane electric potential. As Δψ is increased up to a high steady-state value (200–250 mV), the apparent intermonomer electron transfer accelerates by about two orders of magnitude. The absolute value of the time for MET depends significantly on the unknown reorganization energy and on γ (Fig. 4). For λ ≥ 0.7 eV, the rate under weakly coupled conditions (Δψ ≤ 70 mV) is slower than the corresponding turnover time of the bc1 complex (approx. 1 ms). However, at larger values of Δψ, the rate of MET speeds up and the rate of turnover slows, and the two become comparable. The choice of γ is significant and, for γ = 3.1, the rate of MET at Δψ ≥ 200 mV is only slower than the coupled turnover time for rather large values of λ (≥1.35 eV). For the classical form of the Marcus equation, with γ = 4.2, the MET rate remains slower than the coupled turnover time for λ ≥ 1.0 eV.
Figure 4.
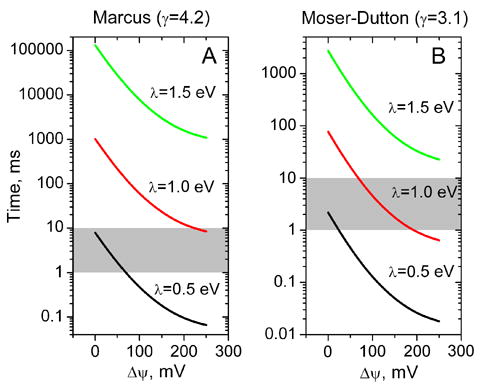
The dependence of the apparent time of electron transfer between monomers, τapp, on the transmembrane electric potential at different value of the reorganization energy, λ, calculated from Eq. 6. Parameters used for calculation are the same as for Fig. 3. Shaded area indicates the characteristic times of bc1 complex turnover.
In spite of the large uncertainty in the appropriate tunneling parameters for electron transfer between the b-hemes, the estimates of Fig. 4 illustrate that the apparent time of monomer-monomer electron transfer is likely to be significantly slower than the optimal turnover time of the bc1 complex under uncoupled conditions (~1 ms when the quinone pool is well poised), and even when the quinone pool is oxidized (~ 10 ms). However, they also suggest that MET can become a significant pathway under conditions of tight coupling, when Δψ achieves maximal levels.
3.5. The transition probability for direct bL-bL electron transfer is small even with a fully developed transmembrane electric potential
The one-step transition probability or branching ratio,
| (7) |
characterizes the fraction of complexes in which bL-bL electron transfer occurs instead of electron transfer to bH. The transmembrane electric potential influences this transition probability through the electric field dependence of kbLbH, but the large value of the ratio of the intrinsic rate constants for bL-bL and bL-bH electron transfer (Fig. 2) means that the probability remains small, even at the largest transmembrane potential. At 250 mV, the transition probability is still substantially less than 0.1 for all considered values of the reorganization energy. Thus, the main mechanism of intermonomer electron transfer necessarily involves pre-equilibration of the electron within one monomer, which is characterized by the observed (apparent) time for the “leaking” of electrons between monomers.
3.6. Direct bL-bL electron transfer is observed only when electron transfer between low and high potential hemes is impaired
The small branching ratio for an electron on bL (even in the presence of a transmembrane electric potential) means that direct bL-bL electron transfer, without prior equilibration within the initial monomer, is significant only when forward electron transfer to bH is impaired. This could occur when bH heme is partially reduced (when one electron is already present within the monomer, i.e., (QibH)−) or fully reduced (when 2 electrons are already present within the monomer, i.e., Qi−bH−). In this case direct bL-bL electron transfer could become significant for the continued function of the complex. Such “impaired” forward electron transfer due to pre-reduction of bH can arise from the Δψ dependence of KbHQ in the highly coupled, energized state, but also under reducing conditions, and in inhibited states. In all other cases, the inter-monomer electron transfer occurs only after the equilibration within the monomer.
3.7. Significance
We find that although the intrinsic rate for bL→bL electron transfer is substantial, it is slower than the forward rate from bL to bH and intermonomer electron transfer occurs significantly only after equilibration within the first monomer. Under uncoupled conditions, the effective rate of MET is then about 2-orders of magnitudes slower than the direct transfer (τapp ~102 ms under uncoupled conditions for λ=1 eV). As a result, MET is likely to be slower than the typical turnover of the bc1 complex (1–10 ms). However, under conditions that substantially decrease the equilibrium constant of electron transfer between bL and bH hemes (e.g., large transmembrane electric potential, or alkaline pH), the net rate of MET can approach and exceed the “respiratory controlled” turnover time of the bc1 complex.
Thus, MET allows the bc1 complex to adapt to tightly coupled conditions, when the membrane potential can lead to partial reduction of bH or even bL. MET facilitates equilibrium distribution of the reducing equivalents to minimize the chance that both b hemes are reduced in one monomer. Without MET, the distribution of electrons is expected to be statistical, with the relative accumulation of singly reduced monomers in which the electron is shared between Qi and bH and, to a lesser extent, bL. Intermonomer electron exchange minimizes the population of singly reduced monomers by allowing the dismutation of semiquinones in adjacent Qi sites to disproportionate. Nevertheless, if bH is pre-reduced in one monomer and a subsequent turnover in the same monomer produces reduced bL, direct bL-bL electron transfer also comes into play. Thus, MET can “relieve” the bc1 complex, by minimizing the chances of accumulating reduced bL. This, in turn, decreases the probability of metastable low-potential semiquinone at the Qo site, produced by transient oxidation of quinol by the iron sulfur center when oxidized bL is not available to take the other electron. This is widely thought to be responsible for the production of superoxide in the bc1 complex [48,49], and MET therefore serves to lower the propensity for superoxide production.
It has been known for some time that the bc1 complex possesses transhydrogenase activity [50], but the mechanism is unknown. MET accounts for this activity by allowing electrons from quinol in the Qi site of one monomer to reduce the quinone in the Qi site of the other monomer in a dimer. Although originally demonstrated with an unphysiological quinone (duroquinone), such transhydrogenase activity of complex III could be functional in the reduction of vitamin E [51,52]. This would be important for vitamin E recycling and antioxidant protection of membrane [53,54].
MET also provides a mechanism for semiquinone dismutase activity, meaning that when two semiquinones are present in each monomer, MET allows oxidation of one semiquinone and reduction of the other and release of Q and QH2 to the pool. This effectively restores the original Q-cycle model of Mitchell [55], in which one electron to Qi comes via the b hemes and the other from an alternate source (a dehydrogenase, originally). According to current versions of the Q-cycle, the first turnover of the bc1 complex, originating from quinol oxidation at the Qo site, leads to semiquinone formation at the Qi site. The only fate for this semiquinone in such models was waiting for the second turnover of the same Qo site. MET provides the additional possibility of semiquinone dismutation within the bc1 complex dimer with release of QH2. In fact, Mitchell and Moyle [56] proposed that the bc1 complex might function as a dimer, with Qi- from one monomer donating to the other, by ”local electron conduction”. MET, as we envision it, is a regulated mechanism to achieve this.
Acknowledgments
We are grateful to Tony Crofts for stimulating discussions. This work was supported by NIH grant GM 53508.
Abbreviations and Notations
- bc1 complex
ubiquinol:cytochrome c oxidoreductase
- bL and bH
low- and high-potential hemes of cytochrome b, respectively
- cyt
cytochrome
- Eh
redox potential of the medium
- Em
midpoint redox potential
- F
the Faraday constant
- ISP
Rieske iron-sulfur protein
- kapp
apparent rate constant of electron transfer between monomers
- KbHQ
the equilibrium constant of electron transfer from cyt bH to Qi
- KbLbH
the equilibrium constant of electron transfer between bL and bH hemes
- K0bLbH
the equilibrium constant of electron transfer between bL and bH hemes at zero transmembrane potential
- KΔψbLbH
the equilibrium constant of electron transfer between bL and bH hemes at transmembrane potential Δψ
- kbLbH
the intrinsic rate constant of electron transfer between bL and bH hemes in the same monomer
- kbLbL
the intrinsic rate constant of electron transfer between bL hemes
- MET
monomer-monomer electron transfer
- Q
coenzyme Q (ubiquinone)
- QH2
dihydroquinone (quinol)
- Qi site (Qo site)
quinone reducing (quinol oxidizing) site of bc1 complex
- r
edge-to-edge distance
- R
the gas constant
- Rb
Rhodobacter
- rbLbH
edge-to-edge distance between bL and bH hemes
- rbLbL
edge-to-edge distance between bL and bH hemes
- SQ
semiquinone
- T
the absolute temperature
- α
the fraction of Δψ applied between bH and bL
- γ
the coefficient in Eq.1, equal to either 4.2, or 3.1
- λ
the reorganization energy in eV
- Δλ
difference of reorganization energies for the reactions bL↔bH and bL↔bL
- Δψ
transmembrane electric potential
- τapp=1/kapp
apparent time of electron transfer between monomers, which takes into account the equilibration within the initial monomer
- τbLbL
intrinsic time of electron transfer between bL hemes
- ΔG0
the standard reaction free energy in eV
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry EA, Guergova-Kuras M, Huang LSARC. Structure and function of cytochrome bc complexes. Annu Rev Biochem. 2000;69:1005–1075. doi: 10.1146/annurev.biochem.69.1.1005. [DOI] [PubMed] [Google Scholar]
- 2.Trumpower BL, Gennis RB. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration - the enzymology of coupling electron-transfer reactions to transmembrane proton translocation. Annual Review of Biochemistry. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 3.Hunte C, Palsdottir H, Trumpower BL. Protonmotive pathways and mechanisms in the cytochrome bc1 complex. Febs Letters. 2003;545:39–46. doi: 10.1016/s0014-5793(03)00391-0. [DOI] [PubMed] [Google Scholar]
- 4.Crofts AR. The cytochrome bc1 complex: Function in the context of structure. Annual Review of Physiology. 2004;66:689–733. doi: 10.1146/annurev.physiol.66.032102.150251. [DOI] [PubMed] [Google Scholar]
- 5.Mulkidjanian AY. Ubiquinol oxidation in the cytochrome bc1 complex: Reaction mechanism and prevention of short-circuiting. Biochim Biophys Acta. 2005;1709:5–34. doi: 10.1016/j.bbabio.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Nalecz KA, Bolli R, Nalecz MJ, Azzi A. Monomeric and dimeric cytochrome c oxidase and bc1-complex - structural and functional analysis. Experientia. 1985;41:787–787. [Google Scholar]
- 7.Musatov A, Robinson NC. Detergent-solubilized monomeric and dimeric cytochrome bc1 Isolated from bovine heart. Biochemistry. 1994;33:13005–13012. doi: 10.1021/bi00248a009. [DOI] [PubMed] [Google Scholar]
- 8.Xia D, Yu CA, Kim H, Xian JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Xia D, Yu CA, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Inhibitor binding changes domain mobility in the iron-sulfur protein of the mitochondrial bc1 complex from bovine heart. PNAS. 1998;95:8026–8033. doi: 10.1073/pnas.95.14.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, et al. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 11.Iwata S, et al. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 12.Hunte C, Koepke J, Lange C, Rossmanith T, Michel H. Structure at 2.3 angstrom resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure. 2000;8:669–684. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 13.Berry EA, Huang LS, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. X-ray structure of Rhodobacter capsulatus cytochrome bc1: comparison with its mitochondrial and chloroplast counterparts. Photosynthesis Research. 2004;81:251–275. doi: 10.1023/B:PRES.0000036888.18223.0e. [DOI] [PubMed] [Google Scholar]
- 14.Huang LS, Cobessi D, Tung EY, Berry EA. Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc1 complex: a new crystal structure reveals an altered intramolecular hydrogen-bonding pattern. J Mol Biol. 2005;351:573–597. doi: 10.1016/j.jmb.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange C, Hunte C. Crystal structure of the yeast cytochrome bc1 complex with its bound substrate cytochrome c. PNAS. 2002;99:2800–2805. doi: 10.1073/pnas.052704699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries S. The pathway of electron transfer in the dimeric QH2: cytochrome c oxidoreductase. J Bioenerg Biomembr. 1986;18:195–224. doi: 10.1007/BF00743464. [DOI] [PubMed] [Google Scholar]
- 17.Nieboer P, Berden JA. Triple inhibitor titrations support the functionality of the dimeric character of mitochondrial ubiquinol-cytochrome-c oxidoreductase. Biochim Biophys Acta. 1992;1101:90–96. doi: 10.1016/0167-4838(92)90472-p. [DOI] [PubMed] [Google Scholar]
- 18.Gopta OA, Feniouk BA, Junge W, Mulkidjanian AY. The cytochrome bc1 complex of Rhodobacter capsulatus: ubiquinol oxidation in a dimeric Q-cycle? FEBS Lett. 1998;431:291–296. doi: 10.1016/s0014-5793(98)00768-6. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez-Cirlos EB, Trumpower BL. Inhibitory analogs of ubiquinol act anti-cooperatively on the yeast cytochrome bc1 complex - Evidence for an alternating, half-of-the-sites mechanism of ubiquinol oxidation. J Biol Chem. 2002;277:1195–1202. doi: 10.1074/jbc.M109097200. [DOI] [PubMed] [Google Scholar]
- 20.Thierbach G, Kunze B, Reichenbach H, Hofle G. The mode of action of stigmatellin, a new inhibitor of the cytochrome bc1 segment of the respiratory chain. Biochim Biophys Acta. 1984;765:227–235. [Google Scholar]
- 21.Schmitt ME, Trumpower BL. Subunit 6 regulates half-of-the-sites reactivity of the dimeric cytochrome bc1 complex in Saccharomyces-cerevisiae. J Biol Chem. 1990;265:17005–17011. [PubMed] [Google Scholar]
- 22.Slater EC. The mechanism of action of the respiratory inhibitor, antimycin. Biochim Biophys Acta. 1973;301:129–154. doi: 10.1016/0304-4173(73)90002-5. [DOI] [PubMed] [Google Scholar]
- 23.Bechmann G, Weiss H, Rich PR. Nonlinear inhibition curves for tight-binding inhibitors of dimeric ubiquinol-cytochrome c oxidoreductases - Evidence for rapid inhibitor mobility. Eur J Biochem. 1992;208:315–325. doi: 10.1111/j.1432-1033.1992.tb17189.x. [DOI] [PubMed] [Google Scholar]
- 24.Rieske JS, Gupta UD. On the sigmoidal relationship between inhibition of respiration and antimycin titer. FEBS Lett. 1972;20:316–320. doi: 10.1016/0014-5793(72)80095-4. [DOI] [PubMed] [Google Scholar]
- 25.Kröger A, Klingenberg M. The kinetics of redox reactions of ubiquinone related to the electron transport activity in the respiratory chain. Eur J Biochem. 1973;34:358–368. doi: 10.1111/j.1432-1033.1973.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 26.Moser CC, Paige CC, Cogdell RJ, Barber J, Wraight CA, Dutton PL. Length, time, and energy scales of photosystems. Adv Protein Chem. 2003;63:71–109. doi: 10.1016/s0065-3233(03)63004-4. [DOI] [PubMed] [Google Scholar]
- 27.Soriano GM, Ponamarev MV, Carrell CJ, Xia D, Smith JL, Cramer WA. Comparison of the cytochrome bc1 complex with the anticipated structure of the cytochrome b6f complex: De plus ca change de plus c'est la meme chose. J Bioenerg Biomembr. 1999;31:201–213. doi: 10.1023/a:1005463527752. [DOI] [PubMed] [Google Scholar]
- 28.Covian R, Trumpower BL. Rapid electron transfer between monomers when the cytochrome bc1 complex dimer is reduced through center N. J Biol Chem. 2005;280:22732–22740. doi: 10.1074/jbc.M413592200. [DOI] [PubMed] [Google Scholar]
- 29.Marres CAM, De Vries S. Reduction of the Q-pool by duroquinol via the 2 quinone-binding sites of the QH2-cytochrome-c oxidoreductase - a model for the equilibrium between cytochrome-b-562 and the Q-pool. Biochim Biophys Acta. 1991;1057:51–63. doi: 10.1016/s0005-2728(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Velasco J, Crofts AR. Complexes or super complexes – inhibitor titrations show that electron-transfer in chromatophores from Rhodobacter sphaeroides involves a dimeric UQH2 - cytochrome-c2 oxidoreductase, and is delocalized. Biochemical Society Transactions. 1991;19:588–593. doi: 10.1042/bst0190588. [DOI] [PubMed] [Google Scholar]
- 31.Crofts A, Guergova-Kuras M, Hong SJ. Chromatophore heterogeneity explains phenomena seen in Rhodobacter sphaeroides previously attributed to supercomplexes. Photosynthesis Research. 1998;55:357–362. [Google Scholar]
- 32.Gong X, Yu L, Xia D, Yu CA. Evidence for electron equilibrium between the two hemes bL in the dimeric cytochrome bc1 complex. J Biol Chem. 2005;280:9251–9257. doi: 10.1074/jbc.M409994200. [DOI] [PubMed] [Google Scholar]
- 33.Marcus RA, Sutin N. Electron transfers in chemistry and biology. Biochim Biophys Acta. 1985;811:265–322. [Google Scholar]
- 34.Moser CC, Page CC, Farid R, Dutton PL. Biological electron transfer. J Bioenerg Biomembr. 1995;27:263–274. doi: 10.1007/BF02110096. [DOI] [PubMed] [Google Scholar]
- 35.Humphrey W, Dalke A, Schulten K. VMD - visual molecular dynamics. J Molec Graphics. 1996;14.1:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 36.Likhtenshtein GI. Chemical Physics of Redox Metalloenzyme Catalysis. Springer-Verlag; Berlin: 1988. [Google Scholar]
- 37.Gray HB, Winkler JR. Electron transfer in proteins. Annu Rev Biochem. 1996;65:537–561. doi: 10.1146/annurev.bi.65.070196.002541. [DOI] [PubMed] [Google Scholar]
- 38.Gray HB, Winkler JR. Electron tunneling through proteins. Quarterly Reviews of Biophysics. 2003;36:341–372. doi: 10.1017/s0033583503003913. [DOI] [PubMed] [Google Scholar]
- 39.Moser CC, Farid TA, Chobot SE, Dutton PL. Electron tunneling chains of mitochondria. Biochim Biophys Acta. 2006;1757:1096–1109. doi: 10.1016/j.bbabio.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Shinkarev VP, Crofts AR, Wraight CA. The electric field generated by photosynthetic reaction center induces rapid reversed electron transfer in the bc1 complex. Biochemistry. 2001;40:12584–12590. doi: 10.1021/bi011334j. [DOI] [PubMed] [Google Scholar]
- 41.Howell N, Robertson DE. Electrochemical and spectral analysis of the longrange interactions between the Qo and Qi sites and the heme prosthetic groups in ubiquinol-cytochrome c oxidoreductase. Biochemistry. 1993;32:11162–11172. doi: 10.1021/bi00092a028. [DOI] [PubMed] [Google Scholar]
- 42.Selak MA, Whitmarsh J. Kinetics of the electrogenic step and cytochrome b6 and f redox changes in chloroplasts: evidence for a Q-cycle. FEBS Lett. 1982;150:286–292. [Google Scholar]
- 43.Robertson DE, Dutton PL. The nature and magnitude of the charge-separation reactions of ubiquinol cytochrome c2 oxidoreductase. Biochim Biophys Acta. 1988;935:273–291. doi: 10.1016/0005-2728(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 44.Glaser EG, Crofts AR. A new electrogenic step in the ubiquinol-cytochrome c2 oxidoreductase complex of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984;766:322–333. doi: 10.1016/0005-2728(84)90248-2. [DOI] [PubMed] [Google Scholar]
- 45.Drachev LA, Kaurov BS, Mamedov MD, Mulkidjanian AJ, Semenov AJ, Shinkarev VP, Skulachev VP, Verkhovsky MI. Flash-induced electrogenic events in the photosynthetic reaction center and bc complex of Rhodobacter sphaeroides chromatophores. Biochim Biophys Acta. 1989;973:189–197. [Google Scholar]
- 46.Jones RW, Whitmarsh J. Inhibition of electron transport and the electrogenic reaction in the cytochrome b/f complex by NQNO and DBMIB. Biochim Biophys Acta. 1988;933:258–268. [Google Scholar]
- 47.Mulkidjanian AY, Mamedov MD, Semenov AY, Shinkarev VP, Verkhovsky MI, Drachev LA. Partial reversion of the electrogenic reaction in the ubiquinol - cytochrome-c2-oxidoreductase of Rhodobacter sphaeroides chromatophores under neutral and alkaline conditions. FEBS Letters. 1990;277:127–130. doi: 10.1016/0014-5793(90)80825-4. [DOI] [PubMed] [Google Scholar]
- 48.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 49.Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 2006;11:473–485. doi: 10.1007/s10495-006-5881-9. [DOI] [PubMed] [Google Scholar]
- 50.Zweck A, Bechmann G, Weiss H. The pathway of the quinol/quinone transhydrogenation reaction in ubiquino1:cytochrome-c reductase of Neurospora mitochondria. Eur J Biochem. 1989;183:199–203. doi: 10.1111/j.1432-1033.1989.tb14913.x. [DOI] [PubMed] [Google Scholar]
- 51.Gille L, Gregor W, Staniek K, Nohl H. Redox-interaction of alpha-tocopheryl quinone with isolated mitochondrial cytochrome bc1 complex. Biochemical Pharmacology. 2004;68:373–381. doi: 10.1016/j.bcp.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 52.Gregor W, Staniek K, Nohl H, Gille L. Distribution of tocopheryl quinone in mitochondrial membranes and interference with ubiquinone-mediated electron transfer. Biochemical Pharmacology. 2006;71:1589–1601. doi: 10.1016/j.bcp.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Lass A, Sohal RS. Electron transport-linked ubiquinone-dependent recycling of alpha-tocopherol inhibits autooxidation of mitochondrial membranes. Arch Biochem Biophys. 1998;352:229–235. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- 54.Kagan VE, Fabisiak JP, Tyurina YY. Independent and concerted antioxidant functions of coenzyme Q. In: Kagan VE, Quinn PJ, editors. Coenzyme Q: Molecular Mechanisms in Health and Disease. CRC Press, Inc; Boca Raton, FL: 2000. pp. 119–129. [Google Scholar]
- 55.Mitchell P. Possible molecular mechanisms of the proton-motive function of cytochrome systems. J Theor Biol. 1976;62:327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell P, Moyle J. The role of ubiquinone and plastoquinone in chemiosmotic coupling between electron transfer and proton translocation. In: Lenaz G, editor. Coenzyme Q. John Wiley & Sons; New York: 1985. pp. 12–19. [Google Scholar]


