Abstract
Background
A complete pathological response to neo-adjuvant chemo-radiation for oesophageal cancer is associated with favourable survival. However, such a benefit is seen in the minority. If one could identify, at diagnosis, those patients who were unlikely to respond unnecessary toxicity could be avoided and alternative treatment can be considered. The aim of this review was to highlight predictive markers currently assessed and evaluate their clinical utility.
Methods
A systematic search of Pubmed and Google Scholar was performed using the following keywords; "neo-adjuvant", "oesophageal", "trimodality", "chemotherapy", "radiotherapy", "chemoradiation" and "predict". The original manuscripts were sourced for further articles of relevance.
Results
Conventional indices including tumour stage and grade seem unable to predict histological response. Immuno-histochemical markers have been extensively studied, but none has made its way into routine clinical practice. Global gene expression from fresh pre-treatment tissue using cDNA microarray has only recently been assessed, but shows considerable promise. Molecular imaging using FDG-PET seems to be able to predict response after neo-adjuvant chemoradiation has finished, but there is a paucity of data when such imaging is performed earlier.
Conclusion
Currently there are no clinically useful predictors of response based on standard pathological assessment and immunohistochemistry. Genomics, proteomics and molecular imaging may hold promise.
Background
Cancer medicine is in the midst of a technological revolution and the way the disease is managed is undergoing enormous change. For the very first time it is becoming increasingly possible to individualise a patient's treatment by predicting those that will and those that will not respond to a chosen therapy. This is being achieved through rapid developments in both advanced diagnostic imaging and translational medicine. Clinical trials incorporating expression array data are already underway in some of the more common tumour sites, such as breast cancer [1]. As a result the foundations have been laid for some of the less frequent, but by no means less serious, pathological types.
Oesophageal cancer is the eighth most common cancer worldwide and more than 80% of cancers occur in less developed countries [2]. The incidence in Europe is 5.4 per 100,000 per year with approximately 4.9 deaths per 100,000 per year. Survival correlates with stage of disease. Five-year survival rates range from 40 to 62 percent for patients treated for localized cancer (stage I and IIA), and from 18 to 25 percent for those with involvement of regional nodes (stage IIB and III) [3]. According to data from the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program, the five-year survival rate for all patients with oesophageal cancer has improved modestly over the last 30 years, from 5 percent in the years 1974 to 1976, to 13 percent during the period 1992 to 1998 [4]. These dismal figures are indicative of the advanced stage of disease (local-regional or metastatic, stages IIB, III and IV) at diagnosis in most individuals [4].
Although the incidence of squamous cell carcinoma has been declining over the past two decades, the incidence of adenocarcinoma has increased markedly. In the US adenocarcinoma now accounts for more than 50% of oesophageal cancer cases [5]. The high mortality rate reflects early lymphatic and haematogenous spread as well as the lack of effective treatments and early therapeutic options.
For many years the standard therapy for localised oesophageal carcinoma has been surgical resection [6]. However local control and overall survival remain poor, and even after radical resection and lymphadenectomy the 5-year survival is at best approximately 40 percent [7,8]. In an effort to improve these figures the management of local-regional oesophageal cancer has undergone a major evolution over the past 15 years. Numerous strategies employing various pre- and post-operative therapies have been studied as well as trials where surgery has been omitted altogether.
To date the optimal therapy for potentially resectable oesophageal cancer remains unclear.
Although somewhat controversial, the use of neo-adjuvant chemoradiation (CRT) has increased outside of clinical trials, and the Patterns of Care studies in the US showed that preoperative CRT therapy increased from 10.4% during 1992–1994 to 26.6% in 1996–1999 for patients with stage IIb and III oesophageal cancer [9]. The same is now true in many European Centres.
However, many patients do not benefit from such an approach and there are now evolving strategies to identify predictive response markers. Several analyses suggest that it is the response to preoperative therapy (particularly the absence of residual disease (pCR) in the surgical specimen) that best predicts disease-free and overall survival [10]. A pCR occurs in approximately 15–30% of cases, and three-year survival rates of approximately 60% irrespective of the applied protocol, type of histology and tumour stage are achieved [10]. A further subdivision of pathological response to neoadjuvant regimens, the tumour regression grade (TRG), may also identify patterns of incomplete response that may impact on treatment outcome [11]. Regression grading stratifies response based on the biological effect of radiation on tumours, dividing it into 5 different grades based on the ratio of fibrosis to tumour (Figure 1). Mandard described complete response as histologic fibrosis with or without inflammation extending through the different layers of the oesophageal wall, but with no viable residual tumour cells (tumour regression grade (TRG) 1). Subtotal response (TRG 2) was characterized by the presence of rare residual cancer cells scattered through the fibrosis. An increase in the number of residual cancer cells, but with fibrosis predominating was termed a partial response (TRG 3). Minimal remission (TRG 4) showed residual cancer outgrowing fibrosis. Absence of any regressive changes (TRG 5) defined no change.
Figure 1.
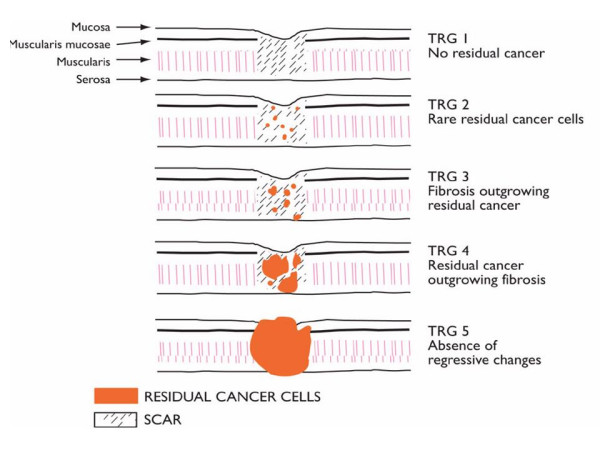
Pathological response grading following neoadjuvant chemoradiation in oesophageal cancer (Mandard [11]).
The addition of the pathological response to standard pTNM has been recently advocated [12]. Where a cohort of patients may benefit from neoadjuvant CRT, with pCR and TRG the surrogate markers, many patients will not be helped, and their prognosis may be worsened by delay in surgery and by the added risks of surgery in patients on multimodal protocols. A predictor of response or resistance based on pre-treatment demographics, imaging, histolopathologic, molecular, or genetic information would have potentially enormous application in optimising outcomes and in the design of clinical trials. However, despite numerous studies to date no clear candidate markers that predict pathological response have emerged.
Predicting response
Conventional patient and histological indices
Numerous clinical and pathological parameters have been analysed in a small number of oesophageal cancer studies with regard to their utility in the prediction of the response to pre-operative CRT. Mandard found that the larger the initial primary tumour the poorer the overall response to neo-adjuvant treatment [11]. Pre-treatment performance status, primary location and age are clearly important factors in terms of tolerating therapy, but they are not known to be associated with the pathological response [13].
Other pre-therapy parameters that some have identified as potentially useful include the patient's nutritional status [14], tumour cell aneuploidy [15] and tumour differentiation [16].
In general, however, many of these factors tend to be relatively crude determinants of the overall management approach, i.e. curative or palliative, rather than being predictors of the molecular response to treatment. It would seem that it is the post-therapy pathological stage that best predicts the survival of patients who receive neo-adjuvant CRT [13] and more precise markers are required in order to determine the most appropriate therapeutic strategy.
Tissue markers
Most studies have correlated the expression of molecular markers in the pre-treatment biopsy with either the biological response to treatment in the oesophagectomy specimen or to survival/recurrence data following treatment.
These markers have usually been identified by immuno-histochemical means.
Apoptosis
P53
The p53 gene is one of the most widely investigated in human cancer, including oesophageal. Several groups have found that the protein it encodes is one of the prognostic indicators in various cancers [17,18]. It is one of the genes responsible for repairing a damaged cells' DNA or triggering apoptosis when this cannot occur (Figure 2[19]) and it is generally accepted that it may be intrinsically involved in the response to CRT [20,21]. Several trials have studied p53 expression as a determinant of response to chemotherapy with or without radiotherapy in oesophageal cancer. In oesophageal adenocarcinoma Duhaylongsod immunostained 42 patients for p53 and c-erb B2 protein. All patients received neoadjuvant CRT followed by resection [22]. They found that 84% of the p53 positive tumours had residual disease as opposed to 44% of the p53 negative (p = 0.01). Similarly in patients with squamous cell carcinoma Seitz et al., identified immunohistochemically a significant association between p53 overexpression and a lower complete response [15]. To counter these results other groups have found no such an association [23]. It may be the small sample sizes or the differences in immunohistochemical staining methods that explain these discrepancies. Equally it has been postulated that p53 overexpression is not necessarily synonymous with p53 mutations [24]. Furthermore absence of p53 staining may occur with gene deletion, failure of transcription, or a non-stabilizing mutation, all of which may be associated with loss of p53 function [25].
Figure 2.
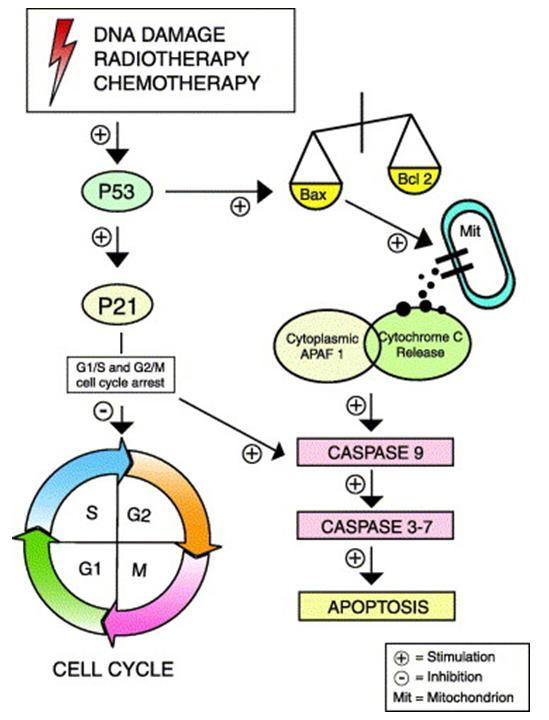
Flow diagram of the P53/apoptosis pathway. Constituents of this pathway are the most commonly assessed predictive markers in oesophageal cancer [19].
P21
The p21 protein is a key member of the p53 signalling pathway (figure 2). It is transcriptionally activated by p53 following DNA damage by ionising radiation, which in turn causes cell cycle arrest, and apoptosis [26,27]. It has been studied as a response predictor because it disrupts regulatory networks, in particular those involved in cell death signaling. It may therefore be a causative factor of radioresistance.
Nakamura et al., found that the survival of patients with p21 positive oesophageal tumours treated with definitive CRT was significantly better than those where no such expression existed (p = 0.0013) [28]. They also identified that the survival of those patients with p53 negative tumours was significantly higher if they were p21 positive than negative (P = 0.0452).
Conversely another Japanese group found that whilst p21 positive expression in the absence of p53 was associated with favourable effects from preoperative chemotherapy there was no such correlation between p21 expression and the clinical effects following CRT [29,30].
Survivin
Survivin is a member of the inhibitor of apoptosis family and is known to be involved in resistance to chemo- and radiation therapy. It is expressed in several cancers particularly rectal cancer where it's expression is associated with a poor survival following CRT [31]. In oesophageal cancer Kato et al., found that that high survivin expression predicted a significantly reduced median survival (9.0 vs 30.0 months, p = 0.0023) in patients receiving pre-operative chemotherapy [32]. Conversely other groups have found the reverse. In tissue samples taken from patients prior to CRT elevated tumour:normal levels of survivin mRNA were significantly associated with improved survival but not histological regression [33].
Cyclo-oxygenase-2 (COX-2)
COX-2 plays an important role in prostaglandin synthesis and mediates angiogenesis and tumour growth. It is overexpressed in various human malignancies and is an important mediator of tumour invasiveness and metastasis [34]. In addition, clinical studies in nasopharyngeal [35] and cervical.
Cancer [36] have demonstrated that endogenous COX 2 expression in pre-treatment biopsies are indicative of a poor response to and an unfavourable prognosis following chemotherapy and ionising radiation and chemotherapy. There is similar data in oesophageal cancer. In 29 biopsies taken from squamous cell carcinoma patients who went on to receive CRT high Cox-2 mRNA levels were significantly associated with a poor response to treatment (p < 0.05) [37]. Whilst Cox-2 levels have not been correlated with response to neoadjuvant CRT in oesophageal adenocarcinoma there is a wealth of literature showing that high levels of expression in this subtype are associated with aggressive disease and a poor survival [38].
Tumour hypoxia
Hypoxic regions within tumours may lead to chemo- and radioresistance by depriving cells of oxygen necessary for the cytotoxic activities of these agents [39]. Furthermore, tumour hypoxia promotes up-regulation of angiogenic and tumour cell survival factors resulting in increased proliferation, radioresistance and angiogenesis. Angiogenesis has an important role in solid tumour growth and metastasis [40]. Vascular endothelial cell growth factor (VEGF) is the main angiogenic factor known to be involved in pathological angiogenesis. Its induction in several solid tumours is thought to be important with respect to the chemotherapy and radiotherapy response [41]. In one study CRT was administered to 52 patients with oesophageal squamous cell carcinoma [42]. Expression of p53, thymidine phosphorylase and VEGF was analysed by immuno-histochemistry. Sixty percent then underwent radical surgery and from these multivariate analysis identified that only VEGF was a significant prognostic indicator (p = 0.0147). Its expression was associated with a high incidence of treatment failure and a significantly worse 5-year survival rate (p = 0.037). These results are further supported by Gorski et al., who found that the anti-tumour effects of ionizing radiation could be enhanced if VEGF activity was blocked [43]. It remains unclear, however, as to whether the expression of VEGF by itself directly or indirectly determines whether a tumour responds to CRT [44].
Growth regulation
Epidermal growth factor receptor (EGFR)
Aberrant activation of the epidermal growth factor receptor (EGFR) is frequently observed in neoplasia, notably in tumours of epithelial origin. In squamous cell oesophageal carcinoma Hickey et al., compared tumour response with expression of EGFR and proliferating cell nuclear antigen [45]. There was a significant survival advantage in those staining negative for one or both markers, while those which stained positive responded poorly. The same would appear to be true for oesophageal adenocarcinoma. Not only was EGFR expression associated with a higher TNM stage, but also with shorter disease-free and overall survival [46].
HER-2
HER-2 protein is a 185 kD transmembrane protein and a member of the EGFR family. It is a proto-oncogene that encodes a tyrosine kinase growth factor receptor and has been associated with the pathogenesis of several human cancers. Its over-expression in breast cancer is associated with a poorer prognosis [47]. In oesophageal cancer the data is conflicting. In adenocarcinoma Duhaylongsod et al., found that over-expression predicted a favourable response to CRT and a 5 year actuarial survival of 60% [22]. However, other groups have found that over-expression in this sub-type was associated with a poor prognosis [48]. In squamous cell carcinoma Akamatsu et al., however, found that immunostaining was useful for predicting chemoradioresistance but this did not correlate with survival [49].
Cyclins
The cyclins are important oncogenic proteins and regulators of the cell cycle.
Sarbia et al., assessed cyclin D1 expression by immuno-histochemistry in squamous cell oesophageal cancer [50]. They identified that in patients treated with multi-modal therapy cyclin D1 expression correlated with a poor response to treatment but not to overall survival. Cyclin E is reportedly overexpressed in adenocarcinoma of the distal oesophagus and in gastric cancer is associated with reduced survival [51]. In oesophageal cancer, however, its prognostic effect has not been elucidated.
Markers of resistance to commonly used chemotherapy agents
Platinum-based compounds, 5-fluorouracil and taxanes are the agents most commonly used in the treatment of oesophageal cancer and advances in molecular pharmacology have enhanced our understanding of their mechanisms of action and modes of resistance.
Joshi et al., measured gene expressions of thymidylate synthase 1 (TS1), glutathione S-transferase π (GSTP1), and excision cross-complementing gene 1 (ERCC1) by quantitative RT-PCR in the pre-treatment biopsies of tumour tissue specimens taken from patients scheduled to receive neoadjuvant 5-FU, cisplatin and radiotherapy. Elevated expression of these genes was significantly associated with a poor survival (p = 0.007) [52].
Metallothionein (MT)
MT is a small protein with a high affinity for divalent heavy metal ions. It is involved in many patho-physiological processes, like metal homeostasis and detoxification, cell proliferation, apoptosis, therapy resistance, and protection against oxidative damage. Alterations in the immuno-histochemical expression of MT have been reported for various human tumours, and a high expression has been found to be associated with a poor clinical outcome [53]. Much of the work in squamous cell oesophageal cancer comes from Japan. MT-positivity in patients treated with neoadjuvant chemotherapy with or without radiotherapy has usually been associated with a worse prognosis [54]. Some studies have, however, shown no such association [55]. There is no data on the effects of MT-positivity and the response to treatment in oesophageal adenocarcinoma, but it is implicated in the malignant transformation of Barrett's epithelium [56].
Nuclear factor-kappa B (NF-KB)
NF-KB regulates several genes involved in inflammatory, immune and apoptotic responses. In patients treated with neoadjuvant CRT for oesophageal adenocarcinoma Abdel-Latif et al., identified that its' expression was inversely related to a major or complete pathological response. 75% of those that did not respond were NFKB negative, whilst only 18% of the responders were positive (p < 0.00001) [57]. More recently Izzo et al., found that activated NF-KB expression was significantly associated with residual disease following neo-adjuvant CRT (p = 0.006), metastatic progression (p = 0.01) and reduced survival (p = 0.01) in 80 oesophageal cancer patients [58].
Serum markers
Serum markers have not proved particularly useful in predicting the response of oesophageal cancer to neo-adjuvant therapy.
Kim et al., evaluated serial CEA levels in 90 patients with potentially resectable oesophageal and gastric adenocarcinoma treated with preoperative chemotherapy [59]. Measurements were taken before treatment and serially thereafter. An increasing CEA level predicted relapse and correlated well with visceral involvement and clinical responses correlated with declining levels of CEA. However, the levels did not predict resectability or survival.
Another group analysed serum VEGF levels in patients with oesophageal cancer before, during and after CRT. Levels did not decline during therapy. They fell following resection but then rose to pre-operative values before falling to normal at three months. They postulated that the tumours were not generating VEGF and therefore levels could not be used as response markers [60].
Quillien et al., examined the serum markers CYFRA 21-1, TPA and SCC in 96 patients with squamous cell oesophageal carcinoma. CYFRA 21-1 was the only marker whose pre-treatment levels significantly correlated with pathological response, but on multivariate analysis treatment was the only independent factor [61].
Nakamura et al., assessed the clinical value of CYFRA 21-1 in comparison to SCC-Ag, CEA and CA19-9 in 112 patients with squamous cell carcinoma. Levels of CYFRA 21-1 correlated closely with stage and with clinical response to both chemotherapy and CRT [62].
These reports suggest that CYFRA 21-1 may be the most useful serum marker currently available, but this has not become widely adopted.
Gene expression arrays
Patients diagnosed with the same stage of cancer by conventional clinical and histopathological criteria may have a completely different course of disease. Since cancer is fundamentally a malfunction of gene expression giving rise to aberrant malignant growth, the most direct classification approach would be to analyse gene expression patterns. To find the relatively small number of genes that are characteristically de-regulated in a given cancer cell, among thousands of genes that are normally expressed, requires high-throughput technologies and sophisticated computational tools.
The first high density microarrays were developed to analyse gene expression by quantitating thousands of mRNAs present in a cell or tissue sample (DNA arrays). Other microarray approaches include the quantitation of proteins (protein arrays), or the analysis of a large number of tissue samples in parallel (tissue arrays). It was clear early on that arrays could be very useful tools in molecular profiling of cancer cells, thus revealing information that cannot be obtained by traditional histological assessment [63].
Over the last few years there have been numerous gene expression studies that have enhanced our understanding of the biology of oesophageal cancer [64-70]. It was hoped that these might identify potential biomarkers for therapeutic targeting, but none of them specifically addressed treatment and pathological outcome data and so their clinical value is so far limited.
There has, however, been only one clinically relevant study. The MD Anderson performed gene expression analyses on 19 patients prior to neo-adjuvant CRT and correlated their findings with the final histopathological response [71]. Unsupervised hierarchical cluster analysis of the cancer biopsies segregated them in to two molecular subtypes. Amongst the adenocarcinomas, most that achieved a complete response clustered in one group and all but one of the poorer responders in the other. They identified a number of genes that were differentially expressed between the two molecular sub-types, several of which have been reported to occur in oesophageal cancer. The authors stress that this was a preliminary study and clearly larger sample numbers and stringent validation is essential before the data generated from array experiments can be evaluated in clinical trials.
Imaging
Despite their widespread use in primary staging, conventional computed tomography (CT) and endoscopic ultrasound (EUS) have not proven beneficial in predicting the response of oesophageal tumours to CRT [72,73]. With the advent of molecular imaging, which has demonstrated superiority over conventional imaging techniques at diagnosis, FDG-PET (18fluorodeoxyglucose positron emission tomography) is frequently being used to predict response in several malignancies [74,75].
Alterations in tissue metabolism often precede anatomical changes and this forms the basis of FDG-PET scanning. In 22 patients with advanced breast cancer, changes in FDG uptake were able to predict the eventual histopathologic response with an accuracy of 88% after the first course of drugs and 91% after the second course [76]. Similar utility has been described in cancers of the lung [77] and colon [78], as well as Hodgkin's [79] and non-Hodgkin's lymphoma [80].
There has been considerable work in oesophageal cancer. Studies evaluating tumour response with PET during and at the completion of neo-adjuvant therapy have yielded encouraging results (Table 1). These studies suggest that changes in FDG uptake in response to therapy correlate with the pathological response as well as predict the risk of local recurrence and survival. Squamous cell oesophageal cancer is more frequently treated with neoadjuvant CRT. Studies have therefore been performed in either squamous cell carcinoma exclusively or in a combination of the two tumour types. There have been no studies investigating the predictive role of FDG-PET in oesophageal adenocarcinoma exclusively and consequently it is not possible to ascertain the usefulness of molecular imaging in predicting response to CRT in one subtype over another.
Table 1.
Studies that have assessed the role of FDG-PET in predicting the response of oesophageal cancer to neoadjuvant chemoradiation
| Study + Reference | Path | Chemo | Radiotherapy | Second PET | Main Results |
| Brucher et al, 2001 [82] | 24 SCC | 5 FU | 30 Gy/15# | 3 weeks after CRT | An SUV reduction of >52% led to sensitivity, specificity, positive and negative predictive values of 100%, 55%, 72% and 100% respectively |
| Kato et al, 2002 [83] | 10 SCC | cis/5 FU | 40 Gy/20# | 2 weeks after CRT | Pathological response did not correlate with rate of reduction of SUV |
| Arslan et al, 2002 [84] | 22 AD 2 SCC |
See notes‡ | 40–50.4 Gy/20–28# | 4 wks after CRT | Change in volume identified responders. Quantitative evaluation of primary tumour pre and post therapy could not separate post therapy inflammation from residual tumour |
| Flamen et al, 2002 [85] | 27 SCC 9 AD |
cis/5 FU | 40 Gy/20# | 4–6 weeks after CRT | When >80% reduction in FDG tumour:liver uptake ratios used to define response sensitivity 71% and specificity 82% |
| Downey et al, 2003 [86] | 26 AD 13 SCC |
cis/taxol | 50.4 Gy/28# (2 had no RT) | After CRT (not specified) | SUV reduction >60% associated with non-significant disease-free and survival advantage compared to when reduction <60% |
| Brink et al, 2004 [87] | 13 AD 7 SCC |
cis/5 FU | 36 Gy/20# | 2–3 weeks after CRT | No correlation |
| Swisher et al, 2004 [88] | 73 AD 10 SCC |
See notes* | 50.4 Gy/28# | After CRT (not specified) | Pathological response correlated with post therapy SUV. Post therapy SUV > 4 was only pre-operative factor to correlate with decreased survival |
| Wieder et al, 2004 [90] | 38 SCC | 5 FU | 40 Gy/20# | During CRT in 27 | Changes in SUV were significantly different between 2 groups |
| Song et al, 2005 [89] | 32 SCC | cis/cape | 45.6/38#(BID) + 46 Gy/23#/5 wks | 4 weeks after CRT | Pathological response could be predicted when analysis limited to initial highly metabolic tumours |
| Levine et al, 2006 [81] | 52 AD 9 SCC 3 UN |
cis/5 FU | 50.4 Gy/28# | After CRT (not specified) | Reduction in SUV ≥ 10 associated with significant response |
| Gillham et al, 2006 [91] | 29 AD 3 SCC |
cis/5 FU | 40.05 Gy/15# 44 Gy/22# | After 1 week of CRT | Changes in SUV during treatment did not predict pathological outcome |
Key: Path – histological sub-type, AD – adenocarcinoma, SCC – squamous cell carcinoma, UN – undifferentiated, CT – computed tomography, 5 FU – 5-fluorouracil, Gy – Gray, # – fractions of radiotherapy, cis – cisplatin, LV – leucovorin, carbo – carboplatin, cape – capecitabine, gem – gemcitabine, BID – twice daily fractionation
‡ Received cisplatin/5 fluorouracil or cisplatin/taxol or carboplatin/5 fluorouracil in combination with radiotherapy
*Patients received either irinotecan/5 FU/docetaxol (up to 2 cycles) prior to CRT with same drugs (reduced dose) or same RT with cisplatin/5 FU or taxol/carboplatin (no pre-CRT chemotherapy)
Levine et al., performed an FDG-PET at diagnosis and following CRT in 31 oesophageal cancer patients [81]. They found that the standardized uptake value (SUV) decreased significantly more in those patients who responded (pathological complete response or microscopic residual disease) than in those who did not (p = 0.05). The MD Anderson study performed CT, EUS and PET before and after CRT in 73 adenocarcinoma and 10 squamous cell carcinoma patients [73]. They found that PET most accurately predicted long-term survival and that by uni- and multivariate Cox regression analysis an SUV ≥4 had the greatest accuracy in predicting pathological response. Similarly there is some evidence to suggest that the initial SUV may be predictive of outcome. Levine's group found that an SUV at diagnosis ≥15 was associated with an observed 77.8% significant response (pathological complete response or microscopic residual disease) compared with 24.2% for patients with a pretreatment SUV < 15 (P = 0.005).
In the majority of studies to date the second PET has been performed after the neo-adjuvant phase of treatment [81-89]. Earlier response prediction, by repeating the PET during neoadjuvant therapy, could potentially differentiate responders from non-responders, minimise the inherent toxicity associated with current regimens and direct non-responders towards alternative therapies. Only two such studies have been performed [90,91]. Wieder et al., reported pre-therapy and early repeat FDG-PET scans at 2 weeks (of a four week neo-adjuvant CRT regime) in 27 patients with squamous cell carcinoma of the oesophagus, a treatment regimen similar to that which was used in the Gillham et al., study. In the former, the change in SUV following CRT reliably separated responders and non-responders; using a reduction in SUV of 30% as the optimal cut off point, they identified a sensitivity and specificity of 93% and 88% respectively with a satisfactory accuracy of 79%. In the latter study the second PET scan was performed after only one week of CRT and included both squamous cell and adenocarcinoma sub-types. Changes in FDG uptake failed to predict the pathological response. Both studies were small and are not directly comparable. However the inflammatory effect of ionising radiation on oesophageal tissue may interfere with the interpretation of the second PET scan.
Future
For many years formalin fixed paraffin embedded tissue samples have provided a wealth of information using immuno-histochemical and DNA based techniques. The process is relatively straightforward and inexpensive and the material can be stored for a number of years. A number of different markers have been identified that seem to be associated with a good or a poor response to treatment in oesophageal cancer. However, comparing the results of different studies is markedly hampered by the differing individual techniques and batch-to-batch variability [92]. As a result none of them have found their way into routine clinical use. Some of these issues may be overcome by the use of tissue microarrays, but such an approach remains very much in its infancy until the appropriate candidate markers are identified.
Advances in microarray technology may mean that, by assessing the transcriptional activity of a large number of genes, the complex gene expression profile may contain more information than any individual molecule that contributes to it. A number of studies have used such an approach to study the different profiles between oesophageal cancer and its pre-malignant components. Unlike in other tumour sites only one study has specifically addressed the issue of response prediction to CRT in oesophageal cancer [71].
The other area of translational medicine that holds promise is that of serum proteomics. It is based on the assumption that cancers shed protein debris into the bloodstream. The technique has been used to differentiate benign from malignant disease [93-95] but, to date, has not been applied in the area of response prediction.
Conclusion
The management of patients with localised oesophageal cancer would be greatly enhanced if predictors of response could be identified.
It would seem there is little to be gained by studying conventional patient and histological indices. At present none of the tissue or serum markers of response to neo-adjuvant treatment are sufficiently accurate on their own to be used to predict response in an individual patient. Genomics and proteomics are fast generating vast amounts of data. In time, it seems likely that these may lead to the detection of stringently validated markers, which become part of routine diagnostic work-up.
Molecular imaging is another evolving science. The cumulative data suggests that changes seen on serial PET scans after neo-adjuvant therapy correlate with the final pathological response and survival. However larger numbers are required and it would be more clinically beneficial if such imaging proved predictive earlier in the course of treatment.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
CG conceived the idea and wrote the manuscript.
JR and DH were involved in the drafting and final approval of the manuscript
All authors read and approved the final manuscript.
Contributor Information
Charles M Gillham, Email: charles.gillham@slh.ie.
John Reynolds, Email: reynoljv@tcd.ie.
Donal Hollywood, Email: dhlywood@tcd.ie.
References
- MINDACT, (Microarray In Node negative Disease may Avoid ChemoTherapy) (EORTC Protocol 10041 – BIG 3-04) http://www.eortc.be/services/unit/mindact/MINDACT_websiteii.asp (Last accessed August 20, 2007)
- Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- Iizuka T, Isono K, Kakegawa T, Watanabe H. Parameters linked to ten-year survival in Japan of resected esophageal carcinoma. Japanese Committee for Registration of Esophageal Carcinoma Cases. Chest. 1989;96:1005–1011. doi: 10.1378/chest.96.5.1005. [DOI] [PubMed] [Google Scholar]
- Daly JM, Karnell LH, Menck HR. National cancer database report on esophageal carcinoma. Cancer. 1996;78:1820–1828. doi: 10.1002/(SICI)1097-0142(19961015)78:8<1820::AID-CNCR25>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–464. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Enzinger PC, Mayer RJ. Esophageal Cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- Altorki N, Kent M, Ferrara RN, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177–183. doi: 10.1097/00000658-200208000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerut T, Coosemans W, De Leyn P, Deneffe G, Topal B, Van de Ven C, Van Raemdonck D. Reflections on 3-field lymphadenectomy in carcinoma of the esophagus and gastroesophageal junction. Hepato-Gastroenterology. 1999;46:717–725. [PubMed] [Google Scholar]
- Suntharalingam M, Moughan J, Coia LR, Krasna MJ, Kachnic L, Haller DG, Willett CG, John MJ, Minsky BD, Owen JB. The national practice for patients receiving radiation therapy for carcinoma of the esophagus: Results of the 1996–1999 Patterns of Care Study. Cancer. 1999;85:2499–2505. doi: 10.1002/(SICI)1097-0142(19990615)85:12<2499::AID-CNCR2>3.0.CO;2-T. [DOI] [Google Scholar]
- Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg. 2001;88:338–356. doi: 10.1046/j.1365-2168.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G, Ollivier J-M, Bonvalot SB, Gignoux M. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::AID-CNCR2820731105>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Swisher SG, Hofstetter W, Wu TT, Correa AM, Ajani JA, Komaki RR, Chirieac L, Hunt KK, Liao Z, Phan A, Rice DC, Vaporciyan AA, Walsh GL, Roth JA. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pPCR) following preoperative CRT (CRT) Ann Surg. 2005;241:810–820. doi: 10.1097/01.sla.0000161983.82345.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, Roth JA, Rashid A, Hamilton SR, Wu TT. Post therapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative CRT. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- Roth JA, Pass HI, Flanagan MM, Graeber GM, Rosenberg JC, Steinberg S. Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. J Cardiovasc Surg. 1988;96:242–248. [PubMed] [Google Scholar]
- Seitz JF, Perrier H, Monges G, Giovannini M, Gouvernet J. Multivariate analysis of the prognostic factors of survival and the response to treatment of squamous cell oesophageal cancer by concomitant radiochemotherapy: Value of p53 immunodetection. Gastroenterol Clin Biol. 1995;19:465–474. [PubMed] [Google Scholar]
- Kitamura K, Kuwano H, Araki K, Egashira A, Kawaguchi H, Saeki H, Morita M, Ohno S, Sugimachi K. Clinicopathologic features of patients with oesophageal cancer obtaining histological complete response for preoperative hyperthermo-chemo-radiotherapy. Int J Hyperthermia. 1998;14:233–243. doi: 10.3109/02656739809018228. [DOI] [PubMed] [Google Scholar]
- Quinlan DC, Davidson AG, Summers CL, Warden HE, Doshi HM. Accumulation of p53 protein correlates with a poor prognosis in human lung cancer. Cancer Res. 1992;52:4828–4831. [PubMed] [Google Scholar]
- Thomas DJ, Robinson M, King P, Hasan T, Charlton R, Martin J, Carr TW, Neal DE. p53 expression and clinical outcome in prostate cancer. Br J Urol. 1993;72:778–781. doi: 10.1111/j.1464-410x.1993.tb16267.x. [DOI] [PubMed] [Google Scholar]
- Smith F, Reynolds J, Miller N, Stephens R, Kennedy M. Pathological and molecular predictors of the response of rectal cancer to neoadjuvant radiochemotherapy. EJSO. 2006;32:55–64. doi: 10.1016/j.ejso.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Ribeiro U, Jr, Finkelstein SD, Safatle-Ribeiro AV, Landreneau RJ, Clarke MR, Bakker A, Swalsky PA, Gooding WE, Posner MC. P53 sequence analysis predicts treatment response and outcome of patients with esophageal carcinoma. Cancer. 1998;83:7–18. doi: 10.1002/(SICI)1097-0142(19980701)83:1<7::AID-CNCR2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Ruley HE, Jacks T, Houseman DE. P53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–67. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Duhaylongsod FG, Gottfried MR, Iglehart JD, Vaughn AL, Wolfe WG. The significance of c-erb B-2 and p53 immunoreactivity in patients with adenocarcinoma of the esophagus. Ann Surg. 1995;221:677–683. doi: 10.1097/00000658-199506000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KY, Law S, Ma LT, Ong SK, Wong J. Pre-operative chemotherapy for squamous cell carcinoma of the oesophagus: Do histological assessment and p53 over-expression predict chemo-responsiveness? Eur J Cancer. 1997;33:1221–1225. doi: 10.1016/S0959-8049(97)00094-4. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D. p53 in tumour pathology: can we trust immunocytometry? J Pathol. 1992;166:329–330. doi: 10.1002/path.1711660402. [DOI] [PubMed] [Google Scholar]
- Catalano V, Baldelli AM, Giordani P, Cascinu S. Molecular markers predictive of response to chemotherapy in gastrointestinal tumors. Crit Rev Oncol Haematol. 2001;38:93–104. doi: 10.1016/S1040-8428(00)00114-1. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Waldman T, Oliner JD, Velculescu VE, Burrell M, Hill DE, Healy E, Rees JL, Hamilton SR. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995;55:2910–2919. [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromized by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hayashi K, Ota M, Ide H, Takasaki K, Mitsuhashi M. Expression of p21(Waf1/Cip1) predicts response and survival of esophageal cancer patients treated by chemoradiotherapy. Dis Oesophagus. 2004;17:315–321. doi: 10.1111/j.1442-2050.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- Nakashima S, Natsugoe S, Matsumoto M, Kijima F, Takebayashi Y, Okumura H, Shimada M, Nakano S, Kusano C, Baba M, Takao S, Aikou T. Expression of p53 and p21 is useful for the prediction of preoperative chemotherapeutic effects in esophageal carcinoma. Anticancer Res. 2000;20:1933–1937. [PubMed] [Google Scholar]
- Okumura H, Natsugoe S, Matsumoto M, Mataki Y, Takatori H, Ishigami S, Takao S, Aikou T. The predictive value of p53, p53R2, and p21 for the effect of CRT therapy on oesophageal squamous cell carcinoma. Br J Cancer. 2005;92:284–289. doi: 10.1038/sj.bjc.6602322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel F, Hoffmann J, Grabenbauer GG, Papadopoulos T, Weiss C, Günther K, Schick C, Sauer R, Rödel C. High survivin expression is associated with reduced apoptosis in rectal cancer and may predict disease-free survival after preoperative radiochemotherapy and surgical resection. Strahlenther Onkol. 2002;178:426–435. doi: 10.1007/s00066-002-1003-y. [DOI] [PubMed] [Google Scholar]
- Kato J, Kuwabara Y, Mitani M, Shinoda N, Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J, Fujii Y. Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer. 2001;95:92–95. doi: 10.1002/1097-0215(20010320)95:2<92::AID-IJC1016>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Warnecke-Eberz U, Hokita S, Xi H, Higashi H, Baldus SE, Metzger R, Brabender J, Bollschweiler E, Mueller RP, Dienes HP, Hoelscher AH, Schneider PM. Overexpression of survivin mRNA is associated with a favourable prognosis following neoadjuvant radiochemotherapy in esophageal cancer. Oncol Rep. 2005;13:1241–1246. [PubMed] [Google Scholar]
- Lagarde SM, ten Kate FJ, Richel DJ, Offerhaus GJ, van Lanschot JJ. Molecular prognostic factors in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 2007;14:977–991. doi: 10.1245/s10434-006-9262-y. [DOI] [PubMed] [Google Scholar]
- Chen WC, McBride WH, Chen SM, Lee KF, Hwang TZ, Jung SM, Shau H, Liao SK, Hong JH, Chen MF. Prediction of poor survival by cyclooxygenase-2 in patients with T4 nasopharyngeal cancer treated by radiation therapy: clinical and in vitro studies. Head Neck. 2005;27:503–512. doi: 10.1002/hed.20178. [DOI] [PubMed] [Google Scholar]
- Kim YB, Kim GE, Pyo HR, Cho NH, Keum KC, Lee CG, Seong J, Suh CO, Park TK. Differential cyclooxygenase-2 expression in squamous cell carcinoma and adenocarcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2004;60:822–829. doi: 10.1016/j.ijrobp.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Takatori H, Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Sasaki K, Tamotsu K, Owaki T, Ishigami S, Aikou T. Cyclooxygenase-2 expression is related to prognosis in patients with esophageal squamous cell carcinoma. Eur J Surg Oncol. 2007. [DOI] [PubMed]
- Bhandari P, Bateman AC, Mehta RL, Stacey BS, Johnson P, Cree IA, Di Nicolantonio F, Patel P. Prognostic significance of cyclooxygenase-2 (COX-2) expression in patients with surgically resectable adenocarcinoma of the oesophagus. BMC Cancer. 2006;6:134. doi: 10.1186/1471-2407-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms and cellular response. Oncologist. 2004;9:4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- Weidner N. Intratumour micro vessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- Volm M, Rittgen W. Cellular predictive factors for the drug response of lung cancer. Anticancer Res. 2000;20:3449–3458. [PubMed] [Google Scholar]
- Shimada H, Hoshino T, Okazumi S, Matsubara H, Funami Y, Nabeya Y, Hayashi H, Takeda A, Shiratori T, Uno T, Ito H, Ochiai T. Expression of angiogenic factors predicts response to chemoradiotherapy and prognosis of oesophageal squamous cell carcinoma. Br J Cancer. 2002;86:552–557. doi: 10.1038/sj.bjc.6600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockade of the vascular endothelial growth factor stress response increases the anti-tumor effects of ionising radiation. Cancer Res. 1999;76:3374–3378. [PubMed] [Google Scholar]
- Imdahl A, Bognar G, Schulte-Monting , Schoffel U, Farthmann EH, Ihling C. Predictive factors for response to neoadjuvant therapy in patients with oesophageal cancer. Eur J Cardio-Thor Surg. 2002;21:657–663. doi: 10.1016/S1010-7940(02)00044-1. [DOI] [PubMed] [Google Scholar]
- Hickey K, Grehan D, Reid IM, O'Briain S, Walsh TN, Hennessy TP. Expression of epidermal growth factor receptor and proliferating cell nuclear antigen predicts response of esophageal squamous cell carcinoma to chemoradiotherapy. Cancer. 1994;74:1693–1698. doi: 10.1002/1097-0142(19940915)74:6<1693::AID-CNCR2820740609>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Wang KL, Wu TT, Choi IS, Wang H, Reseetkova E, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Albarracin CT. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer. 2007;109:658–667. doi: 10.1002/cncr.22445. [DOI] [PubMed] [Google Scholar]
- Wright C, Angus B, Nicholson S, Sainsbury JR, Cairns J, Gullick WJ, Kelly P, Harris AL, Horne CH. Expression of c-erbB-2 oncoprotein: a prognostic indicator in human breast cancer. Cancer Res. 1989;49:2087–2090. [PubMed] [Google Scholar]
- Brien TP, Odze RD, Sheehan CE, McKenna BJ, Ross JS. HER-2/neu gene amplification by FISH predicts poor survival in Barrett's esophagus-associated adenocarcinoma. Hum Pathol. 2000;31:35–39. doi: 10.1016/S0046-8177(00)80195-1. [DOI] [PubMed] [Google Scholar]
- Akamatsu M, Matsumoto T, Oka K, Yamasaki S, Sonoue H, Kajiyama Y, Tsurumaru M, Sasai K. c-erb B-2 oncoprotein expression related to chemoradioresistance in esophageal squamous cell carcinoma. Int J Radiation Oncol Biol Phys. 2003;57:1323–1327. doi: 10.1016/S0360-3016(03)00782-X. [DOI] [PubMed] [Google Scholar]
- Sarbia M, Stahl M, Fink U, Heep H, Dutkowski P, Willers R, Seeber S, Gabbert HE. Prognostic significance of cyclin D1 in esophageal squamous cell carcinoma patients treated with surgery alone or combined therapy modalities. Int J Cancer. 1999;84:86–91. doi: 10.1002/(SICI)1097-0215(19990219)84:1<86::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tenderenda M. A study on the prognostic value of cyclins D1 and E expression levels in respectable gastric cancer and on some correlations between cyclins expression, histoclinical parameters and selected protein products of cell-cycle regulatory genes. J Exp Clin Cancer Res. 2005;24:405–414. [PubMed] [Google Scholar]
- Joshi MB, Shirota Y, Danenberg KD, Conlon DH, Salonga DS, Herndon JE, Danenberg PV, Harpole DH. High gene expression of TS1, GSTP1, and ERCC1 are risk factors for survival in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res. 2005;11:2215–2221. doi: 10.1158/1078-0432.CCR-04-1387. [DOI] [PubMed] [Google Scholar]
- Janssen AM, van Duijn W, Kubben FJ, Griffioen G, Lamers CB, van Krieken JH, van de Velde CJ, Verspaget HW. Prognostic significance of metallothionein in human gastrointestinal cancer. Clin Cancer Res. 2002;8:1889–1896. [PubMed] [Google Scholar]
- Kishi K, Doki Y, Miyata H, Yano M, Yasuda T, Monden M. Prediction of the response to CRT and prognosis in oesophageal squamous cancer. Br J Surg. 2002;89:597–603. doi: 10.1046/j.1365-2168.2002.02057.x. [DOI] [PubMed] [Google Scholar]
- Harpole DH, Jr, Moore MB, Herndon JE, 2nd, Aloia T, D'Amico TA, Sporn T, Parr A, Linoila I, Allegra C. The prognostic value of molecular marker analysis in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res. 2001;7:562–569. [PubMed] [Google Scholar]
- Li Y, Wo JM, Cai L, Zhou Z, Rosenbaum D, Mendez C, Ray MB, Jones WF, Kang YJ. Association of metallothionein expression and lack of apoptosis with progression of carcinogenesis in Barrett's esophagus. Exp Biol Med. 2003;228:286–292. doi: 10.1177/153537020322800307. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif MM, O'Riordan J, Windle HJ, Carton E, Ravi N, Kelleher D, Reynolds JV. NF-kappa B activation in esophageal adenocarcinoma: relationship to Barrett's metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann Surg. 2004;239:491–500. doi: 10.1097/01.sla.0000118751.95179.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo JG, Correa AM, Wu TT, Malhotra U, Chao CK, Luthra R, Ensor J, Dekovich A, Liao Z, Hittelman WN, Aggarwal BB, Ajani JA. Pretherapy nuclear factor-kappa B status, chemoradiation resistance, and metastatic progression in esophageal carcinoma. Mol Cancer Ther. 2006;5:2844–2850. doi: 10.1158/1535-7163.MCT-06-0351. [DOI] [PubMed] [Google Scholar]
- Kim YH, Ajani JA, Ota DM, Lynch P, Roth JA. Value of serial carcinoembryonic antigen levels in patients with resectable adenocarcinoma of the esophagus and stomach. Cancer. 1995;75:451–456. doi: 10.1002/1097-0142(19950115)75:2<451::AID-CNCR2820750207>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- McDonnell CO, Harmey JH, Bouchier-Hayes DJ, Walsh TN. Effect of multimodality therapy on circulating vascular endothelial growth factor levels in patients with esophageal cancer. Br J Surg. 2001;88:1105–1109. doi: 10.1046/j.0007-1323.2001.01838.x. [DOI] [PubMed] [Google Scholar]
- Quillien V, Raoul JL, Laurent JF, Meunier B, Le Prise E. Comparison of cyfra 21-1, TPA and SCC tumor markers in esophageal squamous cell carcinoma. Oncol Rep. 1998;5:1561–1565. doi: 10.3892/or.5.6.1561. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ide H, Eguchi R, Hayashi K, Takasaki K, Watanabe S. CYFRA 21-1 as a tumour marker for squamous cell carcinoma of the esophagus. Dis Esophagus. 1998;11:35–39. [PubMed] [Google Scholar]
- Schmidt U, Begley CG. Cancer diagnosis and microarrays. Int J Biochem Cell Biol. 2003;35:119–124. doi: 10.1016/S1357-2725(02)00124-3. [DOI] [PubMed] [Google Scholar]
- Xu Y, Selaru FM, Yin J, Zou TT, Shustova V, Mori Y, Sato F, Liu TC, Olaru A, Wang S, Kimos MC, Perry K, Desai K, Greenwald BD, Krasna MJ, Shibata D, Abraham JM, Meltzer SJ. Artificial neural networks and gene filtering distinguish between global gene expression profiles of Barrett's esophagus and esophageal cancer. Cancer Res. 2002;62:3493–3497. [PubMed] [Google Scholar]
- Dahlberg PS, Ferrin LF, Grindle SM, Nelson CM, Hoang CD, Jacobson B. Gene expression profiles in esophageal adenocarcinoma. Ann Thorac Surg. 2004;77:1008–1015. doi: 10.1016/j.athoracsur.2003.09.051. [DOI] [PubMed] [Google Scholar]
- McManus DT, Olaru A, Meltzer SJ. Biomarkers of esophageal adenocarcinoma and Barrett's esophagus. Cancer Res. 2004;64:1561–1569. doi: 10.1158/0008-5472.CAN-03-2438. [DOI] [PubMed] [Google Scholar]
- Lu J, Liu Z, Xiong M, Wang Q, Wang X, Yang G, Zhao L, Qiu Z, Zhou C, Wu M. Gene expression profile changes in initiation and progression of squamous cell carcinoma of esophagus. Int J Cancer. 2001;91:288–294. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1063>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Xu SH, Qian LJ, Mou HZ, Zhu CH, Zhou XM, Liu XL, Chen Y, Bao WY. Difference of gene expression profiles between esophageal carcinoma and its precancerous epithelium by gene chip. World J Gastroenterol. 2003;9:417–422. doi: 10.3748/wjg.v9.i3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Hu N, Shih J, Hu Y, Wang QH, Chuang EY, Roth MJ, Wang C, Goldstein AM, Ding T, Dawsey SM, Giffen C, Emmert-Buck MR, Taylor PR. Gene expression analysis of esophageal squamous cell carcinoma reveals consistent molecular profiles related to a family history of upper gastrointestinal cancer. Cancer Res. 2003;63:3872–3876. [PubMed] [Google Scholar]
- Kawamata H, Furihata T, Omotehara F, Sakai T, Horiuchi H, Shinagawa Y, Imura J, Ohkura Y, Tachibana M, Kubota K, Terano A, Fujimori T. Identification of genes differentially expressed in a newly isolated human metastasizing esophageal cancer cell line, T.Tn-AT1, by cDNA microarray. Cancer Sci. 2003;94:699–706. doi: 10.1111/j.1349-7006.2003.tb01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra R, Wu TT, Luthra MG, Izzo J, Lopez-Alvarez E, Zhang L, Bailey J, Lee JH, Bresalier R, Rashid A, Swisher SG, Ajani JA. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative CRT. J Clin Oncol. 2006;24:259–267. doi: 10.1200/JCO.2005.03.3688. [DOI] [PubMed] [Google Scholar]
- Agarwal B, Swisher S, Ajani J, Kelly K, Fanning C, Komaki RR, Putnam JB, Jr, Abu-Hamda E, Molke KL, Walsh GL, Correa AM, Ho L, Liao Z, Lynch PM, Rice DC, Smythe WR, Stevens CW, Vaporciyan AA, Yao J, Roth JA. Endoscopic ultrasound after preoperative chemoradiation can help identify patients who benefit maximally after surgical esophageal resection. Am J Gastroenterol. 2004;99:1258–1266. doi: 10.1111/j.1572-0241.2004.30692.x. [DOI] [PubMed] [Google Scholar]
- Swisher SG, Maish M, Erasmus JJ, Correa AM, Ajani JA, Bresalier R, Komaki R, Macapinlac H, Munden RF, Putnam JB, Rice D, Smythe WR, Vaporciyan AA, Walsh GL, Wu TT, Roth JA. Utility of PET, CT and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–1160. doi: 10.1016/j.athoracsur.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Block MI, Patterson GA, Sundaresan RS, Bailey MS, Flanagan FL, Dehdashti F, Siegel BA, Cooper JD. Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg. 1997;64:770–776. doi: 10.1016/S0003-4975(97)00619-X. [DOI] [PubMed] [Google Scholar]
- Flamen P, Lerut A, Van Cutsem E, De Wever W, Peeters M, Stroobants S, Dupont P, Bormans G, Hiele M, De Leyn P, Van Raemdonck D, Coosemans W, Ectors N, Haustermans K, Mortelmans L. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–3210. doi: 10.1200/JCO.2000.18.18.3202. [DOI] [PubMed] [Google Scholar]
- Schelling M, Avril N, Nährig J, Kuhn W, Römer W, Sattler D, Werner M, Dose J, Jänicke F, Graeff H, Schwaiger M. Positron emission tomography using [18(F)] fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–1695. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Mitchell PL, Berlangieri SU, Tochon-Danguy H, Knight S, Clarke CP, Scott AM. The role of positron emission tomography in assessing response to neoadjuvant chemotherapy for non-small cell lung cancer. Med J Aust. 1998;169:227. doi: 10.5694/j.1326-5377.1998.tb140229.x. Erratum in Med J Aust 1998;169:344. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Fukuda H, Fujiwara T, Iwata R, Ido T, Murakawa Y, Gamo M, Ishioka C, Kanamaru R. FDG PET evaluation of residual masses and regrowth of abdominal lymph node metastases from colon cancer compared with CT during chemotherapy. Clin Nucl Med. 1999;24:261–263. doi: 10.1097/00003072-199904000-00009. [DOI] [PubMed] [Google Scholar]
- Hueltenschmidt B, Sautter-Bihl ML, Lang O, Maul FD, Fischer J, Mergenthaler HG, Bihl H. Whole body positron emission tomography in the treatment of Hodgkin disease. Cancer. 2001;91:302–310. doi: 10.1002/1097-0142(20010115)91:2<302::AID-CNCR1002>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Okada J, Yoshikawa K, Imazeki K, Minoshima S, Uno K, Itami J, Kuyama J, Maruno H, Arimizu N. The use of FDG-PET in the detection and management of malignant lymphoma: correlation of uptake with prognosis. J Nucl Med. 1991;32:686–691. [PubMed] [Google Scholar]
- Levine EA, Farmer MR, Clark P, Mishra G, Ho C, Geisinger KR, Melin SA, Lovato J, Oaks T, Blackstock AW. Predictive value of 18 FDG-PET in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg. 2006;243:472–478. doi: 10.1097/01.sla.0000208430.07050.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brücher BL, Weber W, Bauer M, Fink U, Avril N, Stein HJ, Werner M, Zimmerman F, Siewert JR, Schwaiger M. Neoadjuvant therapy of esophageal squamous cell carcinoma: Response evaluation by positron emission tomography. Ann Surg. 2001;233:300–309. doi: 10.1097/00000658-200103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Masuda N, Fukuchi M, Manda R, Tsukada K, Oriuchi N, Endo K. Usefulness of positron emission tomography for assessing the response of neoadjuvant chemoradiotherapy in patients with oesophageal cancer. Am Surg. 2002;184:279–283. doi: 10.1016/S0002-9610(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Arslan N, Miller TR, Dehdashti F, Battafarano RJ, Siegel BA. Evaluation of response to neoadjuvant therapy by quantitative 2-deoxy-2-[18F]Fluoro-D-Glucose with positron emission tomography in patients with esophageal cancer. Mol Imag Biol. 2002;4:301–310. doi: 10.1016/S1536-1632(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Flamen P, Van Cutsem E, Lerut A, Cambier JP, Haustermans K, Bormans G, De Leyn P, Van Raemdonck D, De Wever W, Ectors N, Maes A, Mortelmans L. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced esophageal cancer. Ann Oncol. 2002;13:361–368. doi: 10.1093/annonc/mdf081. [DOI] [PubMed] [Google Scholar]
- Downey RJ, Akhurst T, Ilson D, Ginsberg R, Bains MS, Gonen M, Koong H, Gollub M, Minsky BD, Zakowski M, Turnbull A, Larson SM, Rusch V. Whole body FDG-PET and the response of esophageal cancer to induction therapy: Results of a prospective trial. J Clin Oncol. 2003;21:428–432. doi: 10.1200/JCO.2003.04.013. [DOI] [PubMed] [Google Scholar]
- Brink I, Hentschel M, Bley TA, Walch A, Mix M, Kleimaier M, Moser E, Imdahl A. Effects of neoadjuvant radio-chemotherapy on 18F-FDG-PET in esophageal carcinoma. Eur J Surg Oncol. 2004;30:544–550. doi: 10.1016/j.ejso.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Swisher SG, Erasmus J, Maish M, Correa AM, Macapinlac H, Ajani JA, Cox JD, Komaki RR, Hong D, Lee HK, Putnam JB, Jr, Rice DC, Smythe WR, Thai L, Vaporciyan AA, Walsh GL, Wu TT, Roth JA. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative CRT in patients with esophageal carcinoma. Cancer. 2004;101:1776–1785. doi: 10.1002/cncr.20585. [DOI] [PubMed] [Google Scholar]
- Song SY, Kim JH, Ryu JS, Lee GH, Kim SB, Park SI, Song HY, Cho KJ, Ahn SD, Lee SW, Shin SS, Choi EK. FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. IJROBP. 2005;63:1053–1059. doi: 10.1016/j.ijrobp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Wieder HA, Brücher BL, Zimmermann F, Becker K, Lordick F, Beer A, Schwaiger M, Fink U, Siewert JR, Stein HJ, Weber WA. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–908. doi: 10.1200/JCO.2004.07.122. [DOI] [PubMed] [Google Scholar]
- Gillham CharlesM, Lucey JulieA, Keogan Mary, Duffy GeorgeJ, Malik Vinod, Raouf AliA, O'Byrne Ken, Hollywood Donal, Muldoon Cian, Reynolds JohnV. Correlation of Early Quantitative changes in FDG-PET Uptake with Tumour Regression grade in patients with Localized Oesophageal cancer undergoing Neo-adjuvant Chemoradiation. Br J Cancer. 2006;95:1174–1179. doi: 10.1038/sj.bjc.6603412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube D. Constants and variables in immunohistochemistry. Arch Histol Cytol. 2004;67:115–134. doi: 10.1679/aohc.67.115. [DOI] [PubMed] [Google Scholar]
- Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, Baggerly KA, Atkinson EN, Skates S, Zhang Z, Lokshin A, Menon U, Jacobs I, Lu K. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15:274–281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- Brown LM, Helmke SM, Hunsucker SW, Netea-Maier RT, Chiang SA, Heinz DE, Shroyer KR, Duncan MW, Haugen BR. Quantitative and qualitative differences in protein expression between papillary thyroid carcinoma and normal thyroid tissue. Mol Carcinog. 2006;45:613–626. doi: 10.1002/mc.20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AL, Espina V, Petricoin EF, Liotta LA, Rosenblatt KP. Use of proteomic patterns to screen for gastrointestinal malignancies. Surgery. 2004;135:243–248. doi: 10.1016/j.surg.2003.08.019. [DOI] [PubMed] [Google Scholar]


