Abstract
When galactose is added to logarithmically growing culture of the yeast Saccharomyces cerevisiae, a set of genes encoding galactose-metabolizing enzymes (GAL genes) starts to be transcribed within a few minutes. This rapid induction involves a serial interplay of Gal3p, Gal80p, and Gal4p. Recent experiments have indicated that a direct interaction between Gal3p and Gal80p plays a pivotal role in an early step of GAL induction. Here we demonstrate that complex of Gal3p and Gal80p, otherwise unstable, is stabilized in the presence of 0.1 mM galactose and 0.5 mM ATP. The requirement for galactose and ATP for stable complex formation is also observed by using highly purified Gal3p and Gal80p from yeast. We further show that thus formed Gal3p/Gal80p complex can easily be dissociated when it is washed with buffer lacking galactose. Finally, we show that mutant proteins encoded by GAL80S or GAL80DE21, which confer galactose-uninducible phenotype, fail to interact with Gal3p. These results strongly suggest that Gal3p functions as the sensor and transducer of galactose signal in the induction pathway of Gal4p-activated genes.
Adaptive responses of microorganisms to the environment have been providing excellent subjects in our understanding of the molecular mechanisms of gene expression, as well as signal transduction of the cell, for nearly the entire latter half of this century. The regulatory circuit of galactose-inducible genes in the yeast Saccharomyces cerevisiae (hereafter called GAL genes) involving Gal4p and Gal80p has been one of the best studied examples of the gene expression (1, 2). Thus, Gal4p is the best characterized transcriptional activator in eukaryotes, and Gal80p regulates the activity of Gal4p directly by the protein–protein interaction in response to galactose in the medium (for review see ref. 2). By contrast, the pathway through which the galactose signal is conveyed to Gal4p has been poorly understood, since the exact role of Gal3p, the key factor in the process of GAL induction, has been unclear.
The most remarkable feature of Gal3p is that its deficiency results in a long delay in inducing GAL genes (3, 4). Once yeast having gal3, loss-of-function mutations in GAL3, adapts to galactose, it can ferment galactose as efficiently as the wild type, suggesting that Gal3p is required only for the establishment of the induced state, and not for its maintenance. Peculiarly, respiratory-deficient gal3 yeast is completely unable to ferment galactose (5). Double mutants, such as gal80gal3 or GAL4Cgal3, can be isolated as galactose-fermenting revertants from gal3 mutants, indicating that the constitutive mutations in the GAL4 or GAL80 loci suppress gal3 mutations (5, 6). In gal3 yeast, mutations in any one of the GAL genes completely eliminate the expression of the other GAL genes (7). In such yeast (i.e., gal3gal1, gal3gal7, or gal3gal10), the Gal3p function is required not only for the establishment but also for maintenance of the induced state (8).
Recent experiments have suggested that a direct interaction between Gal3p and Gal80p plays a pivotal role in GAL induction. Thus, Gal3p, when overexpressed in GAL80 wild-type yeast, causes expression of GAL genes in the absence of galactose (9). This constitutive GAL expression is repressed by concomitant overexpression of GAL80 in the cell (10). The interaction between Gal3p and Gal80p appears to be direct, since Gal3p forms a complex with Gal80p in vitro (10). However, the connection between the galactose signal and Gal3p/Gal80p interaction remained unclear. In this report, we demonstrate that complex of Gal3p and Gal80p is stabilized in the presence of galactose and ATP. This holds true irrespective of the presence or absence of galactose in the medium, in which yeast is grown for preparation of Gal3p and Gal80p. We further show that thus formed Gal3p/Gal80p complex can easily be dissociated when the complex is washed with buffer lacking galactose. These characteristics of the Gal3p/Gal80p interaction can be reproduced by using highly purified Gal3p and Gal80p. Finally, we demonstrate that mutant proteins encoded by GAL80Sconferring galactose-uninducible phenotype (11, 12) fail to interact with Gal3p. These results lead us to propose an advanced model for GAL induction, which can successfully explain some, if not all, of the enigmatic findings on the role of Gal3p mentioned above.
MATERIALS AND METHODS
Yeast Strains.
The strains used were S. cerevisiae NFG1 [MATa gal3::HIS3 gal80::LEU2 ade his3 leu2 trp1 ura3 (10)] and MT81-1 [MATa gal80::LEU2 ade ura3 trp1 his3 leu2 (13)].
Media and Growth Conditions.
Cells were grown at 30°C in enriched synthetic medium (10), supplemented with adenine sulfate at 40 μg/ml and glucose at 2% (ESD), collected by centrifugation, and inoculated in ESGlyLac, which contained 2% each of glycerol and sodium lactate in place of glucose in ESD. Cultures were grown to logarithmic phase and, when necessary, glucose or galactose was added at 2%, and growth was continued for an additional 6 hr.
Plasmids.
Plasmid pHAGAL3, which overexpresses Gal3p tagged with influenza virus hemagglutinin epitope (HA-Gal3p) under the control of the ADH1 promoter, was described (10). The 2.3-kb SphI fragment of pHAGAL3 was subcloned into pTV3 (14) to yield pTH30. The entire open reading frame of wild-type GAL80, GAL80S0, GAL80S1, GAL80S2 (15), and GAL80DE21 (13) were cloned into pVT102U (16) between the PvuII and HindIII sites located downstream of the ADH1 promoter to yield pVTG80, pVTG80S0, pVTG80S1, pVTG80S2, and pVTG80DE21, respectively. The 1.7-kb SphI fragments of pVTG80, pVTG80S0, pVTG80S1, pVTG80S2, and pVTG80DE21 were excised and cloned into pRS316 (17) to yield pRSG80, pRSG80S0, pRSG80S1, pRSG80S2, and pRSG80DE21, respectively. Details of plasmid construction are available upon request. Plasmids were introduced into yeast cells by electroporation (18) by using a Gene Pulser (Bio-Rad).
Immunoprecipitation.
Whole-cell extracts were prepared as reported (10). Five micrograms of 12CA5 anti-HA mAb (Boehringer Mannheim) were reacted with 20 μl of protein G plus/protein A agarose (50% suspension, Oncogene Research Products, Cambridge, MA) at 4°C for 2 hr in Tris-buffered saline (TBS; 20 mM Tris·Cl, pH 7.5/150 mM NaCl) containing 0.02% Tween 20 and 5% nonfat milk. After washing three times with TBS containing 0.02% Tween 20, the antibody conjugated with protein G/A agarose was reacted with 500 μg of the whole-cell extract in basal binding buffer (50 mM Tris·Cl, pH 7.5/100 mM NaCl/5 mM MgCl2/1 mM dithiothreitol/1 μg/ml pepstatin A/1 μg/ml leupeptin/1 mM phenylmethylsulfonyl fluoride, and galactose and ATP at the concentrations indicated in figure legends). The reaction mixture was gently mixed on a microtube rotater (Iuchi, Osaka) at 4°C for 2 hr. After incubation, the immunocomplex was collected by centrifugation at 300 × g for 1 min and washed three times with basal binding buffer containing galactose and ATP at the same concentrations as in the reaction mixture, supplemented with Tween 20 at 0.02% (washing buffer). Proteins were extracted from the precipitates by boiling with a small volume of Laemmli’s sample buffer (19) and then subjected to SDS/PAGE, followed by immunoblotting. Coprecipitated Gal80p was detected with rabbit anti-Gal80p antibody (13) as described previously (10). The membrane blot was reprobed with rabbit anti-Gal3p antibody (10) to detect HA-Gal3p.
Purification of Gal80p and HA-Gal3p.
Gal80p was purified according to the method described by Yun et al. (20), except that the final fraction (Fraction 5) was further subjected to Mono S column chromatography by using the fast protein liquid chromatography (FPLC) system (Pharmacia). HA-Gal3p was purified from YJJ337 [MATa reg1-501 leu2-3, 112 gal1 pep4-3 prb1-1122 (21)] harboring pHAGAL3 grown in ESD or ESGal. Steps from cell disruption to DEAE column chromatography were essentially the same as in purification of Gal80p (20). Eluted samples from the column were successively subjected to phosphocellulose (P11) column chromatography, Bio-Gel 0.5-m gel filtration, and Mono S FPLC. The detailed procedures will be published elsewhere and are available upon request.
Binding Assay of Purified Proteins.
Highly purified HA-Gal3p (150 ng) and Gal80p (50 ng) were reacted in basal binding buffer supplemented with 0.05% Tween 20 at 4°C for 2 hr. Gal80p/HA-Gal3p complex was recovered by the use of 12CA5 anti-HA mAb conjugated with protein G/A agarose and analyzed by immunoblotting as described above.
Assay of UDPGal-4-Epimerase.
Whole-cell extracts were prepared from MT81-1 cells harboring pTH30 and one of pRSG80s. UDPGal-4-epimerase activity was determined by the two-step method described by Fukasawa et al. (22).
RESULTS AND DISCUSSION
Weak Association of Gal3p with Gal80p in the Absence of Specific Cofactor.
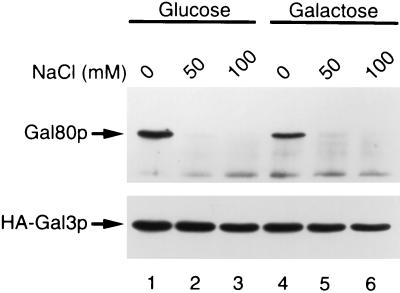
Previously we reported that HA-tagged Gal3p, when overexpressed in GAL80 wild-type yeast grown in the absence of galactose, formed immunoprecipitable complex with Gal80p by using anti-HA antibody (10). The amount of coprecipitated Gal80p increased when the yeast was grown in the presence of galactose. However, when both Gal80p and tagged Gal3p were overexpressed, the amount of coprecipitated Gal80p was not significantly affected by the addition of galactose in the medium. The observed effect of galactose in GAL80 wild-type yeast was accounted for by the increased amount of Gal80p derived from the chromosomal GAL80 by galactose, which facilitated complex formation. [Remember that GAL80 itself is galactose-inducible (23).] Therefore, we were unable to correlate the interaction of Gal3p/Gal80p with the galactose signal. We then undertook a systematic reinvestigation on conditions in which the complex was formed. Thus, we prepared whole-cell extract from gal80gal3 null yeast bearing two multicopy plasmids; one carried HA-tagged GAL3, while the other carried GAL80, both of which were expressed under the control of the ADH1 promoter. The extract was incubated with anti-HA mAb in the presence of varying concentrations of salt without any cofactors. Antigen/antibody complex was bound to protein G/A-immobilized agarose and washed with buffer containing the same concentration of salt as binding buffer. Proteins were then extracted from protein G/A agarose and subjected to SDS/PAGE followed by immunoblot analysis with anti-Gal80p or anti-Gal3p antibodies. As is seen in Fig. 1, Gal80p was found in immunoprecipitates only when immunoreactions were performed in basal buffer containing no NaCl. By contrast, Gal80p was not coprecipitated with HA-Gal3p when reactions were performed in the presence of more than 50 mM NaCl. This holds true irrespective of the presence or absence of galactose in the medium when yeast was grown for preparation of crude extracts. These results have prompted us to reinvestigate the Gal3p/Gal80p interaction under a high salt (100 mM).
Figure 1.
Weak association of Gal80p with HA-Gal3p in the absence of galactose and ATP. Yeast cells (NFG1) overexpressing Gal80p and HA-Gal3p were grown in ESGlyLac supplemented with glucose (lanes 1–3) or galactose (lanes 4–6) at 2%. Whole-cell extracts from these cells were incubated with 12CA5 anti-HA antibody conjugated with protein G/A agarose. NaCl was added to both binding buffer and washing buffer at the indicated concentrations. Immunoprecipitated proteins were subjected to SDS/PAGE, blotted to nitrocellulose membrane, and probed with rabbit anti-Gal80p antibody (Upper) or rabbit anti-Gal3p antibody (Lower).
Requirements for Galactose and ATP in Complex Formation of Gal3p and Gal80p.
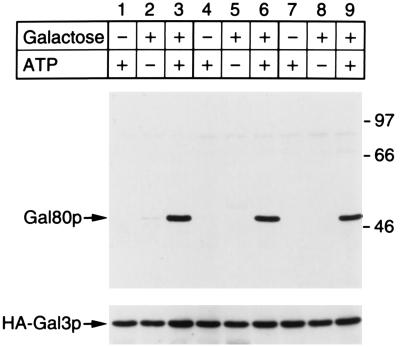
It is known that the yeast Kluyveromyces lactis has a regulatory mechanism of the gene expression controlling galactose metabolism similar to that in S. cerevisiae (24, 25). In the case of K. lactis, galactokinase performs a Gal3p-like regulatory function in addition to the enzymatic function, that is, phosphorylation of galactose in the presence of ATP (26). On the other hand, the S. cerevisiae galactokinase and Gal3p share more than 70% identical amino acids based on the nucleotide sequence of the respective gene (10). These facts strongly suggest that the two proteins evolved from an ancestor like the K. lactis galactokinase. Most recently, Zenke et al. (27) reported that galactokinase and Gal80p of K. lactis form a complex depending on the presence of galactose and ATP. In light of these findings, together with the similarity of Gal3p to galactokinase, we examined whether the interaction between the S. cerevisiae Gal3p and Gal80p was also affected by galactose and ATP. As is seen in Fig. 2, the association of Gal3p and Gal80p was clearly stabilized in the presence of both galactose and ATP even under the high salt concentration.
Figure 2.
Stabilization of Gal80p/HA-Gal3p complex in the presence of both galactose and ATP. Yeast cells (NFG1) overexpressing Gal80p and HA-Gal3p were grown in ESGlyLac (lanes 1–3), in ESGlyLac supplemented with 2% glucose (lanes 4–6), or in ESGlyLac supplemented with 2% galactose (lanes 7–9). Whole-cell extracts prepared from these cells were subjected to immunoprecipitation. ATP (2 mM) and/or galactose (1 mM) were added to both binding buffer and washing buffer as indicated at top. Both binding buffer and washing buffer always contained NaCl at 100 mM. Immunoprecipitated proteins were subjected to SDS/PAGE, blotted to nitrocellulose membrane, and probed with rabbit anti-Gal80p antibody (Upper). The membrane blot was then reprobed with rabbit anti-Gal3p antibody (Lower). Molecular masses of standard markers are shown in kDa to the right (Upper).
Optimal Concentrations of Galactose and ATP for the Interaction Between Gal3p and Gal80p.
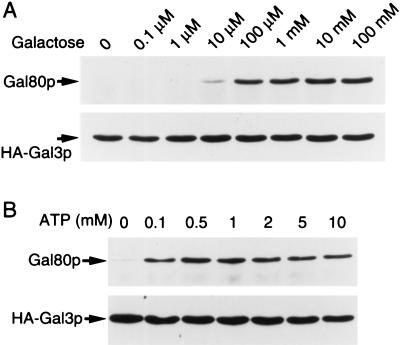
Next we studied optimal concentrations of each of the two cofactors in the formation of Gal3p and Gal80p in crude extracts. Varying concentrations of galactose were added in a crude extract from yeast grown on glycerol and lactate in the presence of a constant concentration of ATP (2 mM). As shown in Fig. 3A, the amount of Gal80p coimmunoprecipitated with Gal3p leveled-off at 100 μM of galactose. Similarly, varying concentrations of ATP were added in the reaction mixture in the presence of a constant concentration of galactose (10 mM). The best coimmunoprecipitation of Gal80p was achieved when the crude extract was incubated in the presence of ATP at a concentration of more than 0.5 mM (Fig. 3B). In these experiments, immunoprecipitates were washed with buffer containing both ATP and galactose at the same concentrations as in the respective reaction mixture.
Figure 3.
Optimal conditions for stable complex formation of Gal80p and HA-Gal3p. Whole-cell extracts were prepared from yeast cells (NFG1) overexpressing Gal80p and HA-Gal3p grown in ESGlyLac. (A) Effect of varying concentrations of galactose on the formation of Gal80p/HA-Gal3p complex. Immunoprecipitation was performed in the presence of 2 mM ATP. Galactose was added to both binding buffer and washing buffer at the concentrations indicated at top. (B) Effect of varying concentrations of ATP on the formation of Gal80p/HA-Gal3p complex. Immunoprecipitation was performed in the presence of 10 mM galactose. ATP was added to both binding buffer and washing buffer at the concentrations as indicated at top.
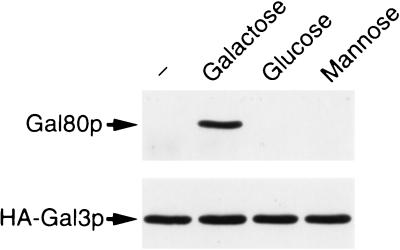
We also examined whether or not isomers of galactose, such as glucose and mannose, can substitute for galactose in stabilization of Gal3p/Gal80p complex. As is clearly seen in Fig. 4, these two isomers could not stabilize the complex formation.
Figure 4.
Effect of optical isomers of galactose on the formation of Gal80p/HA-Gal3p complex. Whole-cell extracts were prepared from yeast cells (NFG1) overexpressing Gal80p and HA-Gal3p grown in ESGlyLac. Immunoprecipitation analysis of extracts from these cells was performed in the presence of 2 mM ATP. Galactose, glucose, or mannose was added to both binding buffer and washing buffer at 10 mM.
Reversible Nature of Interaction Between Gal3p and Gal80p.
As we have shown above, the requirement for galactose and ATP for formation of stable Gal3p/Gal80p complex was observed even when cell extracts were prepared from yeast overexpressing Gal3p and Gal80p grown on galactose. This result may be interpreted to suggest that the presence of galactose in the medium caused no stable modification of either Gal80p or Gal3p. In fact, complex of Gal3p/Gal80p, once formed in the presence of both cofactors, was easily dissociated through washing with buffer devoid of galactose (Fig. 5). From the structural similarity between Gal3p and galactokinase (10), it is reasonable to assume that galactose binds to Gal3p rather than Gal80p. In addition, no evidence has been obtained that galactose binds to purified Gal80p (20). We suggest, therefore, that galactose induces a temporal reversible change in Gal3p in the presence of ATP at a critical concentration, and thereby promotes the complex formation with Gal80p.
Figure 5.
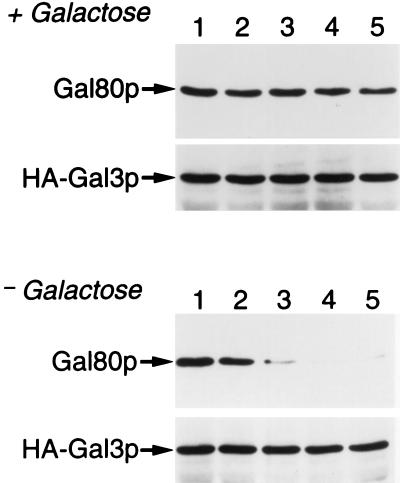
Reversible interaction between Gal80p and HA-Gal3p in a galactose-dependent manner. Whole-cell extracts prepared from cells (NFG1) overexpressing Gal80p and HA-Gal3p grown in ESGlyLac were incubated with anti-HA mAb conjugated with protein G/A agarose in the presence of 1 mM galactose/2 mM ATP. Immunoprecipitates were washed with buffer containing both ATP and galactose (Upper) or with buffer containing ATP only (Lower). After washings for the indicated times shown at top, proteins in the precipitates were analyzed exactly as in Fig. 2.
Analysis of Interaction Between Purified Gal3p and Gal80p.
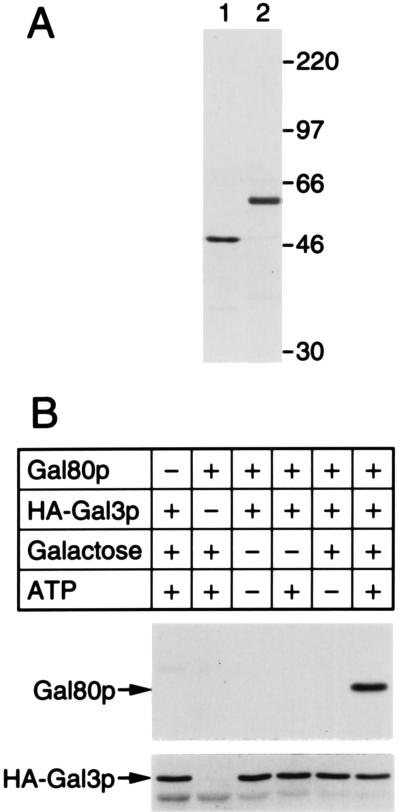
In the above experiments we studied interaction between Gal3p and Gal80p in crude extracts. One might argue therefore that unknown proteins might have been involved in the observed interaction between Gal3p and Gal80p. To address this argument, we purified HA-Gal3p and Gal80p practically to homogeneity from yeast overproducing the respective protein (Fig. 6A). As is seen in Fig. 6B, the purified proteins behaved exactly like those in the crude extracts shown above. These results thus confirmed that Gal3p and Gal80p directly associate without involvement of any other proteins. Precise kinetics and stoichiometry of the Gal3p/Gal80p interaction remain to be elucidated with purified proteins in the near future.
Figure 6.
Interaction between Gal80p and HA-Gal3p at the purified state. (A) One hundred nanograms of purified Gal80p (lane 1) or purified HA-Gal3p (lane 2) were electrophoresed on SDS/polyacrylamide gel followed by silver staining. (B) Purified HA-Gal3p (150 ng), purified Gal80p (50 ng), ATP (2 mM), and galactose (10 mM) were mixed in the binding buffer supplemented with 0.05% Tween 20. The formation of Gal80p/HA-Gal3p complex was analyzed by immunoprecipitation exactly as in Fig. 2.
Failure of Uninducible Mutant Gal80p to Make Contact with Gal3p.
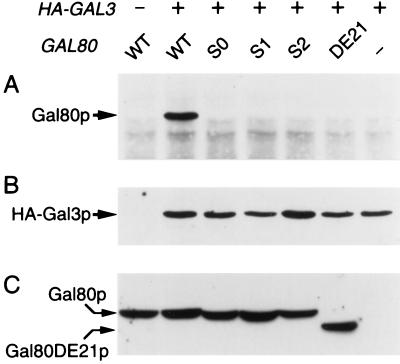
A class of dominant mutations, GAL80S, have been known in the GAL80 allele, which confers uninducibility of GAL genes (11, 12). These mutant proteins had been believed either unable to interact with “inducer” (11, 28), or to bind Gal4p irreversibly (29). In light of the above findings, we suspected if those Gal80p mutant proteins might be anomalous in interaction with Gal3p rather than in interaction with inducer or Gal4p. Whole-cell extracts were then prepared from gal80gal3 null yeast bearing two multicopy plasmids; one carries GAL80S, whereas the other carries tagged GAL3, both of which were expressed under the control of the ADH1 promoter. As shown in Fig. 7, none of three types of Gal80Sp formed complex with tagged Gal3p. This result strongly suggests that the mutational sites in the GAL80S were located in a region through which Gal80p would normally interact with Gal3p. As support for this view, a deletion mutation of Gal80p, Gal80DE21p, encompassing some of the GAL80S sites, has been known to exhibit a dominant uninducible phenotype (13). We then expressed GAL80DE21 along with tagged-Gal3p in gal3gal80 null yeast. As shown in Fig. 7, Gal80DE21p failed to bind Gal3p.
Figure 7.
Failure of uninducible Gal80p mutants in interaction with HA-Gal3p. Whole-cell extracts were prepared from uninduced yeast cells overexpressing HA-Gal3p and the wild-type or one of the mutant Gal80ps; WT, S0, S1, S2, DE21, and − at top represent wild-type GAL80, GAL80S0, GAL80S1, GAL80S2, GAL80DE21, and vacant vector, respectively. Immunoprecipitation was performed in the presence of 1 mM galactose/2 mM ATP. Precipitates were analyzed by immunoblotting with rabbit anti-Gal80p antibody (A) and rabbit anti-Gal3p antibody (B). Twenty micrograms of protein of each whole-cell extract were subjected to immunoblotting using rabbit anti-Gal80p antibody (C).
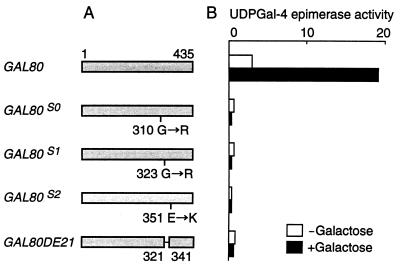
In parallel experiments, we studied the effect of the noninducible mutations used in the above experiments on the expression of UDPGal-4-epimerase in yeast overexpressing HA-Gal3p and wild-type Gal3p. Cells of a gal80 null GAL3 strain (MT81-1) was transformed with pTH30 and one of the pRSG80s. In these cells, either wild-type Gal80p or one of the mutant Gal80ps was expressed under the control of the ADH1 promoter from a centromeric plasmid and, in addition, HA-Gal3p and wild-type Gal3p were expressed from HA-GAL3 on a multicopy plasmid under the control of the ADH1 promoter and from GAL3 on the chromosome under the control of its native promoter, respectively. The activity of UDPGal-4-epimerase encoded by GAL10 was determined in crude extracts from these cells grown in the presence or absence of galactose. As shown in Fig. 8, overexpression of HA-Gal3p and Gal3p caused a partially constitutive or fully induced synthesis of UDPGal-4-epimerase when wild-type Gal80p was expressed in the cell. By contrast, neither constitutive nor induced synthesis of the enzyme was seen when any of the mutant Gal80ps was expressed. These results further strengthen the idea that the interaction between Gal3p and Gal80p plays a crucial role in the pathway of GAL induction.
Figure 8.
Effect of uninducible mutations in GAL80 on the constitutive or induced activity of UDPGal-4-epimerase. (A) Shaded bars represent proteins encoded by wild-type GAL80, GAL80S0, GAL80S1, GAL80S2, or GAL80DE21. The GAL80S-encoded proteins have missense mutations as indicated below the respective protein (28). GAL80DE21 has a deletion encompassing amino acid residues from 322 to 340 (13). (B) The gal80 null yeast (MT81–1) was transformed with a multicopy plasmid overexpressing HA-Gal3p (pTH30) and a centromeric plasmid (pRSG80) carrying one of the uninducible GAL80 mutant genes fused to the ADH1 promoter. Transformant cells were grown in ESGlyLac with or without galactose. The mean values of two independent assays are shown. The activity of UDPGal-4-epimerase (open and solid bars) is expressed as micromoles of UDP-glucose formed per hour per milligram of protein.
A question remains to be addressed as to why GAL80S causes the uninducible phenotype rather than the long-term adaptation like gal3. We tentatively speculate as follows: Because Gal1p (galactokinase), when expressed constitutively by the use of the ADH1 promoter, is capable of complementing gal3 yeast (30), it is reasonable to assume that Gal1p can also interact with Gal80p (but not with Gal80Sp). In a prolonged culture of gal3 yeast Gal1p, along with the other galactose enzymes, could be expressed to a limited extent for an as yet unknown reason; for example, by unbalanced synthesis of Gal4p over Gal80p due to change in intracellular environments. [Unbalanced expression of GAL4/GAL80 is known to result in constitutive expression of GAL genes (31, 32).] Gal1p thus expressed should in turn interact with Gal80p (but not with Gal80Sp) to inhibit its function, resulting in the further amplification of the expression of Gal1p. Such a “chain reaction” would not occur in GAL80S yeast, which would thereby exhibit the uninducible phenotype.
Possible Models for Galactose Induction in S. cerevisiae.
The above findings have advanced the previously proposed model (9, 10) by clarifying the connection of galactose signal with and also the involvement of ATP in the Gal3p/Gal80p interaction: We assume galactose enters the cell and binds to Gal3p, perhaps in the nucleus. Binding of galactose to Gal3p results in a temporal conformational change of Gal3p in the presence of ATP. Gal3p-bound Gal80p in turn changes its affinity to Gal4p, resulting in either dissociation from Gal4p or in an allosteric alteration of Gal4p/Gal80p complex, such that it causes exposure of the transcriptional activation domain of Gal4p (see below). Thus, Gal3p functions not only as the sensor of galactose, but also as the transducer of galactose signal to Gal4p via Gal80p by protein–protein interactions. The requirement of ATP for Gal3p/Gal80p interaction may successfully explain why GAL induction takes place inefficiently in respiratory deficient yeast (see ref. 5). The weak association of Gal3p with Gal80p in the absence of galactose shown in the present as well as in the previous experiments (10) may well account for the fact that overexpression of Gal3p leads to the constitutive expression of GAL genes (9).
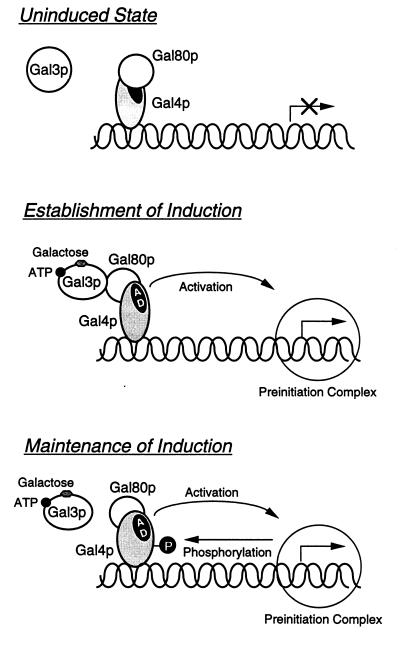
Why do the wild-type S. cerevisiae cells, once adapted to galactose, no longer need the Gal3p function for the maintenance of the induced state? A plausible explanation may be furnished from the recent experiments by Sadowski and his colleagues in which they argue that Gal4p is phosphorylated as a consequence of galactose induction (33), presumably through interaction with the basal transcription complex (34). We assume that the GAL induction process consists of two steps: (i) the establishment of induced state involving the association of Gal3p with Gal80p in the presence of ATP and galactose and (ii) the maintenance of induced state involving phosphorylation of Gal4p (Fig. 9). Sadowski and colleagues demonstrated that Gal4p, once activated in the presence of galactose, is phosphorylated at a critical site, Ser-699, and that Gal4p bearing a mutation of Ser to Ala at the 699 residue, can activate GAL genes only in the gal80 mutant and not in the wild-type yeast (34). These results may imply that the properly phosphorylated Gal4p may no longer be repressed by Gal80p, either by losing affinity to Gal80p or by immobilizing Gal80p at a certain domain of Gal4p such that the activation domain of Gal4p becomes accessible to the transcription machinery. Consequently, Gal3p is no longer needed to maintain the induced state.
Figure 9.
Two-step model for GAL induction. Gal4p is bound to the upstream activating sequence of galactose-inducible genes in a complex with Gal80p in the absence of galactose (35, 36), causing no transcription (uninduced state). Galactose, when added to the medium, binds Gal3p in the presence of ATP resulting in a stable complex formation with Gal80p. Binding of Gal3p to Gal80p in turn causes a structural alteration of Gal80p/Gal4p complex, which makes the activation domain (AD) of Gal4p accessible to the transcription preinitiation complex, and thereby activates the transcription (establishment of induction). Gal4p, once engaged in transcriptional activation, is phosphorylated at a critical site (ref. 34, see text). The properly phosphorylated Gal4p is kept functioning as long as the critical site is phosphorylated (maintenance of induction). For simplicity, the model is tentatively drawn based on the allosteric model (35). This does not necessarily mean that we presently have evidence to rule out the dissociation model.
By contrast, Gal3p is required not only for establishment but also for maintenance of the induced state in galactose-nonfermenting yeast: Thus, the expression of GAL7 in gal3tsgal1 or gal3tsgal10, once induced at a permissive temperature by galactose, is rapidly arrested after shift-up to a restrictive temperature (8). This finding could be explained on the basis of the above model as follows. Suppose the phosphorylation of Gal4p takes place inefficiently in gal3tsgal1 or gal3tsgal10, Gal3p must be kept functioning to maintain GAL7 at the induced state. Indeed, Ser-699 of Gal4p in gal80 null yeast is phosphorylated efficiently when grown on galactose or glucose, but very poorly when grown on nonfermentable carbon sources (see figure 9 in ref. 34). Since gal3tsgal1 or gal3tsgal10 yeast should be growing on nonfermentable carbon sources even when galactose is present in the medium, Gal4p should be phosphorylated but poorly and, therefore, normal function of Gal3p is necessary for GAL7 to be maintained at the induced state.
Our model also explains the deinduction of GAL genes when galactose is exhausted in the medium. As galactose is depleted, Gal3p loses the ability to stably interact with Gal80p. Furthermore, cells begin to use nonfermentable carbon sources that remain in the medium. Dephosphorylation of Gal4p, as well as dissociation of Gal3p from Gal80p, leads to cessation of the transcription of GAL genes.
Finally, a major question to be answered is how the interaction of Gal3p with Gal80p results in activation of Gal4p. Does binding Gal3p to Gal80p cause dissociation of the latter from Gal4p [dissociation model (37, 38)], or does it simply lead to change in the conformation of Gal4p/Gal80p complex [allosteric model (35)]? In our preliminary experiments, Gal4p/Gal80p complex from glucose-grown yeasts did not contain Gal3p. Similarly, Gal3p/Gal80p complex from glucose-grown yeasts did not contain Gal4p. These results rather favor the dissociation model, but do not strictly rule out the allosteric model. In vitro experiments with purified proteins Gal4p, Gal80p, and Gal3p should be performed to address this question.
ABBREVIATION
- HA
hemagglutinin
References
- 1.Johnston M, Carlson M. In: The Molecular and Cellular Biology of the Yeast Saccharomyces: Gene Expression. Jones E W, Pringel J R, Broach J, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. pp. 193–281. [Google Scholar]
- 2.Lohr D, Venkov P, Zlatanova J. FASEB J. 1995;9:777–787. doi: 10.1096/fasebj.9.9.7601342. [DOI] [PubMed] [Google Scholar]
- 3.Winge O, Roberts C. C R Trav Lab Carlsberg Ser Physiol. 1948;24:263–315. [PubMed] [Google Scholar]
- 4.Spiegelman S, Sussman R R, Pinska E. Proc Natl Acad Sci USA. 1950;36:591–605. doi: 10.1073/pnas.36.11.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas H C, Pelroy G. Biochim Biophys Acta. 1963;68:155–156. [Google Scholar]
- 6.Douglas H C, Hawthorne D C. Genetics. 1966;54:911–916. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broach J R. J Mol Biol. 1979;131:41–53. doi: 10.1016/0022-2836(79)90300-0. [DOI] [PubMed] [Google Scholar]
- 8.Nogi Y. J Bacteriol. 1986;165:101–106. doi: 10.1128/jb.165.1.101-106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhat P J, Hopper J E. Mol Cell Biol. 1992;12:2701–2707. doi: 10.1128/mcb.12.6.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki-Fujimoto T, Fukuma M, Yano K-i, Sakurai H, Vonika A, Johnston S A, Fukasawa T. Mol Cell Biol. 1996;16:2504–2508. doi: 10.1128/mcb.16.5.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas H C, Hawthorne D C. J Bacteriol. 1972;109:1139–1143. doi: 10.1128/jb.109.3.1139-1143.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nogi Y, Matsumoto K, Toh-e A, Oshima Y. Mol Gen Genet. 1977;152:137–144. doi: 10.1007/BF00268810. [DOI] [PubMed] [Google Scholar]
- 13.Nogi Y, Fukasawa T. Mol Cell Biol. 1989;9:3009–3017. doi: 10.1128/mcb.9.7.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose M D, Broach J R. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- 15.Nogi Y, Shimada H, Matsuzaki Y, Hashimoto H, Fukasawa T. Mol Gen Genet. 1984;195:29–34. doi: 10.1007/BF00332719. [DOI] [PubMed] [Google Scholar]
- 16.Vernet T, Dignard D, Thomas D Y. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 17.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker D M, Guarente L. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Yun S, Hiraoka Y, Nishizawa M, Takio K, Titani K, Nogi Y, Fukasawa T. J Biol Chem. 1991;266:693–697. [PubMed] [Google Scholar]
- 21.Parthun M R, Jaehning J A. Mol Cell Biol. 1992;12:4981–4987. doi: 10.1128/mcb.12.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukasawa T, Segawa T, Nogi Y. Methods Enzymol. 1982;89:584–592. doi: 10.1016/s0076-6879(82)89101-5. [DOI] [PubMed] [Google Scholar]
- 23.Shimada H, Fukasawa T. Gene. 1985;67:1–9. doi: 10.1016/0378-1119(85)90100-3. [DOI] [PubMed] [Google Scholar]
- 24.Webster T D, Dickson R C. Nucleic Acids Res. 1988;16:8011–8028. doi: 10.1093/nar/16.16.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breunig K D, Kuger P. Mol Cell Biol. 1987;7:4400–4406. doi: 10.1128/mcb.7.12.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer J, Walker-Jonah A, Hollenberg C P. Mol Cell Biol. 1991;11:5454–5461. doi: 10.1128/mcb.11.11.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zenke F T, Engels R, Vollenbroich V, Meyer J, Hollenberg C P, Breunig K D. Science. 1996;272:1662–1665. doi: 10.1126/science.272.5268.1662. [DOI] [PubMed] [Google Scholar]
- 28.Nogi Y, Fukasawa T. Nucleic Acids Res. 1984;12:9287–9298. doi: 10.1093/nar/12.24.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salmeron J M, Jr, Leuther K K, Johnston S A. Genetics. 1990;125:21–27. doi: 10.1093/genetics/125.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhat P J, Hopper J E. Genetics. 1991;128:233–239. doi: 10.1093/genetics/128.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston S A, Hopper J E. Proc Natl Acad Sci USA. 1982;79:6971–6975. doi: 10.1073/pnas.79.22.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto H, Kikuchi Y, Nogi Y, Fukasawa T. Mol Gen Genet. 1983;191:31–38. doi: 10.1007/BF00330886. [DOI] [PubMed] [Google Scholar]
- 33.Sadowski I, Niedbala D, Wood K, Ptashne M. Proc Natl Acad Sci USA. 1991;88:10510–10514. doi: 10.1073/pnas.88.23.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadowski I, Costa C, Dhanawansa R. Mol Cell Biol. 1996;16:4879–4887. doi: 10.1128/mcb.16.9.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leuther K K, Johnston S A. Science. 1992;256:1333–1335. doi: 10.1126/science.1598579. [DOI] [PubMed] [Google Scholar]
- 36.Lue N F, Chasman D I, Buchman A R, Kornberg R D. Mol Cell Biol. 1987;7:3446–3451. doi: 10.1128/mcb.7.10.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perlman D, Hopper J E. Cell. 1979;16:89–95. doi: 10.1016/0092-8674(79)90190-9. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto K, Toh-e A, Oshima Y. J Bacteriol. 1978;134:446–457. doi: 10.1128/jb.134.2.446-457.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]