Abstract
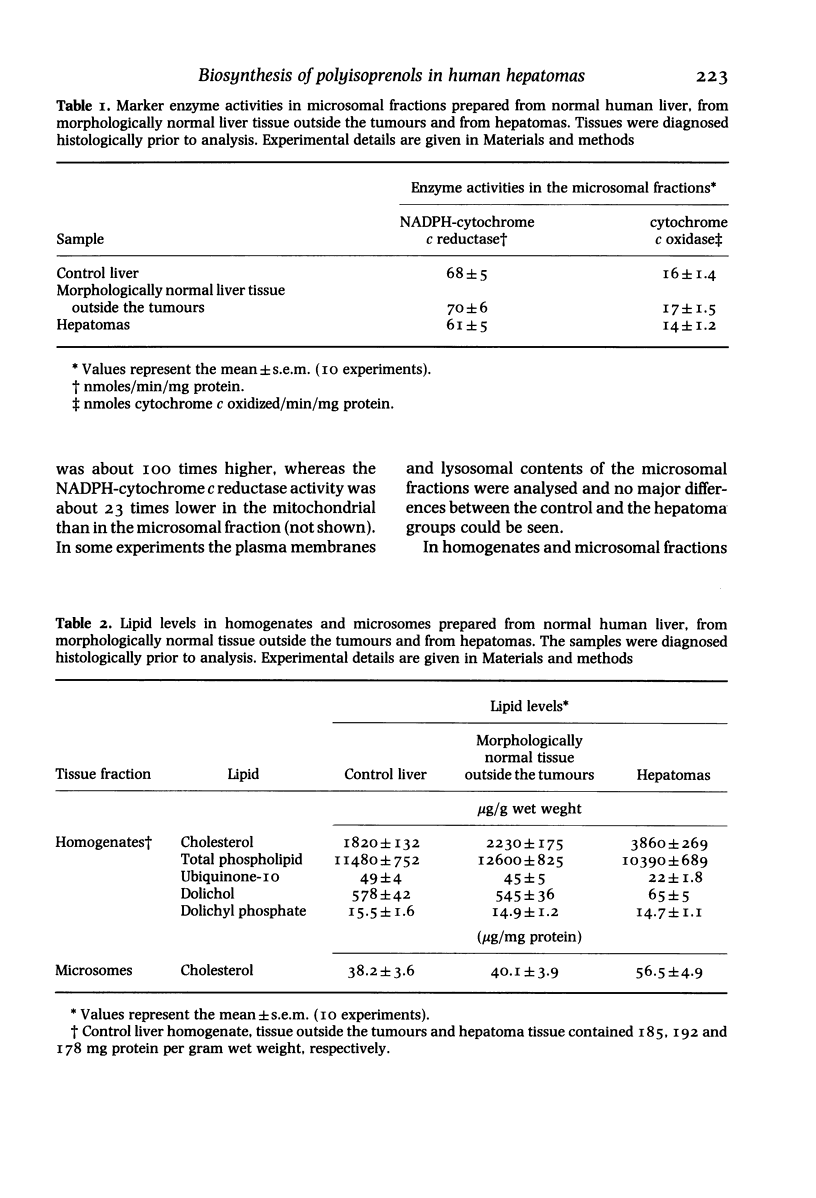
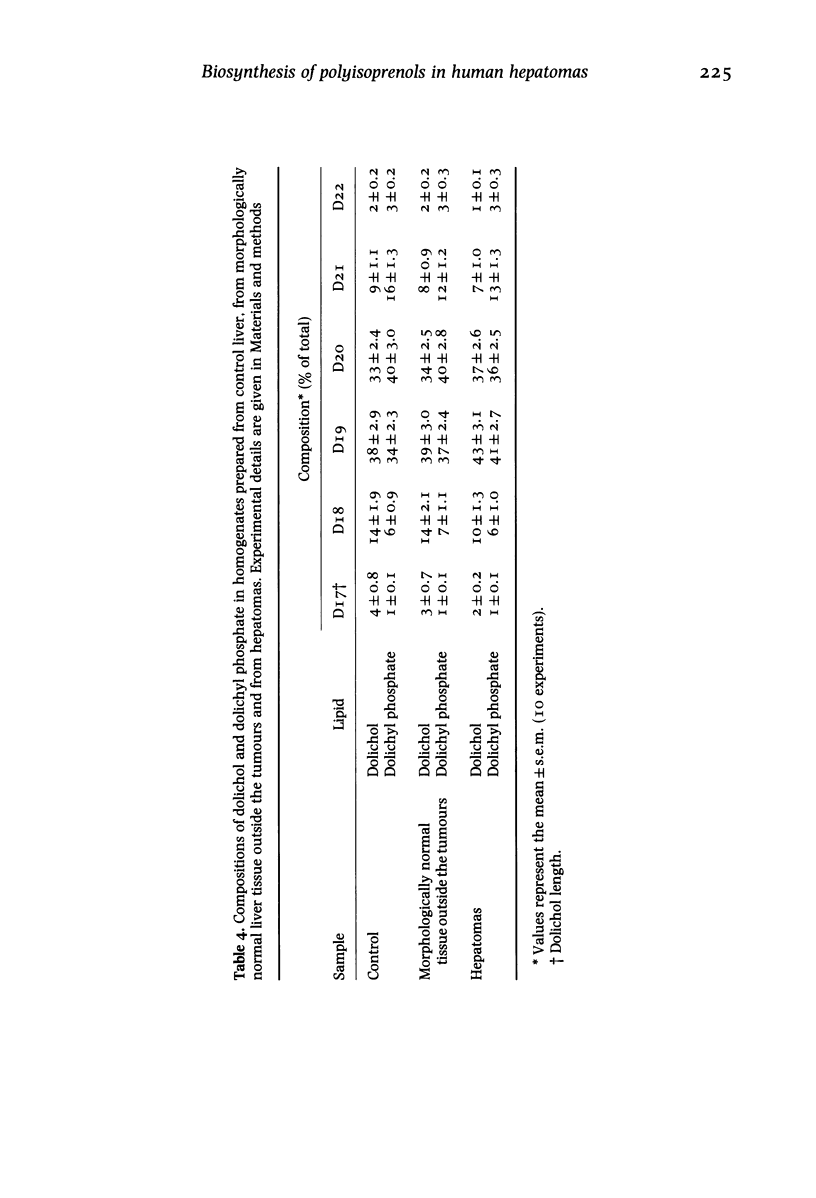
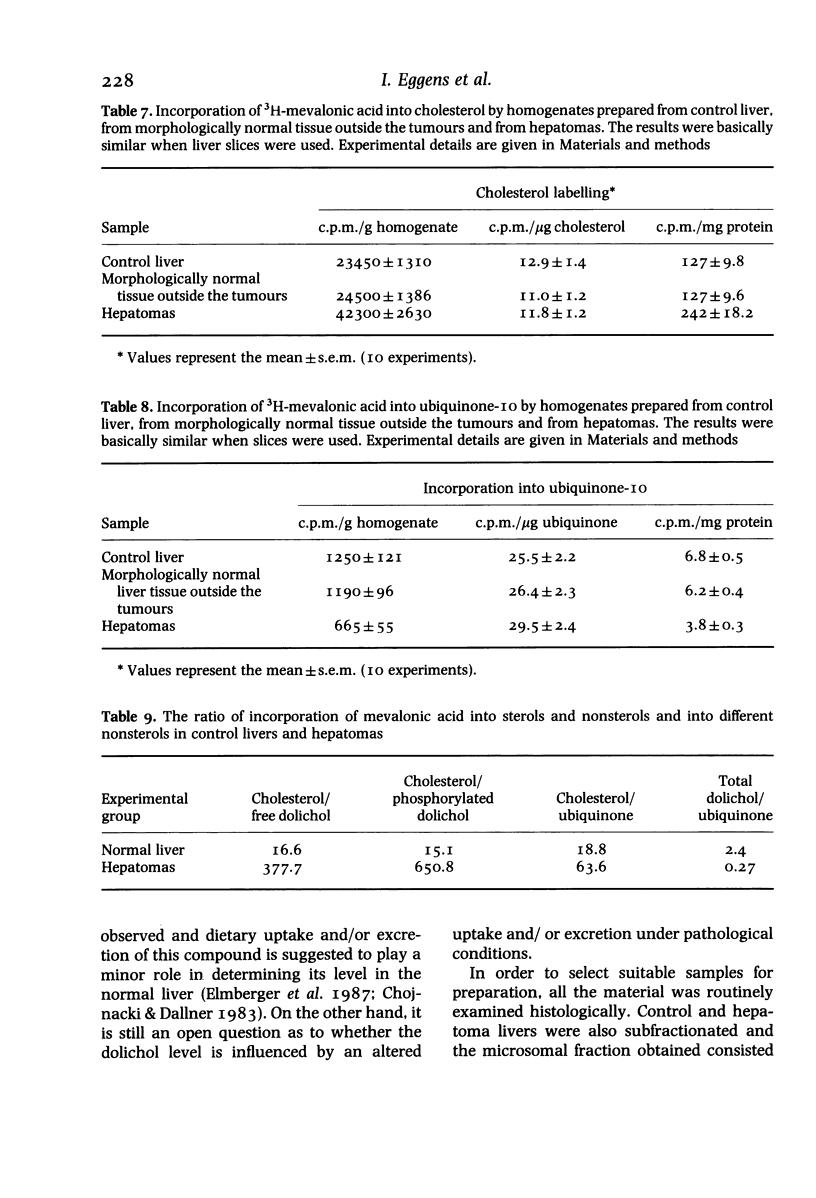
Surgical samples of human hepatic tissue were analysed morphologically and biochemically and highly differentiated hepatomas were compared with two control groups: morphologically normal liver tissue surrounding the tumour, and tissue from normal livers. In tumour homogenates cholesterol levels were more than twice, ubiquinone levels about half and the concentration of free dolichol about 10% of the control value. The levels of dolichyl phosphate were basically similar, whereas the phospholipid level was slightly lower in the tumours. In microsomes isolated from hepatomas, the level of cholesterol was about 30% higher than the control value. HMG-CoA reductase activity in microsomes isolated from hepatomas was elevated almost 100% in comparison to control. In hepatomas, no major alterations in the compositions of dolichol or dolichyl phosphate could be observed. The relative amounts of alpha-saturated and alpha-unsaturated polyprenols were also basically unaltered in hepatomas. Liver samples were incubated with 3H-mevalonic acid and radioactivity was monitored in polyprenols. With control tissue, incorporation was considerably higher in alpha-unsaturated polyprenols than in their alpha-saturated counterparts. In the tumours the rates of incorporation into both polyprenol fractions were much lower, although still higher in the alpha-unsaturated fraction. Labelling of polyisoprenols containing 19 isoprene residues was higher than that of 20 residues. The pattern of labelling in the polyisoprenyl-P fraction was similar. In hepatomas the incorporation into cholesterol and ubiquinone-10 was about 100% higher and 50% lower respectively compared with control tissue. The results in this study of hepatomas indicate that the levels of various lipids may be influenced not only by the regulatory enzyme HMG-CoA reductase, but also by other enzymes catalysing reactions subsequent to this regulatory point. It is also suggested that levels of cholesterol, ubiquinone and dolichol may be regulated independently subsequent to the branch point at farnesylpyrophosphate.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autuori F., Svensson H., Dallner G. Biogenesis of microsomal membrane glycoproteins in rat liver. I. Presence of glycoproteins in microsomes and cytosol. J Cell Biol. 1975 Dec;67(3):687–699. doi: 10.1083/jcb.67.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. D., Earles B. J., Lennarz W. J. Enhancement of protein glycosylation in tissue slices by dolichylphosphate. J Biol Chem. 1981 Nov 25;256(22):11552–11557. [PubMed] [Google Scholar]
- Chen H. W., Kandutsch A. A., Heiniger H. J. The role of cholesterol in malignancy. Prog Exp Tumor Res. 1978;22:275–316. doi: 10.1159/000401203. [DOI] [PubMed] [Google Scholar]
- Eggens I. Biosynthesis of sterols and dolichol in human hepatomas. Acta Chem Scand B. 1987 Jan;41(1):67–69. doi: 10.3891/acta.chem.scand.41b-0067. [DOI] [PubMed] [Google Scholar]
- Eggens I., Eriksson L. C., Chojnacki T., Dallner G. Role of dolichyl phosphate in regulation of protein glycosylation in 2-acetylaminofluorene-induced carcinogenesis in rat liver. Cancer Res. 1984 Feb;44(2):799–805. [PubMed] [Google Scholar]
- Eggens I. Regulation of the level of dolichyl phosphate in human hepatomas. Acta Chem Scand B. 1988 Apr;42(4):247–249. doi: 10.3891/acta.chem.scand.42b-0247. [DOI] [PubMed] [Google Scholar]
- Ekström T. J., Chojnacki T., Dallner G. The alpha-saturation and terminal events in dolichol biosynthesis. J Biol Chem. 1987 Mar 25;262(9):4090–4097. [PubMed] [Google Scholar]
- Elmberger P. G., Eggens I., Dallner G. Conditions for quantitation of dolichyl phosphate, dolichol, ubiquinone and cholesterol by HPLC. Biomed Chromatogr. 1989 Jan;3(1):20–28. doi: 10.1002/bmc.1130030106. [DOI] [PubMed] [Google Scholar]
- Hirayama C., Yamanishi Y., Irisa T. Serum cholesterol and squalene in hepatocellular carcinoma. Clin Chim Acta. 1979 Jan 1;91(1):53–57. doi: 10.1016/0009-8981(79)90470-4. [DOI] [PubMed] [Google Scholar]
- Jaffe N., Traggis D., Das L., Frauenberger G., Hann H. W., Kim B. S., Bishop Y. Favorable remission induction rate with twice weekly doses of L-asparaginase. Cancer Res. 1973 Jan;33(1):1–4. [PubMed] [Google Scholar]
- Kabakoff B. D., Kandutsch A. A. Depression of hepatic dolichol levels by cholesterol feeding. J Lipid Res. 1987 Mar;28(3):305–310. [PubMed] [Google Scholar]
- Kalén A., Appelkvist E. L., Dallner G. Biosynthesis of ubiquinone in rat liver. Acta Chem Scand B. 1987 Jan;41(1):70–72. doi: 10.3891/acta.chem.scand.41b-0070. [DOI] [PubMed] [Google Scholar]
- Keller G. A., Barton M. C., Shapiro D. J., Singer S. J. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase is present in peroxisomes in normal rat liver cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):770–774. doi: 10.1073/pnas.82.3.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. T., Adamany A. M. Impairment of dolichyl saccharide synthesis and dolichol-mediated glycoprotein assembly in the aortic smooth muscle cell in culture by inhibitors of cholesterol biosynthesis. J Biol Chem. 1978 Aug 10;253(15):5270–5273. [PubMed] [Google Scholar]
- NAKAHARA W., FUKUOKA F., NAORA H. Carcinostatic liver factor. II. Discrimination from liver ribonucleic acid. Gan. 1962 Mar;53:1–5. [PubMed] [Google Scholar]
- OSTERBERG K. A., WATTENBERG L. W. Coenzyme Q concerntration in proliferative lesions of liver. Proc Soc Exp Biol Med. 1961 Nov;108:300–303. doi: 10.3181/00379727-108-26920. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Potter J. E., James M. J., Kandutsch A. A. Sequential cycles of cholesterol and dolichol synthesis in mouse spleens during phenylhydrazine-induced erythropoiesis. J Biol Chem. 1981 Mar 10;256(5):2371–2376. [PubMed] [Google Scholar]
- Rudney H., Sexton R. C. Regulation of cholesterol biosynthesis. Annu Rev Nutr. 1986;6:245–272. doi: 10.1146/annurev.nu.06.070186.001333. [DOI] [PubMed] [Google Scholar]
- Rupar C. A., Carroll K. K. Occurrence of dolichol in human tissues. Lipids. 1978 Apr;13(4):291–293. doi: 10.1007/BF02533673. [DOI] [PubMed] [Google Scholar]
- Shinitzky M. Membrane fluidity in malignancy. Adversative and recuperative. Biochim Biophys Acta. 1984;738(4):251–261. doi: 10.1016/0304-419x(83)90007-0. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Yorek M. A. Membrane lipid composition and cellular function. J Lipid Res. 1985 Sep;26(9):1015–1035. [PubMed] [Google Scholar]
- Tollbom O., Dallner G. Dolichol and dolichyl phosphate in human tissues. Br J Exp Pathol. 1986 Oct;67(5):757–764. [PMC free article] [PubMed] [Google Scholar]
- Valtersson C., Dallner G. Compartmentalization of phosphatidylethanolamine in microsomal membranes from rat liver. J Lipid Res. 1982 Aug;23(6):868–876. [PubMed] [Google Scholar]



