Abstract
Phosphorylation of the p53 tumor suppressor protein is known to modulate its functions. Using bacterially produced glutathione S-transferase (GST)-p53 fusion protein and baculovirus-expressed histidine-tagged p53 (Hisp53), we have determined human p53 phosphorylation by purified forms of jun-N-kinase (JNK), protein kinase A (PKA), and β subunit of casein kinase II (CKIIβ) as well as by kinases present in whole cell extracts (WCEs). We demonstrate that PKA is potent p53 kinase, albeit, in a conformation- and concentration-dependent manner, as concluded by comparing full-length with truncated forms of p53. We further demonstrate JNK interaction with GST-p53 and the ability of JNK to phosphorylate truncated forms of GST-p53 or full-length Hisp53. Dependence of phosphorylation on conformation of p53 is further supported by the finding that the wild-type form of p53 (p53wt) undergoes better phosphorylation by CKIIβ and by WCE kinases than mutant forms of p53 at amino acid 249 (p53249) or 273 (p53273). Moreover, shifting the kinase reaction’s temperature from 37°C to 18°C reduces the phosphorylation of mutant p53 to a greater extent than of p53wt. Comparing truncated forms of p53 revealed that the ability of CKIIβ, PKA, or WCE kinases to phosphorylate p53 requires amino acids 97–155 within the DNA-binding domain region. Among three 20-aa peptides spanning this region we have identified residues 97–117 that increase p53 phosphorylation by CKIIβ while inhibiting p53 phosphorylation by PKA or WCE kinases. The importance of this region is further supported by computer modeling studies, which demonstrated that mutant p53249 exhibits significant changes to the conformation of p53 within amino acids 97–117. In summary, phosphorylation-related analysis of different p53 forms in vitro indicates that conformation of p53 is a key determinant in its availability as a substrate for different kinases, as for the phosphorylation pattern generated by the same kinase.
The p53 tumor suppressor protein is a potent transcription factor that is activated in response to a variety of DNA-damaging agents (1–3), leading to cell cycle arrest at the G1/S boundary or to induction of apoptosis (4–6). Disruption of this pathway occurs in a wide variety of human cancers and is highly correlated with the development of the tumorigenic phenotype (3, 7).
Four major functional domains of p53 have been identified (reviewed in ref. 3). At the N terminus is a transcriptional activation domain (amino acids 1–43); transactivation by wild-type p53 has been demonstrated for several regulatory proteins, including GADD45 (8), mdm2 (9), WAF1/p21/CIP1 (10), and cyclin G (11). Within the central part is the sequence-specific DNA-binding domain (amino acids 100–300; ref. 12), which accommodates most of the mutations found so far (3), and the C-terminal portion contains an oligomerization domain and a regulatory domain (amino acids 319–393; ref. 13), the latter of which has been implicated in binding to damaged DNA (14) and in apoptosis (15).
p53 can be phosphorylated in vitro at multiple sites by a variety of protein kinases, including casein kinase II (CKII; ref. 16), cdc2 kinase (17), DNA-dependent protein kinase (18), mitogen-activated protein kinase (19), and protein kinase C (20). Phosphorylation-related function has been demonstrated to affect sequence-specific DNA binding of p53 (9, 13, 21), transcriptional activities (22, 23), simian virus 40 DNA replication (24), growth arrest (25), or to block cellular transformation by dominant oncogenes (26).
We have hypothesized that the complexity of p53 phosphorylation may result from its overall conformation, which is expected to undergo changes due to mutation or association with other proteins. Accordingly, using full-length, truncated, or mutated forms of p53, we have elucidated conformation-related phosphorylation of the human p53 tumor suppressor protein.
MATERIALS AND METHODS
Purification of p53 Proteins.
Recombinant bacterially produced wild-type, truncated, and mutant glutathione S-transferase (GST)-p53 have been described (27). Bacterial extracts were prepared, and p53 was purified from lysates with glutathione beads (Sigma). Baculovirus expressing histidine-tagged p53 (Hisp53) were produced in Sf-9 cells followed by purification on nickel beads (Qiagen, Chatsworth, CA) as previously described (11). The purity of the beads-bound p53 was confirmed by SDS/PAGE followed by silver-staining, revealing single bands of the expected molecular weight (not shown).
Antibodies.
Antibodies against p53 were purchased (Ab-1; Oncogene Science). Antibodies against c-jun and ATF2 were bought (Santa Cruz Biotechnology). Antibodies against jun-N-kinase (JNK) (clone 666), which recognize both JNK1 and JNK2 isoforms (28), were obtained from PharMingen. SDS/PAGE and Western blotting analysis were performed as previously described (28).
Protein Kinase Assays.
Using glutathione-bound GST-p53 or nickel beads-bound Hisp53 as a substrate, p53 phosphorylation was studied in a solid-phase kinase reaction. Briefly, aliquots of p53 (2 μg) were incubated with the respective test kinase, [γ-32P]ATP (50 cpm/fmol; Amersham) for 15 min in the presence of 20 μM ATP and kinase buffer (20 mM Tris·HCl, pH 7.8/5 mM MgCl2/150 mM NaCl/1 mM DTT/0.5 mM phenylmethylsulfonyl fluoride/0.5% Nonidet P-40). After extensive washing with kinase buffer, the phosphorylated p53 was eluted in SDS sample buffer, separated on an SDS-15% polyacrylamide gel, which then was dried and autoradiographed. Quantification was performed with a computerized radioimaging blot analyzer (AMBIS).
For phosphorylation of p53 by PKA, the catalytic subunit (Sigma) was used (80 ng; unless otherwise indicated). p53 phosphorylation by CKIIβ (Sigma) was done in the presence of 40 ng of purified kinase (Sigma). Phosphorylation by JNK was performed by using 200 ng of a purified form of bacterially produced JNK (BioMol, Plymouth, PA). For p53 phosphorylation with whole cell extract (WCE) kinases, 2 μg of WCE prepared from human fibroblasts was used per reaction.
Peptide mapping of p53 phosphorylation was performed on the SDS/PAGE bands after their electroblotting onto poly(vinylidine difluoride) (Millipore) and identification by autoradiography. The band, corresponding to p53, was excised and oxidized by incubation with formic acid (6 M) and H2O2 (1 M) on ice for 1 h. The membrane-bound p53 then was incubated (twice for 2 h at 37°C, and once overnight) with trypsin (2 μg/75 μl). Peptides extracted from the poly(vinylidine difluoride) membrane were concentrated, washed, and separated on isoelectricfocusing PAGE (600 V for 2 h) with a pH range of 3.5 to 10.
In vivo phosphorylation was performed as previously described (28). Briefly, cells were incubated in phosphate-depleted medium followed by addition of [32P]orthophosphate (1 mCi/ml; 1 Ci = 37 Gbq) for 2 h before proteins were isolated, immunoprecipitated with antibodies to p53, and analyzed directly or after their cleavage for peptide mapping.
Peptides Synthesis.
Peptides were synthesized by the solid-phase method using Fmoc chemistry and purified by HPLC as described (29). The identity of the peptide was confirmed by time-of-flight mass spectroscopy (Chemical Analysis Services, Science Applications International Corporation, Frederick, MD) or electron spray mass spectroscopy (kindly performed by Ed Unsworth, U. S. Food and Drug Administration, Bethesda, MD). Peptide purities as assessed by analytical HPLC on a Vydac C18 peptide and protein column were >98%. The p53 peptides synthesized were P7 (amino acids 97–117), VPSQKTYHGSYGFRLGFLHSG; P8 (amino acids 115–135), HSGTAKSVTCTYSPDLNKMFC; P9 (amino acids 133–153), MFCQLAKTCPVQLWVDSTPPP; P10 (amino acids 97–112), VPSQKTYHGSYGFRLG; and P11 (amino acids 102–112), TYHGSYGFRLG.
Conformational Energy Calculations.
Two types of conformational energy calculations were performed. Molecular dynamics calculations, including the effects of solvation, were performed on both proteins that were equilibrated at 300 K. Dynamics trajectories were obtained over a 200-ps time frame (30, 31). The last 50 structures on this trajectory were used for computing the average structure as described previously (31). In addition, electrostatically driven Monte Carlo calculations (based on empirical conformational energies for peptides program; refs. 32, 33) were performed on the same proteins. The energy and rms requirements for saving low-energy structures were calculated as previously described (32).
RESULTS
Phosphorylation of Full-Length and Truncated Forms of GST-p53 by WCE Kinases and CKII.
Protein kinases present in WCE were capable of phosphorylating both full-length (80-kDa) and truncated forms of GST-p53, albeit at different efficiencies. The p53 construct, which lacks amino acids 1–155, exhibited the lowest phosphorylation levels, suggesting that this region is important for phosphorylation of p53 by cellular kinases (Fig. 1A). To elucidate the nature of the kinases that phosphorylate GST-p53 we have tested the ability of CKIIβ, protein kinase (PKA), and JNK to phosphorylate truncated and full-length GST-p53 constructs in vitro.
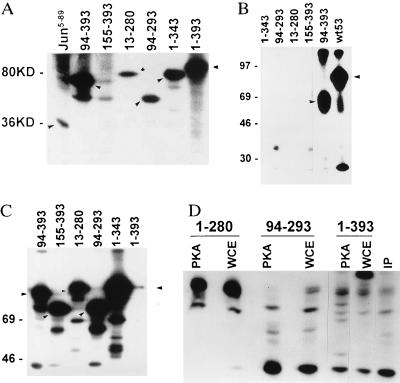
Figure 1.
(A) Effect of WCE kinases on phosphorylation of p53. GST-p53 wild-type (amino acids 1–393; molecular mass 80 kDa) or truncated forms (as indicated by arrows) were incubated with WCE as source of kinases. The solid-phase kinase reaction initiated by addition of [γ-32P]ATP for 15 min at room temperature, followed by washes of glutathione beads-bound GST-p53 and elution of p53 from beads by SDS-loading buffer. Samples were separated on SDS/15% PAGE and autoradiographed. Molecular masses are indicated at left in kDa. GST-c-jun5–89 used as control is shown in the left lane. (B) CKII phosphorylation of p53. Solid-phase kinase assay with respective GST-p53 proteins (position of major phosphorylation site indicated by arrow) was performed in the presence of 40 ng of the catalytic subunit of CKIIβ. (C) PKA phosphorylation of p53. GST-p53 proteins used for solid-phase kinase reaction using catalytic subunit of PKA (80 ng) as source of kinase. Position of major phosphorylation site is indicated by arrows. (D) Mapping of PKA phosphorylation sites on p53. Products of solid-phase kinase reaction using PKA (2 μg of enzyme per reaction) or WCE (2 μg) were washed and then separated on SDS/PAGE. Pattern of endogenous p53 phosphorylation is shown in IP lane, in which cells were labeled with [32P]-orthophosphate (100 μCi) for 4 h before p53 was immunoprecipitated (200 ng of p53 antibody per 1 mg of protein). Bands corresponding to each of the GST-p53 proteins were excised and subjected to trypsinization followed by separation on an isoelectric focusing gel (pH range 3.5–10). Peptides were analyzed by autoradiography.
Using a purified form of CKIIβ, we demonstrated that while full-length GST-p53 was phosphorylated, among the truncated forms tested, only GST-p5394–393 was phosphorylated by CKIIβ (Fig. 1B). Mapping of CKIIβ phosphorylation revealed the C terminus as the primary phosphoacceptor site (not shown). Accordingly, C-terminus-truncated forms of GST-p53 are expected to lack phosphorylation. However, that GST-p53155–393 is among the p53 forms that could not be phosphorylated in vitro by CKIIβ points to the possibility that the region spanning amino acids 94–155 may contribute to phosphorylation of GST-p53 by CKIIβ.
PKA Phosphorylation of p53.
To test the ability of PKA to phosphorylate either full-length or truncated GST-p53, we have used the catalytic subunit of PKA. As shown in Fig. 1C, 80 ng of PKA is capable of phosphorylating each of the truncated forms, but not the full-length GST-p53. A higher amount (2 μg) of PKA was required to achieve phosphorylation of the full-length GST-p53 (Fig. 1D). Mapping p53 phosphorylation sites by tryptic digestion of labeled proteins revealed multiple phosphorylation sites on the full-length protein. In vivo orthophosphate labeling, followed by immunoprecipitation with antibodies to p53, revealed that 6 of the 10 sites found to be phosphorylated by PKA in vitro also are phosphorylated in vivo in fibroblast cells (Fig. 1D).
JNK Binding and Phosphorylation of p53.
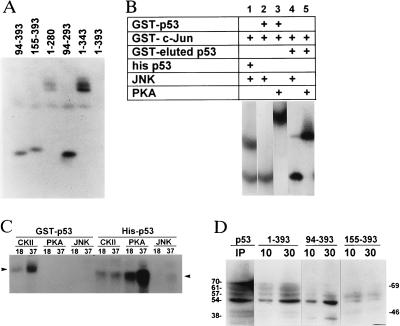
While JNK failed to phosphorylate full-length GST-p53, it was capable of phosphorylating truncated forms of GST-p53. Peptide mapping of JNK phosphorylation reveals at least two phosphorylation sites on p53 within amino acids 1–280 (Fig. 2A). After the p53 was separated from GST (by digestion with thrombin), JNK was capable of phosphorylating the bacterially produced full-length p53 protein (Fig. 2B, lane 4). Similarly, JNK was capable of phosphorylating the insect cell-produced Hisp53 (Fig. 2B, lane 1). To ensure that p53 phosphorylation is mediated by JNK, a bacterially produced and subsequently purified JNK was used. GST-c-jun was used as a control substrate (Fig. 2B). PKA (2 μg of enzyme per reaction) exhibits strong phosphorylation of GST-p53, as of GST-eluted p53, but not of c-jun (Fig. 2B, lanes 3 and 5, respectively).
Figure 2.
(A) JNK phosphorylation of GST-p53 proteins. Full-length or truncated forms of GST-p53 proteins were used for solid-phase kinase reaction using JNK purified from WCE. Respective bands were excised, subjected to digestion with trypsin, and analyzed further by separation on isoelectric focusing gel. (B) p53 phosphorylation by JNK. Solid-phase kinase reactions were performed using bacterially produced active JNK (200 ng) or PKA (2 μg) as the source of kinase and GST p531–393 (lanes 2, 3), thrombin-digested p53 that lacks the GST portion (lanes 4, 5), Hisp53 (lane 1), or GST c-jun5–89 (lanes 1–3) as substrates. (C) CKIIβ, PKA, and JNK phosphorylation of Hisp53 and GST-p53. GST-p53 or Hisp53 substrates were used in solid-phase kinase reaction using CKIIβ (40 ng), PKA (80 ng), or JNK (200 ng) at 18° or 37°C. Arrows point to the position of the GST or histidine-tagged protein. (D) JNK binding to p53. GST-p53 constructs were incubated with protein extracts for either 10 or 30 min at room temperature before beads-bound material was washed and analyzed on Western blots using antibodies to JNK. IP lane represents proteins immunoprecipitated with antibodies to p53 and analyzed by Western blotting with antibodies to JNK. Molecular mass (kDa)is shown at the right, and molecular masses of proteins recognized by JNK antibodies are indicated on the left.
In light of the differences seen in p53 phosphorylation between bacterially produced GST-p53 fusion protein and baculovirus-produced Hisp53, we have compared the ability of PKA, CKIIβ, and JNK to phosphorylate each of those forms. Similarly to CKIIβ, PKA efficiently phosphorylates Hisp53 even when used at low concentration (80 ng) of the kinase, indicating that PKA is a potent p53 kinase (Fig. 2C). In all cases, reactions that took place at 37°C were more efficient than those at 18°C (Fig. 2C). Subjecting Hisp53 to calf intestine phosphatase treatment before phosphorylation revealed the same pattern and degree of phosphorylation when compared with non-calf intestine phosphatases pretreated p53 (not shown), indicating that post-translational processing in the insect cells did not affect the in vitro phosphorylation reactions.
Because JNK phosphorylation of most of its substrates requires physical interaction (i.e., c-jun; ref. 34), we have determined the ability of JNK to interact with p53. Incubation of GST-p53 constructs with protein lysates, followed by immunoblot analysis using antibodies to JNK, revealed the association of JNK isozymes (molecular mass of 54–70 kDa) with full-length GST-p53 (Fig. 2D). The association of JNK with truncated forms of p53 revealed that the binding to p5394–393 is substantially stronger than that found with p53155–393. Furthermore, while the primary form of JNK that interacts with p5394–393 is the 54-kDa JNK2, this isozyme exhibits very weak association with p53155–393. Two bands of higher molecular mass (57 and 61 kDa) were identified as p53155–393-bound JNK-isoforms. In addition, among the p5394–393-bound proteins is a 38-kDa protein that may represent p38 (Fig. 2D). Further confirmation of JNK association with p53 comes from immunoprecipitation of protein extracts with antibodies to p53, which was monitored by immunoblots with antibodies to JNK. Four distinct JNK isozymes in the range of 54–70 kDa were identified, among which the 54-kDa and 70-kDa ones are the prominent p53-bound proteins (Fig. 2D; IP panel). Also identified in this reaction as p53-bound and JNK-recognized proteins is a subset of three proteins with molecular masses of 33, 38, and 43 kDa. Interestingly, JNK association with GST-p53 is not sufficient for mediating its phosphorylation, as shown for the full-length GST-p53 (Fig. 2 A and B).
Effect of Point Mutation on p53 Phosphorylation.
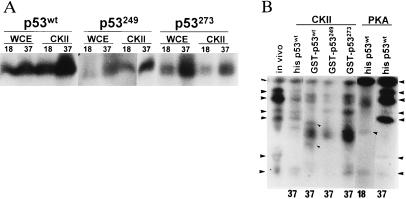
To determine whether point mutations, known to affect p53 stability and activities, would affect its phosphorylation we have compared phosphorylation patterns of two hot-spot mutations at amino acids 249 and 273 with that of p53wt. Comparison was performed at two temperatures, 18°C and 37°C, a change that was shown to affect activity of mutant p53 proteins (35). Higher phosphorylation of p53wt was seen at 37°C than at 18°C by WCE kinases, CKIIβ (Fig. 3A), JNK (not shown), or PKA (Fig. 3B). Peptide mapping revealed the presence of different phosphorylation sites at the two temperatures (shown for PKA in Fig. 3B). Comparison of wild-type p53 with mutant forms revealed that both p53249 and p53273 underwent weaker phosphorylation at the lower temperature (Fig. 3A). Peptide mapping of p53 phosphorylation by CKIIβ revealed both quantitative and qualitative differences in phosphorylation when wt and mutant p53 were compared (i.e. Fig. 3B; indicated by arrowheads).
Figure 3.
(A) Effect of temperature on phosphorylation of wild-type and mutant forms of p53. GST-p53 was subjected to solid-phase kinase reaction using the kinases at 18°C or 37°C. (B) Mapping of mutant p53 phosphorylation. Products of solid-phase p53 phosphorylation at indicated temperatures were separated on SDS/PAGE, and p53 bands, identified through autoradiography, were cut, excised, and digested with trypsin before separation on an isoelectricfocusing gel. Resulting phosphorylated peptides are indicated by the arrowheads on the sides. Phosphorylated peptides identified in wild-type, but not mutant, forms of p53 are indicated by arrowheads on lane 3 (compare with lanes 4 and 5).
Comparison of the phosphorylation pattern seen in vivo (by orthophosphate labeling) with those of GST-p53 and Hisp53 revealed that, when combined, the pattern of phosphorylation obtained in vitro by PKA and CKIIβ consists of most of the phosphorylated peptides seen in vivo (Fig. 3B).
Effect of DNA Binding Domain-Derived Peptides on p53 Phosphorylation.
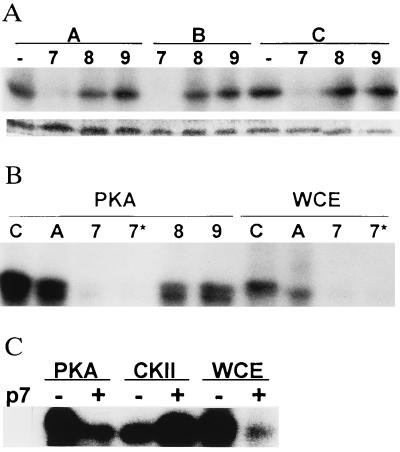
The requirement of the 94–155 region of p53 for its phosphorylation by CKIIβ and the low phosphorylation seen by WCE kinases in p53 that lacks this region (Fig. 1 A and B) prompted experiments in which 20-aa peptides, which span this region (P7, amino acids 97–117; P8, amino acids 115–135; and P9, 133–153) were tested for their effect on in vitro p53 phosphorylation. When WCEs were used as the source of kinases, P7 inhibited phosphorylation of the full-length p53 construct. Inhibition of p53 phosphorylation occurred even when p53 was subjected to extensive washings before the kinase reaction (Fig. 4A), indicating that the peptide affects p53 phosphorylation by contingent association with p53, or through change of p53 conformation. Unlike P7, neither P8 nor P9 was capable of exerting significant changes in p53 phosphorylation by kinases present in the cellular extract (Fig. 4A). Neither of the two truncated forms of P7, (P10, amino acids 97–112 or P11, amino acids 102–112) was capable of inhibiting p53 phosphorylation (not shown).
Figure 4.
(A) Effect of DNA-binding domain-derived peptides on p53 phosphorylation. GST-p531–393 was subjected to solid-phase kinase reaction with WCE, in the presence of peptides 7–9 before addition of [γ32P]ATP (A lanes), before and during kinase reaction (B lanes), or only with the addition of [γ32P]ATP (C). Lower bands represent staining of the GST-p53 to indicate equal loading. (B) Effect of peptides on phosphorylation of p53 by PKA and WCE. GST-p531–393 was subjected to solid-phase phosphorylation (i.e., washes performed before addition of ATP; lanes C and 7–9) or with [γ32P]ATP added without intermediate washes (lanes A and 7*) by PKA or WCE, respectively, in the presence of indicated peptides. (C) Effect of P7 on p53 phosphorylation by CKIIβ, PKA, and WCE. GST-p53 subjected to solid-phase kinase reaction in the presence of P7 and kinases.
Similar to its effect on WCE kinases, P7 elicited 90% inhibition of p53 phosphorylation by PKA, whereas P8 and P9 caused 20% and 10% inhibition, respectively (Fig. 4B).
In contrast to its effects on PKA or WCE kinases, P7 increased in p53 phosphorylation by CKIIβ (Fig. 4C). When tested with Hisp53, P7 was able to exert the same changes as found with GST-p53, whereas P8, P9, and the mutant forms of P7 (P10 and P11) were unable to exert such an effect (not shown).
Adding the P7 peptide, which was synthesized in frame with biotin-tagged penetratin, to fibroblasts grown in culture, allowed us to verify nuclear localization (through immunohistochemistry with antibodies to biotin) and revealed, by orthophosphate labeling, a marked increase in endogenous p53 phosphorylation (monitored through immunoprecipitation with antibodies to p53 followed by SDS/PAGE and autoradiography; not shown).
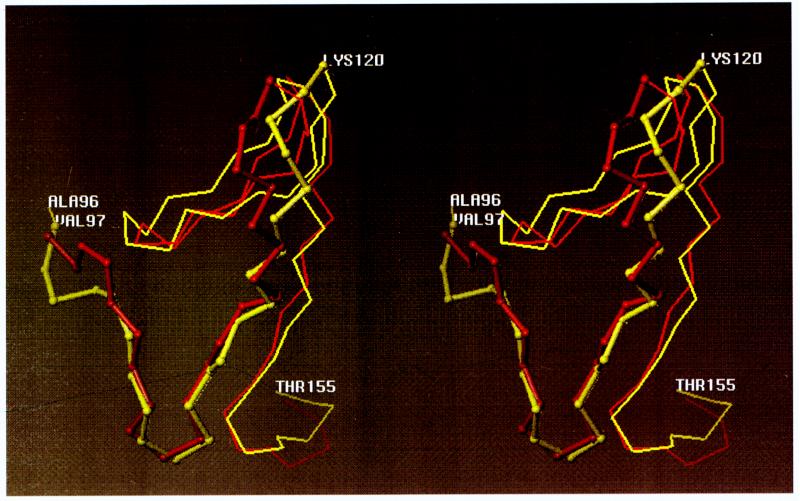
To further elucidate the role of DNA-binding domain in p53 conformation we have used computerized modeling of wild-type and mutant forms of p53. Shown in Fig. 5 is a stereo view of the superposition of the mutant on the wild-type form of p53 within the 96–155 region. The results from the electrostatically driven Monte Carlo calculation, found to be identical to those from molecular dynamics, demonstrate a significant change in the conformation of p53249 within the region of amino acids 96–120, when compared with the p53wt counterpart.
Figure 5.
Stereoview of the Cα trace of the segment 96–155 of two p53 proteins: wild-type (yellow) and Arg249 → Trp mutant (red). The phosphorylation domain from residues 96–117 is shown in a tubular form for both proteins.
DISCUSSION
Using multiple forms of wild-type and mutant p53, the present study demonstrates the role of p53 conformation for its phosphorylation. We have arrived at this conclusion by demonstrating the following. First, PKA is a potent p53 kinase, as revealed from the use of Hisp53 and truncated forms of GST-p53. Crucial for PKA phosphorylation is the conformation of p53, as concluded from the need to use a significantly higher amount of PKA to phosphorylate GST-p53, and from the marked change in Hisp53 phosphorylation pattern by PKA when performed at different temperatures.
Second, we show that JNK is capable of phosphorylating the Hisp53 and truncated forms of the GST-p53. Previous studies have suggested that murine p53 could be phosphorylated at Ser34 by JNK (36). However, due to differences in amino acid sequence between the murine and human p53, and on the basis of the ability of JNK to phosphorylate p53, which lacks its first 94 amino acids (Fig. 3A), it is likely that JNK phosphorylation of human p53 occurs at sites within the amino acids 97–280 region that have yet to be identified. We further demonstrate that JNK physically associates with GST-p53, in spite of its inability to phosphorylate it, indicating that the conformation required for binding is not sufficient for p53 phosphorylation by JNK.
Third, we demonstrate the effect of temperature on phosphorylation of wild-type and mutant p53 forms. Both quantitative and qualitative differences were found, indicative of the conformation-dependent availability of respective sites. Transcriptional activities of these very p53 mutant forms also were shown to be temperature dependent (35).
The effect of point mutation on the conformation of p53 is best illustrated by the computer modeling of p53wt versus the p53249 hot-spot mutation (Fig. 5). The amino acid substitution (Arg → Trp) at position 249 causes a major displacement of the 96–120 segment. Identical results were obtained for p53249 (Arg → Ser). The region spanning amino acids 96–120 not only is important in phosphorylation of DNA-binding domain but also is necessary for CKIIβ phosphorylation in its C-terminal domain. p53 that lacks amino acids 94–155 cannot be phosphorylated by CKIIβ, in spite of the availability of the CKIIβ phosphoacceptor sites. The latter implies that residues 97–117 are critical in affecting the conformation of the C-terminal domain of p53 where CKIIβ phosphoacceptor site is positioned. Our finding that the P7 peptide (amino acids 97–117) alters p53 phosphorylation by multiple kinases is closely corroborated by our computed results (Fig. 5). Because P7 increased p53 phosphorylation by CKIIβ, we attribute the increase seen in in vivo labeling reactions, performed in the presence of P7, primarily to this enzyme. The identification of a peptide that is capable of altering p53 phosphorylation by multiple kinases is an example of means one may consider for altering p53 phosphorylation.
In all, we have demonstrated differences in p53 phosphorylation when insect cell-produced p53 was compared with bacterially produced GST-p53, and similarly, in comparing various truncated forms as well as the wild-type to mutant p53 forms. Collectively, these differences support the role of p53 conformation in its availability to different kinases and to the phosphorylation pattern generated by the same kinase.
On the basis of our observations, we hypothesize that the equilibrium between the different kinases in vivo may play a crucial role in determining the one(s) that will, in fact, phosphorylate p53. Changes due to overexpression or depletion of a kinase would alter this equilibrium and are expected to yield new patterns of phosphorylation.
The possible correlation between p53 phosphorylation and its functions has been extensively addressed, albeit for the most part inconclusively (37, 38). Our present findings contribute to the understanding of the nature of this discrepancy. For example, many of the previous studies have used forms of p53 with mutations at the phosphorylating sites, abrogating not only phosphorylation but also conformation, thereby precluding the ability to properly address function. Phosphorylation-dependent conformation is expected to alter the ability of p53 to associate with other cellular proteins, tetramerization, and interaction with damaged DNA. That mutant forms of p53 are differently phosphorylated than their wild-type counterparts is likely to result in altered function of the protein, which now exhibits different charges at alternate positions.
Acknowledgments
We thank Lisa Dolan and Alla Polotskaya for assistance, Peter Tegtmeyer for Hisp53 baculovirus construct and helpful comments, Craig Monnel for antibodies to JNK, and Roz Alexander and Ilse Hoffmann for manuscript preparation. These studies were supported in part by National Cancer Institute grants to Z.R., M.R.P., and P.B.R.
ABBREVIATIONS
- GST
glutathione S-transferase
- Hisp53
histidine-tagged p53
- p53wt
wild-type p53
- JNK
jun-N-kinase
- PKA
protein kinase A
- CKII
casein kinase II
- CKIIβ
β subunit of casein kinase II
- WCE
whole cell extracts
References
- 1.Donehower L A, Bradley A. Biochim Biophys Acta. 1993;1155:181–205. doi: 10.1016/0304-419x(93)90004-v. [DOI] [PubMed] [Google Scholar]
- 2.Haffner R, Oren M. Curr Opin Genet Dev. 1995;5:84–90. doi: 10.1016/s0959-437x(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 3.Harris C C. J Natl Cancer Inst. 1996;88:1442–1455. doi: 10.1093/jnci/88.20.1442. [DOI] [PubMed] [Google Scholar]
- 4.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 5.Kuerbitz S J, Plunkett B S, Walsh W V, Kastan M B. Proc Natl Acad Sci USA. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko L, Prives C. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 7.Hollstein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–52. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 8.Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 9.Wu X, Levine A J. Proc Natl Acad Sci USA. 1994;91:3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto K, Beach D. EMBO J. 1994;13:4816–4822. doi: 10.1002/j.1460-2075.1994.tb06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hupp T R, Meek D W, Midgley C A, Lane D P. Nucleic Acids Res. 1993;21:3167–3174. doi: 10.1093/nar/21.14.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Prives C. Nature (London) 1995;376:88–91. doi: 10.1038/376088a0. [DOI] [PubMed] [Google Scholar]
- 14.Reed M, Woelker B, Wang P, Wang Y, Anderson M E, Tegtmeyer P. Proc Natl Acad Sci USA. 1995;92:9455–9459. doi: 10.1073/pnas.92.21.9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X W, Vermeulen W, Coursen J D, Gibson M, Lupold S E, Forrester K, Xu G, Elmore L, Yeh H, Hoeijmakers J H J, Harris C C. Genes Dev. 1996;10:1219–1232. doi: 10.1101/gad.10.10.1219. [DOI] [PubMed] [Google Scholar]
- 16.Meek D W, Simon S, Kikkawa U, Eckhart W. EMBO J. 1990;9:3253–3260. doi: 10.1002/j.1460-2075.1990.tb07524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bischoff J R, Friedman P N, Marshak D R, Prives C, Beach D. Proc Natl Acad Sci USA. 1990;87:4766–4770. doi: 10.1073/pnas.87.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lees-Miller S P, Sakaguchi K, Ullrich S J, Appella E, Anderson C W. Mol Cell Biol. 1992;12:5041–5049. doi: 10.1128/mcb.12.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milne D M, Palmer R H, Campbell D G, Meek D W. Oncogene. 1992;7:1361–1369. [PubMed] [Google Scholar]
- 20.Baudier J, Delphin C, Grunwald D, Khochbin S, Lawrence J J. Proc Natl Acad Sci USA. 1992;89:11627–11631. doi: 10.1073/pnas.89.23.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hupp T R, Meek D W, Midgley C A, Lane D P. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 22.Hall S R, Campbell L E, Meek D W. Nucleic Acids Res. 1996;24:1119–1126. doi: 10.1093/nar/24.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer G A, Reed M, Wang P, Wang Y, Schwedes J F, Tegtmeyer P. Cancer Res. 1995;55:10–17. [PubMed] [Google Scholar]
- 24.Paley E L. Carcinogenesis. 1996;17:939–945. doi: 10.1093/carcin/17.5.939. [DOI] [PubMed] [Google Scholar]
- 25.Fiscella M, Zambrano N, Ullrich S J, Unger T, Lin D, Cho B, Mercer W E, Anderson C W, Appella E. Oncogene. 1994;9:3249–3257. [PubMed] [Google Scholar]
- 26.Crook T, Marston N J, Sara E A, Vousden K H. Cell. 1994;79:817–827. doi: 10.1016/0092-8674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 27.Ruppert M J, Stillman B. Mol Cell Biol. 1993;13:3811–3820. doi: 10.1128/mcb.13.6.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler V, Schaffer A, Kim J, Dolan L R, Ronai Z. J Biol Chem. 1995;270:26071–26077. doi: 10.1074/jbc.270.44.26071. [DOI] [PubMed] [Google Scholar]
- 29.Omata Y, Sakamoto H, Robinson R C, Pincus M R, Friedman F K. Biochem Biophys Res Commun. 1994;201:1090–1095. doi: 10.1006/bbrc.1994.1817. [DOI] [PubMed] [Google Scholar]
- 30.Cho Y, Gorina S, Jeffrey P D, Pavletich N P. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 31.Brandt-Rauf P W, Chen J M, Marion M-J, Smith S J, Luo J-C, Carney W, Pincus M R. J Protein Chem. 1996;15:367–375. doi: 10.1007/BF01886863. [DOI] [PubMed] [Google Scholar]
- 32.Ripol D, Scheraga H A. Biopolymers. 1988;27:1283–1305. doi: 10.1002/bip.360270808. [DOI] [PubMed] [Google Scholar]
- 33.Nemety G, Pottle M S, Scheraga H A. J Phys Chem. 1983;87:1883–1887. [Google Scholar]
- 34.Adler V, Franklin C C, Kraft A S. Proc Natl Acad Sci USA. 1992;89:5341–5345. doi: 10.1073/pnas.89.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedlander P, Legros Y, Soussi T, Prives C. J Biol Chem. 1996;271:25468–25478. doi: 10.1074/jbc.271.41.25468. [DOI] [PubMed] [Google Scholar]
- 36.Milne D M, Campbell D G, Caudwell F B, Meek D W. J Biol Chem. 1994;289:9253–9258. [PubMed] [Google Scholar]
- 37.Fuchs B, O’Connor D, Fallis L, Scheidtmann K H, Lu X. Oncogene. 1995;10:789–793. [PubMed] [Google Scholar]
- 38.Milne D M, McKendrick L, Jardine L J, Deacon E, Lord J M, Meek D W. Oncogene. 1996;13:205–211. [PubMed] [Google Scholar]