Abstract
Two closely related β subunit mRNAs (xo28 and xo32) were identified in Xenopus oocytes by molecular cloning. One or both appear to be expressed as active proteins, because: (i) injection of Xenopus β antisense oligonucleotides, but not of sense or unrelated oligonucleotides, significantly reduced endogenous oocyte voltage-gated Ca2+ channel (VGCC) currents and obliterated VGCC currents that arise after injection of mammalian α1 cRNAs (α1C and α1E); (ii) coinjection of a Xenopus β antisense oligonucleotide and excess rat β cRNA rescued expression of α1 Ca2+ channel currents; and (iii) coinjection of mammalian α1 cRNA with cRNA encoding either of the two Xenopus β subunits facilitated both activation and inactivation of Ca2+ channel currents by voltage, as happens with most mammalian β subunits. The Xenopus β subunit cDNAs (β3xo cDNAs) predict proteins of 484 aa that differ in only 22 aa and resemble most closely the sequence of the mammalian type 3 β subunit. We propose that “α1 alone” channels are in fact tightly associated α1β3xo channels, and that effects of exogenous β subunits are due to formation of higher-order [α1β]βn complexes with an unknown contribution of β3xo. It is thus possible that functional mammalian VGCCs, rather than having subunit composition α1β, are [α1β]βn complexes that associate with α2δ and, as appropriate, other tissue-specific accessory proteins. In support of this hypothesis, we discovered that the last 277-aa of α1E have a β subunit binding domain. This β binding domain is distinct from the previously known interaction domain located between repeats I and II of calcium channel α1 subunits.
Xenopus oocytes translate exogenously injected mRNAs and cRNAs with relatively high efficiency. This has made them systems of choice for the functional expression and characterization of many cloned molecules, such as neuronal ligand-gated ion channels, G protein-coupled receptors, and many voltage-gated ion channels, including voltage-dependent Ca2+ channels. Voltage-dependent Ca2+ channels are formed of an α1 pore-forming and voltage-sensing subunit and β and α2δ regulatory subunits. Functional expression in Xenopus oocytes has not only been used to define structure–function relations of voltage-gated calcium channels by assessing the effects of specific mutations of the α1 channel protein, but also to define identity and roles of the regulatory subunits in promoting α1 expression or modifying the properties of the expressed α1 subunit. Several nonallelic genes encoding α1 subunits, termed α1S and α1A–α1E, have been identified by molecular cloning (1–4). Of these, all except α1S have been functionally expressed in the Xenopus oocyte. However, while certain variants of α1C and α1E can be expressed without coinjection of other subunit cRNAs (e.g., refs. 5 and 6), others, particularly α1A, yield only minimal currents in the absence of additional subunits, notably a β subunit (7, 8). The reasons for these differences are not understood.
The interpretation of results obtained expressing cloned Ca2+ channel subunits to define intrinsic properties of α1 and the effects of regulatory subunits have, of course, assumed that oocytes do not express equivalent endogenous subunits. Yet this assumption has not been rigorously tested. We report here the detection of β subunit mRNA in defolliculated, stage IV–VI oocytes, such as are used for α1 subunit expression. We cloned two full-length cDNAs derived from what appear to be two alleles of the same gene and show that the product of this gene is constitutively expressed in oocytes. In addition, we report that α1E has two independent β subunit binding domains.
METHODS
Molecular Cloning of Calcium Channel β Subunit cDNAs from Oocyte mRNA.
Oligo(dT)-purified mRNA (0.3 g) of stage IV–VI oocytes that had been defolliculated by treatment with collagenase was prepared using the FastTrack RNA Isolation Kit (Invitrogen). Presence of mRNA encoding Xenopus calcium channel β subunit homolog(s) was tested using PCR. Full-length cDNAs were cloned using a strategy that combined PCR and rapid amplification of cDNA ends (RACE) PCR with reagents and protocols supplied by the Marathon Amplification Kit (CLONTECH). The reaction products from each of the PCR and RACE PCR reactions were cloned into the Invitrogen pCRII TA cloning vector. Individual clones with inserts of the expected sizes were picked, expanded, and sequenced with Sequenase II (United States Biochemical) by the dideoxynucleotide chain termination method (9), using double-stranded DNA as template. The true nucleotide sequence of the full-length cDNA was deduced from sequencing five PCR clones, each from an independent PCR amplification. Full-length coding sequences of two β subunit-like sequences were obtained, xo28 and xo32, and spanned nucleotide −60, before the ATG initiator codon (A of ATG = 1), through nucleotide 22, after the TGA stop codon. The open reading frames of clones 2-2 of xo28 and 4-3 of xo32 were subcloned into the transcription competent plasmid pAGA2 (10). Taq polymerase purchased from Takara Shuzo (Kyoto) was used throughout. We noted an error rate of 1 nt in 400. Due to the engineering of the NcoI site at the ATG initiation codon, the N termini of xo28 and xo32 from pAGA2 cRNAs were Met-Val instead of Met-Tyr.
cRNAs of the β3xo, β1b (11), human α1E (6), and the high expressor ΔN60 mutant of the rabbit cardiac α1C, which lacks amino acids 1–59 (X. Wei and L.B., unpublished work), were synthesized using mMessage mMachine reagents and protocols purchased in kit form from Ambion (Austin, TX).
Xenopus laevis oocytes were isolated and injected as described (12), and electrophysiological recordings from oocytes were made using the cut-open Vaseline gap voltage-clamp method (13) as modified in ref. 12.
Calcium Channel Currents.
Except when indicated otherwise, the external solution had the following composition: 10 mM Ba2+, 96 mM Na+, and 10 mM Hepes, titrated to pH 7.0 with methanesulfonic acid (CH3SO3H). The solution in contact with the oocyte interior was 110 mM K-glutamate and 10 mM Hepes, titrated to pH 7.0 with KOH. Low access resistance to the oocyte interior was obtained by permeabilizing the oocyte with 0.1% saponin. Activation of Cl− current by Ba2+ influx through the Ca2+ channel was eliminated by injecting 100–150 nl of 50 mM BAPTA-Na4 (1,2-bis(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetate) before recording (12). The BAPTA solution was adjusted to pH 7.0 with methanesulfonic acid. The holding potential was −90 mV. The linear components of the currents, corresponding to the scaled currents elicited by small negative control pulses of one-fourth the amplitude of the stimulating pulse, from −90 mV subtracting holding potential, were subtracted on-line. The data were sampled at 10 kHz and filtered at 2 kHz.
Glutathione S-Transferase (GST) Fusion Proteins, in Vitro Translation of Ca2+ Channel β2a, and Binding of β2a to GST Fusion Proteins.
GST fusion plasmids were based on pGEX-4T-1 (Pharmacia) and were constructed by conventional means using either natural restriction fragments of calcium channel α1 subunits or defined fragments excised by PCR. After transfection into Escherichia coli BL21, synthesis of the fusion proteins was induced with 0.2 mM isopropyl β-d-thiogalactoside (IPTG) in a liquid culture grown to OD of 1.0. After 2–3 hr at 37°C, the cells were collected by centrifugation, resuspended in NETN lysis buffer (0.5% Nonidet P-40/1 mM EDTA/20 mM Tris·HCl, pH 8.0/100 mM NaCl; 1.0 ml per 20 ml of culture) and lysed by sonication. The lysate was cleared by centrifugation at 10,000 × g for 10 min at 4°C. The GST fusion proteins in the supernant were adsorbed for 30 min at room temperature to glutathione-agarose beads in NETN [1 volume of lysate:1 volume of 50% (vol/vol) slurry of agarose-GSH beads (Pharmacia) in NETN]. The last wash was with binding buffer [1% (vol/vol) Lubrol-PX/2 mM EDTA/100 mM NaCl/20 mM Tris·HCl, pH 8.0] instead of NETN. Slurries [50% (vol/vol)] slurries of washed agarose-GSH beads with GST fusion proteins adsorbed to them (agarose-GSH::GST fusions) were then incubated for 30 min at room temperature in an equal volume of binding buffer with 35S-labeled β2a (Promega), synthesized by coupled transcription–translation (Promega), as described in Pragnell et al. (14). After a 50-fold dilution in binding buffer, the beads were centrifuged and washed three times with binding buffer and resuspended in an equal volume of 4× Laemmli’s sample buffer. Proteins released from the beads were analyzed by 10% SDS/PAGE followed by autoradiography to detect retention of β2a by the α1 fragments fused to GST. In vitro-translated [Q212L]Gsα, a constitutively activated human 379-aa human Gsα subunit was used in place of calcium channel β2a to test for specificity in the interaction of β2a with the α1E fragments.
RESULTS
A Calcium Channel β Subunit mRNA in Xenopus Oocytes: β3xo.
We designed mixtures of oligonucleotides based on regions of high amino acid sequence identity among the four known types of mammalian β subunits, and we probed oocyte mRNA for presence of a β subunit-like sequence using reverse transcription-PCR. Two very similar PCR products, xo28 and xo32, were identified. The xo28 and xo32 PCR products were each of 430 nt (excluding the PCR primers) and differed in 25 nt. The sequences in xo28 and xo32 coded for 143 aa of two β subunits that differ in only 6 aa. Full-length cDNA versions of xo28 and xo32 were then cloned by a combination of PCR and RACE PCR techniques and found to be almost identical along their entire length. As determined by sequencing, xo28 has an open reading frame of 1455 nt comprising 484 codons preceded by 89 nt of 5′ untranslated sequence with an in-frame stop codon 42 nt upstream of the putative initiator ATG. The open reading frame is followed by 578 nt of 3′ untranslated sequence. xo32 has an open reading frame of the same length as xo28, differing from xo28 in 74 roughly randomly distributed nucleotides, and encodes a protein that differs from xo28 in 22 aa. The 3′ untranslated sequence of xo32 is of 588 nt and differs from that of xo28 in 15 nt, 14 of which are clustered in the last 50 nt before the poly(A) tail. xo32 lacks nucleotides 1991–1993 of xo28, reads C instead of T at position 2033 of xo28, the last nucleotide before the poly(A) tail, and has an additional 14 nt before the initiation of the poly(A) tract. For xo32, 5′ RACE PCR yielded 126 nt upstream of the initiator ATG, of which the first 89 nt 5′ of the initiator ATG differ from those of xo28 in a single nucleotide at position −11.
Multiple sequence analysis, in which the deduced amino acid sequences of xo28 and xo32 were compared with the amino acid sequences of mammalian β1, β2, β3, and β4, showed that the Xenopus sequences are β3-like. We refer to them as β3xo subunits. Existence of two β3xo genes should not be surprising, as X. laevis is a tetraploid organism (cf. ref. 15). Fig. 1 compares the β3xo sequences to the mammalian β sequences. The high degree of sequence identity of xo28 and xo32 and their similarity to mammalian β3 subunits should be noted.
Figure 1.
Amino acid sequence of Xenopus β subunits as deduced from the xo28 and xo32 cDNAs and comparison to sequences of mammalian β1b, β2a, β2b, β3, and β4 (A) and phylogenetic tree of calcium channel β subunits (B). (A) xoβ3-28 is the reference. –, Same amino acid as in xoβ3-28; –, gap; @, stop; rt, rat; rb, rabbit; hum, human; and xo, X. laevis oocyte. β2a differs from β2b only at the N terminus. β1, β1b splice variant. The phylogram shown in B was calculated by the neighbor-joining technique of Kimura, using version 8.0 of the gcg Sequence Analysis Software Package. Numbers in parenthesis next to names of β subunits are the GenBank accession numbers: for xo28, it is U33217U33217, and for xo32, it is U33218U33218.
Functional Expression of Xenopus β3.
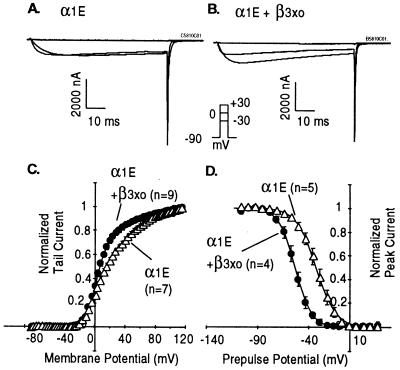
Coinjection of either of the β3xo cRNAs with mammalian α1E mimicked the previously reported effects of coinjecting mammalian β3 (16) in that both variants facilitated voltage induced activation and inactivation of the α1E channel. As illustrated in Fig. 2 for the xo28 Xenopus β subunit, conductance–voltage (G–V) relations obtained in its presence and absence were fitted well by two Boltzmann distributions, the effect of β3xo being primarily to increase the relative amplitude of the proportions of channels activated at the lower voltage. Likewise, steady-state inactivation curves obtained as a function of voltage were shifted to more negative potentials by ≈20 mV by the coexpression of the β3xo. Essentially the same results were obtained with the xo28 and xo32 Xenopus β subunit (data not shown). This showed that xo28 and xo32 each encode a bona fide calcium channel β subunit.
Figure 2.
Effect of β3xo (xo28) on α1E. (A and B) Time courses of activation of α1E in oocytes injected with α1E cRNA alone or cRNAs encoding both α1E and β3xo (clone xo28). (C and D) Effect of β3xo on voltage-dependent activation and inactivation of α1E. G–V curves were obtained from peak tail currents measured by stepping to −50 mV after depolarizing pulses of 25-msec duration from −88 to 116 mV in 4-mV increments. The data were sampled at 10 kHz and filtered at 2 kHz. The data points were fitted by the sum of two Boltzmann distributions. For α1E, the first component had a V1/2 = 2.7 mV, an effective valence (zδ) = 2.9 e, and a relative amplitude of 51%; the second had a V1/2 = 41.8 mV and a zδ = 1.4 e. For α1E + β3xo, the first component had a V1/2 = 2.7 mV, a zδ = 3.4 e and a relative amplitude of 75%; the second had a V1/2 = 51.8 mV and a zδ = 1.3 e. Steady-state inactivation curves were derived from peak currents elicited by a pulse to +20 mV following a conditioning pulse of 10 sec to potentials from −120 to 27 mV in 7 mV increments and a brief (4-msec) pulse to −90 mV. The data were sampled at 500 Hz and filtered at 100 Hz. Sweeps were separated by 20 sec to allow a full recovery from inactivation. Data points were fitted by a Boltzmann distribution. The effective valences were 2.7 ± 0.1 e for α1E and 3.4 ± 0.1 e for α1E + β3xo; the half-inactivation potentials were −32.2 ± 3.4 mV (n = 5) for α1E and −53.5 ± 1.1 mV (n = 4) for α1E + β3xo.
Effects of xo28 and xo32 Antisense Oligonucleotides on Expression of Calcium Channel Currents.
Two sets of experiments were performed to test for a possible role of endogenously expressed oocyte β3 subunits. The first probed for effects of antisense oligonucleotides on expression of endogenous oocyte calcium currents. The second tested for the effects of antisense oligonucleotides on expression of exogenously injected mammalian α1 subunits.
Previous studies by Lacerda et al. (17) had shown enhancement of endogenous oocyte T- and L-type currents by expression of a mammalian β subunit. This indicated that Xenopus Ca2+ channels are susceptible to regulation by β subunits. The finding that oocytes have mRNA encoding a β subunit therefore raised the possibility that endogenous Ca2+ channel currents might be dependent on endogenous β subunits, as has been found for expression of the mammalian α1A. We thus tested for an effect of anti-Xenopus oocyte oligonucleotides on expression of endogenous Ca2+ channel currents. In contrast to Lacerda et al. (17), who found a high proportion of oocyte batches with endogenous calcium channel currents, we were for the most part unable to find batches of oocytes with currents exceeding 5 nA (tested at +30 mV with 74 mM external Ba2+). In fact, we identified only one batch (out of 15 tested) with oocytes displaying peak inward currents of between 5 and 15 nA. These appeared to be calcium channel currents, based on their characteristic current–voltage relationship and their susceptibility to being reversibly blocked by Co2+ (Fig. 3A). As shown in Fig. 3B, injection of the B11 antisense oligonucleotide (complementary to nucleotides 210–235 of the coding sequences of xo28 and xo32; for composition see Table 1), resulted in a significant reduction in their endogenous calcium channel currents, when compared with currents recovered oocytes of the same batch injected with control oligonucleotide B10.
Figure 3.
Inhibition by anti-β3xo oligonucleotide B11 of endogenous Ca2+ channel currents in oocytes of one frog presenting such currents. Expression of Ca2+ channel currents in Xenopus oocytes was determined on the day after isolation and collagenase treatment of oocytes. Oocytes from one frog expressing 5–10 nA at +30 mV with 74 mM Ba2+ in the external solution were injected with 50 nl of 100 μM B11 (antisense) or B10 (sense) oligonucleotide. The injected oocytes were then tested 3, 5, and 7 days later for Ca2+ channel activity. (A) Representative IBa recorded from one uninjected oocyte. (B) I–V relations obtained 7 days after injection of sense or antisense oligonucleotides. The external solution was 80 mM Ba2+/10 mM Hepes, titrated to pH 7.0 with methanesulfonic acid (CH3SO3H).
Table 1.
Effect of oocyte β subunit antisense oligonucleotide on functional expression of coinjected calcium channel α1 subunit
| Injections
|
Ion channel activity,† frequency of success | |
|---|---|---|
| α1 subunit cRNA | Oligonucleotide,* 20 nl per oocyte | |
| α1C‡ | — | 20/22 |
| α1C | B10 sense, 100 μM | 6/6 |
| α1C | B11 antisense, 100 μM | 0/10 |
| α1C | B11 antisense, 10 μM | 0/14 |
| α1E | — | 27/29 |
| α1E | B10 sense, 100 μM | 10/10 |
| α1E | B11 antisense, 100 μM | 0/4 |
| α1E | B8 antisense, 100 μM | 3/16§ |
| α1E | B24 antisense, 100 μM | 0/12 |
| α1E | B24 antisense, 10 μM | 20/20¶ |
| α1E | B11 antisense, 10 μM | 1/9‖ |
Sense and antisense refer to coding strand of common sequences of Xenopus oocyte xo-28 and xo-32 cDNAs. B8, 5′TTGAGCC[A/T]GCCTCTCCACCTC; B10, 5′AGATTTCCTACATATCAAGGAGAA; B11, 5′GCACTCCTCATCCAGCGCTCCACAG; and B24, 5′TGAACCCACTTCTGAGTCTTCAAA.
x of y oocytes from 2 to 3 frogs.
All α1C experiments were performed with the ΔN60 mutant of α1C lacking amino acids 1–60 at the N terminus.
1–5% of control in the three positives.
16 ± 8% of control at 30 mV.
3% of control for the only positive.
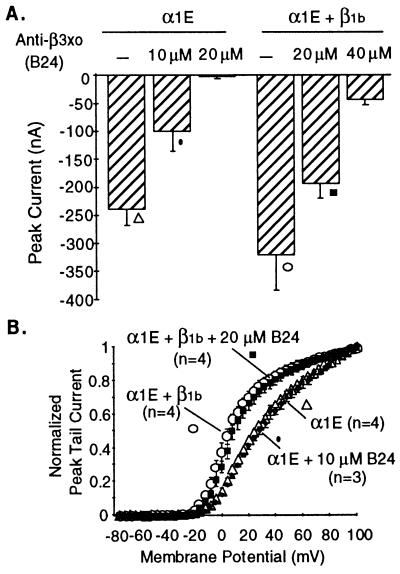
For the second approach, we coinjected antisense oligonucleotides together with mammalian α1C or α1E subunits, which according to our previous experience do not require coexpression of β subunits for their expression. We found that anti-xo28/xo32 oligonucleotides completely suppressed the functional expression of the type C and E α1 subunits (Table 1 and Fig. 4). This suppression of expression of α1C and α1E was not due to a poisoning effect of the antisense oligonucleotides because: (i) the same effect was obtained with three different antisense oligonucleotides (B8, B11, and B24); (ii) sense oligonucleotides synthesized and purified in parallel failed to affect expression of exogenous α1 subunits; (iii) β3xo antisense oligonucleotides that prevented α1C and α1E expression did not interfere with expression of the Shaker K+ channel—i.e., of an unrelated ion channel (data not shown); and (iv) α1 currents could be rescued from suppression by antisense oligonucleotide by coinjection of rat β1b cRNA (Fig. 4). Table 1 summarizes our results on effects of xo28 and xo32 antisense oligonucleotides on the functional expression of mammalian α1 subunits.
Figure 4.
Inhibition by anti-β3xo oligonucleotide B24 of expression of α1E currents and rescue of α1E currents by coinjection of cRNA encoding the rat β1b subunit. Oocytes were injected with 50 nl of a solution containing 100 μg/ml of α1E cRNA alone or in combination with 100 μg/ml β1b cRNA and the indicated concentrations of B24 oligonucleotide. Ca2+ channel currents were recorded 6 days after injection. The results presented on this figure were obtained with oocytes obtained from a single frog. Similar results but varying in the concentration of B24 needed to affect α1E currents, were obtained in two other experiments. (A) Peak inward α1E currents recorded from oocytes after a 250-msec test pulse from a holding potential of −90 mV. Injection of cRNAs and B24 oligonucleotide are indicated above the bars, which are means ± SEM of 4–5 oocytes. (B) Averaged G–V curves (mean ± SEM) of α1E currents in oocytes coinjected or not with B24 and B24 plus β1b cRNA. The composition of B24 is given in Table 1.
Two Sites for Interaction with a β Subunit on α1E.
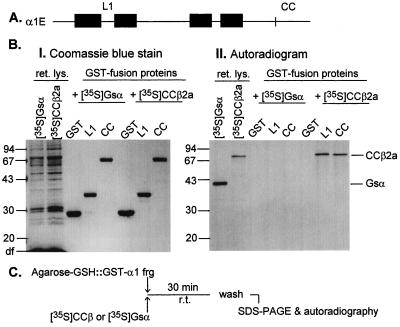
Campbell and coworkers (14) identified the existence of a β subunit binding domain within the L1 loop that connects the homology repeats I and II of α1 subunits. We used a very similar technique to test for the possible existence of a second β subunit binding domain by incubating fragments of α1E fused to GST (GST fusions) with a Ca2+ channel β subunit (CCβ) made and labeled by in vitro translation in the presence of [35S]methionine as described by Pragnell et al. (14). As shown in Fig. 5, we found that β2a, in addition to binding to L1, binds to a domain contained within the last 277 aa of the >400-aa C terminus of α1E (fragment CC). It follows that, at least in vitro, α1E has two interaction domains able to interact with and forming stable complexes with a calcium channel β subunit.
Figure 5.
Identification of two sites on α1E that interact with β subunits. (A) Ideogram of a Ca2+ channel α1 subunit with linear N and C termini, four homologous repeats (filled boxes), and connecting loops. (B) 12% SDS/PAGE analysis of GST fusion proteins and bound calcium channel β2a or G protein αs subunits synthesized by reticulocyte lysates in the presence of [35S]methionine. BI, Coomassie blue stain; BII, autoradiogram of the gel shown in BI. (C) Experimental design of the test for protein–protein interaction. The figure shows binding of β2a to the L1 and the CC regions of α1E. L1, α1E[356–451]; and CC, α1E[2036–2312]. Note that both α1E L1 and α1E CC bound [35S]CCβ2a but not an unrelated protein, [35S]Gsα.
DISCUSSION
Natural Expression of a Calcium Channel β Subunit in Stage IV–VI Xenopus Oocytes.
Throughout these studies, we gathered four types of evidence indicating that Xenopus oocytes express an endogenous β subunit that is active and functional. First, we identified by molecular cloning two mRNAs encoding type-3 β subunits (xo28 and xo32). These sequences were found in mRNA isolated from oocytes that had been thoroughly defolliculated by the collagenase treatment routinely used by us in the preparation of oocytes for injection of cRNAs (see Methods). This makes it unlikely that xo28 and xo32 were derived from non-oocyte mRNA. Second, coinjection into oocytes of α1E cRNA and either one of the newly cloned xoβ cRNAs led to expression of Ca2+ channel currents that were indistinguishable from those obtained upon coexpression of α1E with mammalian β3, indicating that the cloned sequences encode a β subunit that is functional in an assay that we and others have used previously to identify β subunit function (cf. 8, 18, and 19). Third, injection of the B11 antisense oligonucleotide (Table 1), which was designed to be complementary to a region of the coding sequence of xo28 and xo32 showing no differences in nucleotide composition, resulted in partial but significant suppression of expression of endogenous voltage-dependent Ca2+ currents, as would be expected if endogenous Ca2+ channel function were dependent on presence of a regulatory β subunit. Fourth, injection of xo28/xo32 antisense oligonucleotides (B11, B8 and B24) resulted in suppression of the functional expression of exogenously injected α1 subunits. B11, B8, and B24 are complementary to nucleotides 210–235, nucleotides 127–147, and nucleotides 28–51, respectively, of xo28 and xo32. Coinjection of sense type xo28/xo32 oligonucleotides (e.g., B10) did not mimic the effects of B11, B8, and B24.
The role of this endogenous β subunit has not been explored, but is likely to relate to the fact that there are batches of oocytes expressing endogenous voltage-dependent Ca2+ currents. Type-3 Xenopus β is thus likely to be part of the molecular makeup of endogenous oocyte Ca2+ channels.
Implications for Interpretation of Calcium Channel Properties Observed in Xenopus Oocytes.
The most significant finding of the present work is not that oocytes express a calcium channel β subunit, but rather that β3xo antisense oligonucleotides completely obliterated expression of exogenous calcium channel currents that would otherwise have been expressed, and also the apparent inability of the endogenous xoβ subunits to affect the gating properties of the channel they are helping to express. Thus, there was no expression of α1 without an endogenous β, but on the other hand, the level of expression of the endogenous β was not sufficient to regulate the activity of the channel. Related to this is the question of whether calcium channel currents measured in oocytes that were injected with α1 subunits alone are currents mediated by calcium channels formed of α1 alone (i.e., devoid of β) or whether these currents are mediated by complexes formed of the injected α1 plus an endogenous Xenopus β, an xoβ.
One possibility is that α1 subunits are expressed only in association with a β (i.e., as α1β complexes) and that differences in properties between α1·xoβ and α1·exogenous β reflect differences in the regulatory abilities of xoβ vs. those of the exogenous mammalian β. The result with B11 (the anti-xoβ oligonucleotide) on endogenous calcium channel currents would be consistent with this possibility, as the I–V relationships in B11 injected oocytes were the same as those found in oocytes injected with inactive control oligonucleotide. In this case, the effect of the antisense oligonucleotide is well explained by assuming that residual currents reflect currents due to the residual α1·xoβ complexes expressed on the surface of the oocyte. On the other hand, by the same reasoning, injection of xoβ should not have affected the properties of currents of mammalian α1 subunits, but merely increased them. Yet, Xenopus β3 (i.e., βxo28 or βxo32), rather than merely augmenting α1E currents, modified the properties of α1E currents changing both their G–V relations and voltage-induced inactivation. Since in the absence of xoβ (in B11-, B8-, or B24-injected oocytes) there was no expression of a functional α1 whatsoever as seen from absence of gating currents at the beginning of the time courses of channel activation (data not shown), one would have to argue that the endogenous xoβ must have acted as a “chaperone” aiding in the folding and transit of α1 to the surface of the oocyte, but that once at the cell surface, formation of stable α1β complexes requires “high” levels of β, higher than naturally supplied by the oocyte. This raises the possibility that currents in α1E-injected oocytes, generally referred to as α1 alone are in reality the result of mixtures of α1E alone plus α1E·βxo complexes. Analysis of G–V curves of α1E alone oocytes could indeed be interpreted in this way: (i) the channel behaves as if it existed in two main states, one, ≈50% of the total, responding to voltage with a V1/2 of −5 mV, and the other responding with a V1/2 of +50 mV; and (ii) the effect of exogenous β subunits (mammalian β or β3xo; Figs. 2 and 4) on activation is essentially to increase the proportion of channels responding to voltage with a V1/2 of −5 mV. We could presume that the channels responding at low voltage represent α1β complexes, while channels responding at the higher voltage represent α1 alone, which however required xoβ for their successful targeting to the plasma membrane. Against this reasoning is a consistent failure to observe a decrease in the proportion of the channel molecules responding to low voltage coincident with reduction in the level of residual current. As shown in Fig. 4, the G–V curves of α1E alone are indistinguishable from the G–V curves of oocytes in which α1E expression was reduced by 50% by injection of 10 μM of B24, an anti-β3xo oligonucleotide.
Taking our findings into account, it would seem that the maturing and regulation of a voltage-gated calcium channel under the influence of a β subunit should be envisaged as depending on two independent actions of β subunits: one, requiring only low levels of β, would be to serve as an organizer of the secondary and tertiary structure of α1 and to help in one or more of the many steps that intervene between its initial translation and its appearance on the cell surface; the second, requiring higher concentrations of β, would be to regulate the activity of the assembled channel. The steps between translation and localization to the plasma membrane may include simple folding, membrane insertion, aid in co- and posttranslational modification, and targeting of the inserted α1 to the particular plasma membrane domain where it is to function—e.g., cell body, axon, and synapse. It is possible that once inserted and targeted to the cell surface, the site or sites of α1–β interaction lose affinity for each other so that further actions of β subunits require higher levels of the β subunit. Such higher concentrations would of course be readily attained upon injection of massive amounts of cRNA into an oocyte.
The thus far known regulatory effects of β subunits include: (i) facilitating the coupling between the voltage sensor and pore opening, thus facilitating channel activation (5); (ii) modulating switching between gating modes (20); (iii) conferring sensitivity to prepulse potentiation to α1C, which is an effect seen with β1b but not with β2a subunits (21, 22); (iv) facilitating or decreasing, in an also β subunit-specific manner, voltage-induced inactivation (16); and (v) interfering with inhibitory regulation of dihydropyridine-insensitive Ca2+ channels by activated G proteins (23, 24). Work by Pragnell et al. (14) and Witcher et al. (25) has led to the identification of a high-affinity binding site for β within the L1. This site is present on all α1 subunits and binds to all β subunits. Its existence does not easily explain diverse effects of β subunits on Ca2+ channel activity that vary with the type of α1 and/or the type of β. Moreover, its high affinity does not predict easy dissociation of β from α1 to allow for appearance of the α1 alone-type channels seen in Xenopus oocytes in the absence of exogenous injection of a β subunit cRNA.
An alternative explanation for the observations at hand is that the interaction between α1 and β mediated by the loop I–II site is permanent and has a structural role and that the regulatory effects result from interaction of α1β complexes with a second and possibly more β subunits. Our finding that the C-terminal half of α1E is able to form a stable complex with a β subunit in vitro without the participation of the L1 loop (Fig. 5), while not proving participation of more than one β subunit in the normal functioning of a channel in the environment of an intact cell, is at least compatible with this possibility. The interaction and regulation of phosphorylase kinase with calmodulin is an example of this type of regulation (26). Phosphorylase kinase has subunit composition (αβγδ)4 (Mr, 1,280,000), of which the δ subunit is calmodulin, which remains associated to the complex even in 8 M urea, provided Ca2+ is present. The activity of this complex is dependent on Ca2+ and is stimulated 5-to 6-fold by addition of exogenous calmodulin, which reaches a maximum at molar ratios of calmodulin to phosphorylase kinase of 20 (26).
In conclusion, our findings are consistent with the hypothesis that β subunits may regulate α1β complexes: (i) inhibition of β subunit biosynthesis prevents channel formation (Table 1 and Fig. 4); (ii) biosynthesis of “low levels” of β, such as might constitutively be made by Xenopus oocytes, allows for assembly of functional channels that functionally appear unaffected by a β subunit (e.g., α1E alone traces in Fig. 2 A, C, and D and Fig. 4B may in fact be due to α1β); and (iii) expression of high(er) levels of β subunits, as obtained upon injection of cRNA encoding a β (e.g., Fig. 2 B, C, and D and Fig. 4B) allows for development of Ca2+ channels with properties that may vary not only with the type of α1 but also the type of β subunit. Higher-order regulatory complexes, based on lower affinity interactions, may not withstand prolonged purification procedures and could therefore have been missed in previous biochemical studies. Specific studies analyzing immunoprecipitates obtained under nondenaturing conditions that favor preservation of multicomponent complexes will have to be carried out to substantiate the formation of complexes of α1β with β in a normal cellular environment.
Acknowledgments
E.T. was a recipient of a postdoctoral fellowship from the Deutsche Forschungsgesellschaft. This work was supported in part by National Institutes of Health Grants AR-43411 (L.B.) and AR-38970 (E.S.).
ABBREVIATIONS
- RACE
rapid amplification of cDNA ends
- GST
glutathione S-transferase
References
- 1.Catterall W A. Science. 1991;253:1499–1500. doi: 10.1126/science.1654596. [DOI] [PubMed] [Google Scholar]
- 2.Snutch T P, Reiner P B. Curr Opin Neurobiol. 1992;2:247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Reyes E, Schneider T. Drug Dev Res. 1994;33:295–318. [Google Scholar]
- 4.Birnbaumer L, Campbell K P, Catterall W A, Harpold M M, Hofmann F, Horne W A, Mori Y, Schwartz A, Snutch T P, Tanabe T, Tsien R W. Neuron. 1994;13:505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 5.Neely A, Wei X, Olcese R, Birnbaumer L, Stefani E. Science. 1993;262:575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- 6.Schneider T, Wei X, Olcese R, Constantin J, Neely A, Palade P, Perez-Reyes E, Qin N, Zhou J, Crawford G D, Smith G R, Appel S H, Stefani E, Birnbaumer L. Recept Channels. 1994;2:255–270. [PubMed] [Google Scholar]
- 7.DeWaard M, Campbell K. J Physiol. 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 9.Sanger F, Nicklen S, Coulson A B. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanford J, Codina J, Birnbaumer L. J Biol Chem. 1991;266:9570–9579. [PubMed] [Google Scholar]
- 11.Perez-Reyes E, Castellano A, Kim H S, Bertrand P, Baggstrom E, Lacerda A E, Wei X, Birnbaumer L. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 12.Neely A, Olcese R, Wei X, Birnbaumer L, Stefani E. Biophys J. 1994;66:1895–1903. doi: 10.1016/S0006-3495(94)80983-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taglialatela M, Toro L, Stefani E. Biophys J. 1992;61:78–82. doi: 10.1016/S0006-3495(92)81817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch T P, Campbell K P. Nature (London) 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 15.Hughes M K, Hughes A L. Mol Biol Evol. 1993;10:1360–1369. doi: 10.1093/oxfordjournals.molbev.a040080. [DOI] [PubMed] [Google Scholar]
- 16.Olcese R, Qin N, Neely A, Stefani E, Birnbaumer L. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 17.Lacerda A E, Perez-Reyes E, Wei X, Castellano A, Brown A M. Biophys J. 1994;66:1833–1843. doi: 10.1016/S0006-3495(94)80977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. J Biol Chem. 1993;268:3450–3455. [PubMed] [Google Scholar]
- 19.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. J Biol Chem. 1993;268:12359–12366. [PubMed] [Google Scholar]
- 20.Costantin J, Qin N, Birnbaumer L, Stefani E, Neely A. Biophys J. 1995;68:258. (abstr.). [Google Scholar]
- 21.Bourinet E, Charnet P, Tomplinson W J, Stea A, Snutch T, Margeot J. EMBO J. 1994;13:5032–5029. doi: 10.1002/j.1460-2075.1994.tb06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costantin J L, Qin N, Birnbaumer L, Stefani E. Biophys J. 1996;70:A13. [Google Scholar]
- 23.Campbell V, Berrow N S, Fitzgerald E M, Brickley K, Dolphin A C. J Physiol. 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roche J P, Anantharam V, Treistman S N. FEBS Lett. 1995;371:43–46. doi: 10.1016/0014-5793(95)00860-c. [DOI] [PubMed] [Google Scholar]
- 25.Witcher D R, De Waard M, Liu H, Pragnell M, Campbell K P. J Biol Chem. 1995;270:18088–10093. doi: 10.1074/jbc.270.30.18088. [DOI] [PubMed] [Google Scholar]
- 26.Shenolikar S, Cohen P T, Cohen P, Nairn A C, Perry S V. Eur J Biochem. 1979;100:329–337. doi: 10.1111/j.1432-1033.1979.tb04175.x. [DOI] [PubMed] [Google Scholar]