Abstract
Daidzin is the major active principle in extracts of radix puerariae, a traditional Chinese medication that suppresses the ethanol intake of Syrian golden hamsters. It is the first isoflavone recognized to have this effect. Daidzin is also a potent and selective inhibitor of human mitochondrial aldehyde dehydrogenase (ALDH-2). To establish a link between these two activities, we have tested a series of synthetic structural analogs of daidzin. The results demonstrate a direct correlation between ALDH-2 inhibition and ethanol intake suppression and raise the possibility that daidzin may, in fact, suppress ethanol intake of golden hamsters by inhibiting ALDH-2. Hamster liver contains not only mitochondrial ALDH-2 but also high concentrations of a cytosolic form, ALDH-1, which is a very efficient catalyst of acetaldehyde oxidation. Further, the cytosolic isozyme is completely resistant to daidzin inhibition. This unusual property of the hamster ALDH-1 isozyme accounts for the fact we previously observed that daidzin can suppress ethanol intake of this species without blocking acetaldehyde metabolism. Thus, the mechanism by which daidzin suppresses ethanol intake in golden hamsters clearly differs from that proposed for the classic ALDH inhibitor disulfiram. We postulate that a physiological pathway catalyzed by ALDH-2, so far undefined, controls ethanol intake of golden hamsters and mediates the antidipsotropic effect of daidzin.
The identification of pharmaceutical agents that selectively suppress compulsive human ethanol consumption has been a principal aim of biochemical and pharmacological studies of alcohol abuse, its causes, and control. However, the lack of a specific and well identified molecular target (receptor) for ethanol and a suitable animal model for human alcoholism have made this an elusive goal (1). While many central nervous system and ethanol-sensitizing agents have been shown to suppress the ethanol intake of animals commonly employed as models for the human problem, only acamprosate and naltrexone have been judged suitable for the treatment of this human ailment. Even those drugs are only marginally effective in a relatively minor segment of the afflicted population and then only when used in conjunction with psychotherapy (2–4). The manifestations of alcoholism are complex, encompassing both physical and psychiatric features seemingly unique to humans. Hence, it is not surprising that the extension of results from animal studies to the human situation has not resulted in the identification of agents that cure alcohol abuse.
The search for new antidipsotropic agents, nevertheless, calls for a laboratory animal that voluntarily consumes ethanol, ideally in significant amounts. For this purpose, we have chosen the Syrian golden hamster (Mesocricetus auratus) because of its known natural preference to consume large quantities of ethanol compared with water and its predictive validity (5). Neither selective breeding, special training, nor the addition of dietary sweeteners is required to manipulate or induce it to prefer ethanol. The physiological basis for this preference is unknown, but we have found that in addition to the mitochondrial aldehyde dehydrogenase isozyme (ALDH-2), hamster liver contains large amounts of a cytosolic isozyme (ALDH-1), which is also a very efficient catalyst of acetaldehyde metabolism (6). This feature has allowed us to differentiate the physiological function of ALDH-2 from its role in the detoxification of acetaldehyde in the course of ethanol metabolism.
To identify hitherto unknown antidipsotropic agents, we have elected a relatively unconventional approach by taking advantage of the vast clinical experience of earlier generations in China, where for millennia folk medicines have been used safely and effectively to treat humans who either abused or were addicted to ethanol. We have thoroughly searched the ancient Chinese pharmacopoeias and compiled a list of traditional remedies that include the 16 “stop drinking” formulae of Sun Simiao (about 600 A.D.) and the “anti-alcohol addiction” medicine of Li Dongyuan (about 1200 A.D.). However, like those employed by the early Romans, many of these remedies were based largely on psychological aversion and have long been abandoned, presumably because of their ineffectiveness or undesirable side effects. The only medications that have survived historical trial-and-error scrutiny are those based on flos puerariae (FP) and radix puerariae (RP), the flower and root of Pueraria lobata (the kudzu plant), respectively, which we have adopted as the basis of our search for a source of new therapeutically effective agents and their identities.
Our first experimental results, reported in 1993 (5, 7), confirmed the antidipsotropic effect of a crude RP extract in golden hamsters and identified daidzin and daidzein as the major active constituents of this source. Moreover, we also found that these isoflavones potently and selectively inhibit human ALDH-2 (8) and the γ-type alcohol dehydrogenase, respectively (9). We further established that daidzin, at a dose that significantly suppresses the ethanol intake of these animals, does not affect their overall ethanol and acetaldehyde metabolism (10). Thus, daidzin is clearly not an ethanol-sensitizing agent. However, we conjectured that it and other antidipsotropic isoflavones might act by inhibiting an as-yet-undefined physiological pathway that is catalyzed by ALDH-2 and/or γ-type alcohol dehydrogenase.
In the same study, we detected high concentrations of daidzin (70 μM) in the liver mitochondria of daidzin-treated hamsters. Moreover, daidzin potently inhibited acetaldehyde oxidation catalyzed by mitochondria isolated from hamster livers. Accordingly, we proposed that hamster ALDH-2, like its human counterpart, is also sensitive to daidzin inhibition much as it may not be the sole hamster isozyme that catalyzes acetaldehyde metabolism (10). The present study was undertaken to examine this hypothesis by characterizing the acetaldehyde-oxidizing activities of purified hamster liver ALDH isozymes and their inhibition by daidzin. Moreover, we have examined a series of structural analogs of daidzin to explore links between ALDH-2 inhibition and antidipsotropic activity. The results suggest that daidzin suppresses ethanol intake of Syrian golden hamsters by inhibiting ALDH-2.
MATERIALS AND METHODS
Human, hamster, and rat liver mitochondrial and cytosolic ALDH isozymes were purified as described (6). Puerarin (4′,7-dihydroxy-8-C-glucosylisoflavone) was a product of Indofine Chemical (Somerville, NJ), and chrysin (5,7-dihydroxyflavone) and 7,8-dihydroxyflavone were from Aldrich. Synthesis of daidzin and daidzein, 6-dimethylamino-2-naphthaldehyde (6-DMA-2-NA) and the 7-0-substituted daidzein derivatives were carried out in this laboratory by Werner Dafeldecker (10), Jacek Wierzchowski (11), and Barton Holmquist, respectively.
ALDH activity was assayed by monitoring the increase in absorbance at 340 nm due to the formation of NADH (ɛ340 = 6.22 mM−1·cm−1) in a Varian Cary 219 spectrophotometer when acetaldehyde was used as the substrate, or by monitoring the increase in fluorescence at 430 nm on formation of 6-DMA-2-NA (λex = 330 nm) in a Perkin–Elmer MPF3 spectrofluorimeter when 6-DMA-2-NA was used as the substrate. Stock solutions of all water-soluble substrates and test compounds were made in water purified by a Milli-Q system (Millipore), and all others were in methanol. The final concentrations of methanol in the assay mixtures were ≤1% and had no effect on the activities of the ALDH isozymes. The kinetic parameters Km and Vmax for cytosolic ALDHs were determined from Lineweaver–Burk plots derived from duplicate determinations of initial velocities, whereas those for the mitochondrial isozymes were determined from progress kinetic curves, details of which have been described (12).
RESULTS
Effect of Daidzin on Hamster, Human, and Rat Liver ALDH-2.
Low concentrations of daidzin (<0.1 μM) appear to inhibit human ALDH-2 competitively with respect to acetaldehyde (8). At higher concentrations, inhibition is clearly mixed: Vmax decreases and Km for acetaldehyde increases. The inhibition kinetics can be treated according to Eq. 1
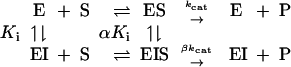 |
1 |
for which
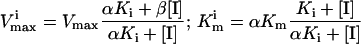 |
2 |
and
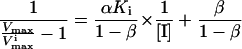 |
3 |
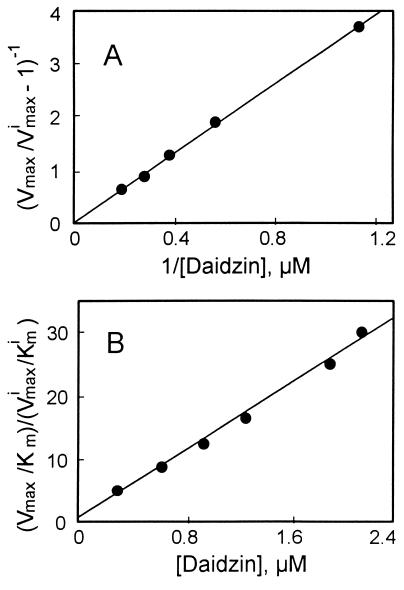
A replot of the kinetic constants according to Eq. 3 (Fig. 1A) shows that β is equal to zero, indicating that EIS does not form product. The noncompetitive inhibition constant αKi is determined from the slope in Fig. 1A where α is the “interference coefficient,” a measure of the degree of interference between substrate and inhibitor binding. The competitive inhibition constant Ki can be determined from Eq. 4 (see also Fig. 1B):
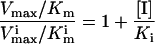 |
4 |
At pH 9.5, Ki and αKi for daidzin are 0.042 and 0.65 μM, respectively (Table 1), and the interference coefficient is 15.5, indicative of partially overlapping substrate and inhibitor binding sites. Thus, it would be expected that α will vary with different substrates and/or inhibitors. Daidzin does not compete with NAD+ binding at concentrations up to 3 μM.
Figure 1.
Daidzin is a mixed inhibitor of hamster liver ALDH-2 oxidation of acetaldehyde. Conditions: pH 9.5, 3 mM NAD+. (A) Replot of kinetic constants obtained from progress kinetic curves based on Eq. 3. The y-intercept is equal to 0 and hence β (Eq. 3) is equal to 0; the slope of the straight line is equal to αKi (0.65 μM), the noncompetitive inhibition constant. (B) Replot of kinetic constants based on Eq. 4; the slope of the straight line is equal to Ki−1 (23.8 μM).
Table 1.
Inhibition constants of daidzin with hamster, human, and rat liver (mitochondrial) ALDH-2
| Source | Km,* μM | pH 9.5
|
pH 7.5
|
||
|---|---|---|---|---|---|
| Ki, μM | αKi, μM | Ki, μM | αKi, μM | ||
| Hamster | 0.2 | 0.082 | 3.5 | 0.39 | 12 |
| Human | 0.2 | 0.042 | 0.65 | 0.29 | 3.1 |
| Rat | 0.2 | 0.052 | 1.2 | ND | ND |
ALDH activity was determined in 0.1 M sodium pyrophosphate (pH 9.5) or 0.1 M sodium phosphate (pH 7.5) containing 3 mM NAD+ and various concentrations of acetaldehyde and daidzin. ND, not determined.
Taken from ref. 6.
Daidzin also potently inhibits hamster and rat ALDH-2 and, as for the human enzyme, the inhibition is mixed. The daidzin Ki values for hamster and rat ALDH-2 are 0.082 and 0.052 μM, respectively, slightly higher than that for the human enzyme (Table 1). The αKi value for the hamster enzyme (3.5 μM) is more than 5 times higher than that of the human, with an α value of 42.7. Daidzin inhibition of both human and hamster ALDH-2 is pH dependent. The competitive and noncompetitive inhibition constants measured at pH 7.5 are about 3 to 7 times higher than those obtained at pH 9.5 (Table 1).
Effect of Daidzin on Hamster, Human, and Rat ALDH-1.
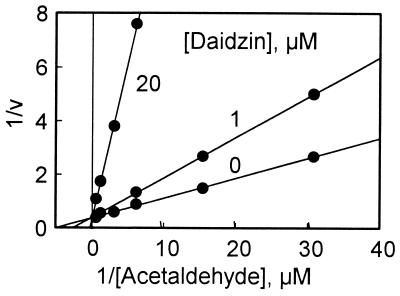
Compared with ALDH-2, the Km values of hamster and rat ALDH-1 for acetaldehyde are much higher (12 and 15 μM, respectively) and both enzymes are completely resistant to daidzin inhibition. Daidzin at a concentration up to 50 μM does not affect the activity of hamster or rat ALDH-1. Hamster liver cytosol also contains a high Km ALDH isozyme (ALDH-3), which is also resistant to daidzin inhibition (Table 2). Unlike the hamster and rat enzymes, human ALDH-1 has a substantially higher Km (180 μM) for acetaldehyde and is more susceptible to daidzin inhibition. Nevertheless, the inhibition constant, Ki, determined at pH 9.5 with acetaldehyde as substrate, is 28 μM, almost 700 times higher than that for ALDH-2 (Tables 1 and 2). Further, daidzin inhibition of human ALDH-1 is purely competitive with respect to acetaldehyde (Fig. 2).
Table 2.
Effect of daidzin on hamster, human, and rat cytosolic ALDH isozymes
| Source | Isozyme | Km,* μM | Ki, μM |
|---|---|---|---|
| Hamster | ALDH-1 | 12 | NI |
| ALDH-3 | 3000 | NI | |
| Human | ALDH-1 | 180 | 28 |
| Rat | ALDH-1 | 15 | NI |
ALDH activity was determined in 0.1 M sodium pyrophosphate (pH 9.5) containing 3 mM NAD+ and various concentrations of acetaldehyde and daidzin. NI, no inhibition up to 50 μM daidzin.
Taken from ref. 6.
Figure 2.
Competitive inhibition of human liver ALDH-1-catalyzed acetaldehyde oxidation by daidzin. Conditions: pH 7.5, 3 mM NAD+.
Acetaldehyde-Oxidizing Capacities of Mitochondrial and Cytosolic ALDHs.
The potential inhibitory effect of daidzin on overall acetaldehyde metabolism in hamster, human, and rat liver can be estimated from the acetaldehyde-oxidizing capacities of the respective mitochondrial and cytosolic ALDHs (Table 3). Among the three species studied, the total acetaldehyde-oxidizing capacity of hamster liver is much higher (3.5 μmol/min per g of liver), relative to that of either the rat (0.64 μmol/min per g of liver) or the human (0.45 μmol/min per g of liver). About half of the hamster liver acetaldehyde-oxidizing capacity is attributable to the daidzin-sensitive ALDH-2 and the other half to the daidzin-insensitive ALDH-1. The Km of hamster ALDH-3 for acetaldehyde is so high (Table 2) that under the conditions specified its contribution to acetaldehyde oxidation is negligible (Table 3). Only a small amount of ALDH-1 is present in the cytosol of human and rat livers. Thus, daidzin-sensitive ALDH-2 accounts for virtually all of the acetaldehyde-oxidizing capacity of these tissues.
Table 3.
Acetaldehyde-oxidizing capacities of human, hamster, and rat liver ALDH isozymes
| Source | Isozyme | Acetaldehydeoxidizing capacity, μmol/min per g of liver | Sensitive to daidzin inhibition |
|---|---|---|---|
| Hamster | |||
| Mitochondrial | ALDH-2 | 1.9 | Yes |
| Cytosolic | ALDH-1 | 1.6 | No |
| Cytosolic | ALDH-3 | 0 | No |
| Human | |||
| Mitochondrial | ALDH-2 | 0.43 | Yes |
| Cytosolic | ALDH-1 | 0.02 | No |
| Rat | |||
| Mitochondrial | ALDH-2 | 0.59 | Yes |
| Cytosolic | ALDH-1 | 0.05 | No |
Acetaldehyde-oxidizing capacities of ALDH-1 and ALDH-2 were calculated on the basis of the amounts of each of these isozymes found in freshly prepared liver homogenates, the Km and kcat values determined at pH 7.5 (taken from ref. 6), and an acetaldehyde concentration of 20 μM. The amounts of ALDH-1 and ALDH-2 in these homogenates were determined by the disulfiram titration method using 6-DMA-2-NA as substrate as described (6).
Inhibition of ALDH-2 by Synthetic 7-O-Substituted Daidzein Derivatives.
Our 1993 report noted that isoflavones with a blocked 7-hydroxyl group were the most potent inhibitors of ALDH-2 (8). Daidzein, the aglycone of daidzin, also inhibits ALDH-2, but its potency is at least 200 times less than that of daidzin. Therefore, a series of 7-O-substituted daidzein derivatives were synthesized and their effects on ALDH-2 were tested (Table 4). The results indicate that all of the 7-O-substituted daidzein derivatives are better ALDH-2 inhibitors than daidzein, and 10 of them (compounds 3–12) are even better than daidzin. It appears that the long chain 7-O-(ω-carboxyalkyl) derivatives of daidzein are the most potent inhibitors of ALDH-2 (compounds 3–6). Puerarin, the 8-C-glucosyl derivative of daidzein, and the two flavonoid compounds, chrysin (5,7-dihydroxyflavone) and 7,8-dihydroxyflavone, are poor inhibitors of human ALDH-2.
Table 4.
ALDH-inhibitory and ethanol intake-suppressive activities of daidzein and its 7-O-substituted analogs
| Analog no. | ALDH-2†
|
Ethanol intake suppression,‡ % | ||
|---|---|---|---|---|
| Ki, μM | αKi, μM | |||
| 1 | H— (daidzein) | 9.2 | 180 | 32 |
| 2 | Glc— (daidzin) | 0.042 | 0.65 | 62 |
| 3 | HOOC(CH2)5— | 0.009 | 0.15 | 70 |
| 4 | HOOC(CH2)6— | 0.009 | 0.14 | 69 |
| 5 | HOOC(CH2)9— | 0.004 | 0.05 | ND |
| 6 | HOOC(CH2)10— | 0.003 | 0.04 | ND |
| 7 | CH3CH2— | 0.035 | 0.56 | ND |
| 8 | Br(CH2)6— | 0.003 | 0.07 | ND |
| 9 | (CH3CH2)3N+(CH2)6— | 0.005 | 0.08 | ND |
| 10 | NH2(CH2)6— | 0.036 | 0.60 | ND |
| 11 | Br(CH2)6CO— | 0.02 | 0.26 | ND |
| 12 | (CH3CH2)3N+(CH2)2— | 0.08 | 1.6 | ND |
| 13 | ClCH2CO— | 1.06 | 10.6 | ND |
| 14 | BrCH2CO— | 1.8 | 20 | ND |
| 15 | (CH3)2N(CH2)3CO— | 1.75 | 24 | ND |
| 16 | Puerarin | 15 | 440 | 0 |
| 17 | Chrysin | 35 | 110 | 0 |
| 18 | 7,8-Dihydroxyflavone | 35 | 270 | 0 |
R refers to the 7-O-substituents. Compounds 1-7 were synthesized in our laboratory and identified by NMR and mass spectrometry. Compounds 8-15 were synthesized in our laboratory and their structures were deduced from the chemistry of synthesis. Puerarin was purchased from Sigma, and chrysin and 7,8-dihydroxyflavone were from Aldrich.
ALDH-2 activity was assayed at pH 9.5 using acetaldehyde as the substrate. At pH 7.5, the inhibition constants Ki and αKi of compounds 2, 3, 4, and 5 for ALDH-2 are 0.29 and 3.6, 0.016 and 270, 87 and 760, and 53 and 780 μM, respectively; the Ki values for compounds 2 and 4 for ALDH-1 are 30 and 370 μM, respectively.
Ethanol intake-suppressive activity of each compound was measured as described in ref. 8. Dose = 70 meq per hamster per day, i.p. ND, not determined.
Antidipsotropic Activity of 7-O-Substituted Daidzeins.
The antidipsotropic effect of two of the strongest [7-O-(ω-carboxypentyl)- and 7-O-(ω-carboxyhexyl)-daidzeins] and three of the weakest (puerarin, chrysin, and 7,8-dihydroxyflavone) ALDH-2 inhibitors were tested in ethanol-preferring golden hamsters. Daidzin and daidzein also were tested at equivalent doses for comparison. As previously reported, both daidzin and daidzein effectively suppress ethanol intake, the former being more potent (5, 7). An i.p. dose of daidzin or daidzein (70 meq per hamster per day) suppresses hamster ethanol intake by 62% or 32%, respectively (Table 4). At equivalent doses, puerarin, chrysin, and 7,8-dihydroxyflavone do not exert any significant effect on ethanol intake, whereas the two 7-O-(ω-carboxyalkyl)-daidzeins are somewhat better than daidzin (about 70% suppression).
DISCUSSION
The discovery of a new and effective antidipsotropic agent depends heavily on the availability of a suitable and reliable experimental animal. An ideal animal model would mimic the metabolic, physical, and cerebral responses of humans to ethanol and serve as a guide for the therapeutic effectiveness of new agents to modify human ethanol consumption. Such an experimental system has long been sought by, e.g., selective breeding, extensive training, and other experimental variations. Numerous animal (primarily rat) models have been created, but none has proved to be completely satisfactory. Most importantly, none has led to a suitable antidipsotropic agent effective in controlling alcohol abuse. For reasons detailed elsewhere, we have adopted Syrian golden hamsters as the species for our experiments. They have a prodigious appetite for ethanol, which on the one hand facilitates identification of active agents, and on the other directs attention to the biochemical mechanisms underlying this behavior, which is very unusual among rodents.
The choice of a source from which to isolate a potential antidipsotropic agent for detailed study is equally critical. RP was chosen as a consequence of our intensive, anthropological search of information contained in the ancient Chinese pharmacopoeias. RP- and FP-based medications are the only ones to have survived a millennium of trial and error in humans. Interviews with traditional Chinese physicians (see footnote in ref. 5), identified these medications as the only principal ones to continue to be used safely and effectively to treat alcohol abuse in China. This, of course, is the reason for our expectation that extracts of RP and/or FP would contain safe and efficacious antidipsotropic principles.
Chromatography of RP extracts led to the identification of daidzin and daidzein as potent inhibitors of ALDH-2 and γ-type alcohol dehydrogenase, respectively (8, 9). Both compounds later were shown to suppress ethanol intake of golden hamsters. These isoflavones are major chemical constituents of RP extracts, and to our knowledge they are the first isoflavones found to exhibit these properties. The concentration of daidzin in RP extract is at least 8 times higher than that of daidzein (13). Further, daidzin is about 3 times more potent in suppressing ethanol intake of golden hamsters (7). Hence, we have focused our research attention on daidzin and its ALDH-2 inhibitory and ethanol intake-suppressive activity.
To identify more potent ALDH-2 inhibitors and to establish a potential link between ALDH-2 inhibition and ethanol-intake suppression, we examined a number of structural analogs of daidzin for their effects on both ALDH-2 activity and hamster ethanol consumption and found a positive correlation (Table 4)—the stronger the ALDH-2 inhibition, the greater the ethanol-intake suppression. The poorest ALDH-2 inhibitors do not detectably affect ethanol consumption at the doses tested. Although the precise site and mechanism of action of these antidipsotropic isoflavones are unknown, these findings strongly implicate the involvement of ALDH-2.
In principle, inhibition of ALDH-2 could affect ethanol consumption in at least two ways. It might serve as an ethanol-sensitizing agent by blocking acetaldehyde metabolism subsequent to drinking and thereby allow acetaldehyde to reach toxic levels. Alternatively, it could disrupt an as-yet-undefined physiological pathway involving ALDH-2 and alter the concentration of some intrinsic metabolite that suppresses the appetite for ethanol directly. To determine if daidzin acts as an ethanol-sensitizing agent, we studied its effect on acetaldehyde and ethanol metabolism in hamsters. A dose of daidzin that significantly suppresses ethanol intake does not inhibit either acetaldehyde or ethanol metabolism (10). This clearly eliminates an ethanol-sensitizing mechanism. It should be noted that had we used rats for these studies we would not have been able to differentiate between these two alternatives because the physiological function of ALDH-2 is unknown and because in rat liver ALDH-2 is the only enzyme that catalyzes the oxidation of acetaldehyde efficiently. In contrast, hamster liver contains not only mitochondrial ALDH-2 but also large amounts of cytosolic ALDH-1, which plays a major role in the detoxification of acetaldehyde (Table 3) even in the presence of daidzin, to which it is completely insensitive (Table 2).
Mitochondria have been shown to catalyze monoamine metabolism (14) and, hence, it is possible that mitochondrial ALDH-2 plays an important role in serotonin and dopamine metabolism owing to its low Km values toward the aldehyde metabolites of these neurotransmitters (15). The antidipsotropic isoflavones may act via the serotoninergic and/or dopaminergic pathways by inhibiting the metabolism of serotonin and/or dopamine. The role of ALDH-2 in mitochondria-catalyzed serotonin and dopamine metabolism and its inhibition by antidipsotropic isoflavones now are being investigated.
Daidzin very potently inhibits ALDH-2 not only in human and hamster livers but also in those of the rat (Table 1). On this basis, one would expect that daidzin also would suppress ethanol intake in rats. Indeed, we have demonstrated an antidipsotropic effect of daidzin in rats employing a two-lever, free-choice (ethanol vs. an isocaloric starch solution), self-administration procedure (16). This study was the first to demonstrate that daidzin remains effective in an experimental setting in which the animal must work to obtain ethanol, and further that suppression of ethanol intake by daidzin does not reflect an overall suppression of appetite. This is of particular importance because calories play only a minor role, if any, in the regulation of human ethanol consumption. The antidipsotropic effect of daidzin, daidzein, and a crude extract containing both of them subsequently has been confirmed by others using Fawn Hooded and P rats (17–19) under various conditions, including the two-bottle free-choice, limited-access, and alcohol-deprived paradigms. These findings further reinforce our belief that RP and its isoflavones can be used safely and effectively in the treatment of human ethanol addiction.
Rats and golden hamsters respond differently to puerarin, another isoflavone isolated from RP. Puerarin appears to suppress ethanol intake in rats (14, 19) but has little or no effect on that in golden hamsters (5, 7). This could reflect differences in pharmacokinetic and/or pharmacodynamic processes of this isoflavone in the two species, or it also could indicate differences in the molecular mechanisms that govern their ethanol drinking. The fact that puerarin and two structurally related analogs, chrysin and 7,8-dihydroxyflavone, do not suppress ethanol intake in golden hamsters suggests that a specific mechanism might be involved in the suppression of ethanol drinking in this species. The effects of chrysin and 7,8-dihydroxyflavone on ethanol intake of rats have not been tested (16–19). Much as comparative studies with hamsters and rats might prove helpful in elucidating the mechanism(s) of action of these antidipsotropic compounds, the ultimate proof of efficacy and safety of the antidipsotropic isoflavones can be achieved only by trials in humans.
Unlike most antidipsotropic agents described previously, the isoflavones were discovered based solely on empirical clinical information rather than on preconceived theories or hypotheses derived from animal experiments. Thus, whatever is learned about the mechanism of action of these compounds not only will provide clues crucial to the development of safer and more efficacious drugs for alcoholism but also may hold the key to the delineation of the specific biochemical pathway(s) that influence ethanol drinking.
Acknowledgments
We thank Drs. D. J. Li for assistance in the animal drinking experiments, Barton Holmquist for synthesizing the daidzin derivatives, Werner Dafeldecker for synthesizing daidzin and daidzein, and J. F. Riordan and T. C. French for valuable discussions. This work was supported by the Endowment for Research in Human Biology, Inc.
ABBREVIATIONS
- FP
flos puerariae
- RP
radix puerariae
- ALDH-1
cytosolic aldehyde dehydrogenase
- ALDH-2
mitochondrial aldehyde dehydrogenase
- 6-DMA-2-NA
6-dimethylamino-2-naphthaldehyde
References
- 1.Vallee B L. In: Exploring the Universe: Essays on Science and Technology. Day P, editor. Oxford: Oxford Univ. Press; 1996. in press. [Google Scholar]
- 2.Lhuintre J P, Moore N, Tran G, Steru L, Langrenon S, Daoust M, Parot P, Ladure P, Libert C, Boismare F, Hillemand B. Alcohol Alcohol. 1990;25:613–622. doi: 10.1093/oxfordjournals.alcalc.a045057. [DOI] [PubMed] [Google Scholar]
- 3.Volpicelli J R, Alterman A L, Hayachida M, O’Brien C P. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 4.O’Malley S S, Jaffe A J, Chang G, Schottenfeld R S, Meyer R E, Rounsaville B. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 5.Keung W M, Vallee B L. Proc Natl Acad Sci USA. 1993;90:10008–10012. doi: 10.1073/pnas.90.21.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klyosov A A, Rashkovetsky L G, Tahir M K, Keung W M. Biochemistry. 1996;35:4445–4456. doi: 10.1021/bi9521093. [DOI] [PubMed] [Google Scholar]
- 7.Keung W M, Vallee B L. EXS. 1994;71:371–381. doi: 10.1007/978-3-0348-7330-7_37. [DOI] [PubMed] [Google Scholar]
- 8.Keung W M, Vallee B L. Proc Natl Acad Sci USA. 1993;90:1247–1251. doi: 10.1073/pnas.90.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keung W M. Alcohol Clin Exp Res. 1993;17:1254–1260. doi: 10.1111/j.1530-0277.1993.tb05238.x. [DOI] [PubMed] [Google Scholar]
- 10.Keung W M, Lazo O, Kunze L, Vallee B L. Proc Natl Acad Sci USA. 1995;92:8990–8993. doi: 10.1073/pnas.92.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wierzchowski J, Dafeldecker W P, Holmquist B, Vallee B L. Anal Biochem. 1989;178:57–62. doi: 10.1016/0003-2697(89)90356-4. [DOI] [PubMed] [Google Scholar]
- 12.Rashkovetsky L G, Maret W, Klyosov A A. Biochim Biophys Acta. 1994;1205:301–307. doi: 10.1016/0167-4838(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 13.Keung W M, Lazo O, Kunze L, Vallee B L. Proc Natl Acad Sci USA. 1996;93:4284–4288. doi: 10.1073/pnas.93.9.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tank A W, Weiner H. Biochem Pharmacol. 1979;28:3139–3148. doi: 10.1016/0006-2952(79)90624-5. [DOI] [PubMed] [Google Scholar]
- 15.Ambroziak W, Pietruszko R. J Biol Chem. 1991;266:13011–13018. [PubMed] [Google Scholar]
- 16.Heyman G M, Keung W M, Vallee B L. Alcohol Clin Exp Res. 1996;20:1083–1087. doi: 10.1111/j.1530-0277.1996.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 17.Overstreet D H, Lee Y W, Rezvani A H, Pei Y H, Criswell H E, Janowsky D S. Alcohol Clin Exp Res. 1996;20:221–227. doi: 10.1111/j.1530-0277.1996.tb01633.x. [DOI] [PubMed] [Google Scholar]
- 18.Overstreet D H, Rezvani A H, Lee Y W. Alcohol Clin Exp Res. 1996;20:16A. doi: 10.1111/j.1530-0277.1996.tb01633.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin R C, Guthrie S, Xie C-Y, Mai K, Lee D Y, Lumeng L, Li T-K. Alcohol Clin Exp Res. 1996;20:659–663. doi: 10.1111/j.1530-0277.1996.tb01668.x. [DOI] [PubMed] [Google Scholar]