Abstract
The epithelia from the crypts of the intestine are exquisitely sensitive to metabolic perturbation and undergo cell death with the classical morphology of apoptosis. Administration of 40 mg/kg 5-fluorouracil (5-FU) to BDF-1 p53+/+ mice resulted in an increase in p53 protein at cell positions in the crypts that were also those subjected to an apoptotic cell death. In p53−/− mice apoptosis was almost completely absent, even after 24 hr. 5-FU is a pyrimidine antimetabolite cytotoxin with multiple mechanisms of action, including inhibition of thymidylate synthase (TS), which gives rise to DNA damage, and incorporation into RNA. The inhibition of TS can be increased by coadministration of folinic acid and can be abrogated by administration of thymidine. The incorporation of 5-FU into RNA is inhibited by administration of uridine. p53-Dependent cell death induced by 5-FU was only inhibited by administration of uridine. Uridine had no effect on the apoptosis initiated by 1 Gy of γ-radiation. Although thymidine abrogated apoptosis induced by the pure TS inhibitor Tomudex, it had no effect on 5-FU-induced apoptosis, and coadministration of folinic acid did not increase apoptosis. The data show that 5-FU-induced cell death of intestinal epithelial cells is p53-dependent and suggests that changes in RNA metabolism initiate events culminating in the expression of p53.
Keywords: epithelia
Among a repetoire of cellular responses to stress, the tumor suppressor protein p53 has been shown to play a critical role in determining the fate of cells with genomic damage (1). One of these fates is cell death by apoptosis (2). How p53 “senses” DNA damage in intact cells is not clear. Studies in vitro have suggested that it may physically bind to broken strands of DNA (3), but what topological damage it is recognizing and how it initiates the engagement of apoptosis is uncertain. γ-Irradiation of mice results in cell position-specific deletion, by apoptosis, of crypt epithelial cells (4). In the small intestine, apoptosis occurs rapidly (4 hr) and at the highest frequency in what are considered to be the stem cells (5). In the colon, radiation also induces rapid apoptosis but in a less positionally dependent manner, with apoptotic cells being observed along the length of the crypt. Interestingly, the colonic stem cells, located at the base of the crypt, are not a particular focus of cell deletion by apoptosis after irradiation. Immunohistochemical analysis of p53 protein in intestinal crypts after irradiation showed that it was positionally coincident with morphologically apoptotic cells (6). Indeed, irradiation of p53 null (−/−) mice showed that the wave of irradiation-induced apoptosis observed at 4.5 hr was entirely p53-dependent (6). Cytotoxic drugs and chemicals also initiate apoptosis in intestinal epithelia after administration in vivo (7, 8). The positional pattern of cell death induced after a single bolus injection of the pyrimidine antimetabolite 5-fluorouracil (5-FU) differed from that observed after radiation. Stem cells in the small intestine were largely spared, and rapidly dividing early transit cells underwent the most frequent apoptosis (7, 8).
5-FU cytotoxicity depends on conversion to the nucleotides 5-fluoroUTP, 5-fluoro-dUMP, and, possibly, 5-fluoro-dUTP. These metabolites can act in a number of ways to induce cytotoxicity. Binding of 5-fluoro-dUMP to the enzyme thymidylate synthase (TS) in the presence of reduced folate cofactor, 5,10-methylenetetrahydrofolic acid, inhibits the synthesis of thymidine nucleotides, while concomitantly increasing dUMP and dUTP. Inhibition of TS gives rise to DNA strand breaks (9), believed to be the result of ribosylthymine 5′-triphosphate depletion and probably the misincorporation of dUTP into DNA (reviewed in ref. 10). Incorporation into RNA has been shown to be responsible for the gastrointestinal toxicity of 5-FU in mice (11) and in vitro mechanistic studies have shown that 5-FU incorporation into RNA but not DNA was associated with cell death (12). Because the topological pattern of apoptosis induced by 5-FU in the crypt was different from that induced by γ-radiation, we have investigated the role that p53 plays in the 5-FU-induced apoptosis of intestinal epithelial cells in vivo.
MATERIALS AND METHODS
Materials.
5-FU was purchased from Roche (Welwyn Garden City, U.K.), and Tomudex (ZD1694) was a gift from Zeneca Pharmaceuticals (Macclesfield, U.K.) and was dissolved in 0.05 M sodium bicarbonate; the pH was altered to 8.5–9.0. Folinic acid (calcium salt) was from Lederle Laboratories (Gosport, U.K.); thymidine and uridine were from Sigma.
Animals.
The following mouse strains were used: BDF1 (C57BL × DBA/2), p53 wild-type (+/+), and p53 homozygously null (−/−) (13). Male mice ages 10–12 weeks were used in all cases, and at least four mice were used in each experimental group. The mice were fed RM1 expanded diet (Special Diet Services, Waltham, Essex, U.K.) and water ad libitum. They were housed in a 12-hr dark/12-hr light cycle with lights on at 0600.
Measurements of Apoptosis.
5-FU was administered to groups of 4–6 mice by bolus i.p. injection at 0900 to 1000. Groups of four mice were γ-irradiated with 1 Gy using a Caesium137 source. After specified time intervals, mice were killed by cervical dislocation and intestines dissected and fixed in Carnoy’s fixative. Transverse sections of small intestine and the middle third of the colon (midcolon) were prepared and stained by hematoxylin and eosin (5, 14). Fifty half-crypts were scored for apoptosis on a cell-positional basis per mouse as described in detail (7, 8, 14–16). Briefly, cells were numbered from the base of the crypt, designated cell position 1, and scored as normal, apoptotic (containing one or more apoptotic fragments) or mitotic, up to the crypt-villus junction. Most of the scoring was performed by one observer (D.M.P.), but blinded checking of scoring by others was performed. Data are presented either as the total number of apoptotic events per mouse (50 half-crypts) or as an apoptotic index percentage (percentage of counted cells that were apoptotic). Statistical analysis was by a two-tailed Student’s t test, assuming unequal variance of the groups being compared.
Immunohistochemistry.
The intestines of BDF1 mice were fixed in 4% formal saline, and routine 3-μm paraffin sections were prepared. Staining for p53 protein was performed as described (6). The primary antibody used was CM5 raised in a rabbit to recombinant mouse p53 (a gift from David Lane, University of Dundee, Dundee, U.K.).
RESULTS
Induction of Apoptosis in Small Intestine and Colon by 5-FU.
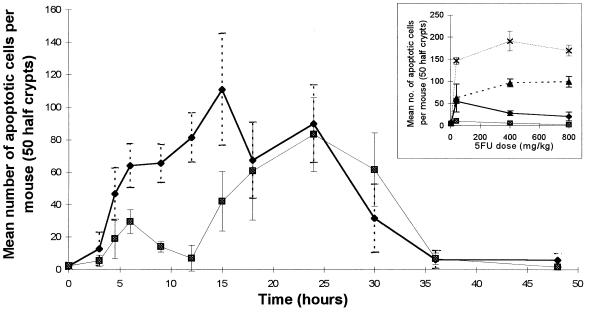
Fig. 1 shows a time course of the induction of apoptosis in small intestine and midcolon after administration of 40 mg/kg 5-FU to BDF-1 mice. There was a significant induction of apoptosis 4.5 hr after 5-FU administration, and this time point was used thereafter to estimate what we consider to be the acute induction of drug-induced cell death. Apoptosis in both small intestinal and colonic crypts reached a peak by ≈24 hr (Fig. 1) and began to decline thereafter; 24 hr therefore was chosen as the second fixed time point at which to assess apoptosis under various conditions. Dose increases of 40–800 mg/kg 5-FU showed only modest increases in apoptotic yield (Fig. 1 Inset) and subsequent experiments all utilized 40 mg/kg 5-FU.
Figure 1.
Time course showing total number of apoptotic events counted in 50 half-crypts (4 experimental animals at each time point) of small intestine (⧫) and midcolon (▪) of BDF1 mice, following 40 mg/kg i.p. injection of 5-FU. (Inset) Levels of apoptosis induced in BDF1 intestine with 5-FU doses ranging from 4 to 800 mg/kg. The total number of apoptotic events occurring in 50 half-crypts (4 experimental animals at each point) are shown. ⧫ and ▴, Small intestine at 4.5 and 24 hr, respectively; ▪ and ×, midcolon at 4.5 and 24 hr, respectively, after drug administration. (Error bars = SD.)
Induction of p53 in Small Intestinal and Colonic Crypts by 5-FU.
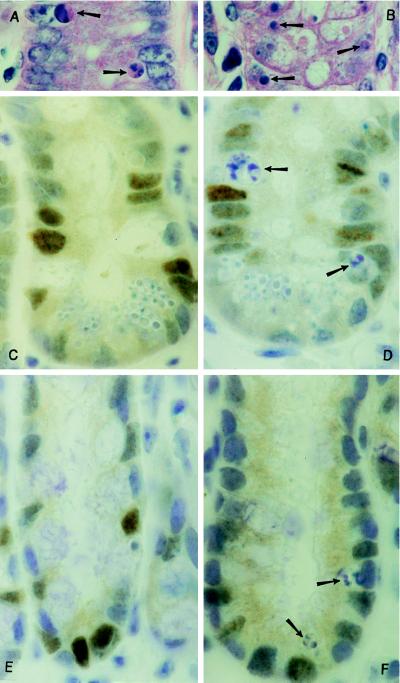
Fig. 2 shows immunohistochemical staining of p53 in the epithelial cells from both small intestine and colon of BDF-1 (p53+/+) mice treated with 40 mg/kg 5-FU. As has been noted by us previously (6), apoptotic cells did not stain positively for p53 protein, although analysis of the cell positional distribution suggested that there was a coincidence between p53 expression and apoptosis.
Figure 2.
Examples of apoptotic figures (arrows) in (A) small intestine and (B) midcolon of BDF1 mice following 40 mg/kg 5-FU (staining of 3-μm paraffin sections; hematoxylin/eosin, ×1000). p53 protein expression identified by the affinity-purified CM5 polyclonal antibody in BDF1 mouse small intestine (C and D) and midcolon (E and F), 24 hr following the i.p. injection of 40 mg/kg 5-FU. p53-Positive cells are darkly stained; arrows, apoptotic cells. (×1000).
5-FU-Induced Apoptosis in Crypt Epithelia Is p53-Dependent but Positionally Different from Radiation-Induced Apoptosis.
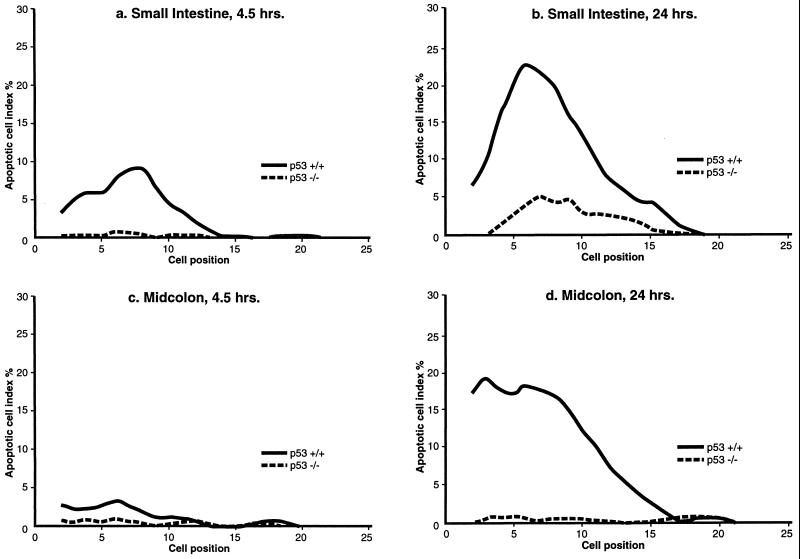
Analysis of the positional dependency of 5-FU-induced apoptosis in vivo is shown in Fig. 3. The data have been smoothed over three cell positions (14). At both 4.5 hr and 24 hr, apoptosis in the small intestinal crypts was most frequent at cell positions 6 to 8, where the early transit cells are located. This is in agreement with previously published work (7, 8). In the p53 homozygously null (−/−) animals, no apoptotic cells above background levels were observed 4.5 hr after 40 mg/kg 5-FU in comparison to the +/+ animals (Fig. 3a). At 24 hr there was a small increase in apoptosis in the small intestine of p53−/− animals, but this was significantly less than in the +/+ animals (Fig. 3b).
Figure 3.
Cell positional distribution of apoptosis in the intestinal crypts of p53 wild-type (+/+) and homozygously null (−/−) mice (four animals in each experimental group, with data smoothed over three cell positions; ref. 13), following the i.p. administration of 40 mg/kg 5-FU. (a) Small intestine, after 4.5 hr. (b) Small intestine, after 24 hr. (c) Midcolon, after 4.5 hr. (d) Midcolon, after 24 hr.
Fig. 3 c and d show that 5-FU treatment of p53+/+ animals caused very little early cell death in midcolonic crypts, at 4.5 hr (see also Fig. 1), but that apoptosis at 24 hr was considerable. The highest frequency of apoptosis was in the cell positions at the base of the colon. This position of maximum cell death is coincident with the positions considered to harbor the stem cells (positions 1 and 2). In the p53−/− animals there was a complete abrogation of apoptosis in the midcolon both at 4.5 and 24 hr after treatment with 40 mg/kg 5-FU (Fig. 3 c and d).
Effects of Thymidine and Uridine on 5-FU-Induced Apoptosis in Vivo.
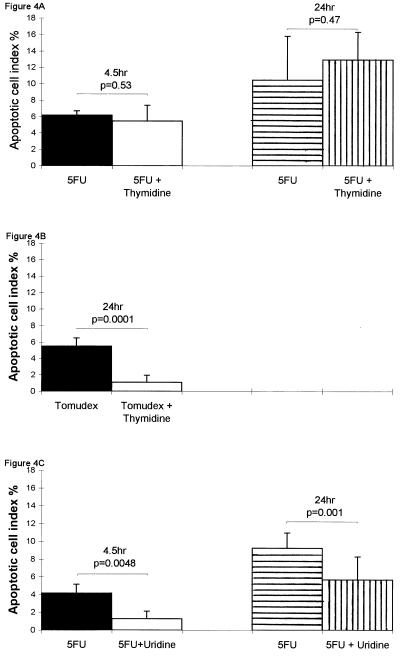
5-FU metabolites may induce cytotoxicity by either inhibition of TS, which is prevented by administration of excess thymidine, or by incorporation into RNA, which can be prevented by administration of uridine (see Introduction). Fig. 4A shows that administration of thymidine (500 mg/kg) with, and hourly after (at 8 and 16 hr) 40 mg/kg 5-FU did not significantly change the apoptosis observed at either 4.5 or 24 hr. To confirm that thymidine was able to relieve inhibition of TS in vivo, the highly specific quinazoline TS inhibitor Tomudex (10 mg/kg; reviewed in ref. 17) was administered alone or with thymidine. Fig. 4B shows that thymidine efficiently relieved the apoptosis induced by Tomudex. In other experiments 30 mg/kg of folinic acid was administered 1 hr prior to the 40 mg/kg 5-FU. This procedure has been shown to increase the inhibition of TS by 5-FU (18), but here it produced no significant change in the patterns of apoptosis induced by 5-FU (data not shown). 5-FU was administered with 3500 mg/kg of uridine to relieve the incorporation of 5-FU into RNA. By giving the uridine 2 hr after 5-FU, there was a highly significant reduction of 5-FU-induced apoptosis in crypt epithelia both at 4.5 and 24 hr (Fig. 4C). Administration of uridine did not change any feature of the cell death pattern induced by the specific TS inhibitor Tomudex (data not shown). Importantly, administration of uridine did not change the position or incidence of apoptosis induced by 1 Gy of γ-radiation, a process that we have previously shown to be entirely p53-dependent (6). Thus, the apoptotic cell index percentage 4.5 hr following 1 Gy of γ-radiation was 10.2 (n = 4), and for those given 3500 mg/kg uridine 2 hr after radiation it was 10.0 (n = 4).
Figure 4.
Effects on apoptotic cell index percentage in the small intestinal crypts of BDF1 mice. (Error bars represent SD; statistical analysis was by a two-tailed Student’s t test.) (A) ▪, 4.5 hr after 40 mg/kg 5-FU (n = 3); □, 4.5 hr after 40 mg/kg 5-FU and contemporaneous 500 mg/kg thymidine (n = 4); ▤, 24 hr after 40 mg/kg 5-FU (n = 4); ▥, 24 hr after 40 mg/kg 5-FU and contemporaneous 500 mg/kg thymidine with further 500 mg/kg thymidine at 8 and 16 hr (n = 4). (B) ▪, 24 hr after 10 mg/kg Tomudex (n = 4); □, 24 hr after 10 mg/kg Tomudex and contemporaneous 500 mg/kg thymidine with further 500 mg/kg thymidine at 8 and 16 hr (n = 4). (C) ▪, 4.5 hr after 40 mg/kg 5-FU (n = 4); □, 4.5 hr after 40 mg/kg 5-FU with 3500 mg/kg uridine given 2 hr after 5-FU (n = 4); ▤, 24 hr after 40 mg/kg 5-FU (n = 11); ▥, 24 hr after 40 mg/kg 5-FU with 3500 mg/kg uridine given 2 hr after 5-FU (n = 12).
DISCUSSION
The induction of apoptosis in intestinal epithelia in vivo by 5-FU reported here was characterized by a pattern of cell death that was different from that produced by γ-radiation, previously described by us (6, 14). This raised questions about the mechanism of its induction, because we had expected the inhibition of TS by 5-FU to lead to DNA strand breaks, which would be “sensed” in a p53-dependent manner, as those generated by γ-radiation. In the small intestine the incidence of 5-FU-induced apoptosis was, unlike radiation, not maximal at the position of the stem cell population (cells 4 to 6 from the base of the crypt) but rather had a peak of incidence in the rapidly proliferating transit cell population, from cell positions 6–8 (Fig. 3 a and b). In the colon the greatest incidence of apoptosis after 5-FU was observed at the base of the crypt (Fig. 3 c and d), whereas γ-radiation spared the stem cells at the base of the crypt (6, 14). The sensitivity of colonic stem cells to 5-FU is congruent with the effects of the drug causing colitis (19). Whether the acute apoptosis observed here in both colon and small intestine translates directly into the toxicological profile of the drug must be defined in more detail.
5-FU-induced damage in vivo led to a significant increase in p53 protein (Fig. 2), and apoptosis was dependent on p53 (Fig. 3). Although in the small intestine there appeared to be a small but significant amount of p53-independent apoptosis there was a very considerable reduction in apoptosis observed in the absence of p53. Surprisingly, the failure to relieve cytotoxicity with thymidine or to increase it with folinic acid suggested that TS was not the major locus of action of 5-FU (Fig. 4A). This was supported by the observation that we were able to relieve the apoptosis induced by the quinazoline-based antifolate Tomudex, a pure TS inhibitor (17) with thymidine, but not the apoptosis induced by 5-FU (Fig. 4B).
It has been suggested, from the experiments of many laboratories, that the toxicity of 5-FU to some cell types is not due to inhibition of TS but to its incorporation into RNA (reviewed in refs. 10, 20, and 21). For example, in a comprehensive study by Geoffroy et al. (12) it was shown that [3H]5-FU was incorporated in vitro into RNA but not DNA from HCT116 colon cancer cells. Incubation of HCT116 cells with 1 mM uridine inhibited the incorporation of [3H]5-FU into RNA and relieved toxicity, as measured by a clonogenic assay, whereas thymidine had no effect on 5-FU toxicity (12). Similarly, we have found that the p53-dependent apoptosis induced in vivo was very significantly reduced by administration of uridine (Fig. 4C), a classical method of relieving toxicity (21–23), but not by thymidine. Taken together, our data and that published by others strongly support the idea that cell death in intestinal epithelia requires that 5-FU metabolites be incorporated into RNA. This death is by apoptosis and is p53-dependent.
Recent in vitro studies of a human embryonic fibroblast cell line have suggested that a p53-dependent G1 cell cycle checkpoint could be induced by N-phosphonacetyl-l-aspartate (PALA), an inhibitor of UMP synthesis, when <3% of the cells were synthesizing DNA and in which it was claimed that no DNA damage was detected (24). It was proposed that p53 was able to sense the termination of synthesis of specific RNA molecules after rNTP depletion, perhaps affecting ribosome/poysome integrity (24). rRNA (5.8S) has been shown to be associated with the CKII region of both human and murine p53 (25), and 5S rRNA with p53 in a complex with the negative regulator of p53, MDM-2, together with L5 ribonucleoproteins (26). In addition, the inhibition of transcription by α-amanitin increased nuclear accumulation of p53 in normal human fibroblasts treated in vitro, suggesting the possibility that aberrant mRNAs might activate p53 (27). These possible effects on mRNA, and their sensing by p53, do not readily explain the topological restriction of 5-FU-induced apoptosis to rapidly dividing transit cells and the sparing of what are presumed to be transcriptionally active differentiated cells further up the crypts. It is possible that DNA replication requires an intact RNA template of some fidelity, but this is speculative at present.
As in the study of Linke et al. (24), in which it was claimed that no DNA damage had occurred after PALA, we cannot entirely discount the possibility that direct DNA damage has occurred after 5-FU treatment, although its selective relief by uridine and not by thymidine would be most puzzling, particularly when we found that uridine did not relieve the toxicity of the specific TS inhibitor Tomudex nor of 1 Gy of γ-radiation (see Results). It currently is not possible to measure DNA damage in single, topologically restricted cells from epithelial crypts, and further studies are underway using cell lines. In HCT116 colon cancer cells, 5-FU treatment, although clearly primarily perturbing RNA metabolism, later induced alkali-sensitive DNA strand breaks (12). Although these DNA strand breaks may be secondary to perturbations of RNA, they could be the ultimate signal for the induction of a p53-induced cell death. Whether perturbations in RNA structure and/or metabolism per se or DNA damage, which is in some way secondary to the effects on RNA, is sensed by p53 is under active investigation.
Acknowledgments
We thank Dr. Tom Boyle at Zeneca Pharmaceuticals for the gift of Tomudex, and for his interest in this work, and Dr. Keith Caldecott and Prof. David Thompson for valuable discussion. We thank members of the Department of Epithelial Biology at the Paterson Institute for technical assistance. We are grateful for funding from the British Digestive Foundation and the Cancer Research Campaign.
ABBREVIATIONS
- 5-FU
5-fluorouracil
- TS
thymidylate synthase
References
- 1.Lane D P. Nature (London) 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 2.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Nature (London) 1991;353:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Bayle J H, Elenbaas B, Pavletich N P, Levine A J. Mol Cell Biol. 1995;15:497–504. doi: 10.1128/mcb.15.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potten C S. Cancer Metastasis Rev. 1992;11:179–195. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- 5.Potten C S. In: Radiation and Gut. Potten C S, Hendry J H, editors. Amsterdam: Elsevier Science; 1995. pp. 1–31. [Google Scholar]
- 6.Merritt A J, Potten C S, Kemp C J, Hickman J A, Ballmain A, Lane D P, Hall P A. Cancer Res. 1994;54:614–617. [PubMed] [Google Scholar]
- 7.Ijiri K, Potten C S. Br J Cancer. 1983;47:175–185. doi: 10.1038/bjc.1983.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ijiri K, Potten C S. Br J Cancer. 1987;55:113–123. doi: 10.1038/bjc.1987.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtin N J, Harris A L, Aherne G W. Cancer Res. 1991;51:2346–2352. [PubMed] [Google Scholar]
- 10.Parker W B, Cheng Y C. Pharmacol Ther. 1990;48:381–395. doi: 10.1016/0163-7258(90)90056-8. [DOI] [PubMed] [Google Scholar]
- 11.Houghton J A, Houghton P J, Wooten R S. Cancer Res. 1979;39:2406–2413. [PubMed] [Google Scholar]
- 12.Geoffroy F J, Allegra C J, Sinha B, Grem J L. Oncol Res. 1994;6:581–591. [PubMed] [Google Scholar]
- 13.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Nature (London) 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 14.Merritt A J, Potten C S, Watson A J M, Loh D Y, Nakayama K, Nakayama K, Hickman J A. J Cell Sci. 1995;108:2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- 15.Li Y Q, Fan C Y, O’Connor P J, Winton D J, Potten C S. Carcinogenesis. 1992;13:361–368. doi: 10.1093/carcin/13.3.361. [DOI] [PubMed] [Google Scholar]
- 16.Merritt A J, Jones L S, Potten C S. In: Techniques in Apoptosis. Cotter T G, Martin S J, editors. London: Portland Press; 1996. pp. 269–300. [Google Scholar]
- 17.Jackman A L, Farrugia D C, Gibson W, Kimbell R, Harrap K R, Stephens T C, Azab M, Boyle F T. Eur J Cancer. 1995;31:1277–1282. doi: 10.1016/0959-8049(95)00166-g. [DOI] [PubMed] [Google Scholar]
- 18.Peters G J. In: The Scientific Basis for Cancer Treatment. Peckham M, Pinedo H, Veronesi U, editors. Oxford: Oxford Univ. Press; 1995. pp. 524–552. [Google Scholar]
- 19.Floch M H, Hellman L. Gastroenterology. 1965;48:430–437. [PubMed] [Google Scholar]
- 20.Klubes P, Leyland-Jones B. Pharmacol Ther. 1989;41:289–302. doi: 10.1016/0163-7258(89)90111-3. [DOI] [PubMed] [Google Scholar]
- 21.Bagrij T, Kralovansky J, Gyergyay F, Kiss E, Peters G J. Anticancer Res. 1993;13:789–794. [PubMed] [Google Scholar]
- 22.Martin D S, Stoffi R L, Sawyer R C, Spiegelman S, Young C W. Cancer Res. 1982;42:3964–3970. [PubMed] [Google Scholar]
- 23.Kralovansky J, Prajda N, Kerpel-Fronius S, Bagrij T, Kiss E, Peters G J. Cancer Chemother Pharmacol. 1993;32:243–248. doi: 10.1007/BF00685843. [DOI] [PubMed] [Google Scholar]
- 24.Linke S P, Clarkin K C, Di Leonardo A, Tsou A, Wahl G M. Genes Dev. 1996;10:934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- 25.Fontoura B M A, Sorokina E A, David E, Carroll R B. Mol Cell Biol. 1992;12:5145–5151. doi: 10.1128/mcb.12.11.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marechal V B, Elenbas J, Piette J C, Nicolas J C, Levine A J. Mol Cell Biol. 1994;14:7414–7420. doi: 10.1128/mcb.14.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaizumi M, Sugano T. Oncogene. 1994;9:2775–2784. [PubMed] [Google Scholar]