Abstract
Heterotrimeric G proteins, composed of Gα and Gβγ subunits, transmit signals from cell surface receptors to cellular effector enzymes and ion channels. The Gαo protein is the most abundant Gα subtype in the nervous system, but it is also found in the heart. Its function is not completely known, although it is required for regulation of N-type Ca2+ channels in GH3 cells and also interacts with GAP43, a major protein in growth cones, suggesting a role in neuronal pathfinding. To analyze the function of Gαo, we have generated mice lacking both isoforms of Gαo by homologous recombination. Surprisingly, the nervous system is grossly intact, despite the fact that Gαo makes up 0.2–0.5% of brain particulate protein and 10% of the growth cone membrane. The Gαo−/− mice do suffer tremors and occasional seizures, but there is no obvious histologic abnormality in the nervous system. In contrast, Gαo−/− mice have a clear and specific defect in ion channel regulation in the heart. Normal muscarinic regulation of L-type calcium channels in ventricular myocytes is absent in the mutant mice. The L-type calcium channel responds normally to isoproterenol, but there is no evident muscarinic inhibition. Muscarinic regulation of atrial K+ channels is normal, as is the electrocardiogram. The levels of other Gα subunits (Gαs, Gαq, and Gαi) are unchanged in the hearts of Gαo−/− mice, but the amount of Gβγ is decreased. Whichever subunit, Gαo or Gβγ, carries the signal forward, these studies show that muscarinic inhibition of L-type Ca2+ channels requires coupling of the muscarinic receptor to Gαo. Other cardiac Gα subunits cannot substitute.
Heterotrimeric G proteins, composed of Gα and Gβγ subunits, transmit signals from cell surface receptors to cellular effector enzymes and ion channels. One type of Gα subunit, Gαo, is extremely abundant in the brain, where it was first identified (1, 2), but it is also expressed in heart, pituitary, and pancreas. In addition to Gαo, both the brain and the heart contain other closely related Gα subunits (for example, members of the Gαi group that are, like Gαo, substrates for ADP ribosylation by pertussis toxin), as well as Gαs (which stimulates adenylyl cyclase) and Gαq (which stimulates phospholipase Cβ).
The exact function of Gαo in heart and brain is not known. It is an extremely abundant protein in the nervous system, making up 0.2–0.5% of brain particulate protein (3, 4) and 10% of the growth cone membrane (5). In the nervous system, Gαo has been postulated to play several roles. The ability of Gαo to bind GTPγS can be modulated by GAP43 (neuromodulin), an abundant growth cone protein that is important for neuronal pathfinding (5). Potentially, Gαo could be part of the signaling cascade that regulates neuronal guidance. Its appearance in the mouse central nervous system is consistent with such a role, since it begins to appear as neurons terminally differentiate and increases as they send out processes (6). The Gαo protein is conserved in Drosophila, where it is found predominantly in the nervous system and the ovaries (7–10). In Drosophila, the Go protein is present at all stages of embryonic development, but increases significantly after 10 hr of embryogenesis, when there is rapid development of axonal tracts (11). Gαo levels are modestly increased in certain memory mutants of Drosophila, but the significance of the finding is not yet clear (12).
Genetic analysis of Gαo function in the nematode Caenorhabditis elegans revealed that Gαo is needed to transmit signals from serotonin receptors (13, 14). In C. elegans, Gαo is expressed in the nervous system and in muscle. Worms lacking Gαo are hyperactive, have abnormal feeding and egg-laying behaviors, and are partially resistant to exogenous serotonin. Transgenic worms expressing permanently activated mutants of Gαo have the opposite phenotype. Surprisingly, in C. elegans, mutation of Gαo does not lead to gross disruption of neuronal development, but to a rather subtle defect in a defined pathway.
Another proposed function for Go is regulation of calcium channels. In the pituitary-derived cell line, GH3, antisense oligonucleotides directed against alternately spliced forms of Gαo specifically blocked somatostatin and muscarinic cholinergic receptor inhibition of an N-type Ca2+ channel (15). In the nervous system, Gαo has also been thought to activate neuronal Ca2+ and K+ channels (16–20). The Gαo subunit interacts with a number of cell surface receptors, such as γ-aminobutyric acid type B (GABAB; ref. 21), muscarinic (22), opioid (23), and α2-adrenergic receptors (24), although its exact role in signal transduction through those receptors is not known.
There is little information about the function of Go in the heart. The amount of Gαo in cardiac myocytes is 30- to 120-fold lower than in brain (25, 26). Unlike the brain, in the rat heart, the Gαo is not the major Gα subunit expressed. In rat heart, the amount of Gαs is 0.2 μg per mg of protein, while Gαo is 0.06 μg per mg of protein (25). Gαo is half as abundant as its close relative, Gαi2 (26). It has not been clear whether it specifically regulated any pathway, nor how redundant is its function with other, more abundant Gα subunits. We find that it is absolutely required for normal Ca2+ channel regulation in the heart.
METHODS
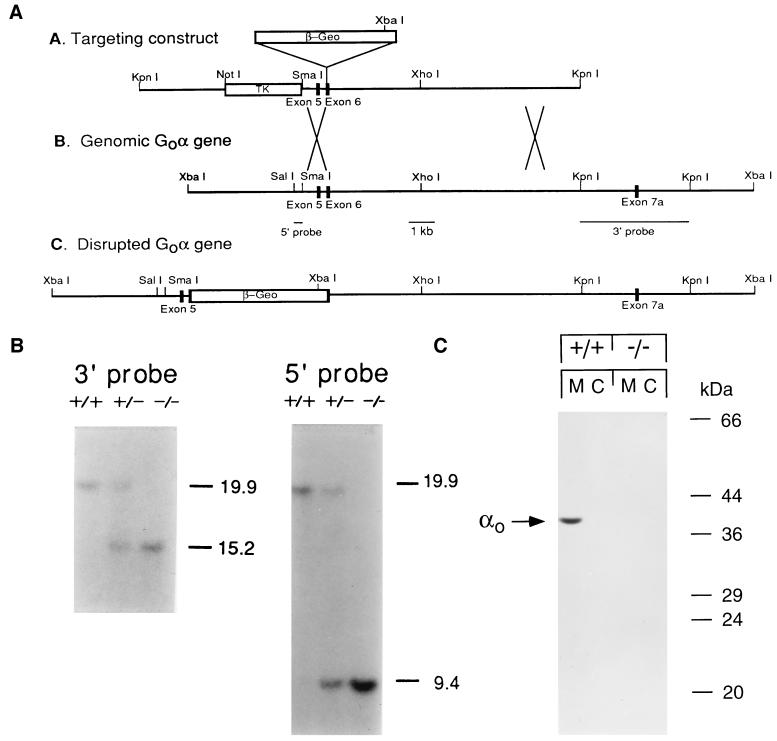
The targeting vector was generated by inserting a blunt-ended 4.7-kb XhoI fragment from the β-geo cassette, which contains the genes for lac Z and neomycin resistance in the same translational frame (27), into the BamHI site of the sixth exon of Gαo. Homologous recombination in J1 embryonic stem cells gave the desired recombinants, two of which were used to transmit the mutation through the germ line. Chimeric mice produced were mated with C57BL/6 mice, and their offspring were interbred to obtain Gαo−/− mice. Southern blot analysis and immunoblotting analysis followed standard procedures. Genomic DNAs were cut with XbaI. The probes used for Southern blot analysis are a 440-bp SalI–SmaI fragment from the 5′ end and a 3.7-kb KpnI fragment from the 3′ end. Two polyclonal rabbit anti-Gαo antibodies were used in Western blots: R4 (against purified bovine brain Gαo; ref. 3) and GC/2 (against the N terminus of Gαo; DuPont/NEN).
Litters from heterozygous Gαo+/− F1 crosses, in the 129 Sv-ter/C57BL/6 hybrid genetic background, were monitored from day of birth until day 125, when the last (n = 24) surviving Gαo−/− mouse died. Litters were inspected twice daily for a period of 3 weeks, and animals found dead were genotyped by PCR of leg tissue. After 3 weeks, animals were ear-tagged, and tail biopsies were used for PCR genotyping. To detect the wild-type Gαo allele, two deoxyoligonucleotides with the following sequences were used: 5′-AGGGGATGAGAGCCGCCTGCAGTC-3′ and 5′-ATGATGGCCGTGACATCCTCGAAGCA-3′. These oligonucleotides amplify a 196-nt fragment spanning the BamHI site in Gαo exon 6. In the β-geo disrupted allele, the size of the PCR product is 4.9 kb. This much larger fragment is undetectable under our PCR conditions. To detect the β-geo gene, a deoxynucleotide pair complementary to sequences in the neo portion of the gene was used. The oligonucleotide pair with sequences: 5′-ATGAACTGCAGGACGAGGCAGCG-3′ and 5′-GGCGATAGAAGGCGATGCGCTG-3′ amplify a 603-nt sequence. The PCR mixture contained the four oligodeoxynucleotides at 25 pmol each in 1× Taq polymerase buffer B containing 3 mM MgCl2 (Promega). To 90 μl of PCR mixture, ≈500 μg of proteinase K-digested tissue was added. Samples were denatured at 95°C for 5 min and kept at 72°C, and 0.5 μl of Taq polymerase (5 units/μl) was added. This was followed by 30 cycles of 30 sec at 95°C and 1 min at 72°C. Aliquots of the PCR were electrophoresed in 2% agarose/ethidium bromide TAE (40 mM Tris·acetate/2 mM EDTA) gel under standard conditions.
Western Blot Analysis.
Brains from 32-day-old mice were excised and homogenized in 10 volumes (wet weight) of TMSDE buffer (50 mM Tris·HCl, pH 7.6/6 mM MgCl2/75 mM sucrose/1 mM DTT/1 mM EDTA and proteinase inhibitors). Homogenates were centrifuged at 800 × g for 10 min at 4°C. The supernatant was then centrifuged at 100,000 × g for 30 min at 4°C.
Heart ventricles from 31- to 32-day-old mice were excised and homogenized in a volume equal to 10 times the wet weight of KCl extraction buffer (20 mM Tris·HCl, pH 7.6/1 mM EDTA/1 M KCl/250 mM sucrose and proteinase inhibitors), incubated on ice for 1 hr, and centrifuged in a tabletop centrifuge at Vmax for 30 min at 4°C. The supernatant containing cytosolic proteins and extracted contractile proteins was removed, and the pellet washed twice in TMSDE buffer and finally resuspended in four volumes of TMSDE buffer. Protein content of both particulate and cytosolic fractions were determined according to Bradford (28) with BSA as standard. Proteins (10–60 μg) were separated on 11% SDS/PAGE (29), followed by semidry blotting for 3 hr. The nitrocellulose strips were blocked with 5% milk powder in PBS and incubated with antibodies against various G protein subunits: αo R4 antibody (3), β R7 antibody (3), β(common) antibody (UBI), αi AS/7 antibody (DuPont, NEN), αs 3A-155 antibody (Gramsch Laboratories), and αq X384-1 antibody (30). After washing with PBS containing 0.1% Tween 20, blots were developed with horseradish peroxidase-conjugated second antibody and chemiluminescence. All G protein subunits tested were present in the particulate fraction, except for traces of Gαq and Gβ in the cytosolic fraction, which did not differ between wild-type, Gαo+/−, and Gαo−/−. Each genotype was represented by three different animals. For each experiment, three Gαo+/+ samples were averaged and taken to equal 100%. Gαo+/− and Gαo−/− samples were expressed as percent of Gαo+/+ mice. The data from the three independent experiments was averaged and expressed in mean ± SD.
Optic Nerve Labeling.
Optic nerve labeling was done as in Strittmatter et al. (31). Proteins were fixed and 10 mg/ml 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI; Molecular Probes) in dimethylformamide was applied to the cut end of the optic nerve, and the sample was incubated at 37°C in 4% formaldehyde for 7 days. Three wild-type and three Gαo−/− were examined after labeling at embryonic day 15, and three wild-type and four Gαo−/− were examined after labeling on the day of birth.
Isolation of Cardiac Myocytes.
Hearts from 6- to 9-day-old (for atrial myocytes) or 21- to 41-day-old mice (for ventricular myocytes) were excised and Langendorff-perfused with Ca2+-free Tyrode’s solution containing: 126 mM NaCl, 4.4 mM KCl, 18 mM sodium bicarbonate, 1 mM MgCl2, 30 mM 2,3-butane-dione monoxime (BDM; Sigma), 4 mM Hepes, 0.13 units/ml insulin, and 11 mM glucose for 3–5 min to wash out the blood, followed by collagenase II (95 units/ml; Worthington) and hyaluronidase (172.5 units/ml; Sigma) containing Tyrode’s solution for 15 min. Tissues from the atrium and ventricle were removed separately and minced in Ca2+-free Tyrode’s solution containing trypsin (0.02 mg/ml) and DNase (60 units/ml). Agitation of the tissue chunks at 37°C for 10 min led to isolation of the majority of the cells. The cell suspension was centrifuged at 50 × g for 3 min, and the cells were suspended in DMEM/Tyrode’s solution (1:1). The final CaCl2 concentration was 0.9 mM.
Analysis of K+ and Ca2+ Channel Function.
Recordings from cardiomyocytes from wild-type and Gαo−/− mice were performed using the membrane-ruptured patch whole-cell configuration (32). The DC resistance of the glass electrode was in the range of 2–4 MΩ when filled with the internal solution containing: 95 mM K-aspartate, 30 mM KCl, 5.0 mM Hepes, 3 mM Na2-phosphocreatine, 0.1 mM GTP (Na), 3 mM K2-ATP, 1 mM MgCl2, 10 mM EGTA, and 1 mM CaCl2; pCa was in the range of 7.4–7.5 and pH was 7.2, adjusted with KOH. A liquid junction potential of approximately −10 mV was corrected electronically. The atrial acetylcholine-sensitive K+ channel (IK-Ach) was studied in the atrial myocytes. Cells were perfused in a solution containing: 145 mM NaCl, 5.4 mM KCl, 1.0 mM MgCl2, 1.0 mM Na2HPO4, 5.0 mM Hepes, 1.8 mM CaCl2, and 10 mM glucose; pH was adjusted to 7.4 with NaOH when gassed with 100% O2. Tetrodotoxin (TTX; 30 μM) and verapamil (2 μM) were added to this solution to block the fast Na+ channel (INa) and the L-type (dihydropyridine-sensitive) ventricular calcium channel (ICa-L), respectively, when recording the IK-Ach. ICa-L were studied in ventricular myocytes. To isolate ICa-L in ventricular myocytes, the electrode solution contained: 90 mM aspartic acid, 30 mM CsCl, 110 mM CsOH, 10 mM EGTA, 3 mM Mg-ATP, 5 mM Hepes, and 1 mM CaCl2, pH 7.2 adjusted with CsOH. Cells were perfused with a solution that contained: 110 mM Tris·HCl, 30 mM CsCl, 5 mM Hepes, 1 mM MgCl2, 5 mM 4-aminopyridine, 1.8 mM CaCl2, and 10 mM glucose, pH 7.4 adjusted with CsOH. ICa-L was measured by subtracting the steady-state current level at −40 mV from the peak inward current (at 0 mV). Data were discarded from experiments in which rundown of ICa-L was >10%. Cells from wild-type and Gαo−/− mice were coded, and the code was not broken until after the experiments were analyzed.
Electrocardiogram of Wild-Type and Gαo−/− Mice.
The electrocardiograms of wild-type Gαo+/− and Gαo−/− mice, ranging in body weight from 9 to 25 g, were acquired daily from weaning (17–24 days old) to 6 weeks of age, then weekly for up to 6 more weeks. The animals were sedated with intraperitoneal valium (10–22 ml/kg, 5 mg/ml). Needle electrodes (27 gauge) were placed subcutaneously, and standard limb leads were obtained with the mouse placed in a prone position 5, 10, and 15 min after sedation. Heart rates were averaged to provide a daily value. The heart rates (≈700 beats per min) of the two Gαo−/− mice (one male, one female) were within the range of those of the wild type. In two conscious, unethered mice (one 22-week-old female wild type and one 19-week-old male Gαo−/−; both weighed 32 g), a continuous single lead electrocardiogram signal was emitted for 24 hr from a 4-g radiotransmitter implanted subcutaneously at the nape of the neck and captured by a receiver placed under the animal’s cage (33). There were no arrhythmic beats. The wild-type and Gαo−/− mice had similar heart rates.
RESULTS
We inactivated the Gαo gene in 129/Sv J1 embryonic stem cells by insertion of the neomycin resistance gene, β-geo, at the BamHI site in exon 6 (Fig. 1A). Interrupting the protein at this point would inactivate both Gαo1 and Gαo2, the two alternatively spliced polypeptide products of the Gαo gene. Incorporation of the mutated gene is revealed by Southern blotting (Fig. 1B). No Gαo polypeptide was detected by immunoblotting membranes and cytosol of homozygous mutants (Fig. 1C). The antibody used in these studies is a polyclonal antibody raised against purified Gαo (a mixture of Gαo1 and Gαo2) that recognizes epitopes throughout the α subunit. We detected no truncated forms of Gαo subunits in brain homogenates from the Gαo−/− mice. Similar results were obtained with an antibody against the N terminus of Gαo (data not shown).
Figure 1.
Targeted disruption of the mouse Gαo gene and its molecular analysis. (A) Scheme of targeting vector, genomic XbaI fragment spanning the Gαo targeting site, and predicted structure of the disrupted gene. β-geo, β-galactosidase and neomycin phosphotransferase gene; TK, herpes virus thymidine kinase gene. (B) Southern blot analysis of XbaI-digested genomic DNA from wild-type, Gαo+/−, and Gαo−/− mice. As predicted from A, the wild type displays a single 19.9-kb band when probed with the 3′ and 5′ flanking probe, and the Gαo−/− lacks the 19.9-kb band but has new bands at 15.2 and 9.4 kb when hybridized to the 3′ and 5′ flanking probes, respectively. Gαo+/− displays the bands found in both wild-type and the Gαo−/− animal. (C) Immunoblot of brain membrane and cytosol from wild-type and Gαo−/− mice probed with R4 antibody. The size of molecular weight markers are indicated at the right.
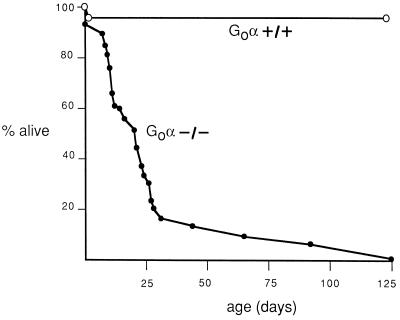
Transmission of the Gαo mutation shows a distribution close to Mendelian at birth, indicating no major preferential death of mutant mice in utero. Of 85 mice analyzed immediately after birth, 48% were heterozygotes, 38% were wild type, and 14% were homozygotes. However, Gαo−/− mice died prematurely starting at day 5 after birth, and, by day 25, only 20% of the Gαo−/− animals were still alive (Fig. 2). We do not yet know why the animals die prematurely. They are not hypoglycemic (data not shown), as might be expected if absence of Gαo mimicked inhibition of Gi and Go function by pertussis toxin. Although there are no gross abnormalities in the nervous system, the universal development of tremors suggests that subtle connectivity aberrations may occur and lead to consequent motor and feeding abnormalities. Whatever the cause of death, absence of Gαo has a more deleterious effect on survival than was noted in C. elegans (13, 14).
Figure 2.
Postnatal survival of wild-type and Gαo null mutants. The figure shows the percentage of surviving wild-type (•) and Gαo−/− mice (○) as a function of time, starting at day of birth.
Neuronal Function in Gαo−/− Mice.
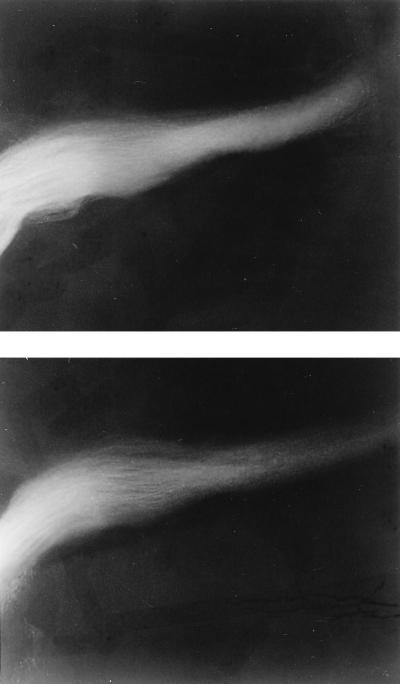
The mildness of the neural phenotype is surprising, given the abundance of Gαo in the brain, particularly in nerve growth cones. The ability of Gαo to bind GTPγS is enhanced by the growth cone-associated protein, GAP43, suggesting that Go may be involved in growth cone function (5). Mice lacking GAP43 have abnormal neural pathfinding at the developing optic chiasm (31). Microscopic examination at many levels of the neuraxis after Nissl staining with cresyl violet, as well as specific examination of the optic nerve with DiI, showed no differences between wild type and Gαo−/− (Fig. 3). Because Gαo might be important for normal growth cone collapse (34), the behavior of growth cones from dorsal root ganglion neurons from Gαo−/− mice was compared with wild type. In three Gαo−/− and four Gαo+/+ mice, there was no difference in the dose response of growth cone collapse in response to brain membrane extract. In both, the fraction of collapsed growth cones went from 30% at basal to 70% at the maximal dose of brain membrane extract (data not shown). Nevertheless, it is likely that there are subtle changes in the structure of the nervous system of Gαo−/− mice, because they exhibit tremors when grasped by the tail. Occasionally, the Gαo−/− mice have been observed to have seizures.
Figure 3.
Optic nerves labeled with DiI appear normal. Whole mount views of the base of brain of wild-type (Upper) and Gαo−/− littermate (Lower) in the region of the optic chain. The brains were fixed and labeled at embryonic day 15 and examined 7 days later. No difference was noted with embryonic day 15 labeling or for animals labeled the day of birth (data not shown).
Cardiac Function in Gαo−/− Mice.
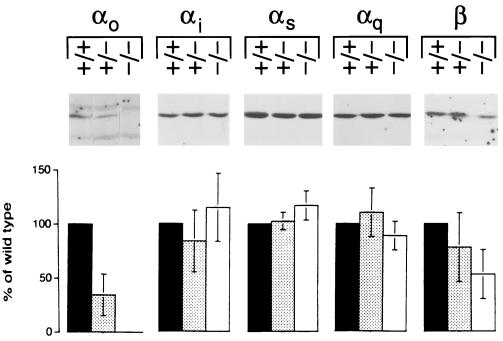
Fig. 4 shows that Gαo subunit is decreased to 35 ± 19% (n = 9) of wild type in the hearts of Gαo+/− mice and undetectable in Gαo−/− hearts Gαi, Gαs, and Gαq are unchanged in Gαo−/− and in Gαo+/− hearts. In hearts of Gαo−/− animals, the level of Gβ is 53 ± 23% (n = 8) of wild type. A similar decrease in Gβ was found in transformed embryonic fibroblasts from Gαi2 −/− mice (35).
Figure 4.
Expression of G protein α and β subunits in heart membranes from Gαo+/+, Gαo+/−, and Gαo−/− mice. Gαo protein is absent and Gβ is reduced in ventricular membranes from Gαo−/− mice. Gαi, Gαs, and Gαq are unchanged. Shown are the bands of interest from representative immunoblots and the densitometric evaluation of the chemiluminescence signal.
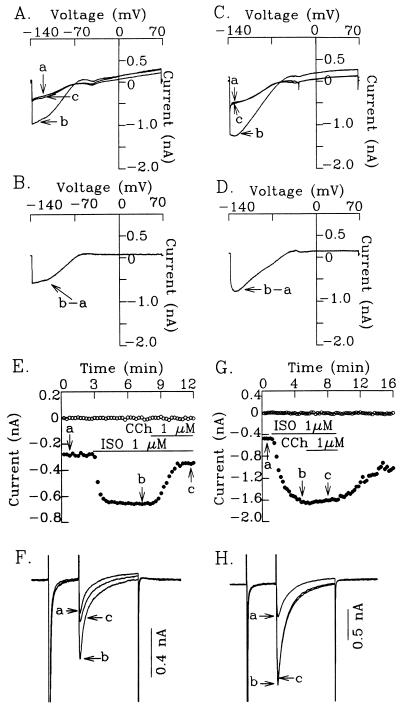
The electrocardiograms of Gαo−/− mice did not show any consistent abnormality. One Gαo−/− mouse that survived to be big enough for cardiac monitoring by telemetry showed no abnormality of heart rate over 24 hr of observation. To dissect the function of cardiac ion channels more precisely, we analyzed the function of IK-ACh and of ICa-L in the hearts of Gαo−/− mice. The IK-ACh channel is directly regulated by Gβγ subunits released from a pertussis toxin-sensitive G protein activated by the muscarinic cholinergic receptor (36, 37). Fig. 5 A–D show that carbamylcholine, an agonist for the muscarinic receptor, activates IK-ACh similarly in atrial myocytes from wild-type and Gαo−/− mice, confirming that Gαo is not involved in direct activation of IK-Ach. Furthermore, the normal regulation of IK-Ach in Gαo−/− mice also shows that the Gβγ released by the muscarinic receptors to activate IK-ACh does not come from a Gαβγ complex that contains Gαo.
Figure 5.
Muscarinic cholinergic activation of IK-ACh and inhibition of isoproterenol (ISO)-stimulated ICa-L in cardiomyocytes from wild-type and Gαo−/− mice. (A–D) Normal activation of IK-ACh in atrial myocytes from wild-type (A and B) and Gαo−/− (C and D) mice. (A and C) Superimposed current traces showing control (a), effect of 1 μM carbamylcholine (CCh) (b), and effect of 1 μM atropine in the presence of CCh (c). (B and D) IK-ACh obtained by subtracting b. A ramp protocol (−140 to +70 mV) was applied to the cells at 0.09 V/sec. (E–H) Significant attenuation of CCh inhibition of ISO-stimulated ICa-L in murine ventricular myocytes from Gαo−/− mice. (E and G) Time course showing inhibition of ISO-stimulated ICa-L by CCh in murine ventricular cells from wild-type (E) and Gαo−/− mice (G). (F and H) Superimposed current traces showing control (a), effect of ISO (b), and effect of CCh in the presence of ISO (c). Current traces in F and H were taken from the time points marked in E and G, respectively. Holding potential was −80 mV. A prepulse to −40 mV was given to inactivate the fast inward sodium current (INa) and a further depolarizing pulse to 0 mV was applied to the cell to activate ICa-L. The horizontal lines in E and G indicate the time during which each test agent was applied. ○, Steady-state current level at −40 mV; and •, ICa-L.
ICa-L is activated by the β-adrenergic receptor (reviewed in ref. 38). In myocytes from the wild-type mice, the isoproterenol-stimulated current is completely blocked by 1 μM nifedipine or 100 μM Cd2+ (data not shown). N-type Ca2+ channels are probably not expressed in the heart, since the N-type Ca2+ current cannot be identified in cardiac myocytes of all species studied to date (39). Muscarinic agonists antagonize the effect of β-adrenergic stimulation by reducing ICa-L. As shown in Fig. 5 E–H and Table 1, there is no difference in the effect of isoproterenol on ICa-L in ventricular myocytes from Gαo+/+ and Gαo−/− mice. However, the inhibitory effect of carbamylcholine was almost completely abolished in myocytes from Gαo−/− mice. This result demonstrates that in the heart, the muscarinic inhibition of ICa-L requires the Gαo protein. The Gβγ subunit enhances reassembly of Gα subunits with the receptor. Therefore, a decrease in Gβγ might have a universal damaging effect on G protein-mediated signal transduction. The finding that activation of ICa-L by isoproterenol is normal shows that not all hormone responses are blunted. Adenosine receptors also inhibit isoproterenol-stimulated ICa-L. However, the response to adenosine in myocytes from wild-type mice was very variable, so we could not evaluate the effect of adenosine in myocytes from αo−/− mice.
Table 1.
Response of ICa-L to isoproterenol and carbachol in ventricular myocytes from wild-type and Gαo−/− mice
| Condition | % ICa-L activity (n)
|
|
|---|---|---|
| Gαo+/+ | Gαo−/− | |
| No agonist | 100 | 100 |
| Isoproterenol | 241 ± 27 (9) | 244 ± 29 (10) |
| Isoproterenol plus carbachol | 106 ± 14 (9) | 212 ± 37 (10) |
The data are tabulated from experiments performed as described in Fig. 5 and given as mean ± SD for the number of cells tested (n). The cells came from four Gαo+/+ and five Gαo−/− mice.
DISCUSSION
This study demonstrates a clear role for Gαo mediating the muscarinic regulation of L-type calcium channels in the heart. There is no muscarinic inhibition of the isoproterenol-stimulated ICa-L in ventricular myocytes from Gαo−/− mice, even though Gαo is a minor component of the G protein repertoire in the heart. Other cardiac Gα proteins cannot substitute for Gαo in this function. This discrete action provides an example in normal tissue of the specificity of action of individual Gα subtypes. Although there is considerable crosstalk among purified receptors and Gα subunits in reconstitution assays, such promiscuity does not always occur in cells (reviewed in ref. 40).
Regulation of ICa-L by G protein-coupled receptors is believed to be important to cardiac function. Activation of the channel by the β-adrenergic receptor is a consequence of that receptor’s activation of adenylyl cyclase through Gs. Therefore, cAMP levels rise, protein kinase A is activated and phosphorylates the channel. Calcium entry through this channel causes the release of sequestered calcium from the sarcoplasmic reticulum, which in turn activates the contractile apparatus (reviewed in ref. 41). The mechanism of muscarinic inhibition of the channel is not yet completely understood. The channel could be directly regulated by Gα or Gβγ. The Gβγ subunit modulates another type of Ca2+ channel, the N-type Ca2+ channel (42, 43), but this channel is not present in the heart (39). In the heart, the regulation of ICa-L is probably indirect. In rat ventricular myocytes (44), rabbit sinoatrial node (45), and atrial ventricular node (46), muscarinic antagonism of β-adrenergic agonist stimulation of ICa-L depends on activation of a constitutively expressed NO synthetase. It is not known how muscarinic stimulation would increase NO production, but one possible route is that release of Gβγ from Gαo βγ activates phospholipase Cβ, causing release of Ca2+ from internal stores and activation of Ca2+-dependent NO synthase. Further studies will delineate the function of the NO system in myocytes from αo−/− mice. Stehno-Bittel et al. (47) showed in frog oocytes that, even though the Gβγ subunit was the primary activator of phospholipase Cβ, the specificity of the reaction was regulated by the type of Gα subunit in the heterotrimer. In ventricular myocytes from Gαo−/− mice, there is a total lack of Gαo and a secondary decrease in Gβγ. At the present time, we cannot tell which subunit directly activates the relevant effector. Nevertheless, our results show that whichever subunit directly or indirectly regulates the cardiac L-type Ca2+ channel, it must be released from a Gαoβγ heterotrimer. To avoid cellular chaos, signal transduction in vivo must be a tightly controlled process. Our studies in Gαo−/− mice show that Gαo is specifically required for transmission of signals from the muscarinic receptor to ICa-L. Its function cannot be taken over by other more abundant Gα subunits in the heart.
Acknowledgments
We thank Allison Cohen for expert technical assistance, Dr. Yingzi Zhao for preparing mouse ventricular myocytes, and Dr. Shobita Sundar and Dr. Peter Finn for carrying out the electrocardiogram and telemetric analysis. We also thank Dr. Thomas W. Smith and Dr. Ralph A. Kelly for helpful discussions. We are especially grateful to Paula McColgan for her excellent secretarial help. This work was partially supported by National Institutes of Health Grants HL52320 and GM36359 to E.J.N.
References
- 1.Neer E J, Lok J M, Wolf L G. J Biol Chem. 1984;259:14222–14229. [PubMed] [Google Scholar]
- 2.Sternweis P C, Robishaw J D. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 3.Huff R M, Axton J M, Neer E J. J Biol Chem. 1985;260:10864–10871. [PubMed] [Google Scholar]
- 4.Asano T, Semba R, Kamiya N, Ogasawara N, Kato K. J Neurochem. 1988;50:1164–1169. doi: 10.1111/j.1471-4159.1988.tb10588.x. [DOI] [PubMed] [Google Scholar]
- 5.Strittmatter S M, Valenzuela D, Kennedy T E, Neer E J, Fishman M C. Nature (London) 1990;344:836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt C J, Zubiaur M, Valenzuela D, Neer E J, Drager U C. J Neurosci Res. 1994;38:182–187. doi: 10.1002/jnr.490380208. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt C J, Garen-Fazio S, Chow Y-K, Neer E J. Cell Regul. 1989;1:125–134. doi: 10.1091/mbc.1.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon J, Shortridge R D, Bloomquist B T, Schneuwly S, Perdew M H, Pak W L. J Biol Chem. 1989;264:18536–18543. [PubMed] [Google Scholar]
- 9.deSousa S M, Hoveland L L, Yarfitz S, Hurley J B. J Biol Chem. 1989;264:18544–18551. [PubMed] [Google Scholar]
- 10.Thambi N C, Quan F, Wolfgang W J, Spiegel A, Forte M. J Biol Chem. 1989;264:18552–18560. [PubMed] [Google Scholar]
- 11.Guillen A, Semeriva M, Bockaert J, Homburger V. Cell Signalling. 1991;3:341–352. doi: 10.1016/0898-6568(91)90063-z. [DOI] [PubMed] [Google Scholar]
- 12.Guillen A, Jallon J-M, Fehrentz J-A, Pantaloni C, Bockaert J, Homburger V. EMBO J. 1990;9:1449–1455. doi: 10.1002/j.1460-2075.1990.tb08261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendel J E, Korswagen H C, Liu K S, Hajdu-Cronin Y M, Simon M I, Plasterk R H A, Sternberg P W. Science. 1995;267:1652–1655. doi: 10.1126/science.7886455. [DOI] [PubMed] [Google Scholar]
- 14.Segalat L, Elkes D A, Kaplan J M. Science. 1995;267:1648–1651. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- 15.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Nature (London) 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 16.Hescheler J, Rosenthal W, Trautwein W, Schultz G. Nature (London) 1987;325:445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- 17.Ewald D A, Sternweis P C, Miller R J. Proc Natl Acad Sci USA. 1988;85:3633–3637. doi: 10.1073/pnas.85.10.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris-Warrick R M, Hammond C, Paupardin-Tritsch D, Homburger V, Rouot B, Bockaert J, Gerschenfeld H M. Neuron. 1988;1:27–32. doi: 10.1016/0896-6273(88)90206-1. [DOI] [PubMed] [Google Scholar]
- 19.van Dongen A M J, Codina J, Olate J, Mattera R, Joho R, Birnbaumer L, Brown A M. Science. 1988;242:1433–1434. doi: 10.1126/science.3144040. [DOI] [PubMed] [Google Scholar]
- 20.McFadzean I, Mullaney I, Brown D A, Milligan G. Neuron. 1989;3:177–182. doi: 10.1016/0896-6273(89)90030-5. [DOI] [PubMed] [Google Scholar]
- 21.Asano T, Ui M, Ogasawara M. J Biol Chem. 1985;260:12653–12658. [PubMed] [Google Scholar]
- 22.Kurose H, Katada T, Haga T, Haga K, Ichiyama A, Ui M. J Biol Chem. 1986;261:6423–6428. [PubMed] [Google Scholar]
- 23.Carda B D, Medzihradsky F. Proc Natl Acad Sci USA. 1993;90:4062–4066. doi: 10.1073/pnas.90.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerione R A, Regan J W, Nanata H, Codina J, Benoric J L, Gierschik P, Somers R L, Spiegel A M, Birnbaumer L, Lefkowitz R J. J Biol Chem. 1986;261:3901–3909. [PubMed] [Google Scholar]
- 25.Li Y, Mende U, Lewis C, Neer E J. Biochem J. 1996;318:1071–1077. doi: 10.1042/bj3181071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asano T, Morishita R, Semba R, Itoh H, Kaziro Y, Kato K. Biochemistry. 1989;28:4749–4754. doi: 10.1021/bi00437a035. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich G, Sonano P. Gene Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 28.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Gutowski S, Smrcka A, Nowak L, Wu D, Simon M I, Sternweis P C. J Biol Chem. 1991;266:20519–20524. [PubMed] [Google Scholar]
- 31.Strittmatter S M, Fankhauser C, Huang P L, Mashimo H, Fishman M C. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- 32.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–90. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 33.Opitz C F, Mitchell G F, Pfeffer M A, Pfeffer J M. Circulation. 1995;92:253–261. doi: 10.1161/01.cir.92.2.253. [DOI] [PubMed] [Google Scholar]
- 34.Igarashi M, Strittmatter S M, Vartanian T, Fishman M C. Science. 1993;259:77. doi: 10.1126/science.8418498. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph U, Spicher K, Birnbaumer L. Proc Natl Acad Sci USA. 1996;93:3209–3214. doi: 10.1073/pnas.93.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logothetis D E, Kurachi Y, Galper J, Neer E J, Clapham D E. Nature (London) 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 37.Krapivinsky G, Krapivinsky L, Wickman K, Clapham D E. J Biol Chem. 1995;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- 38.Wickman K, Clapham D E. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 39.McDonald T F, Pelzer S, Trautwein W, Pelzer D. Physiol Rev. 1994;72:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 40.Neer E J. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 41.Bers, D. M. (1991) Dev. Cardiovasc. Med. 122.
- 42.Herlitze S, Garcia D E, Mackle K, Hille B, Scheuer T, Catterall W A. Nature (London) 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda S R. Nature (London) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 44.Balligand J-L, Kobzik L, Han X, Kaye D M, Belhassen L, O’Hara D S, Kelly R A, Smith T W, Michel T. J Biol Chem. 1995;270:14582–14586. doi: 10.1074/jbc.270.24.14582. [DOI] [PubMed] [Google Scholar]
- 45.Han X, Shimoni Y, Giles W R. J Physiol (London) 1994;476.2:309–314. doi: 10.1113/jphysiol.1994.sp020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han X, Kobzik L, Balligand J-L, Kelly R A, Smith T W. Circ Res. 1996;78:998–1008. doi: 10.1161/01.res.78.6.998. [DOI] [PubMed] [Google Scholar]
- 47.Stehno-Bittel L, Krapivinsky G, Krapivinsky L, Peol Z, Perez-Terzic C, Clapham D E. J Biol Chem. 1995;270:30068–30074. doi: 10.1074/jbc.270.50.30068. [DOI] [PubMed] [Google Scholar]