Abstract
Maintenance of fluid homeostasis is critical to establishing and maintaining normal physiology. The landmark discovery of membrane water channels (aquaporins; AQPs) ushered in a new area in osmoregulatory biology that has drawn from and contributed to diverse branches of biology, from molecular biology and genomics to systems biology and evolution, and from microbial and plant biology to animal and translational physiology. As a result, the study of AQPs provides a unique and integrated backdrop for exploring the relationships between genes and genome systems, the regulation of gene expression, and the physiologic consequences of genetic variation. The wide species distribution of AQP family members and the evolutionary conservation of the family indicate that the control of membrane water flux is a critical biological process. AQP function and regulation is proving to be central to many of the pathways involved in individual physiologic systems in both mammals and anurans. In mammals, AQPs are essential to normal secretory and absorptive functions of the eye, lung, salivary gland, sweat glands, gastrointestinal tract, and kidney. In urinary, respiratory, and gastrointestinal systems, AQPs are required for proper urine concentration, fluid reabsorption, and glandular secretions. In anurans, AQPs are important in mediating physiologic responses to changes in the external environment, including those that occur during metamorphosis and adaptation from an aquatic to terrestrial environment and thermal acclimation in anticipation of freezing. Therefore, an understanding of AQP function and regulation is an important aspect of an integrated approach to basic biological research.
Keywords: Nephrogenic Diabetes Insipidus, Wood Frog, Major Intrinsic Protein, Constriction Region, Membrane Water Permeability
Mechanisms of fluid homeostasis and aquaporins
The maintenance of fluid homeostasis is critical to all life processes. Vertebrates have evolved intricate physiologic mechanisms for sensing and responding to changes in fluid composition and volume that are caused by environmental variables such as diet, health, hydration, injury, disease, temperature, and other stressors. The biological mechanisms that regulate fluid homeostasis in amphibians and mammals are complex and highly coordinated processes involving the precise regulation of ensembles of ion and water transporters. While osmotically driven transmembrane water movement can occur via simple diffusion through the lipid bilayer, selective membrane water permeability required for rapid and regulated physiologic processes such as secretion and reabsorption requires facilitation through proteinaceous water pores. Aquaporins (AQPs) are water-selective channels that function to increase plasma membrane water permeability in response to osmotic gradients. AQP1 (CHIP28), the first mammalian water channel to be functionally characterized, was isolated from the membranes of red blood cells as a 28-kDa protein and was shown to increase membrane permeability in response to osmotic gradients (Preston and Agre 1991; Preston et al. 1992). Amino acid homology with the major intrinsic protein (MIP) of the lens indicated that AQP1 is a member of the MIP family of membrane proteins (Gorin et al. 1984). In 2003, Dr. Peter Agre was awarded the Nobel Prize in Chemistry to signify the importance of this discovery (Agre 2004). To date, more than 450 members of the MIP superfamily of integral membrane proteins have since been identified in a wide range of organisms, including mammals (reviewed in Krane and Kishore 2003), plants (reviewed in Chaumont et al. 2005), yeast (reviewed in Pettersson et al. 2005), bacteria (reviewed in Tanghe et al. 2006), and anurans (reviewed in Suzuki et al. 2007).
Aquaporin structure
MIP superfamily members are typically 28–30 kDa in size and construct an integral membrane pore, characterized topographically by six transmembrane spanning domains, with cytosolic amino and carboxy termini (Fig. 1). Structural and amino acid similarities between the first and second half of the protein and comparative analysis of gene structure in paralogous sequences indicate that AQPs likely arose by way of an intragenic duplication that occurred relatively early in evolution (Pao et al. 1991). Intracellular loop B and extracellular loop E contain highly conserved asparagine-proline-alanine (NPA) motifs which are inserted into the membrane to create the functional water pore, generating what is referred to as the “hourglass model” (Jung et al. 1994). In some family members, a cysteine residue in the extracellular loop E (Cys 189 in human AQP1) that is situated close to the pore confers functional channel inhibition by mercurials through physical blockage of the pore (Preston et al. 1993; Zhang et al. 1993). Recent studies have indicated that the water permeability of a subset of aquaporins can be blocked by quaternary ammonium compounds such as tetraethyl ammonium (TEA) (Detmers et al. 2006). AQPs assemble as homotetramers in the membrane; however, each monomer is a functional water pore that supports bidirectional, osmotically driven transmembrane water flow. In vivo, the direction of water flow through AQPs is determined by the osmotic gradient that exists across the membrane during specific physiologic processes such as absorption or secretion driven by active ion transport.
Fig. 1.
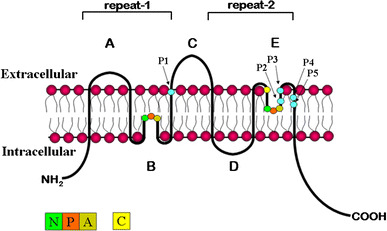
Transmembrane structure of MIP family of integral membrane proteins. Hydropathy plots of primary amino acid sequence of the major intrinsic membrane protein family identified a six-transmembrane-spanning topology. X-ray crystallography and electron microscopy confirmed this structure and provided further proof of homotetramer membrane assembly. The conserved NPA motifs are indicated in loops B and E. The cysteine shown in loop E confers mercury sensitivity in many MIP proteins. Amino acid positions P1-P5 confer functional permeability for either water or glycerol in the AQP and GLP subfamilies, respectively
The NPA motifs are present in nearly all MIP family members, with a few exceptions. Among the 13 known mammalian MIP family members, AQP7, AQP11, and AQP12 encode variant versions of the NPA box in loop B, and AQP7 also has a variant second NPA box in loop E. The proline in the NPA box of loop B of AQP7 is changed to an alanine, thereby changing the first NPA motif to NAA, whereas a serine replaces the alanine in loop E, resulting in an NPS motif (reviewed in Zardoya 2005). The first NPA box in the B loops of AQP 11 and AQP12 is changed to NPC and NPT, respectively (Gorelick et al. 2006; Itoh et al. 2005).
Aquaporins vs. aquaglyceroporins
Aquaporins
Other primary amino acid sequence differences that exist between members of the MIP family have given rise to two structural classes of proteins whose functions are distinctive. Aquaporins (AQPs) are water-selective members of the MIP family, whereas aquaglyceroporins (GLPs) transport both water and organic compounds such as glycerol, urea (reviewed in Hara-Chikuma and Verkman 2006), and potentially other small solutes (e.g., NH3 and NH+4; Holm et al. 2005). The determination of pore selectivity for water in the AQP subclass has been examined through a variety of experimental methods, including site-directed mutagenesis, chimeric domain swaps, membrane permeability assays, electron crystallography, X-ray crystallography, and molecular dynamic simulations (reviewed in Gonen and Walz 2006). X-ray crystallographic analysis of bovine AQP1 from red blood cells at a 2.2-Å resolution identified extracellular and cytosolic pore entry/exit passageways for water molecules that are separated by a central constriction region of the channel (Sui et al. 2001). The constriction region is formed by the interactions of four amino acids within the pore (His 182, Arg 197, Cys 191, Phe 58), which limits the pore size to a 2.8-Å diameter (Sui et al. 2001). Three of the amino acids found within the constriction site are conserved in all water-specific MIP members (Arg 197, His 182, Phe 58; Park and Saier 1996) and contribute to the water selectivity seen in the AQP subclass of the MIP family. In addition, a “selectivity filter,” consisting of six amino acids, was identified. It forces water to make and break hydrogen bonds as molecules pass single file through the pore (Sui et al. 2001). Using real-time molecular dynamic simulations of water movement through human AQP1, De Groot and Grubmüller (2001) proposed a two-stage filter model in which the NPA motif forms a selectivity-determining region, and a second region termed the aromatic/arginine (ar/R) region functions as a proton filter.
Aquaglyceroporins
Transport of glycerol has been studied in a few cell types, vertebrate and otherwise. In some cells glycerol crosses the membrane readily, whereas others are quite impermeable (Vom Dahl and Häussinger 1997). In liver, permeation of glycerol across the membrane (as opposed to its phosphorylation and metabolism) is the rate-limiting step in glycerol utilization (Li and Lin 1983). Characteristics described for this transport include a combination of simple diffusion and H+- or Na+-coupled cotransport (Carlsen and Wieth 1976; Lages and Lucas 1995; Lucas et al. 1990). The mechanism is distinct from the glucose transporter and may be phloretin-sensitive (vom Dahl and Häussinger 1997). These studies may well have incorporated characteristics of multiple transport mechanisms, which remain poorly defined. One mechanism that is common to cells ranging from E. coli (Heller et al. 1980) to insect (Farinas et al. 1995) to mammalian kidney involves glycerol transport via proteins from the aquaporin family. The “selectivity” of the AQP subclass was determined based on comparisons made with members of the GLP subclass. The best studied member of the GLP subclass is GlpF, a glycerol facilitator isolated from E. coli that is permeable to glycerol, urea, and glycine, with very low water permeability (Borgnia and Agre 2001; Maurel et al. 1994). Although GlpF assembles a transmembrane structure that is roughly similar to AQP1, the channel is asymmetric, and the lengths of the extracellular loops and constituents at several amino acid positions within the constriction region and selectivity filter differ significantly from AQP1 (Lu et al. 2003; Sui et al. 2001; reviewed in Gonen and Walz 2006). Five amino acid positions (P1-P5) located in transmembrane helix 3 (P1), extracellular loop E (P2, P3), and transmembrane helix 6 (P4, P5) differ consistently between the AQP and GLP subfamilies in both mammalian and nonmammalian species (Table 1; Fig. 1) (Froger et al. 1998; Heymann and Engel 2000; Lagree et al. 1999). X-ray crystallographic images in the absence of glycerol confirmed the results of the functional studies showing that GlpF does facilitate water transport (Sui et al. 2001; Tajkhorshid et al. 2002), although GlpF-mediated water permeability is far less than glycerol permeability. Since then the structure of several members of both the AQP and GLP subfamilies in a variety of organisms has been resolved at the atomic level (reviewed in Gonen and Walz 2006).
Table 1.
Amino acid conservation at sites determinative of AQP vs. GLP selectivity
| Position | Location | AQP | GLP | HC-1 | HC-2 | HC-3 |
|---|---|---|---|---|---|---|
| P1 | Transmembrane Helix 3 | Nonaromatic | Aromatic | Thr | Thr | Tyr |
| P2 | Extracellular Loop E | Small and uncharged | Asp | Ser | Ser | Asp |
| P3 | Extracellular Loop E | Small and uncharged | Lys or Arg | Ala | Ala | Arg |
| P4 | Transmembrane Helix 6 | Aromatic | Pro | Phe | Phe | Pro |
| P5 | Transmembrane Helix 6 | Aromatic | Nonaromatic | Trp | Trp | Iso |
Amino acids at positions P1-P5 determine AQP vs. GLP function and are conserved in mammalian and nonmammalian species (Froger et al. 1998; Heymann and Engel 2000; Lagree et al. 1999). Amino acids at positions P1-P5 for AQP members HC-1, HC-2, and HC-3, the latter a glyceroporin, from the anuran H. chrysoscelis (HC) are included as examples of conservation in a nonmammalian species (Zimmerman et al. 2007).
Functional and phylogenetic classes of mammalian aquaporins/aquaglyceroporins
Thirteen functionally and phylogenetically distinct mammalian water channels (AQP0–AQP12) have been identified on the basis of sequence homology to AQP1. Evolutionary comparison of mammalian MIP sequences classify AQP0, AQP1, AQP2, AQP4, AQP5, AQP6, and AQP8 as members of the water-selective aquaporin subgroup, whereas AQP3, AQP7, AQP9, and AQP10 are evolutionarily grouped as aquaglyceroporins (Gonen and Walz 2006; Gorelick et al. 2006; Zardoya 2005) (Fig. 2, Table 2). AQP11 and AQP12 are the most distantly related paralogs (Morishita et al. 2005). They have only approximately 20% homology with the MIP family and may constitute a third functionally distinct evolutionary branch of the MIP superfamily (Gorelick et al. 2006; Itoh et al. 2005; Morishita et al. 2004, 2005).
Fig. 2.
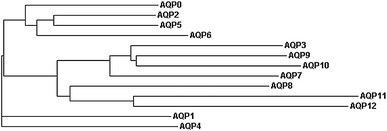
Phylogram of human AQP/GLPs. A phylogram was generated using human AQP/GLP protein sequences available through NCBI/Swiss-PROT and the multisequence alignment program ClustalW available at http://www.ebi.ac.uk/clustalw/index.html. The phylogenetic relationships shown here are consistent with previously published trees (Gorelick et al. 2006; Itoh et al. 2005)
Table 2.
Phylogenetic/functional classifications and tissue distribution of mammalian MIPs
| Gene name | Phylogenetic AQP/GLP | Functional permeability | Tissue/cellular localization |
|---|---|---|---|
| AQP0 | AQP | Water | Lens of the eye |
| AQP1 | AQP | Water | Kidney (proximal tubule and thin descending limb of the loop of Henle), erythrocytes, capillary endothelium, choroid plexus, corneal epithelium, ear, lung, GI tract, skeletal muscle, heart muscle |
| AQP2 | AQP | Water | Kidney (principal cells of the collecting duct and connecting tubules; apical surface and subapical vesicles) |
| AQP3 | GLP | Urea and glycerol; water | Kidney (principal cells of the collecting duct and connecting tubules; basolateral surface), airways, lung, GI tract, brain, ear, urinary bladder, cornea, epidermis |
| AQP4 | AQP | Water | Kidney (collecting duct principal cells; basolateral), retina, ear, airways, lung, GI tract, fast-twitch skeletal muscle, glial cells at blood brain barrier, astrocytes |
| AQP5 | AQP | Water | Salivary gland, lacrimal gland, trachea, epithelia of nasopharynx and airways, alveolar type 1 cells, ear, eye, placenta, pancreas |
| AQP6 | AQP | Anions (NO−3 and Cl−; water | Kidney (intracellular vesicles in type A intercalated cells of the collecting duct) |
| AQP7 | GLP | Urea and glycerol; water, arsenite | Testis, sperm, kidney (proximal tubule), adipose tissue, skeletal muscle |
| AQP8 | AQP | Urea and NH3, water | Testis, sperm, GI tract, placenta, kidney (proximal tubule and collecting duct), airways, liver, salivary glands, glial and neuronal cells, pancreas |
| AQP9 | GLP | Urea and glycerol; water, arsenite | Liver, testis, sperm, spleen, brain, leukocytes, kidney, lung, brain (astrocytes and ependymal cells) |
| AQP10 | GLP | Urea and glycerol; water | Duodenum, jejunum |
| AQP11 | *SuperAQP | Unknown | Kidney (intracellular localization in proximal tubule), liver, testis, brain |
| AQP12 | *SuperAQP | Unknown | Pancreas (acinar cells) |
Typically, MIP family members are functionally characterized for osmotic water permeability by expressing the candidate AQP/GLP cRNA in Xenopus oocytes and assessing cell volume changes upon hypotonic challenge (Preston et al. 1992). Likewise, solute (i.e., urea/glycerol) permeability can be determined by measuring solute uptake in isotonic solution. In some instances, AQP/GLP function has also been assessed in reconstituted proteoliposomes (Zeidel et al. 1992) or by expression in yeast (Lagree et al. 1998). Functionally, AQPs 0–10 support various levels of transmembrane water permeability (Table 2). AQP0, AQP6, AQP9, and AQP10 show low water permeability compared with AQP1, AQP2, AQP3, AQP4, AQP5, AQP7, and AQP8. The water permeability of AQP3 and AQP6 is affected by pH. AQP3, AQP7, AQP9, and AQP10 are also permeable to both urea and glycerol, whereas AQP8 has been reported to exhibit urea and ammonia permeability (Saparov et al. 2007). Interestingly, both AQP7 and AQP9 have been reported to facilitate arsenite uptake (Liu et al. 2002), and AQP6 functions as an anion channel, with permeability to nitrate and chloride (Ikeda et al. 2002; Yasui et al. 1999). The membrane transport properties of AQP11 and AQP12 are currently unknown. Evaluation of AQP11 function in Xenopus oocytes failed to identify any water, urea, glycerol, or ion permeability under various pH conditions (Gorelick et al. 2006). The transport properties of AQP12 remain unstudied because of the inability to obtain adequate plasma membrane expression in Xenopus oocytes (Itoh et al. 2005).
Mammalian AQP/GLP expression
The subsets of organ, tissue, cellular, and subcellular expression patterns of the 13 known mammalian MIP family members are unique for each protein (Table 2). For example, whereas AQP1 is constitutively expressed in diverse tissues, AQP2, AQP10, and AQP12 show a narrow range of tissue-specific expression: AQP2 is predominantly present in principal cells of the renal collecting duct, with minor functionally significant expression in epididymis (Nelson et al. 1998) and inner ear (Merves et al. 2000), AQP10 in the small intestine, and AQP12 in the pancreas (Table 2). Moreover, most if not all MIP genes are subject to temporal and tissue-selective expression via mechanisms that control transcription and translation, and in some cases through post-translational modifications, vesicular trafficking, and polar membrane insertion. AQP2 is perhaps the best-studied MIP with regard to mechanisms that regulate its expression. In the renal collecting duct, AQP2 vesicular trafficking is regulated by arginine vasopressin (AVP), the mammalian antidiuretic hormone (ADH), in response to dehydration (Kishore et al. 1996; Nielsen et al. 1995). In addition, AQP2 mRNA and protein expression are upregulated in response to chronic dehydration (DiGiovanni et al. 1994; Terris et al. 1996). Other signaling cues responsible for altering MIP gene expression include cAMP (Yang et al. 2003), cGMP (Ishikawa et al. 1998, 2002) NO (Ishikawa et al. 2002; Nagai et al. 2007), hypertonicity (Hoffert et al. 2000), cholinergic stimulation (Ishida et al. 1997), TNF-α (Towne et al. 2001), and steroids (King et al. 1996). It is clear that MIP family gene expression is highly regulated at multiple levels in response to a variety of physiologic triggers. The list of regulators will no doubt continue to expand as more is learned about the importance of MIP function during development and in normal physiologic and pathophysiologic states.
Physiologic function of AQP/GLPs in mammals
Some of what is known of the physiologic function of AQPs has been learned by examining the clinical manifestation and associated pathology of AQP deficiency in human disease. Human disorders whose pathogeneses are associated with defects in water channel proteins include inherited cataracts (AQP0; Berry et al. 2000; Francis et al. 2000) and nephrogenic diabetes insipidus (AQP2; Deen et al. 1994; reviewed in Knoers and Deen 2001), and water channel dysfunctions have been implicated in the etiology of Sjogren’s syndrome (AQP5; Beroukas et al. 2001; Steinfeld et al. 2001; Tsubota et al. 2001). Individuals lacking functional AQP1 have a decrease in pulmonary vascular permeability (King et al. 2002) and fail to concentrate urine maximally when dehydrated (King et al. 2001). The list of AQP involvement in human disease is likely to increase as an understanding of the complexity of AQP function becomes better realized.
Targeted disruptions of individual water channel proteins in knockout mouse models have been useful in further elucidating the role of AQP/GLPs in whole-animal physiology. Table 3 summarizes the phenotypes reported to date for the AQP-deficient mice currently available. The information gleaned from the knockout strains implicates abnormal AQP function/regulation as a potential contributor to other urine-concentrating defects (AQP3; Ma et al. 2000b) and to human diseases such as disorders of the skin (AQP3; Ma et al. 2002), impaired wound healing (AQP3; Hara et al. 2002), hearing loss (AQP4; Li and Verkman 2001), salivary gland secretory defects (Ma et al. 1999; Krane et al. 2001b), impaired sweat gland function (Nejsum et al. 2002), asthma (AQP5; Krane et al. 2001a), diabetes and insulin resistance (AQP7; Hibuse et al. 2005), obesity (AQP7; Hara-Chikuma et al. 2005) and polycystic kidney disease (AQP11; Morishita et al. 2005). As such associations emerge for human pathologies, designed pharmacologic inhibition of AQP function may be of specific clinical utility, such as for preventing cellular migration during metastatic tumor progression (AQP1; Saadoun et al. 2005) or in response to brain edema or ischemic injury (AQP4; Manley et al. 2000).
Table 3.
Phenotypes of MIP-deficient mouse strains
| Gene Name | Phenotype of MIP-deficient mouse strains | Reference |
|---|---|---|
| AQP0 | Cataracts | Shiels and Bassnett 1996 |
| AQP1 | Polyuria, defective proximal tubule fluid absorption | Ma et al. 1998 |
| Decreased osmotic water permeability across endothelium | Bai et al. 1999 | |
| AQP2 | Severe polyuria; failure to thrive | Rojek et al. 2006 |
| AQP3 | Urinary concentrating defect—NDI | Ma et al. 2000b |
| Reduced skin hydration and elasticity | Ma et al. 2002 | |
| Delayed wound healing | Hara et al. 2002 | |
| AQP4 | Mild urine-concentrating defect | Ma et al. 1997 |
| Reduced injury-induced brain edema | Manley et al. 2000 | |
| Hearing defects | Li and Verkman 2001 | |
| AQP5 | Impaired salivary secretion | Krane et al. 2001b; Ma et al. 1999 |
| Airway hyperresponsiveness to cholinergic stimulation | Krane et al. 2001a | |
| Impaired stimulated sweat secretion | Nejsum et al. 2002 | |
| Decreased osmotic water permeability across alveolar epithelium | Ma et al. 2000a | |
| Impaired secretion in airway submucosal glands | Song and Verkman 2001 | |
| AQP6 | Unknown | |
| AQP7 | Increased body fat with adipocyte hypertrophy | Hara-Chikuma et al. 2005 |
| Increased body weight and age-dependent insulin resistance | Hibuse et al. 2005 | |
| AQP8 | Mild hypertriglyceridemia | Yang et al. 2005 |
| AQP9 | Unknown | |
| AQP10 | Unknown | |
| AQP11 | Polycystic kidney disease (proximal tubule) | Morishita et al. 2005 |
| AQP12 | Unknown |
AQPs/GLPs in thermal tolerance: the importance of comparative analyses of nonmammalian subjects
Aquaporin proteins are found ubiquitously among the kingdoms of living things. Many of these organisms experience extremes of thermal and osmotic stress far beyond those tolerated by mammals. One such circumstance is the possibility of substantial cellular dehydration elicited by freezing; organisms ranging from bacteria through certain vertebrates tolerate subfreezing temperatures, and they do so using a combination of water and solute transport mechanisms.
Recently, the importance of AQP/GLP in the process of glycerol-facilitated cryopreservation has been shown under natural and experimental conditions. Glycerol is an organic solute commonly used in biomedicine as a cryoprotectant to enable bacterial, fungal, and embryonic cells to freeze at ultralow temperatures without compromised viability. Enhanced AQP/GLP expression correlates with improved freeze tolerance in baker’s yeast (Tanghe et al. 2002) and sperm (Dibas et al. 1998), and artificial AQP/GLP expression improves viability following cryopreservation of fish embryos (Hagedorn et al. 2002) and mouse oocytes (Edashige et al. 2003), suggesting that facilitated glycerol transport through AQP/GLP may participate in the physiology of freeze tolerance in animals. Clinical cryopreservation is currently most successful with small or single-celled tissues. Therefore, insights into how a multicellular organism survives freezing could yield important clues to the cryopreservation of larger tissues and organs. For example, modeling studies have suggested that cryopreservation of whole mammalian kidneys, which would entail perfusion of cryopreservative solution such as glycerol through the vasculature, would succeed best if tissue permeability to the cryopreservative agent were high, thereby minimizing osmotically induced changes in cellular and extracellular volumes (Lachenbruch et al. 1998). Thus, the comparative analysis of AQPs/GLPs in amphibians, a subset of which undergo a physiologic process of cryopreservation and freeze tolerance (sometimes involving glycerol accumulation), may serve as a model for testing such ideas.
Role of the kidneys in conserving organic solutes in anurans
Renal conservation of cryoprotective solutes may be critical to freeze-tolerant anurans. Repeated cycles of freezing and thawing deplete glycogen stores in anuran liver (Lee and Costanzo 1993), quite likely because glucose is lost in the urine: The renal tubules have a limited capacity for glucose reabsorption, and this may be overwhelmed at high glucose concentrations occurring after freezing (Layne et al. 1996). Wood frogs may compensate for this loss by cutaneous uptake of excreted glucose.
In wood frogs, glycogenesis is initiated promptly upon thawing, thereby minimizing the duration of high plasma glucose and so its potential urinary loss. In contrast, gray treefrogs retain high plasma glycerol concentrations for weeks, before and after freezing. How do they avoid urinary loss of this solute? Presumably glycerol is filtered, albeit at a reduced rate, in cold-acclimated frogs, which have reduced rates of glomerular filtration (Zimmerman et al. 2007). Thus, reabsorption of filtered glycerol should be at a premium.
In other circumstances when glomerular filtration rate (GFR) is reduced in amphibians, plasma arginine vasotocin (AVT), the amphibian antidiuretic hormone, is elevated (Nouwen and Kuhn 1983; Rosenbloom and Fisher 1974). AVT may act on the renal vasculature to reduce GFR (Pang 1983), on the bladder to increase water permeability, and on the renal tubules (Uchiyama 1994). The physiologic response of the latter structures to AVT is not known, but one response of vertebrate kidneys to ADH is upregulation and membrane insertion of aquaporins, which could mediate reabsorption of water and/or glycerol.
Aquaporins in amphibians
Historically, amphibians have played a critical role in the conceptualization of and suggestion for the existence of water channels long before the first water channel was cloned. Indeed, even before aquaporins were understood as such, careful study of toad urinary bladder suggested the “shuttle hypothesis,” based on visualization of “particle aggregates” that appeared to be inserted into and retrieved from the apical membrane under conditions of changing water reabsorption (e.g., Wade 1989; Wade et al. 1981). Additional evidence has confirmed a role for AQPs and GLPs in amphibian osmoregulation. Aquaporins have been identified in amphibian skin, bladder, fat body, and elsewhere (Ma et al. 1996; Virkki et al. 2002; Zimmerman et al. 2007), and several proteins of the aquaporin family have been sequenced from anurans. Phylogenetic analysis of 17 anuran AQP mRNA sequences deposited in public databases has revealed six classes of anuran AQPs, two of which are distinct to anurans (reviewed in Suzuki et al. 2007). “FA-CHIP” in Rana esculenta (Abrami et al. 1994), “AQP-t1” in Bufo marinus (Ma et al. 1996), AQP-h1 in Hyla japonica (Hasegawa et al. 2003), and HC-1 in Hyla chrysoscelis (Zimmerman et al. 2007) resemble each other in both sequence and wide tissue distribution patterns. These proteins are also similar to mammalian AQP1 (76%–98% sequence identity), and expression cloning has confirmed that AQP-t1, AQP-h1, and HC-1 function as water but not as glycerol channels (Hasegawa et al. 2003; Ma et al. 1996; Zimmerman et al. 2007) (Table 1). Temperature-sensitive regulation of HC-1 expression was seen in brain, kidney, and liver; frogs acclimated to cold conditions (4°C) had higher HC-1 mRNA expression in the liver than did warm-acclimated frogs, whereas in brain and kidney warm-acclimated frogs expressed higher levels of HC-1 (Zimmerman et al. 2007) (Fig. 3). A second AQP, HC-2, has recently been isolated from urinary bladder cDNA from H. chrysoscelis and shows strong amino conservation compared with mammalian AQP2 (69% identity, 85% similarity; Zimmerman et al. 2007). Like hAQP2, HC-2 was functionally determined to be within the AQP subclass, and it supported osmotically driven water transport (Zimmerman et al. 2007) (Table 1). HC-2 mRNA was detected primarily in organs of osmoregulation (skin, bladder, and kidney; Zimmerman et al. 2007). Interestingly, like HC-1, HC-2 expression varied depending on thermal conditions, i.e., hydrated frogs that were acclimated to cold conditions (4°C) had high levels of HC-2 expression in skin, whereas no HC-2 expression was observed from the ventral skin of hydrated warm-acclimated frogs (Zimmerman et al. 2007).
Fig. 3.
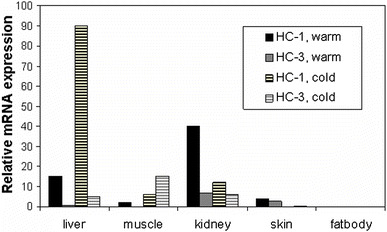
Relative HC-1 and HC-3 mRNA expression in warm- vs. cold-acclimated tissues from H. chrysoscelis. Relative mRNA expression (real-time PCR, expression of HC-1 or HC-3 mRNA normalized to expression of β-actin mRNA) in an aquaporin (HC-1) and a glyceroporin (HC-3) from the anuran Hyla chrysoscelis. Note that expression varies both among tissues and depending on acclimation to either warm (20°C) or cold (4°C) conditions
To date, four anuran sequences similar to mammalian AQP3 have been identified. They include AQP3 from X. laevis (Schreiber et al 2000), AQP from X. tropicalis (unpublished; GenBank Accession number CR855446), AQP-h3BL from H. japonica (Akabane et al. 2007), and HC-3 from H. chrysoscelis (Zimmerman et al. 2007). HC-3 from H. chrysoscelis shows 82% identity and 94% amino acid similarity with mammalian AQP3, and functionally it performs as a GLP, with low water permeability and high glycerol permeability (Zimmerman et al. 2007) (Table 1). HC-3 mRNA exhibited both tissue-specific and thermal-selective patterns of expression. Of special note, tissue glycerol concentrations increased in the liver and skeletal muscle in cold-acclimated frogs compared with warm-acclimated frogs (Fig. 3). The increase in glycerol concentration in these tissues corresponds well with an increase in HC-3 mRNA abundance in muscle, liver, and bladder in cold-acclimated frogs (Zimmerman et al. 2007). Studies of mammalian GLPs are just beginning to elucidate potential physiologic roles for their facilitation of glycerol transport. These roles include glycerol export from adipocytes (Hara-Chikuma et al. 2005) and a contribution to pliability in skin (Ma et al. 2002). Amphibians that naturally accumulate glycerol represent a natural model for studying the roles and regulation of glycerol-transporting aquaporins.
Two aquaporins from H. japonica (AQP-h2 and AQP-h3) have been sequenced (Hasegawa et al. 2003; Tanii et al. 2002) that have high homology to each other and to AQP-t2 and AQP-t3 from B. marinus. Suzuki et al. (2007) have suggested that these four genes form an anuran-specific, phylogenetically distinct MIP subclass (type AQPa2). AQP-h2 and AQP-h3 are both expressed in ventral skin, whereas AQP-h2 is also expressed in the urinary bladder. Expression of both is upregulated by AVT (Hasegawa et al. 2005). Coexpression of the AVT receptor, AQP-h2, and AQP-h3 during metamorphosis, when the animals are undergoing a transition from aquatic to terrestrial environment, suggests a role for AVT-regulated AQPs in this process (Hasegawa et al. 2004). A second anuran-specific phylogenetic class, type-a1, has been assigned for a novel aquaporin identified from oocytes of X. laevis; that protein exhibits unique mercury sensitivity and less than 50% amino acid identity to the most closely related mammalian aquaporins (Virkki et al. 2002).
Conclusion
Studies of mammalian aquaporins have revealed many details of structure and function and are beginning to yield insights into pathogenic mechanisms. Nevertheless, mammalian aquaporins comprise a relatively limited subset of proteins from this large class of molecules. Proteins from the MIP family are present in every sort of organism, from bacteria through fungi, plants, and animals. It is likely that novel insights into structure-function relationships, into mechanisms of regulation, and into physiologic roles will derive from this diversity of organisms. Amphibians present particularly attractive models for studies of aquaporin function in vertebrate osmoregulation and thermoregulation; transitions from water to land and tolerance of tissue freezing are the epitomes of combined osmotic and thermal demands. Thus, just as studies of amphibians contributed to the original elucidation of aquaporin function, before we knew of aquaporins per se, we suggest that such studies will continue to contribute to a fundamental understanding of these ubiquitous proteins.
Acknowledgments
The authors thank Dr. B.K. Kishore for reviewing the manuscript. This work was supported in part by research grant NSF IOB-0517301 to DLG and CMK.
References
- Abrami L, Simon M, Rousselet G, Berthonaud V, Buhler J-M, et al. Sequence and functional expression of an amphibian water channel, FA-CHIP: a new member of the MIP family. Biochim Biophys Acta. 1994;1192:147–151. doi: 10.1016/0005-2736(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Agre P. Aquaporin water channels (Nobel lecture) Angew Chem Int Ed Engl. 2004;43:4278–4290. doi: 10.1002/anie.200460804. [DOI] [PubMed] [Google Scholar]
- Akabane G, Ogushi Y, Hasegawa T, Suzuki M, Tanaka S. Gene cloning and expression of an aquaporin, AQP-h3BL, in the basolateral membrane of water-permeable epithelial cells in osmoregulatory organs of the tree frog. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2340–2351. doi: 10.1152/ajpregu.00905.2006. [DOI] [PubMed] [Google Scholar]
- Bai C, Fukuda N, Song Y, Ma T, Matthay MA, et al. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J Clin Invest. 1999;103:555–561. doi: 10.1172/JCI4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukas D, Hiscock J, Jonsson R, Waterman SA, Gordon TP. Subcellular distribution of aquaporin 5 in salivary glands from Sjogren’s syndrome. Lancet. 2001;358:1875–1877. doi: 10.1016/S0140-6736(01)06900-8. [DOI] [PubMed] [Google Scholar]
- Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Missense mutations in MIP underlie autosomal dominant polymorphic and lamellar cataracts linked to 12q. Nat Genet. 2000;25:15–17. doi: 10.1038/75538. [DOI] [PubMed] [Google Scholar]
- Borgnia MJ, Agre P. Reconstitution and functional comparison of purified GlpF and AqpZ, the glycerol and water channels from Escherichia coli. Proc Natl Acad Sci U S A. 2001;98:2888–2893. doi: 10.1073/pnas.051628098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen A, Wieth JO. Glycerol transport in human red cells. Acta Phys Scand. 1976;97:501–513. doi: 10.1111/j.1748-1716.1976.tb10290.x. [DOI] [PubMed] [Google Scholar]
- Chaumont F, Moshelion M, Daniels MJ. Regulation of plant aquaporin activity. Biol Cell. 2005;97:749–764. doi: 10.1042/BC20040133. [DOI] [PubMed] [Google Scholar]
- De Groot BL, Grubmüller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1066115. [DOI] [PubMed] [Google Scholar]
- Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, et al. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Detmers FJM, de Groot BL, Müller EM, Hinton A, Konings IBM, et al. Quaternary ammonium compounds as water channel blockers. J Biol Chem. 2006;281:14207–14214. doi: 10.1074/jbc.M513072200. [DOI] [PubMed] [Google Scholar]
- Dibas AI, Mia AJ, Yorio T. Aquaporins (water channels): role in vasopressin-activated water transport. Proc Soc Exp Biol Med. 1998;219:183–199. doi: 10.3181/00379727-219-44332. [DOI] [PubMed] [Google Scholar]
- DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci U S A. 1994;91:8984–8988. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edashige K, Yamaji Y, Kleinhans FW, Kasai M. Artificial expression of aquaporin-3 improves the survival of mouse oocytes after cryopreservation. Biol Reprod. 2003;68:87–94. doi: 10.1095/biolreprod.101.002394. [DOI] [PubMed] [Google Scholar]
- Farinas J, Simanek V, Verkman AS. Cell volume measured by total internal reflection microfluorimetry: application to water and solute transport in cells transfected with water channel homologs. Biophys J. 1995;68:1613–1620. doi: 10.1016/S0006-3495(95)80335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis P, Chung JJ, Yasui M, Berry V, Moore A, et al. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum Mol Genet. 2000;9:2329–2334. doi: 10.1093/oxfordjournals.hmg.a018925. [DOI] [PubMed] [Google Scholar]
- Froger A, Tallur B, Thomas D, Delamarche C. Prediction of functional residues in water channels and related proteins. Protein Sci. 1998;7:1458–1468. doi: 10.1002/pro.5560070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen T, Walz T. The structure of aquaporins. Q Rev Biophys. 2006;39:361–396. doi: 10.1017/S0033583506004458. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Praetorius J, Tsunerai T, Nielsen S, Agre P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006;7:1–14. doi: 10.1186/1471-2091-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin MB, Yancey SB, Cline J, Revel JP, Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984;39:49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Lance SL, Fonseca DM, Kleinhans FW. Altering fish embryos with aquaporin-3: an essential step toward successful cryopreservation. Biol Reprod. 2002;67:961–966. doi: 10.1095/biolreprod.101.002915. [DOI] [PubMed] [Google Scholar]
- Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3 deficient mice may account for impaired skin hydration, elasticity and barrier recovery. J Biol Chem. 2002;277:46616–46621. doi: 10.1074/jbc.M209003200. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins. Cell Mol Life Sci. 2006;63:1386–1392. doi: 10.1007/s00018-006-6028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, et al. Progressive adipocyte hypertrophy in aquaporin 7 deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J Biol Chem. 2005;280:15493–15496. doi: 10.1074/jbc.C500028200. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Tanii H, Suzuki M, Tanaka S. Regulation of water absorption in the frog skins by two vasotocin-dependent water-channel aquaporins, AQP-h2 and AQP-h3. Endocrinology. 2003;144:4087–4096. doi: 10.1210/en.2003-0418. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Sugawara Y, Suzuki M, Tanaka S. Spatial and temporal expression of the ventral pelvic skin aquaporins during metamorphosis of the tree frog, Hyla japonica. J Membr Biol. 2004;199:119–126. doi: 10.1007/s00232-004-0677-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Suzuki M, Tanaka S. Immunocytochemical studies on translocation of phosphorylated aquaporin-h2 protein in granular cells of the frog urinary bladder before and after stimulation with vasotocin. Cell Tissue Res. 2005;322:407–415. doi: 10.1007/s00441-005-0037-8. [DOI] [PubMed] [Google Scholar]
- Heller KB, Lin EC, Wilson TH. The substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980;144:274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann JB, Engel A. Structural clues in the sequences of the aquaporins. J Mol Biol. 2000;295:1039–1053. doi: 10.1006/jmbi.1999.3413. [DOI] [PubMed] [Google Scholar]
- Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci U S A. 2005;102:10993–10998. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffert JD, Leitch V, Agre P, King LS. Hypertonic induction of aquaporin-5 expression through an ERK-dependent pathway. J Biol Chem. 2000;275:9070–9077. doi: 10.1074/jbc.275.12.9070. [DOI] [PubMed] [Google Scholar]
- Holm LM, Jahn TP, Møller AL, Schjoerring JK, Feeri D, et al. NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pflug Arch. 2005;450:415–428. doi: 10.1007/s00424-005-1399-1. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, et al. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem. 2002;277:39873–39879. doi: 10.1074/jbc.M207008200. [DOI] [PubMed] [Google Scholar]
- Ishida N, Hirai SI, Mita S. Immunolocalization of aquaporin homologs in mouse lacrimal glands. Biochem Biophys Res Commun. 1997;238:891–895. doi: 10.1006/bbrc.1997.7396. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Skowronshi MT, Inoue N, Ishida H. Alpha(1)-adrenoceptor-induced trafficking of aquapoin-5 to the apical plasma membrane of rat parotid cells. Biochem Biophys Res Commun. 1998;265:194–200. doi: 10.1006/bbrc.1999.1630. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Iida H, Ishida H. The muscarinic acetylcholine receptor-stimulated increase in aquaporin 5 levels in the apical plasma membrane in rat parotid acinar cells is coupled with activation of nitric oxide/ cGMP signal transduction. Mol Pharmacol. 2002;61:1423–1434. doi: 10.1124/mol.61.6.1423. [DOI] [PubMed] [Google Scholar]
- Itoh T, Rai T, Kuwahara M, Ko SBH, Uchida S, et al. Identification of a novel aquaporin AQP12, expressed in pancreatic acinar cells. Biochem Biophys Res Commun. 2005;330:832–838. doi: 10.1016/j.bbrc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- King LS, Nielsen S, Agre P. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat. J Clin Invest. 1996;97:2183–2191. doi: 10.1172/JCI118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Choi M, Fernandez PC, Cartron JP, Agre P. Defective urinary-concentrating ability due to a complete deficiency of aquaporin -1. New Engl J Med. 2001;345:175–179. doi: 10.1056/NEJM200107193450304. [DOI] [PubMed] [Google Scholar]
- King LS, Nielsen S, Agre P, Brown RH. Decreased pulmonary vascular permeability in aquaporin-1-null humans. Proc Natl Acad Sci USA. 2002;99:1059–1063. doi: 10.1073/pnas.022626499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore BK, Terris JM, Knepper MA. Quantitation of aquaporin-2 abundance in microdissected collecting ducts: Axial distribution and control by AVP. Am J Physiol. 1996;271:F62–F70. doi: 10.1152/ajprenal.1996.271.1.F62. [DOI] [PubMed] [Google Scholar]
- Knoers NV, Deen PM. Molecular and cellular defects in nephrogenic diabetes insipidus. Pediatr Nephrol. 2001;16:1146–1152. doi: 10.1007/s004670100051. [DOI] [PubMed] [Google Scholar]
- Krane CM, Kishore BK. Aquaporins: membrane water channels of the biological world. Biologist. 2003;50:81–86. [Google Scholar]
- Krane CM, Fortner CN, Hand AR, McGraw DW, Lorenz JN, et al. Aquaporin 5-deficient mouse lungs are hyperresponsive to cholinergic stimulation. Proc Natl Acad Sci U S A. 2001;98:14114–14119. doi: 10.1073/pnas.231273398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, et al. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276(26):23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- Lachenbruch CA, Diller KR, Pegg DE. Sensitivity of kidney perfusion protocol design to physical and physiological parameters. Ann N Y Acad Sci. 1998;858:298–309. doi: 10.1111/j.1749-6632.1998.tb10164.x. [DOI] [PubMed] [Google Scholar]
- Lages F, Lucas C. Characterization of a glycerol/H+ symport in the halotolerant yeast Pichia sorbitolophila. Yeast. 1995;11:111–119. doi: 10.1002/yea.320110203. [DOI] [PubMed] [Google Scholar]
- Lagree V, Pellerin I, Hubert JF, Tacnet F, Le Caherec F, et al. A yeast recombinant aquaporin mutant that is not expressed or mistargeted in Xenopus oocyte can be functionally analyzed in reconstituted proteoliposomes. J Biol Chem. 1998;273:12422–12426. doi: 10.1074/jbc.273.20.12422. [DOI] [PubMed] [Google Scholar]
- Lagree V, Froger A, Deschamps S, Hubert JF, Delamarche C, et al. Switch from an aquaporin to a glycerol channel by two amino acids substitution. J Biol Chem. 1999;274:6817–6819. doi: 10.1074/jbc.274.11.6817. [DOI] [PubMed] [Google Scholar]
- Layne JR, Lee RE, Cutwa MM. Post-hibernation excretion of glucose in urine of the freeze tolerant frog Rana sylvatica. J Herp. 1996;30:85–87. [Google Scholar]
- Lee RE, Costanzo JP (1993) Integrated physiological responses promoting anuran freeze tolerance. In: Life in the Cold, Carey C, et al. (eds.) (Boulder, CO: Westview Press), pp 501–510
- Li C-C, Lin EC. Glycerol transport and phosphorylation by rat hepatocytes. J Cell Physiol. 1983;117:230–234. doi: 10.1002/jcp.1041170214. [DOI] [PubMed] [Google Scholar]
- Li J, Verkman AS. Impaired hearing in mice lacking aquaporin-4 water channels. J Biol Chem. 2001;276:31233–31237. doi: 10.1074/jbc.M104368200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, et al. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Grayson P, Schulten K. Glycerol conductance and physical asymmetry of the Escherichia coli glycerol facilitator GlpF. Biophys J. 2003;85:2977–2987. doi: 10.1016/S0006-3495(03)74718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C, Costa MD, VanUden N. Osmoregulatory active sodium-glycerol co-transport in the halotolerant yeast Debaryomyces hansenii. Yeast. 1990;6:187–192. [Google Scholar]
- Ma T, Baoxue Y, Verkman AS. cDNA cloning of a functional water channel from toad urinary bladder epithelium. Am J Phys. 1996;271:C1699–C1704. doi: 10.1152/ajpcell.1996.271.5.C1699. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, et al. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, et al. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, et al. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci U S A. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem. 2002;277:17147–17153. doi: 10.1074/jbc.M200925200. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Maurel C, Reizer J, Schroeder JI, Chrispeels MJ, Saier MH., Jr Functional characterization of the Escherichia coli glycerol facilitator, GlpF,in Xenopus oocytes. J Biol Chem. 1994;269:11869–11872. [PubMed] [Google Scholar]
- Merves M, Bobbitt B, Parker K, Kishore BK, Choo D. Developmental expression of aquaporin 2 in the mouse inner ear. Laryngoscope. 2000;110:192–1930. doi: 10.1097/00005537-200011000-00030. [DOI] [PubMed] [Google Scholar]
- Morishita Y, Sakube Y, Sasaki S, Ishibashi K. Molecular mechanisms and drug development in aquaporin water channel diseases: aquaporin superfamily (superaquaporins): expansion of aquaporins restricted to multicellular organisms. J Pharmacol Sci. 2004;96:276–279. doi: 10.1254/jphs.fmj04004x7. [DOI] [PubMed] [Google Scholar]
- Morishita Y, Matsuzaki T, Hara-Chikuma M, Andoo A, Shimono M, et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol. 2005;25:7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Watanabe M, Seto M, Hisatsune A, Miyata T, et al. Nitric oxide decreases cell surface expression of aquaporin-5 and membrane water permeability in lung epithelial cells. Biochem Biophys Res Commun. 2007;354:579–584. doi: 10.1016/j.bbrc.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Nejsum LN, Kwon TH, Jensen UB, Fumagalli O, Frokiaer J, et al. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci U S A. 2002;99:511–516. doi: 10.1073/pnas.012588099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RD, Stricklett P, Gustafson C, Stevens A, Ausiello D, et al. Expression of an AQP2 Cre recombinase transgene in kidney and male reproductive system of transgenic mice. Am J Physiol. 1998;275:C216–C226. doi: 10.1152/ajpcell.1998.275.1.C216. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Chou C-L, Marples D, Chirstensen EI, Kishore BK, et al. Vasopressing increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen EJ, Kuhn ER. Radioimmunoassay of arginine vasotocin and mesotocin in serum of the frog Rana ridibunda. Gen Comp Endocrinol. 1983;50:242–251. doi: 10.1016/0016-6480(83)90224-1. [DOI] [PubMed] [Google Scholar]
- Pang PKT. Evolution of control of epithelial transport in vertebrates. J Exp Biol. 1983;106:283–299. doi: 10.1242/jeb.106.1.283. [DOI] [PubMed] [Google Scholar]
- Pao GM, Wu LF, Johnson KD, Hofte H, Chrispeels MJ, et al. Evolution of the MIP family of integral membrane transport proteins. Mol Microbil. 1991;5:33–37. doi: 10.1111/j.1365-2958.1991.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Park JH, Saier MH., Jr Phylogenetics characterization of the MIP family of transmembrane channel proteins. J Membr Biol. 1996;153:171–180. doi: 10.1007/s002329900120. [DOI] [PubMed] [Google Scholar]
- Pettersson N, Filipsson C, Becit E, Brive L, Hohmann S. Aquaporins in yeasts and filamentous fungi. Biol Cell. 2005;97:487–500. doi: 10.1042/BC20040144. [DOI] [PubMed] [Google Scholar]
- Preston GM, Agre P. Isolation of a cDNA from erythrocyte integral membrane protein of 28 kilodalton: member of an ancient channel family. Proc Nat Acad Sci U S A. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 proteins. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston GM, Jung JS, Guggino WB, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP 28 water channel. J Biol Chem. 1993;268:17–20. [PubMed] [Google Scholar]
- Rojek A, Fuchtbauer EM, Kwon TH, Frokiaer J, Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci U S A. 2006;103:6037–6042. doi: 10.1073/pnas.0511324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom AA, Fisher DA. Radioimmunoassay of arginine vasotocin. Endocrinology. 1974;95:1726–1732. doi: 10.1210/endo-95-6-1726. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Saparov SM, Liu K, Agre P, Pohl P. Fast and selective ammonia transport by aquaporin-8. J Biol Chem. 2007;282:5296–5301. doi: 10.1074/jbc.M609343200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Pavenstadt H, Greger R, Kunzelmann K. Aquaporin 3 cloned from Xenopus laevis is regulated by the cystic fibrosis transmembrane conductance regulator. FEBS Lett. 2000;475:291–295. doi: 10.1016/s0014-5793(00)01689-6. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bassnett S. Mutations in the founder of the MIP gene family underlie cataract development in the mouse. Nat Genet. 1996;12:212–215. doi: 10.1038/ng0296-212. [DOI] [PubMed] [Google Scholar]
- Song Y, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem. 2001;276:41288–41292. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- Steinfeld S, Cogan E, King LS, Agre P, Kiss R, et al. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjogren’s syndrome patients. Lab Invest. 2001;81:143–148. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- Sui H, Han B-G, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hasegawa T, Ogushi Y, Tanaka S (2007) Amphibian aquaporins and adaptation to terrestrial environments: A review. Comp Biochem Physiol A Mol Integr Physiol (epub ahead of print) [DOI] [PubMed]
- Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O’Connell J, et al. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 2002;296:525–530. doi: 10.1126/science.1067778. [DOI] [PubMed] [Google Scholar]
- Tanghe A, Van Dijck P, Dumortier F, Teunissen A, Hohmann S, et al. Aquaporin expression correlates with freeze tolerance in baker’s yeast, and overexpression improves freeze tolerance in industrial strains. Appl Environ Microbiol. 2002;6:5981–5989. doi: 10.1128/AEM.68.12.5981-5989.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanghe A, van Dijck P, Thevelien JM. Why do microorganisms have aquaporins? Trends Microbiol. 2006;14:78–85. doi: 10.1016/j.tim.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tanii H, Hasegawa T, Hirakawa N, Suzuki M, Tanaka S. Molecular and cellular characterization of a water-channel protein, AQP-h3, specifically expressed in the frog ventral skin. J Membr Biol. 2002;188:43–53. doi: 10.1007/s00232-001-0172-4. [DOI] [PubMed] [Google Scholar]
- Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rat. Am J Physiol. 1996;271:F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- Towne JE, Krane CM, Bachurski CJ, Menon AG. Tumor necrosis factor-β inhibits aquaporin 5 expression in mouse lung epithelial cells. J Biol Chem. 2001;276:18657–18664. doi: 10.1074/jbc.M100322200. [DOI] [PubMed] [Google Scholar]
- Tsubota K. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet. 2001;357:688–689. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- Uchiyama M. Sites of action of arginine vasotocin in the nephron of the bullfrog kidney. Gen Comp Endocrinol. 1994;94:366–373. doi: 10.1006/gcen.1994.1092. [DOI] [PubMed] [Google Scholar]
- Vom Dahl S, Häussinger D. Evidence for a phloretin-sensitive glycerol transport mechanism in the perfused rat liver. Am J Physiol. 1997;272:G563–G574. doi: 10.1152/ajpgi.1997.272.3.G563. [DOI] [PubMed] [Google Scholar]
- Virkki LV, Franke C, Somieski P, Boron WF. Cloning and functional characterization of a novel aquaporin from Xenopus laevis oocytes. J Biol Chem. 2002;277:40610–40616. doi: 10.1074/jbc.M206157200. [DOI] [PubMed] [Google Scholar]
- Wade JB. Dynamics of apical membrane responses to ADH in amphibian bladder. Am J Physiol. 1989;257:R998–R1003. doi: 10.1152/ajpregu.1989.257.5.R998. [DOI] [PubMed] [Google Scholar]
- Wade JB, Stetson DL, Lewis SA. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]
- Yang F, Kawedia JD, Menon AG. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J Biol Chem. 2003;278:32173–32180. doi: 10.1074/jbc.M305149200. [DOI] [PubMed] [Google Scholar]
- Yang B, Song Y, Zhao D, Verkman AS. Phenotype analysis of aquaporin-8 null mice. Am J Physiol Cell Physiol. 2005;288:C1161–C1170. doi: 10.1152/ajpcell.00564.2004. [DOI] [PubMed] [Google Scholar]
- Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, et al. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 1999;402:184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- Zardoya R. Phylogeny and evolution of the major intrinsic protein family. Biol Cell. 2005;97:397–414. doi: 10.1042/BC20040134. [DOI] [PubMed] [Google Scholar]
- Zeidel ML, Ambudkar SV, Smith BL, Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992;31:7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- Zhang R, van Hoek AN, Biwersi J, Verkman AS. A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel CHIP28k. Biochemistry. 1993;32:2938–2941. doi: 10.1021/bi00063a002. [DOI] [PubMed] [Google Scholar]
- Zimmerman SL, Frisbie J, Goldstein DL, West J, Rivera K, et al. Excretion and conservation of glycerol, and expression of aquaporins and glyceroporins, during cold acclimation in Cope’s gray tree frog Hyla chrysoscelis. Am J Physiol Regul Integr Comp Physiol. 2007;292:R544–R555. doi: 10.1152/ajpregu.00434.2006. [DOI] [PubMed] [Google Scholar]


