Abstract
Rodent cells resistant to N-phosphonacetyl-l-aspartate (PALA) invariably contain amplified carbamyl-P synthetase/aspartate transcarbamylase/dihydro-orotase (CAD) genes, usually in widely spaced tandem arrays present as extensions of the same chromosome arm that carries a single copy of CAD in normal cells. In contrast, amplification of CAD is very infrequent in several human tumor cell lines. Cell lines with minimal chromosomal rearrangement and with unrearranged copies of chromosome 2 rarely develop intrachromosomal amplifications of CAD. These cells frequently become resistant to PALA through a mechanism that increases the aspartate transcarbamylase activity with no increase in CAD copy number, or they obtain one extra copy of CAD by forming an isochromosome 2p or by retaining an extra copy of chromosome 2. In cells with multiple chromosomal aberrations and rearranged copies of chromosome 2, amplification of CAD as tandem arrays from rearranged chromosomes is the most frequent mechanism of PALA resistance. All of these different mechanisms of PALA resistance are blocked in normal human fibroblasts.
Keywords: chromosome rearrangements, genomic stability, fluorescence in situ hybridization, induced amplification, isochromosome 2
In rodent cells, amplified genes are often seen as ladder-like repeats, usually on the same chromosome arm that carries a single copy of the gene in wild-type cells (1–4). The presence of dicentric chromosomes in newly selected cells (3, 5) has led to the conclusion that chromosome breakage or telomere fusion initiates bridge–breakage–fusion cycles, leading to formation of dicentric sister chromatids, dicentric chromosomes, and eventually the amplified structures observed (reviewed in ref. 6). Selection with N-phosphonacetyl-l-aspartate (PALA) has been especially useful in analyzing amplification in rodent cells because the only mechanism of PALA resistance observed is amplification of the target gene carbamyl-P synthetase/aspartate transcarbamylase/dihydro-orotase (CAD) (6). Studies of gene amplification in human cells have been reported less frequently. Yin et al. (7) and Livingstone et al. (8) showed the importance of p53 in regulating CAD gene amplification in normal human fibroblasts, which are not permissive for amplification (9, 10) but become so when p53 is lost, as in immortal derivatives of fibroblast cell strains from Li–Fraumeni patients. Both groups selected these p53-null cell lines with PALA and found a high frequency of resistant clones, all of which were reported to contain CAD gene amplifications, consistent with the results that had been obtained with rodent cells previously. Schaefer et al. (11) reported that many PALA-resistant clones of simian virus 40-infected human fibroblasts contained no amplified CAD genes and achieved resistance by increasing the CAD gene copy number through accumulation of more than two copies of chromosome 2 and through formation of an isochromosome 2p. White et al. (12) selected PALA-resistant cells from human fibroblasts expressing the E6 protein of human papillomavirus type 16 (13). In these cells, CAD gene amplification was the only mechanism of resistance found. We now have studied human cell lines that do not harbor known viral oncogenes and are not virus-infected. Chromosomal CAD gene amplification, formation of isochromosome 2p, accumulation of extra copies of chromosome 2, and increased CAD gene expression without amplification all contribute to PALA resistance. The preponderant mechanism depends on the cell line, and chromosomal amplification is rare unless chromosome 2 is rearranged.
MATERIALS AND METHODS
Cells and Tissue Culture.
HT-1080 cells (14) were from Peter Goodfellow (Imperial Cancer Research Fund, London), U-2 OS, Saos-2, and WI-38 cells (passage 13) were from the American Type Culture Collection. MDAH041 cells (15) were from Michael Tainsky (M.D. Anderson Cancer Center, Houston), and LIM-1215 cells (16) were from Boris Kopnin (Russian Academy of Medical Sciences, Moscow). All cells were grown in DMEM (GIBCO) with 10% (vol/vol) fetal calf serum (GIBCO), 100 units/ml penicillin, and 100 μg/ml streptomycin.
Selection with PALA.
PALA (NSC 224131-F) was obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute (Bethesda). Selections were done with 10% (vol/vol) dialyzed fetal calf serum (17). For each cell line, the ID50 was determined as described by Perry et al. (17). Rates were determined with independent populations of cells: ≈103 cells were grown to ≈105 cells in each well of a 24-well plate, dispersed as single cells, and selected on a 10-cm plate. The medium was changed every 4–5 days until colonies of 100–200 cells were observed in ≈3 weeks. Preexisting mutant cells present in the 103-cell aliquots were excluded by choosing clones only from plates with fewer than 10 colonies of resistant cells. Rates were calculated as described by Perry et al. (17) and Luria and Delbruck (18).
Nomenclature of clones.
The first number is the concentration of PALA used for selection (μM), and the second identifies each independent plate of cells. A and B represent independent colonies from the same plate. Twenty plates of normal human WI-38 cells were selected with 60 μM of PALA (3 × ID50) for 10 days at 2 × 105 cells/10-cm dish. The arrested cells were placed into PALA-free medium containing 1 mM uridine for 2 days, trypsinized, pooled, and replated at the same density. The entire procedure was repeated four times. After each cycle, the cells were tested for incorporation of 10 μM of BrdUrd in a 2-h pulse (19).
Aspartate Transcarbamylase Assays.
Cells frozen in 40 mM Hepes buffer (pH 8.5) and 10% glycerol were thawed and disrupted by sonication (20). Aspartate transcarbamylase activity was measured using 14C-labeled aspartate (21, 22). Activities were standardized against total protein (23).
Probes.
Duplicate filters of human cosmid library H2 (derived from adult male leukocytes and kindly supplied by Anna–Maria Frischauf, Imperial Cancer Research Fund, London) were used. The library, made in pCos2 EMBL (24) using Sau3a partial digests inserted into the BamH1 site, was screened using a 32P-labeled, 6.5-kb cDNA fragment of pCAD142, encoding most of Syrian hamster CAD (25) labeled as described by Sambrook et al. (26). Cosmid HU-CAD-69, 31 kb long, was used as a fluorescence in situ hybridization (FISH) probe. To prepare the telomere-proximal probe 2p25, the end of the p arm of chromosome 2 (2p24–25) was microdissected and amplified in a PCR using a universal primer (27). COS-106, a human 2p centromere-proximal (2p11–12) probe (28), was kindly provided by Hans Zachau (Munich University). The chromosome 2 painting probe from Oncor was used according to the manufacturer’s protocol.
FISH.
Slides of metaphase cells were prepared and analyzed, essentially as described by Pinkel et al. (29) and Smith et al. (2). HU-CAD-69 was labeled with digoxigenin-11-dUTP (Boehringer Mannheim) or biotin-11-dUTP (BioNick Labeling System, GIBCO). The COS-106 and 2p25 probes were labeled with biotin-11-dUTP. Colcemid (40 ng/ml; GIBCO) was added for 40 min before collection of mitotic cells. Biotin-labeled probes were detected by incubating slides containing mitotic chromosome spreads with fluorescein isothiocyanate-labeled avidin (5 μg/ml; Vector Laboratories), anti-avidin (5 μg/ml; Vector Laboratories), and fluorescein isothiocyanante–avidin for 20 min each. Digoxigenin-labeled probes were detected with fluorescein isothiocyanate-labeled sheep anti-digoxigenin (10 μg/ml; Boehringer Mannheim). The DNA was counterstained with 0.2 μg/ml propidium iodide. The slides were mounted in fluorescence antifade solution (Vector Laboratories). Images were obtained with a Nikon Optiphot epifluorescence microscope coupled to a cooled charge-coupled device camera controlled by a computer (Oncor).
Quantitative Southern Blot Analysis.
Genomic DNA (10 μg) was cut with BamH1, and the fragments were separated by electrophoresis in 0.8% agarose gels, depurinated, and transferred to a Hybond N+ membrane (Millipore) in alkali (26). The membrane was probed with a mixture of the 32P-labeled, 6.5-kb HindIII fragment of pCAD142 (25) and the 1.5-kb XhoI fragment of p53 cDNA (internal control). Hybridization was at 60°C overnight in 6× SSC, and washes were at 60°C for 30 min each in 2× SSC (twice) followed by 60°C for 30 min each in 0.2× SSC (twice). The CAD and p53 DNA bands were quantitated using a PhosphorImager (Molecular Dynamics).
RESULTS
Selection of Human Cell Lines and FISH Analysis of PALA-Resistant Clones.
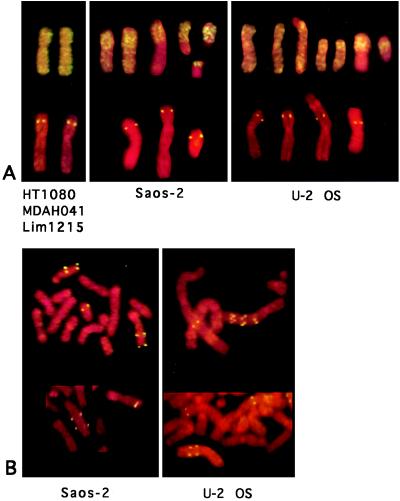
Five different cell lines were analyzed (Table 1). In each case, colonies representing new events responsible for PALA resistance were isolated with a protocol that excludes preexisting mutant cells (see Materials and Methods). In Saos-2 and U-2 OS cells, the predominant mechanism of PALA resistance was amplification of the CAD gene as chromosomal arrays, superficially similar to those obtained in PALA-resistant rodent cells whereas PALA-resistant HT-1080, LIM-1215, and MDAH041 cells rarely contained such structures (Table 1). CAD is on human chromosome 2p 21–22 (30), so a chromosome “painting” probe was used to analyze the various cell lines. Chromosome painting is a low resolution technique that reveals only gross cytogenetic abnormalities. HT-1080 and LIM-1215 cells carry two unrearranged copies of chromosome 2 in a nearly diploid background whereas MDAH041 cells are polyploid, with five or six unrearranged copies of chromosome 2 (Fig. 1A). Nearly triploid Saos-2 and U-2 OS cells have 60–70% of all chromosomes rearranged (as described in the American Type Culture Collection catalog), and our chromosome painting experiment shows that some of the copies of chromosome 2 are rearranged (Fig. 1A). Both Saos-2 and U-2 OS have two or three “normal” copies of chromosome 2, plus fragments of chromosome 2, separate or translocated to other chromosomes (about four per cell). In Saos-2 and U-2 OS cells, CAD is amplified as ladder-like structures on the rearranged and not on the normal chromosomes (Fig. 1B). The unselected Saos-2 populations we used contained ≈1% of cells with a preexisting CAD gene amplification (Table 1).
Table 1.
Analysis of the basis of PALA resistance in human cell lines
| Cell line | Karyotype | Rearranged chromosome 2 | Selective concentration of PALA, μM* | Rate of PALA resistance, ×10−5 | Colonies examined by FISH, n | Colonies with the specified mechanism of PALA resistance, %†
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Chromosomal amplification | Isochromosomes 2p or 2p arms | More copies of chromosome 2 | No increase in CAD copies | ||||||
| HT-1080 | pseudodiploid; | no | 20–60 (rate) | 5 | 23 | 4 | 39 | 0 | 57 |
| few rearrangements | 40 (frequency) | 15 | 7 | 27 | 0 | 67 | |||
| MDAHO41 | hypertetraploid | no | 170–190 (rate) | 16 | 13 | 0 | 15 | 0 | 85 |
| 170 (frequency) | 14 | 7 | 14 | 0 | 79 | ||||
| LIM-1215 | pseudodiploid; few rearrangements | no | 50–60 (rate) | 7 | 14 | 0 | 7‡ | 100‡ | 0 |
| Saos-2 | hypotriploid; >60% of chromosomes rearranged | yes | 130–170 (rate) | 140§ | 11 | 100 | 0 | 0 | 0 |
| U-2 OS | hypertriploid; >70% of chromosomes rearranged | yes | 400 (rate) | 1.4¶ | 15 | 33 | 0 | ND | ND |
Before selection with PALA, 500-cell aliquots were grown to ≈105 cells each and dispersed into 10-cm dishes. Selections were performed using PALA concentrations near 3 × ID50 determined separately for each cell line. Independent PALA-resistant colonies, isolated from separate plates, were grown to ≈5 × 105 cells in PALA before analysis. ND, not determined.
The colonies examined were obtained in either rate or frequency selections, as noted.
The predominant mechanism is shown. Clones often contained a small number of cells that displayed a different mechanism.
One clone displayed both an isochromosome and an additional copy of chromosome 2.
We had to select Saos-2 cells with a high concentration of PALA (8–10 × ID50). There were too many colonies at 3 × ID50 because the parental cell line included many cells that have already amplified the CAD gene. FISH analysis revealed unselected cells with one or two extra copies of CAD on a rearranged marker chromosome, similar to the pattern in PALA-resistant clones.
The selective concentration of PALA was ≈6 × ID50 in this case.
Figure 1.
FISH analysis of cells with a painting probe for chromosome 2 and a CAD probe. (A) Typical examples of chromosomes from unselected cells tested with both probes. The chromosomes from HT-1080, MDAH041, and LIM-1215 appeared to be identical, and only the example from HT-1080 cells is shown. (B) Chromosomes from PALA-resistant cells tested with the CAD probe. Two examples from each cell line are shown. CAD is amplified on the rearranged copies of chromosome 2.
Our results with MDAH041 cells differ from those of Livingstone et al. (8) and Yin et al. (7), who found CAD gene amplification to be the predominant or exclusive mode of PALA resistance. Both groups used the “frequency” method of selecting PALA-resistant cells, which includes analysis of resistant cells that preexist in the unselected population, so we explored the possibility that the difference might be due to our use of “rate” selections, which exclude preexisting resistant cells. However, we did not observe any significant difference in the distribution of resistance mechanisms in MDAH041 or HT-1080 cells selected in the two modes (Table 1).
Further Analysis of PALA-Resistant HT-1080 Clones.
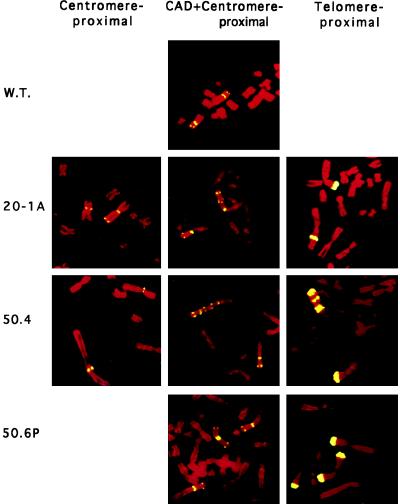
Twenty HT-1080 colonies obtained in rate–mode selections were analyzed to determine the basis of their PALA resistance (Table 2). As noted above, the PALA-resistant cells present in steady state are similar to those that arise during a short period of growth in the rate experiment. Also, some of the events leading to PALA resistance are induced by PALA in both rate and frequency experiments (31, 32). The ladder-like structure in two PALA-resistant HT-1080 clones was analyzed in more detail using a centromere-proximal probe, CAD, and a telomere-proximal probe (Fig. 2). These probes give considerable insight into the events that may have generated the amplified structures. Clone 20–1A may have arisen when the 2p arms of sister chromatids fused at or near the telomeres, followed by a highly asymmetric break between the locus marked by the pericentric probe and the centromere. These events can account for the observed marker chromosome, in which both CAD and the telomere-proximal locus are found only near the center of the modified p arm at or near the original site of fusion (Fig. 2 Center and Right), with the pericentric locus both in its normal position and also near the new chromosome end (Fig. 2 Left), which has probably acquired a new telomere. Note that the interstitial signal with the telomere-proximal probe on the marker chromosome is about half as intense as the signal on the normal copy of 2p (Fig. 2 Right), suggesting that fusion may have been accompanied by deletion of much of the telomere-proximal sequence and probably occurred within that sequence.
Table 2.
Different types of events responsible for PALA resistance in HT-1080 cells
| Status of chromosome 2 | PALAr clones, n |
|---|---|
| Two copies only | 3 |
| Extra copies | 3 |
| Isochromosome 2 | 3 |
| Amplification ladder on chromosome 2 | 1 |
| Mixture of (1) and (2) | 5 |
| Mixture of (1), (2), and (3) | 5 |
Resistant colonies were selected using 20, 30, 40, 50, or 60 μM PALA, and independent, newly formed colonies were analyzed by FISH (see Figs. 1 and 2 for examples). We did not observe a significant difference in the distribution of events responsible for PALA resistance in the clones selected with different concentrations of PALA. PALAr, PALA-resistant.
Figure 2.
FISH analysis of wild-type HT-1080 cells and PALA-resistant clones with probes for centromere-proximal, CAD, and telomere-proximal sequences. W.T., wild type.
In the amplified array of clone 50.4 (obtained in a rate–mode selection), the pericentric sequence was retained only in its normal position on the marker 2p arm (Fig. 2 Left, bottom chromosome), indicating that breakage probably occurred between this sequence and the telomere. Again, the telomere-proximal sequence is found interstitially, in multiple copies interspersed with multiple copies of CAD (Fig. 2 Right). This structure may have arisen through an initial event that involved fusion in the region of the telomere-proximal locus, followed by additional bridge–breakage–fusion cycles to yield the complex structures observed. See Stark (6) and Toledo et al. (4) for a more detailed discussion and for diagrams of similar events.
Analysis with the same probes of clone 50–6P, which contains an isochromosome 2p, made it clear that this isochromosome still retains a centromere, presumably comprised of two copies of the 2p-proximal part of the original centromere (Fig. 2 Center). Note that, in Fig. 2, the two copies of the pericentric probe are separate. On the right, there is (bottom to top) an isolated 2p arm, a normal chromosome 2, and a 2p isochromosome. Several clones with isochromosome 2p, maintained in the original concentration of PALA for 6 more weeks, were analyzed by FISH. No change was observed, demonstrating that these structures are stable. Clones with no extra copies of CAD were indistinguishable from PALA-sensitive, wild-type HT-1080 cells when analyzed with all three probes.
A careful quantitative analysis of gene copy numbers in PALA-resistant clones without CAD gene amplification was carried out by comparing the relative copy numbers of restriction fragments from within the CAD gene and from elsewhere in the genome (Table 3). Clones 30–9A and 40–2A showed no evidence of CAD gene amplification in this assay compared with the clone 50.4 control and were in agreement with the results of FISH analysis. Therefore, FISH is a reliable method of analyzing CAD gene copy numbers in these cells. The number of CAD signals in interphase nuclei correlated well with the number observed in metaphase spreads in the same clonal populations (data not shown). DNA is not likely to be lost from interphase cells, so these observations argue against failure to observe extrachromosomal, amplified CAD genes for technical reasons.
Table 3.
CAD copy number, specific aspartate transcarbamylase activity, and levels of PALA resistance in HT-1080 clones
| Cells | Amplification mode, FISH | Relative copy no., Southern | Relative ATCase activity* | PALA resistance, ID50, μM |
|---|---|---|---|---|
| Wild type | None | 1.0 | 1.0 | 10 |
| 30-9A | None | 1.0 | 1.6 | NA |
| 40-2A | None | 1.1 | 1.7 | 75 |
| 40-6P | None | NA | NA | 60 |
| 50.4 | Ladder | 2.6 | 3.9 | 75 |
| 50-4P | Isochromosome | NA | NA | 50 |
| 20-1A | Ladder | NA | 2.1 | NA |
| 50-6P | Isochromosome | NA | 2.2 | 75 |
| 60-1B | Isochromosome | NA | NA | 100 |
NA, not analyzed; ATCase, aspartate transcarbamylase.
The value for 30-9A is an average of duplicate assays (1.5 and 1.7); the values for 40-2A (1.7, 2.1, and 1.2) and 50.4 (3.3, 6.0, and 2.4) are averages of triplicate assays; 20-1A and 50-6P were assayed only once.
All PALA-resistant clones assayed had more aspartate transcarbamylase activity than wild-type HT-1080 cells (Table 3). This increase was large enough to account for the levels of resistance observed (Table 3; see Discussion), and, therefore, there was no suggestion that PALA-resistant HT-1080 cells are altered in PALA transport. Because the relative increases in enzyme activity were small, it would be very difficult to be certain of a corresponding difference in the levels of the CAD protein or mRNA although we think that increased CAD gene expression is likely to account for the increased enzyme activity and resistance to PALA.
Second-Step Selection of PALA-Resistant Clones.
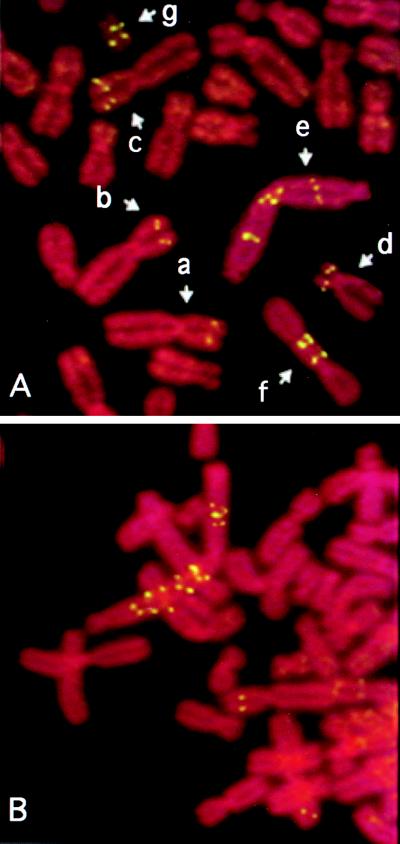
Can amplification be forced by a second step of selection in first-step clones that have no CAD gene amplification? None of the second-step selections gave PALA-resistant clones in which extension of the short arm of chromosome 2 was observed, in striking contrast to results with Syrian hamster cells (2). The concentrations of PALA used were 160–220 μM (≈3 × ID50 for the first-step clones). Clone 20–2B, in which about 5% of cells had ladders initially, exclusively gave second-step colonies in which all of the resistant cells had similar ladders present on a marker chromosome that was not chromosome 2 (data not shown). Second-step clones from 30–9A all had a rearranged marker carrying two copies of CAD on each short arm (Fig. 3A). Most second-step colonies from clone 40–2A had two copies of chromosome 2 and no marker with amplification of CAD. One did have a marker with amplified CAD genes, again not an extension of the normal chromosome 2 (Fig. 3B).
Figure 3.
FISH analysis of cells resistant to a second step of PALA selection. (A) Examples of chromosomes in the second-step clone 30–9A/5. Chromosomes a–c were present in the first step population of 30–9A cells. c is a marker chromosome 2 with two copies of CAD detected in many second-step clones. This chromosome may have been present at low abundance in the first step population, followed by selection in the second step. d–g are rearranged chromosomes with amplified CAD, found only in clone 30–9A/5. Chromosomes e and f probably result from the fusion and breakage of chromosome c. (B) Chromosomal CAD gene amplification in second-step clone 40–2A/4.
Normal Human Fibroblasts Do Not Give Rise to PALA-Resistant Colonies.
PALA-resistant clones are not obtained from normal senescent human cells (9, 10). Normal cells arrest stably and reversibly when exposed to PALA (7, 8) and are strongly contact-inhibited, so failure to observe colonies might be due to a lack of space for colonies of PALA-resistant cells to grow. To test this, we exposed normal human fibroblasts at a low density to repeated cycles of PALA selection and replating (see Materials and Methods). BrdUrd-positive cells were observed in the initial population at a low frequency (≈10−4), which did not increase as the cells were cycled through PALA selections. Therefore, there was no enrichment for PALA-resistant normal human fibroblasts, so such cells either were not formed or did not grow.
DISCUSSION
No defects in PALA transport have been reported; therefore, in mammalian cells, the only known mechanisms of PALA resistance lead to an increase in the activity of aspartate transcarbamylase, the target enzyme. We observed that the relative number of CAD genes per human cell can increase in several different ways. Amplification of the chromosome arm that carries the CAD gene, the mechanism predominant in rodent cells, was seen only rarely in three of the five human cell lines we examined. CAD gene amplification (as chromosomal ladders) always involves rearranged chromosomes carrying CAD, when these are present initially. Another mechanism involves the centromere of chromosome 2, which appears to be fragile, leading to the formation of isochromosome 2p and free 2p arms. PALA resistance through an increase in the number of copies of chromosome 2 is seen convincingly only in LIM-1215 cells, where it predominates. LIM-1215 cells may have lost control over the normal disjunction of chromosome 2 during mitosis, or endoreduplication of chromosome 2 may occur more often in LIM-1215 cells than in the other cell lines we have studied.
It is not obvious that a relatively small increase in CAD should result in a large increase in resistance to PALA, but this is indeed the case. In HT-1080 cells, 5- to 10-fold increases in PALA resistance accompany 2-fold or smaller increases in CAD-specific activity or copy number (Table 3). In Syrian hamster cells, we noted that doubling the number of CAD genes per cell leads to a ≈20-fold increase in resistance to PALA (33, 34). The lack of correlation between an increase in CAD expression and PALA resistance is likely to result from the fact that PALA inhibits the second, but not the first, step of UMP biosynthesis. Overproduction of CAD, a trifunctional enzyme, leads not only to more aspartate transcarbamylase (which binds more PALA) but also to more carbamyl-P synthetase and thus to more carbamyl-P, which is competitive with PALA (35).
To analyze those PALA-resistant clones in which there was no increase in CAD gene copy number (as shown by internally controlled quantitative Southern analysis and by FISH), we chose aspartate transcarbamylase activity assays rather than quantitative analyses of CAD mRNA or protein, which are not precise enough to detect a 50% increase per cell. More enzyme activity is present in PALA-resistant cells that show no evidence of CAD gene amplification than in unselected parental cells (Table 3). Schaefer et al. (11) and Sharma and Schimke (36) also observed that human cells can achieve PALA resistance without CAD gene amplification but concluded, in contrast to our results, that increased expression of CAD was not responsible. The resistant cells studied in these two laboratories might harbor small increases in aspartate transcarbamylase sufficient to account for the increases in PALA resistance but not to be detected by the assays used.
We do not know the basis of increased expression of CAD in those PALA-resistant cells with no increase in gene copy number. However, alteration in regulation of CAD gene expression mediated by a change in DNA methylation is an attractive possibility. Many genes in mammalian cell lines are silenced by de novo methylation of CpG islands in regions that are not methylated in normal cells (37). One copy of CAD may be silent in those cell lines that can achieve increased expression without an increase in CAD gene copy number, and activation may occur at a low frequency either spontaneously or through the action of PALA.
The predominant mechanism of PALA resistance varies widely among different cell lines. Even though they are pseudodiploid, LIM-1215 cells readily increase the relative copy number of chromosome 2, both in straightforward selections and in response to an induction protocol. For unknown reasons, this pathway is not used by the other cell lines studied. Amplification of CAD as chromosomal ladders is observed often in Saos-2 and U-2 OS cells, but the amplification takes place only on rearranged chromosomes carrying CAD. The rearrangements may either predispose the altered chromosomes toward amplification or remove a constraint that prevents the CAD gene from being amplified at its normal location on chromosome 2p. In HT-1080 or LIM-1215 cells, second-step selection of first-step, PALA-resistant clones that had not achieved resistance through amplification of the p arm of chromosome 2 still did not yield resistant cells by this mechanism. When amplification was observed in a second step (from clone 30–9A), the extra copies of CAD were present on a rearranged marker chromosome and not on a chromosome formed by extension of the 2p arm.
In many PALA-resistant cells, formation of isochromosome 2p accounts for resistance, a mechanism also observed by Schaefer et al. (11). An important clue about how the 2p isochromosome might arise is provided by the occasional observation of isolated 2p arms (Fig. 2, 50–6P, Right, top chromosome). The centromere of chromosome 2 probably is induced to break by treatment with PALA. A 2p chromosome arm so formed could segregate normally if it retained centromere function, and the broken ends of the replicated sister chromatids would be likely to fuse, producing the observed isochromosome 2p, which would then be stable in the absence of further treatment, as observed. PALA has not been studied as an inducer of fragile sites, but other well characterized inducers, including methotrexate, aphidicolin, and BrdUrd, have potent effects on DNA synthesis or DNA stability (38).
Normal human cells do not give rise to PALA-resistant colonies (refs. 9 and 10; data shown above). How do they regulate so many diverse mechanisms? p53-mediated pathways play a vital role in this regulation (7, 8), so an important aspect is the response of normal cells to broken DNA, which can trigger p53-dependent permanent cell cycle arrest (39) or apoptosis (40, 41). Formation of chromosomal arrays of amplified CAD genes is likely to involve bridge–breakage–fusion cycles, which can trigger the p53-dependent normal response to DNA damage (3, 4, 42). Also, if the initial step in isochromosome 2p formation involves cleavage at a fragile site in the centromere of chromosome 2, this event will also trigger the arrest or death of normal cells. LIM-1215 cells have overcome whatever constraint is used by normal cells and the other cell lines studied to prevent an increase in the relative copy number of chromosome 2, but it is not yet clear how maintenance of the normal complement of chromosomes is regulated in a p53-dependent manner. Finally, we do not yet understand enough about the basis of increased CAD expression without any increase in copy number to speculate about how a p53-dependent mechanism might be involved.
Acknowledgments
We appreciate greatly the essential reagents provided by Hans Zachau and Anna–Maria Frischauf. This work was supported by Grants GM-49345 (G.R.S.) and GM-47644 (J.N.D.) from the National Institutes of Health.
ABBREVIATIONS
- PALA
N-phosphonacetyl-l-aspartate
- CAD
carbamyl-P synthetase/aspartate transcarbamylase/dihydro-orotase
- FISH
fluorescence in situ hybridization
References
- 1.Trask B J, Hamlin J L. Genes Dev. 1989;3:1913–1925. doi: 10.1101/gad.3.12a.1913. [DOI] [PubMed] [Google Scholar]
- 2.Smith K A, Gorman P A, Stark M B, Groves R P, Stark G R. Cell. 1990;63:1219–1227. doi: 10.1016/0092-8674(90)90417-d. [DOI] [PubMed] [Google Scholar]
- 3.Toledo F, Le Roscouet D, Buttin G, Debatisse M. EMBO J. 1992;11:2665–2673. doi: 10.1002/j.1460-2075.1992.tb05332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toledo F, Buttin G, Debatisse M. Curr Biol. 1993;3:255–264. doi: 10.1016/0960-9822(93)90175-n. [DOI] [PubMed] [Google Scholar]
- 5.Smith K A, Stark M B, Gorman P A, Stark G R. Proc Natl Acad Sci USA. 1992;89:5427–5431. doi: 10.1073/pnas.89.12.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stark G R. Adv Cancer Res. 1993;61:87–113. doi: 10.1016/s0065-230x(08)60956-2. [DOI] [PubMed] [Google Scholar]
- 7.Yin Y, Tainsky M A, Bischoff F Z, Strong L C, Wahl G M. Cell. 1992;70:937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- 8.Livingstone L R, White A, Sprouse J, Livanos E, Jacks T, Tlsty T D. Cell. 1992;70:923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- 9.Wright J A, Smith H S, Watt F M, Hancock M C, Hudson D L, Stark G R. Proc Natl Acad Sci USA. 1990;87:1791–1795. doi: 10.1073/pnas.87.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tlsty T D. Proc Natl Acad Sci USA. 1990;87:3132–3136. doi: 10.1073/pnas.87.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaefer D I, Livanos E M, White A E, Tlsty T D. Cancer Res. 1993;53:4946–4951. [PubMed] [Google Scholar]
- 12.White A E, Livanos E M, Tlsty T D. Genes Dev. 1994;8:666–677. doi: 10.1101/gad.8.6.666. [DOI] [PubMed] [Google Scholar]
- 13.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 14.Brown R, Marshall C J, Penne S G, Hall A. EMBO J. 1984;3:1321–1326. doi: 10.1002/j.1460-2075.1984.tb01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischoff F, Yim S O, Pathak S, Grant G, Siciliano M J, Giovanella B C, Strong L C, Tainsky M A. Cancer Res. 1990;50:7979–7984. [PubMed] [Google Scholar]
- 16.Whitehead R H, Macrae F A, St. John D J, Ma J. J Natl Cancer Inst. 1985;74:759–765. [PubMed] [Google Scholar]
- 17.Perry M E, Rolfe M, McIntyre P, Commane M, Stark G R. Mutat Res. 1992;276:189–197. doi: 10.1016/0165-1110(92)90008-w. [DOI] [PubMed] [Google Scholar]
- 18.Luria S E, Delbruck M. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal M L, Agarwal A, Taylor W R, Stark G R. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao G N, Davidson J N. DNA. 1988;7:423–432. doi: 10.1089/dna.1.1988.7.423. [DOI] [PubMed] [Google Scholar]
- 21.Shoaf W F, Jones M E. Biochemistry. 1973;12:4039–4051. doi: 10.1021/bi00745a004. [DOI] [PubMed] [Google Scholar]
- 22.Patterson D, Carnright D V. Somat Cell Genet. 1977;3:483–495. doi: 10.1007/BF01539120. [DOI] [PubMed] [Google Scholar]
- 23.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Ehrich E, Craig A, Poustka A, Frischauf A M, Lehrach H. Gene. 1987;57:229–237. doi: 10.1016/0378-1119(87)90126-0. [DOI] [PubMed] [Google Scholar]
- 25.Shigesada K, Stark G R, Maley J A, Niswander L A, Davidson J N. Mol Cell Biol. 1985;5:1735–1742. doi: 10.1128/mcb.5.7.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 27.Meltzer P S, Guan X, Burgess A, Trent J M. Nat Genet. 1992;1:24–28. doi: 10.1038/ng0492-24. [DOI] [PubMed] [Google Scholar]
- 28.Zimmer F J, Hameister H, Schek H, Zachau H G. EMBO J. 1990;9:1535–1542. doi: 10.1002/j.1460-2075.1990.tb08272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinkel D, Landegent J, Collins C, Fuscoe J, Segraves R, Lucas J, Gray J. Proc Natl Acad Sci USA. 1988;85:9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K C, Vannais D B, Jones C, Patterson D, Davidson J N. Hum Genet. 1989;82:40–44. doi: 10.1007/BF00288269. [DOI] [PubMed] [Google Scholar]
- 31.Almasan A, Linke S P, Paulson T G, Huang L, Wahl G M. Cancer Metastasis Rev. 1995;14:59–73. doi: 10.1007/BF00690212. [DOI] [PubMed] [Google Scholar]
- 32.Poupon M-F, Smith K A, Chernova O B, Gilbert C, Stark G R. Mol Biol Cell. 1996;7:345–354. doi: 10.1091/mbc.7.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempe T D, Swyryd E A, Bruist M, Stark G R. Cell. 1976;9:541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- 34.Zieg J, Clayton C E, Ardeshir F, Giulotto E, Swyryd E A, Stark G R. Mol Cell Biol. 1983;3:2089–2098. doi: 10.1128/mcb.3.11.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins K D, Stark G R. J Biol Chem. 1971;246:6599–6605. [PubMed] [Google Scholar]
- 36.Sharma R C, Schimke R T. Mutat Res. 1994;304:243–260. doi: 10.1016/0027-5107(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 37.Antequera F, Boyes J, Bird A. Cell. 1990;62:503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland G R, Richards R I. Curr Opin Genet Dev. 1995;5:323–327. doi: 10.1016/0959-437x(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 39.Di Leonardo A, Linke S P, Clarkin K, Wahl G M. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 40.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. Nature (London) 1993;362:847–852. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 41.Merritt A J, Potten C S, Kemp C J, Hickman J A, Balmain A, Lane D P, Hall P A. Cancer Res. 1994;54:614–617. [PubMed] [Google Scholar]
- 42.Ishizaka Y, Chernov C M, Burns C M, Stark G R. Proc Natl Acad Sci USA. 1995;92:3224–3228. doi: 10.1073/pnas.92.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]