Abstract
Promoter selectivity for all three classes of eukaryotic RNA polymerases is brought about by multimeric protein complexes containing TATA box binding protein (TBP) and specific TBP-associated factors (TAFs). Unlike class II- and III-specific TBP–TAF complexes, the corresponding murine and human class I-specific transcription initiation factor TIF-IB/SL1 exhibits a pronounced selectivity for its homologous promoter. As a first step toward understanding the molecular basis of species-specific promoter recognition, we cloned the cDNAs encoding the three mouse pol I-specific TBP-associated factors (TAFIs) and compared the amino acid sequences of the murine TAFIs with their human counterparts. The four subunits from either species can form stable chimeric complexes that contain stoichiometric amounts of TBP and TAFIs, demonstrating that differences in the primary structure of human and mouse TAFIs do not dramatically alter the network of protein–protein contacts responsible for assembly of the multimeric complex. Thus, primate vs. rodent promoter selectivity mediated by the TBP–TAFI complex is likely to be the result of cumulative subtle differences between individual subunits that lead to species-specific properties of RNA polymerase I transcription.
Keywords: protein–protein interactions/ribosomal DNA/sequence comparison/cDNA
Transcription initiation by all three classes of eukaryotic nuclear RNA polymerases is a complex process, requiring concerted interactions between multiple protein factors and RNA polymerase. Each class of RNA polymerase uses a distinct assortment of transcription factors that are thought to nucleate the assembly of transcription initiation complexes at specific promoters. For transcription governed by RNA polymerase I (pol I), the murine transcription initiation factor (TIF) IB and its human homologue SL1 have been shown to direct the assembly of productive initiation complexes at the mouse and human rDNA promoter (1–3). TIF-IB/SL1 is thought to communicate with the upstream binding factor (UBF) and to recruit pol I together with the associated factors TIF-IA and TIF-IC to the template (4).
Earlier studies had revealed that rDNA transcription is species-specific, requiring factors from either the same or very closely related species (5). Most of the factors, i.e., UBF, pol I, TIF-IA, and TIF-IC, are interchangeable between human and mouse (3, 6–9) whereas TIF-IB/SL1 has been found to be the species-specific component in the preinitiation complex (3, 9). A significant advance toward a functional characterization of this selectivity factor was the discovery that TIF-IB/SL1 is a multiprotein complex consisting of TATA box binding protein (TBP) and three TBP-associated factors (TAFs) (10, 11). Given the low abundance of TIF-IB/SL1 in the cell, studies on the molecular mechanism of promoter recognition and species-specific transcription require the isolation and functional characterization of the individual subunits of TIF-IB and SL1. Recently, this was accomplished for the components of SL1 (12, 13). Here we report the cloning and expression of the cDNAs encoding the mouse pol I-specific TBP-associated factors (TAFIs) subunits. We have characterized the interaction between TBP and each of the three mouse TAFIs, between the TAFIs themselves, and between mouse and human TAFIs. Our results suggest that, despite differences in the primary structure, the interactions between mouse and human TAFIs appear to be conserved and that multimeric complexes can be assembled using either human or mouse TAFIs in any combination. The assembly of chimeric TIF-IB/SL1 complexes, together with the availability of specifically tagged TAFIs and the respective antibodies, represents powerful new tools to analyze the species specificity of human and mouse rDNA transcription.
MATERIALS AND METHODS
Cloning of Murine TAFIs.
TIF–IB was purified from Ehrlich ascites cells (HD34K) as described (11). The peptide sequence KLAVAEDNPETSVL from the 48-kDa subunit was used to design degenerate oligonucleotides (Ampli A, 5′-AAG/A C/TTG/A/T/C GCA/T/C GTI GC-3′; Ampli B1, 5′-AG/AC/A/GGCT/A/GGA IGTC/TTC-3′; and Ampli B2, 5′-AG/AC/A/GGCA/GCTIGT C/TTC-3′) to perform an intrapeptide “touchdown PCR” from cDNA. A 41-bp fragment encoding the expected peptide was generated and used to screen a mouse embryo cDNA library. Two cDNA clones (2.4 and 1.56 kb) containing an identical 1.4 kb ORF were isolated. cDNAs encoding mTAFI68 and mTAFI95 were isolated from mouse cDNA libraries using DNA fragments derived from the respective human TAFI cDNA (12). The full length ORFs encoding mTAFI68 and mTAFI95 were reconstructed by fusion of two partial cDNAs.
Expression and Purification of Recombinant Proteins.
Individual cDNA were tagged at their 5′-end with sequences encoding the hemagglutinin (HA) epitope, the FLAG epitope [peptide DYKDDDDK, a specific epitope recognized by mAb M2 (Kodak)], or 10 histidine residues, respectively, to facilitate affinity purification and immunoprecipitation. Details of the cloning strategies are available on request. Histidine-tagged TAFIs (His-mTAFI68 and His-hTAFI63) and HA-tagged TAFIs (HA-mTAFI95 and HA-hTAFI110) were expressed in Escherichia coli, and FLAG-tagged mTAFI48 and hTAFI48 were expressed in Sf9 cells. Recombinant proteins were purified from inclusion bodies by sequential extraction with a buffer containing 25 mM Tris·HCl (pH 7.7), 0.5 mM EDTA, 1 mM DTT, 10 mM methionine, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium metabisulfide, and 1 M NaCl followed by extraction in the same buffer containing 3 M, 5 M, and finally 7 M urea. The 7-M urea fraction containing the majority of solubilized TAFIs was dialyzed against 5 M urea, 50 mM Tris·HCl (pH 7.9), 0.1 M NaCl, 10% glycerol, and 5 mM β-mercaptoethanol, passed through a 0.22-μm filter and loaded onto a POROS HS column (Perspective Biosystems, Cambridge, MA) using the SMART fast protein liquid chromatography system (Pharmacia). Bound proteins were eluted with a linear gradient from 0.1 to 0.7 M NaCl in the presence of 5 M urea. The peak fractions were pooled and stored in aliquots at −70°C. hTBP expressed in E. coli was purified on phosphocellulose as described (14). mTBP was expressed as a glutathione S-transferase (GST) fusion protein in E. coli. After purification on glutathione–Sepharose (Pharmacia), mTBP was removed from the GST moiety by thrombine cleavage and was purified further on a phosphocellulose column.
Protein–Protein Interaction Studies.
GST–mTBP “pull-down” assays were performed as described (12). For TAF–TAF interaction studies, M2 antibody beads (Kodak) were incubated with extracts from Sf9 cells containing FLAG-tagged mTAFIs at 4°C in buffer TM-400 (400 mM KCl/50 mM Tris·HCl, pH 7.9/12.5 mM MgCl2/10% glycerol/1 mM DTT/0.2 mM PMSF/1 mM sodium metabisulfide/0.1% Nonidet P-40). As a control, the antibody resin incubated with extracts from uninfected Sf9 cells was used. After washing, the resins were equilibrated in buffer TM-200 and incubated for 2 h at 4°C with [35S]methionine-labeled TAFIs or TBP. Bound proteins were separated by SDS/PAGE and were visualized by autoradiography.
Assembly of TIF-IB from Recombinant Subunits.
To assemble TIF-IB from individual subunits in vitro, purified TAFIs were mixed in buffer TMCZ {50 mM Tris·HCl, pH 7.9/12.5 mM MgCl2/10% glycerol/0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/5 μM Zn-acetate/1 mM DTT/0.2 mM PMSF/1 mM sodium metabisulfide} containing 1 M NaCl and 4 M urea. The urea concentration was stepwise lowered by dialysis against TMCZ/1 M NaCl containing 2, 1, 0.5, and 0.1 M urea. After addition of recombinant TBP, the complexes were dialyzed against TMCZ with 0.1 M urea/0.5 M NaCl followed by TMCZ with 0.05 M urea/0.2 M NaCl. TBP–TAFI complexes were immunoprecipitated with M2 antibody beads, washed in buffer TMZ-700 {TMCZ containing 0.2% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/0.1% Nonidet P-40/0.7 M NaCl}, equilibrated in TMZ-200, and eluted in the same buffer with the FLAG peptide (0.4 mg/ml). Aliquots of the eluates were analyzed by SDS/PAGE and silver staining.
To assemble TIF-IB from recombinant subunits in vivo, Sf9 cells were infected simultaneously with four baculoviruses encoding individual TAFIs and TBP. Extracts were prepared in buffer AM-500 (20 mM Tris·HCl, pH 7.9/0.1 mM EDTA/20% glycerol/5 mM MgCl2/1 mM PMSF/1 mM PMSF/500 mM KCl) containing 0.5% Nonidet P-40 and 1 mM sodium metabisulfide, 10 mM leupeptin, 1 mM pepstatin, and 5 μg/ml aprotinin. TBP–TAF complexes were immunopurified from the soluble fraction using mAb 3G3, an mAb directed against TBP (15), washed with buffers AM-1000/0.1% Nonidet P-40, AM-500/0.1% Nonidet P-40, and AM-300/0.1% Nonidet P-40 and eluted in buffer AM-300 containing 0.1% Nonidet P-40 and 1 mg/ml 3G3 epitope peptide. Complexes were reimmunoprecipitated with M2 antibodies directed against FLAG mTAFI95, and were eluted in AM-300 containing 0.1% Nonidet P-40, 0.4 mg/ml FLAG epitope peptide, and 0.1 mg/ml insulin.
In Vitro Transcription Assay.
pol I, TIF-IA, and TIF-IC were purified as described (4, 16). TIF-IB was immunopurified using mAb 3G3 (11, 15). FLAG-tagged UBF was immunopurified from extracts of baculovirus-infected Sf9 cells. For run-off transcription assays, 35 ng of linearized plasmid pMrWT containing mouse ribosomal wild-type DNA sequences from −170155) was incubated in a 25-μl assay with either 6 μl of nuclear extract from cultured Ehrlich ascites cells or 4 μl of pol I (MonoS fraction), 2.5 μl of TIF-IA/TIF-IC (poly-lysin-agarose fraction), and 5 ng of UBF. After incubation for 1 h at 30°C, transcripts were analyzed by gel electrophoresis and autoradiography (2).
Immunoprecipitation of TIF-IB.
IgGs covalently coupled to protein A–Sepharose were incubated in buffer AM-100 (100 mM KCl/20 mM Tris·HCl, pH 7.9/0.1 mM EDTA/20% glycerol/5 mM MgCl2/1 mM PMSF/1 mM dithioerythritol) supplemented with 2 mg/ml BSA and 2 mg/ml phosphatidylcholin to block nonspecific interactions. Packed beads (15 μl) were incubated with 90 μl of nuclear extract (800 μg of protein) in AM-300 for 2 h at 4°C, washed with AM-1000/0.1% Nonidet P-40, AM-700/0.1%Nonidet P-40, and AM-300/0.1%Nonidet P-40, and finally suspended in AM-100. Aliquots of the beads were either assayed for transcriptional activity or analyzed by Western blotting.
RESULTS
Cloning and Sequence Analysis of cDNAs Encoding Murine pol I-Specific TAFs.
To isolate the cDNA encoding mTAFI48, peptide sequences derived from affinity-purified TIF-IB were used to generate a homologous probe for screening of mouse cDNA libraries. Two cDNA clones were identified that contain an identical 1.4-kb ORF encoding a protein of 453 amino acids with a calculated molecular mass of 52.7 kDa. cDNAs for mTAFI68 and mTAFI95 were obtained by low stringency hybridization with human TAFI63 or TAFI110 cDNA fragments. The sequence of the mTAFI68 cDNA predicts an ORF of 586 amino acids and specifies a 68-kDa polypeptide. The mTAFI95 cDNA encodes a polypeptide of 837 amino acids with a calculated molecular mass of 92 kDa.
In Fig. 1, the deduced amino acid sequences of the murine TAFIs are aligned with their respective human homologues. Comparison of the sequence of the murine with the human TAFIs demonstrates a strong conservation of these proteins. Differences between mouse and human TAFIs are not confined to specific regions but are scattered throughout the entire length of the proteins. As summarized in Table 1, TAFI48 is the most conserved of the three polypeptides. Human and mouse TAFI68/63 reveal pronounced differences in their N- and C-terminal portions. hTAFI63 has a 40-amino acid extension at its N terminus that the mouse protein lacks whereas mTAFI68 contains an additional 66 amino acids at the C terminus. The two putative zinc finger motifs present in human TAFI63 (12) are conserved in the murine protein, and the C-terminal extension in mTAFI68 allows for the possibility of a third zinc finger (amino acids 486–490 and 518–522). This additional zinc finger in mTAFI68 could explain why TIF-IB can form a stable, committed complex in the absence of UBF and SL1 cannot (3, 4). TAFI95/110 is the least conserved TAFI exhibiting 66% identity at the amino acid level. Although the calculated molecular masses of TAFI95 and TAFI110 are very similar (Table 1), they exhibit a pronounced difference in electrophoretic mobility. This is likely to be a consequence of structural differences rather than posttranslational modifications because both proteins expressed in E. coli also migrate anomalously.
Figure 1.
Alignment of the deduced amino acid sequences of murine and human TAFIs. The sequence of murine TAFIs is shown in the upper line; the sequence of human TAFIs is shown in the lower line. Identical amino acids are boxed, and gaps introduced for best alignment are indicated by hyphens.
Table 1.
Comparison of mouse and human TAFIs
| mTAFI48 | hTAFI48 | mTAFI68 | hTAFI63 | mTAFI95 | hTAFI110 | |
|---|---|---|---|---|---|---|
| Amino acids | 453 | 450 | 586 | 556* | 837 | 869 |
| Calculated mm, kDa | 53 | 53 | 68 | 64 | 92 | 95 |
| Apparent mm, kDa | 48 | 48 | 68 | <63 | 95 | 110 |
| Identity, % | 80 | 74 | 66 | |||
| Similarity, % | 89 | 82 | 77 | |||
| Noncolinear amino acids | 3 | 102 | 36 | |||
Similarity was calculated based on the rules: P = A = S, G = A = S, T = A = S, D = E = Q = N, K = R = H, V = I = L = M = F, and F = Y. mm, molecular mass.
*Not full length.
Characterization of the Murine TAFIs.
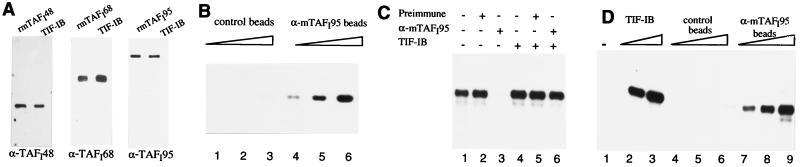
To establish that the proteins encoded by the three cDNAs are integral components of TIF-IB, polyclonal antibodies raised against the individual mTAFIs were used for comparison of recombinant and endogenous TAFIs. The immunoblots shown in Fig. 2A demonstrate that the electrophoretic mobilities of the recombinant proteins are indistinguishable from those of the TIF-IB subunits, indicating that the cDNAs encode full length murine TAFIs.
Figure 2.
Characterization of the recombinant TAFIs. (A) Recombinant TAFIs are indistinguishable from the endogenous subunits of TIF-IB. Recombinant mTAFIs and TIF-IB were analyzed by immunoblotting using antisera against the individual TAFIs as indicated. (B) Antibodies against mTAFI95 precipitate a protein complex containing TBP. Nuclear extract was incubated with immobilized IgGs from preimmune serum (lanes 1–3) or α-mTAFI95 serum (lanes 4–6). The immunoprecipitates were analyzed by immunoblotting using αTBP antibodies (mAb 3G3). (C) α-mTAFI95 antibodies deplete TIF-IB activity from nuclear extract. Transcription was assayed in untreated extract (lanes 1 and 4) or in extract treated with bead-bound preimmune (lanes 2 and 5) or α-mTAFI95 serum (lanes 3 and 6) either in the absence of additional factors (lanes 1–3) or in the presence of immunopurified TIF-IB (lanes 4–6). (D) TIF-IB precipitated by α-mTAFI95 antibodies is transcriptionally active. Nuclear extract was incubated with bead-bound preimmune (lanes 4–6) or α-mTAFI95 serum (lanes 7–9), and aliquots of the suspended beads (1, 3, and 5 μl) were assayed for TIF-IB activity in a reconstituted transcription system. Transcripts synthesized after addition of 40 and 80 pg of immunopurified TIF-IB are shown in lanes 2 and 3.
To test whether α-mTAFI antibodies can precipitate native TIF-IB, nuclear extracts were incubated with bead-bound control or α-mTAFI95 antibodies. The immunoprecipitates were analyzed on Western blots for the presence of TBP, and the supernatants were assayed for transcriptional activity. Significant amounts of TBP were found in the α-mTAFI95 immunoprecipitates, indicating that these antibodies precipitate TIF-IB (Fig. 2B). Moreover, the transcriptional activity of the supernatants was severely impaired after incubation with the α-mTAFI95 antiserum but not with the preimmune serum (Fig. 2C, lanes 1–3). Addition of immunopurified TIF-IB specifically restored the transcriptional activity of the depleted extract (lane 6), indicating that the decrease in transcriptional activity was caused by depletion of TIF-IB activity.
In a reciprocal experiment, the immunoprecipitates were assayed for TIF-IB activity. Immobilized control or α-mTAFI95 antibodies were incubated with nuclear extract, and the washed beads were added to a reconstituted transcription system lacking TIF-IB. In the absence of TIF-IB, this system is transcriptionally inactive, and addition of immunopurified TIF-IB strongly augments transcription (Fig. 2D, lanes 1–3). Proteins bound to the control antibodies did not complement transcriptional activity (lanes 4–6) whereas transcription was stimulated by increasing amounts of the α-mTAFI95 immunoprecipitates (lanes 7–9). This result demonstrates that the α-mTAFI95 antibodies have depleted TIF-IB activity from the extract and that bead-bound TIF-IB is transcriptionally active.
TBP–TAFI and TAFI–TAFI Interactions Are Conserved in TIF-IB/SL1.
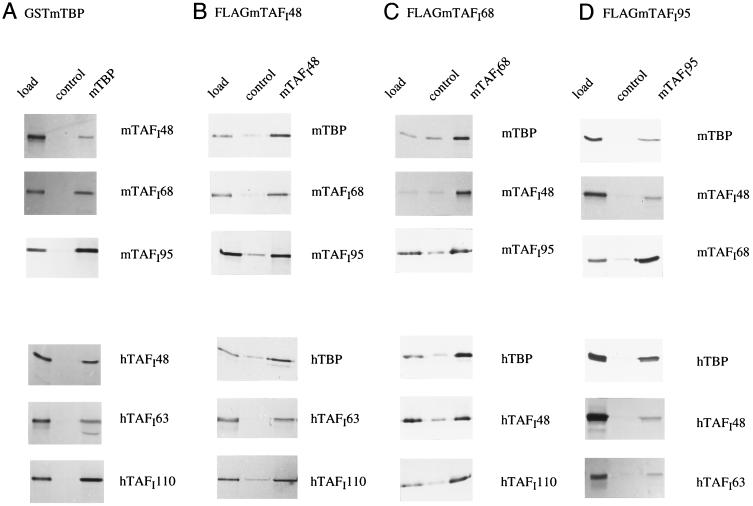
Protein–protein binding studies were performed to determine the ability of the individual subunits of TIF-IB and SL1 to interact with each other. First, immobilized GST–mTBP was incubated with radiolabeled TAFIs, and bound proteins were visualized by autoradiography. As shown in Fig. 3A, each of the mouse and human TAFIs is able to bind to GST–mTBP but not to GST alone, indicating that the interactions between TBP and TAFIs are conserved. This finding is in accord with previous data showing that differences in the N termini of human and mouse TBP do not contribute to the promoter selectivity of TIF-IB/SL1 (9).
Figure 3.
TAFI–TBP and TAFI–TAFI interactions are conserved between mouse and human. GST and GST–mTBP were immobilized on glutathione–Sepharose and incubated with [35S]Met-labeled TAFIs. FLAG epitope-tagged mouse TAFIs were immobilized on M2 antibody beads directed against the FLAG epitope and incubated with [35S]Met-labeled TAFIs or TBP. As a control, the antibody beads preincubated with crude Sf9 cell extract were used. Bound complexes were analyzed by SDS/PAGE and autoradiography. The “load” shows 10% of the input proteins.
To further analyze the protein–protein interactions within the TBP–TAFI complex, FLAG-tagged mTAFIs immobilized on M2 antibody beads were incubated with radiolabeled human and mouse TBP and TAFIs. Binding was monitored by SDS/PAGE and autoradiography (Fig. 3 B–D). These interaction studies revealed that each of the immobilized TAFIs can specifically interact with every other TIF-IB/SL1 subunit from either human or mouse origin. To control the specificity of the interactions, radiolabeled UBF was tested in parallel for binding to TBP and the mTAFIs. Consistent with earlier results (17), UBF interacted with TBP and mTAFI48 but not with mTAFI68 or mTAFI95 (data not shown). This preferential interaction of UBF with only one of the TAFIs argues for the specificity of the multiple TAFI–TAFI interactions and suggests that the protein surfaces involved in TBP–TAFI as well as in TAFI–TAFI interactions are evolutionary conserved. Although this kind of pull-down assay is not quantitative and does not permit conclusions about the affinities between the different interacting partners, we have consistently observed that mTAFI68 binds stronger to both mTAFI95 and hTAFI110 than hTAFI63 (Fig. 3D; data not shown). This higher affinity of murine TAFI68 to the largest TAFI may reflect subtle species-specific structural and/or functional differences among these TAFIs.
Assembly of Chimeric TBP–TAFI Complexes in Vitro.
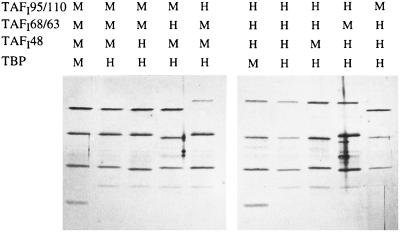
Each of the mTAFIs is able to bind the other subunits of TIF-IB and SL1, so we attempted to reconstitute chimeric TBP–TAF complexes from purified proteins. Human and mouse TAFIs were expressed in E. coli or in insect cells and were purified under denaturing conditions. The purified proteins were combined in all possible combinations, renatured by a stepwise dialysis procedure, and complemented with mouse or human TBP. Assembled complexes were immunopurified with M2 antibodies that recognize the FLAG epitope-tagged TAFI48 subunit. The complexes were eluted with the epitope peptide and analyzed on silver-stained polyacrylamide gels. As shown in Fig. 4, the homologous subunits form complexes whose composition is almost indistinguishable from the polypeptide pattern of TIF-IB and SL1. Significantly, all possible permutations of mouse and human TAFIs were able to form chimeric TBP–TAF complexes. When FLAG–TAFI48 was omitted from the assembly reactions, none of the other subunits was precipitated by the M2 antibodies (data not shown). This result demonstrates that the TBP–TAFI complexes are specifically immunoprecipitated via the FLAG epitope-tagged TAFI48 and do not simply stick to the antibody beads. The finding that any combination of TAFIs could be used to assemble chimeric complexes suggests that differences in the primary structure of the human and mouse TAFIs do not appreciably affect the network of subunit contacts within the multimeric TBP–TAFI complex and do not interfere with the formation of stable multiprotein complexes.
Figure 4.
Assembly of chimeric TIF-IB/SL1 complexes from recombinant subunits in vitro. Recombinant TBP and TAFIs were combined as indicated and assembled into TBP–TAFI complexes. Complexes were immunopurified with α-FLAG antibodies, eluted with the epitope peptide, and analyzed on a silver-stained SDS/polyacrylamide gel.
Recombinant TIF-IB Is Transcriptionally Inactive.
Having succeeded in assembling stoichiometric TBP–TAFI complexes from recombinant subunits, we tested their ability to functionally complement for TIF-IB in a reconstituted transcription system containing partially purified pol I, TIF-IA, TIF-IC, and recombinant UBF.
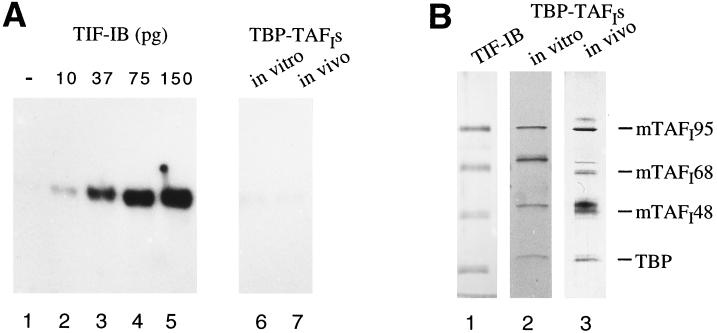
This system is extremely sensitive and is activated by picogram amounts of immunopurified TIF-IB (Fig. 5A, lanes 2–5). The system was used to assay the transcriptional activity of recombinant TBP–TAFI complexes that were either assembled in vitro or in Sf9 cells after quadruple infection with baculoviruses encoding the three TAFIs and TBP. However, despite considerable efforts, we failed to observe any transcriptional activity with the recombinant complexes (lanes 6, 7). Both the complexes reconstituted from purified subunits and those assembled in vivo proved to be transcriptionally inactive although the stoichiometry of the reconstituted TBP–TAFI complexes resembled that of cellular TIF-IB (Fig. 5B). This finding suggests that either a critical posttranslational modification or an additional protein factor is required for promotion of transcription.
Figure 5.
Recombinant TBP–mTAFI complexes do not reconstitute TIF-IB activity. (A) Affinity-purified TIF-IB, but not recombinant TBP–TAFI complexes, is transcriptionally active. Lanes: 1, transcriptional activity in the absence of TIF-IB; 2–5, transcriptional activity in the presence of picogram amounts of immunopurified cellular TIF-IB; 6 and 7, the reactions that have been complemented by addition of 100 pg of the recombinant TBP–mTAFI complexes shown in B. The recombinant complexes also were inactive if added at higher or lower amounts (not shown). (B) Subunit composition of cellular TIF-IB and recombinant TBP–TAFI complexes. Silver-stained SDS/polyacrylamide gels showing the subunits of cellular TIF-IB (lane 1), recombinant TBP–mTAFI complexes assembled from purified subunits (lane 2), and complexes assembled in baculovirus-infected Sf9 cells (lane 3). The individual complexes contained differently tagged subunits (see Materials and Methods) and were analyzed on separate gels. Therefore, the electrophoretic mobility of the subunits is not identical.
DISCUSSION
Previous studies revealed that TIF-IB and SL1 are functionally equivalent and share a similar overall structure. Nevertheless, they exhibit different template specificities in that they require the homologous template to promote initiation complex formation, indicating that subtle structural differences account for these functional differences. TBP has been shown to be exchangeable between the human and mouse factor, and therefore differences in the variable N-terminal domains in human and mouse TBP do not appear to play a significant role in rDNA promoter selectivity (9, 18). Consequently, the molecular basis for species-specific promoter recognition should reside in differences between the human and mouse TAFIs.
As a first step toward comparing the structure and function of rodent and human TBP–TAFI complexes, we cloned and characterized the TAF subunits of TIF-IB. The murine and human TAFIs are 66–80% identical, and amino acid exchanges are scattered throughout the proteins. mTAFI68 could potentially encode an additional zinc finger motif when compared with the human protein. This putative third zinc finger may be functionally relevant because this protein motif has been implicated in DNA binding and thus could affect the DNA binding characteristics of TIF-IB/SL1. hTAFI63 can be cross-linked to the rDNA promoter (17) and has been shown to be involved in the binding of SL1 to the promoter in the presence of UBF. Consistent with species-specific differences between TIF-IB and SL1, SL1 requires UBF to interact efficiently with the human rDNA promoter (3, 19, 20) whereas TIF-IB forms a committed complex in the absence of UBF (4). Thus, the ability of mouse TIF-IB to bind promoter sequences even without UBF may be partly due to the presence of an additional DNA contact mediated by the third zinc finger.
To investigate functional differences between mouse and human TAFIs, protein–protein interaction studies were performed. As has been shown for the human TAFIs (12), the three mouse TAFIs can bind individually and specifically to TBP, and each of the TAFIs can interact with every other TAFI subunit to form a stable TBP–TAFI complex. Furthermore, each mouse TAFI can contact every human TAFI, demonstrating that the domains mediating the complex network of TAF–TAF and TBP–TAF interactions are conserved between human and mouse. Consistent with this finding, all possible combinations of chimeric TBP–TAFI complexes could be assembled in vitro.
Unfortunately, despite considerable efforts, we did not succeed in generating functionally active complexes from the recombinant subunits and, therefore, species specificity of the chimeric complexes could not be tested. We have used two complementary approaches to assemble TIF-IB, i.e., in vitro assembly from purified subunits and in vivo assembly by coexpression of the four subunits in Sf9 cells. Although both methods yielded TBP–TAFI complexes that had a similar subunit composition as TIF-IB, they were transcriptionally inactive. This was an unexpected result because similar procedures reconstituted active SL1 from recombinant subunits (13). Possible explanations for our failure to reconstitute TIF-IB activity from recombinant subunits could be that the assembled complexes are stable but have assumed inappropriate or inactive conformations. Alternatively, phosphorylation by a specific kinase could be required for transcriptional activity. In this scenario, recombinant SL1 is either active per se, or the human transcription system used contains the activating kinase that is missing in the reconstituted mouse system. Furthermore, it is conceivable that a protein that is associated in substoichiometric levels with immunopurified cellular TIF-IB, but not with the recombinant complex, is required for mediating initiation complex formation. Experimental support for either hypothesis will require studies on the functional relevance of posttranslational modifications of TAFIs or the identification of a putative mediator protein. Nevertheless, the full set of cDNAs encoding murine pol I-specific TAFs, the availability of the recombinant proteins, and the corresponding antibodies should prove valuable tools to allow more detailed studies of the mechanisms responsible for promoter specificity and gene regulation.
Acknowledgments
We thank S. Ruppert, K. Kästner, and J. M. Garnier for providing cDNA libraries, H. Beckmann for baculovirus encoding FLAG-tagged hUBF, K. Goodrich for sequencing, L. Tora for mAb 3G3, F. Lottspeich for microsequencing mTAFI48, and Bettina Erny for extract preparation. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 229) and the Fonds der Chemischen Industrie.
ABBREVIATIONS
- pol
RNA polymerase
- TBP
TATA box binding protein
- TAF
TBP-associated factor
- UBF
upstream binding factor
- TIF
transcription initiation factor
- HA
hemagglutinin
- GST
glutathione S-transferase
- PMSF
phenylmethylsulfonyl fluoride
Footnotes
References
- 1.Learned R M, Cordes S, Tjian R. Mol Cell Biol. 1985;5:1358–1369. doi: 10.1128/mcb.5.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clos J, Buttgereit D, Grummt I. Proc Natl Acad Sci USA. 1986;83:604–608. doi: 10.1073/pnas.83.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell S P, Jantzen H M, Tjian R. Genes Dev. 1990;4:943–954. doi: 10.1101/gad.4.6.943. [DOI] [PubMed] [Google Scholar]
- 4.Schnapp A, Grummt I. J Biol Chem. 1991;266:24588–24595. [PubMed] [Google Scholar]
- 5.Grummt I, Roth E, Paule M R. Nature (London) 1982;296:173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- 6.Mishima Y, Financsek I, Kominami R, Muramatsu M. Nucleic Acids Res. 1982;10:6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnapp A, Schnapp G, Erny B, Grummt I. Mol Cell Biol. 1993;13:6723–6732. doi: 10.1128/mcb.13.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnapp G, Schnapp A, Rosenbauer H, Grummt I. EMBO J. 1994;13:4028–4035. doi: 10.1002/j.1460-2075.1994.tb06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudloff U, Eberhard D, Tora L, Stunnenberg H, Grummt I. EMBO J. 1994;13:2611–2616. doi: 10.1002/j.1460-2075.1994.tb06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comai L, Tanese N, Tjian R. Cell. 1992;68:965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- 11.Eberhard D, Tora L, Egly J M, Grummt I. Nucleic Acids Res. 1993;21:4180–4186. doi: 10.1093/nar/21.18.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comai L, Zomerdijk J C B M, Beckmann H, Zhou S, Admon A, Tjian R. Science. 1994;266:1966–1972. doi: 10.1126/science.7801123. [DOI] [PubMed] [Google Scholar]
- 13.Zomerdijk J C B M, Beckmann H, Comai L, Tjian R. Science. 1994;266:2015–2018. doi: 10.1126/science.7801130. [DOI] [PubMed] [Google Scholar]
- 14.Peterson M G, Tanese N, Pugh B F, Tjian R. Science. 1990;248:1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- 15.Brou C, Chaudhary S, Davidson I, Lutz Y, Wu J, Egly J M, Tora L, Chambon P. EMBO J. 1993;12:489–499. doi: 10.1002/j.1460-2075.1993.tb05681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnapp A, Grummt I. Methods Enzymol. 1996;273:346–359. doi: 10.1016/s0076-6879(96)73023-9. [DOI] [PubMed] [Google Scholar]
- 17.Beckmann H, Chen J L, O’Brien T, Tjian R. Science. 1995;270:1506–1509. doi: 10.1126/science.270.5241.1506. [DOI] [PubMed] [Google Scholar]
- 18.Rudloff U, Eberhard D, Grummt I. Proc Natl Acad Sci USA. 1994;91:8229–8233. doi: 10.1073/pnas.91.17.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Learned R M, Learned T K, Haltiner M M, Tjian R. Cell. 1986;45:847–857. doi: 10.1016/0092-8674(86)90559-3. [DOI] [PubMed] [Google Scholar]
- 20.Bell S P, Learned R M, Jantzen H M, Tjian R. Science. 1988;241:1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]